Abstract
Apoptosis has been suggested as a mechanism by which dengue (DEN) virus infection may cause neuronal cell death (P. Desprès, M. Flamand, P.-E. Ceccaldi, and V. Deubel, J. Virol. 70:4090–4096, 1996). In this study, we investigated whether apoptotic cell death occurred in the central nervous system (CNS) of neonatal mice inoculated intracerebrally with DEN virus. We showed that serial passage of a wild-type human isolate of DEN virus in mouse brains selected highly neurovirulent variants which replicated more efficiently in the CNS. Infection of newborn mice with these neurovirulent variants produced fatal encephalitis within 10 days after inoculation. Virus-induced cell death and oligonucleosomal DNA fragmentation were observed in mouse brain tissue by day 9. Infected mouse brain tissue was assayed for apoptosis by in situ terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and for virus replication by immunostaining of viral antigens and in situ hybridization. Apoptotic cell death and DEN virus replication were restricted to the neurons of the cortical and hippocampal regions. Thus, DEN virus-induced apoptosis in the CNS was a direct result of virus infection. In the murine neuronal cell line Neuro 2a, neuroadapted DEN virus variants showed infection patterns similar to those of the parental strain. However, DEN virus-induced apoptosis in these cells was more pronounced after infection with the neurovirulent variants than after infection with the parental strain.
Dengue (DEN) virus, a member of the Flavivirus genus (family Flaviviridae) is a mosquito-borne virus of which there are four serotypes (DEN-1, -2, -3, and -4) which cause severe disease in tropical and subtropical regions (12). DEN virus produces a spectrum of illness in humans, ranging from a flu-like disease (DEN fever) to hemorrhagic fever, a fulminating illness which can progress to DEN shock syndrome and death (12). The pathogenesis of severe DEN disease remains poorly understood. Virus-induced cell death in cases of infection of vital tissues may be a crucial event leading to host morbidity and mortality. The nature of host cell injury and pathological response to DEN virus infection in vivo remains unknown. Apoptotic cell death has been implicated as a mechanism for cytopathology in response to DEN virus infection in vitro (7, 25). Apoptosis is an active process of cell destruction. Apoptotic cells display characteristic morphological and biochemical features, including cell shrinkage, formation of apoptotic bodies, condensation of chromatin, nuclear fragmentation, and extensive internucleosomal DNA fragmentation. Necrosis is accompanied by inflammation, whereas apoptosis is not. In this study, we have investigated the possible involvement of apoptotic cell death in the pathophysiological response to DEN virus infection in intracerebrally inoculated newborn mice. DEN virus infection of newborn mice has been used as a model system for the characterization of viral factors involved in pathogenesis (2, 6, 15, 19, 21, 31). Neurons are the target cells in the central nervous system (CNS) of susceptible newborn mice, and infected mice succumb shortly after neuropathological changes become visible in the CNS (1, 7, 15, 16, 35). Thus, virus-induced neuronal death in the mouse CNS may be detrimental to the host. Neuronal damage and neuronal loss in the CNS may involve apoptosis or necrosis (28).
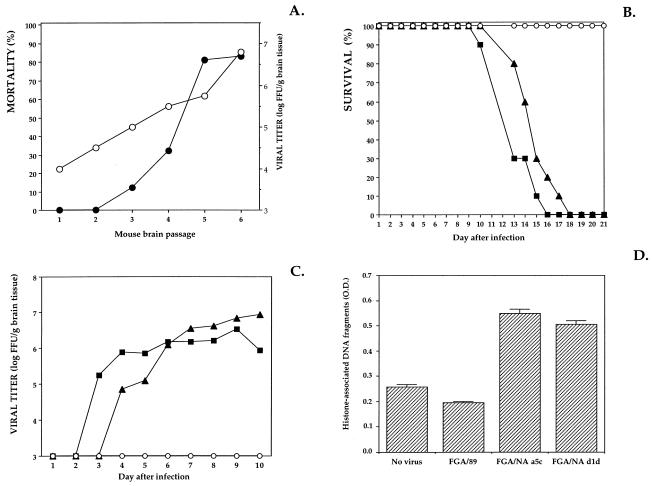
Newborn mice are fairly insensitive to intracerebral (i.c.) inoculation with nonneuroadapted mouse DEN virus strains. The process of neuroadaptation is critical to the emergence of DEN virus variants with increased neurovirulence in newborn mice (5, 15, 19, 21, 35). Repeated passages of DEN virus in mouse brain may allow selection of highly neurovirulent mutants (21, 35), so we first adapted the human isolate of DEN-1 virus strain FGA/89 by serial passage in the mouse CNS. The derivation and characterization of the DEN-1 virus strain FGA/89, isolated from human viremic serum, have been described previously (6, 7). Production of DEN virus on the mosquito cell line AP61, purification, and virus titration by focus immunodetection assay (FIA) were performed as previously described (6). Virus titers are expressed in focus-forming units (FFU) on AP61 cells per milliliter of inoculum. Mouse-passaged variants of DEN virus were amplified by serial i.c. passages of FGA/89 in newborn mice. Because the neuroadaptation was carried out in immunologically immature mice, antibody-induced selection pressure was not involved in the evolution of neurovirulent DEN virus phenotypes. Two-day-old Swiss mice were inoculated i.c. with 10−2 i.c. 50% lethal doses (LD50s) of FGA/89 (i.c. LD50 = 107 FFU). Eight days after i.c. inoculation, brains from three suckling mice were collected and homogenized, and the 10% tissue suspension obtained represented the first passage of FGA/89, FGA/NA-P1. The remaining mice were observed for 13 more days, and deaths were recorded (Fig. 1A). To quantify the amount of new infectious DEN virus produced in mouse brain, FGA/NA-P1 was titrated by FIA (Fig. 1A). The virus was amplified in the AP61 cell line for 10 days before a second passage to avoid the effects of factors such as cytokines, which may be present in crude tissue suspensions. The virus was then titrated, and a dose of 105 FFU was used to inoculate i.c. mice. Brain tissue suspensions were prepared 8 days after infection. The experimental procedure was repeated until passage six, with a fixed virus dose of 105 FFU. At passage 6, the rate of mortality stabilized at 80%, and the amount of virus in the brain peaked at 107 FFU/g (Fig. 1A). It appeared therefore that neurovirulent variants had been generated during the adaptation of FGA/89 to growth in newborn mouse brains, which may result from a quasispecies phenomenon (4, 21). However, neuroadaptation was correlated with greater efficiency of viral replication in neural tissues. To select mouse-neurovirulent DEN virus variants of FGA/89, FGA/NA-P6 was amplified in AP61 cells, and the resulting virus (i.c. LD50 = 102.5 FFU) was cloned. Monolayers of AP61 cells grown in 24-well tissue culture plates were incubated with 1 FFU of mouse-passaged DEN virus per well for 10 days. After two rounds of selection, several variant clones were evaluated for virulence by i.c. inoculation of newborn mice. Two FGA/89 virus variants, FGA/NA a5c and FGA/NA d1d, had i.c. LD50s of 102 and 102.5 FFU, respectively. Thus, both mouse-passaged DEN-1 viruses were 10,000 times more neurovirulent for mice than their parental strain, FGA/89. The neurovirulence of FGA/NA a5c and FGA/NA d1d was essentially restricted to newborn mice. The age dependence of DEN virus neurovirulence may be correlated with restriction of virus propagation in the CNS. This may be attributed to developmentally regulated host cell factors affecting virus replication (20, 23, 29, 30). However, both mouse-neurovirulent DEN virus variants were essentially nonneuroinvasive in 2-day-old mice inoculated by the peripheral route (intraperitoneal LD50 of >106 FFU).
FIG. 1.
Replication of DEN virus in mouse brain. Litters of 2-day-old Swiss mice (Breeding Centre R. Janvier, Le Genest St-Isle, France) were inoculated i.c. with 20 μl of DEN virus in Leibovitz L-15 growth medium containing 2% heat-inactivated fetal bovine serum. (A) Mice were inoculated with 105 FFU of mouse-passaged DEN-1 virus (strain FGA/89) and observed daily for 21 days, and mortality was recorded (•). Eight days after inoculation, brains from three suckling mice were collected and weighed. Brain tissues were prepared as 10% (wt/vol) suspensions, and their infectivity was titrated by FIA (○). Titers are expressed as FFU per gram of brain tissue. (B and C) Newborn mice were inoculated with 5,000 FFU of FGA/89 (○), FGA/NA a5c (▪), or FGA/NA d1d (▴). For panel B, infected mice were observed daily for 21 days and mortality was recorded. For panel C, viral growth in the brains of infected mice was titrated. Each point represents the titration of pooled brain tissues extracted from three DEN virus-infected mice. (D) Oligonucleosomal DNA fragments in brain tissue suspensions were quantified by ELISA. Mouse brains were harvested in triplicate 9 days after infection. Brain tissue suspensions (20 mg) were incubated with lysis buffer, and the histone-associated DNA fragments released into the cytoplasmic fractions were quantified with a cell death detection ELISA kit according to the manufacturer’s protocol (Boehringer Mannheim Biochemicals). Optical density (O.D.) was measured at 405 nm.
A time course study of the viral growth in mice inoculated i.c. was carried out. Two-day-old mice were inoculated with 5,000 FFU of virus to determine the course of DEN virus infection in the CNS (Fig. 1B). Mice inoculated with FGA/89 were observed for a total of 21 days and showed no neurological symptoms. FGA/NA a5c- and FGA/NA d1d-infected mice developed clinically apparent encephalitis 10 days after infection and did not survive for more than 18 days (Fig. 1B). The mean day of death was not significantly different between infection with FGA/NA d1d (mean ± standard deviation, 14.8 ± 1.3 days) and infection with FGA/NA a5c (13.1 ± 1.3 days). The spread of the virus in brain tissues was assessed by virus titration at daily intervals (Fig. 1C). Infectious FGA/89 virus was not detected in the CNS of newborn mice at any time after inoculation (detection threshold, 103 FFU/g of brain tissue). Analysis of the growth of the DEN virus variants in mouse brain showed new virus formation within 3 days of infection with FGA/NA a5c and within 4 days of infection with FGA/NA d1d (Fig. 1C). There was transient peripheral viremia 8 days after infection (<105 FFU/ml). By day 6 of infection, the amounts of FGA/NA a5c in the brain reached a plateau at approximately 106 FFU/g, whereas FGA/NA d1d continued to increase, reaching 107 FFU/g by day 10. These results indicate that the mouse-neurovirulent DEN virus variants replicate differently from the parental strain in the CNS of newborn mice.
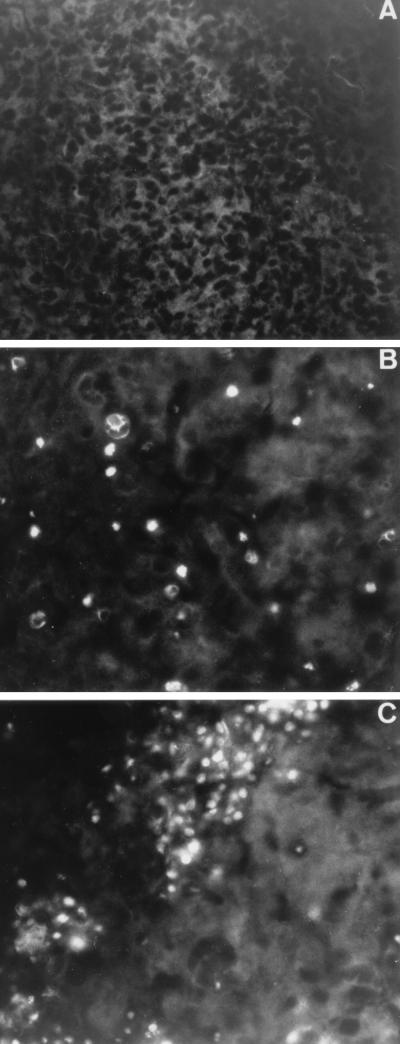
To assess whether apoptotic cell death occurred in vivo, fragmented DNA was examined with brain tissue suspensions collected daily between 5 and 10 days after infection. Oligonucleosome-sized DNA fragments were detected in mouse brains up to 8 days after infection with mouse-neurovirulent DEN virus variants (data not shown). The release of histone-associated cytoplasmic DNA generated by internucleosomal degradation of genomic DNA was demonstrated 9 days after infection by immunoassay (Fig. 1D). This result suggests that DEN virus infection in the CNS was associated with cell damage characterized by apoptotic DNA degradation, before mice showed clinical signs of encephalitis. By day 9 of infection, DEN virus-infected mouse brains were tested in situ by the nick end labeling of DNA. Apoptotic DNA fragmentation was assessed in brain cryostat sections by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method. TUNEL histochemical analysis was performed with sequential sections from the striatum to the cerebellum, and TUNEL-positive cells were detected by fluorescence (Fig. 2). No TUNEL-positive cells were detected in brain sections of mice inoculated with FGA/89. TUNEL-positive cells in brain sections infected with FGA/NA a5c or FGA/NA d1d were not distributed randomly throughout the CNS, but were clustered in the cortical and hippocampal regions. The nuclei of TUNEL-positive cells in the cortex showed the morphological features characteristic of apoptosis, such as nuclear condensation and fragmentation. The pattern of DNA degradation was clearly more complex in the hippocampal region, where TUNEL-positive cells showed an irregular pattern of chromatin condensation. This variability probably reflects a range of DNA degradation (34). There were only a few TUNEL-positive cells in the thalamus and striatum (data not shown). By day 9, histopathological investigation of cortical and hippocampal regions showed minimal perivascular cuffings without visible tissue damage or inflammation (data not shown). The lack of a significant early inflammatory response is characteristic of the apoptotic process in infected tissues. Sections with TUNEL-positive cells were tested for the presence of viral antigens by immunodetection with DEN anti-E monoclonal antibody (MAb) 8C2 (data not shown) (6, 7). There was a correlation between the distribution of TUNEL-stained cells and DEN virus antigen-positive cells (Table 1), which was determined as follows.
FIG. 2.
Distribution of apoptosis in the brain regions of DEN virus-infected mice. Mouse brains were harvested 9 days after inoculation. For mouse brain sections, the brains were removed, covered in Tissue-Tek O.C.T. (Miles) embedding medium, and stored at −80°C. Parasagittal sections of the brain blocks (15-μm thickness) were cut on a cryostat (Jung Frigocut) and mounted onto Vectabond-precoated slides (Vector Laboratories). Sections were fixed in 3% paraformaldehyde in phosphate-buffered saline and stored at 4°C in 70% ethanol. Tissue sections mock infected (A) or infected with DEN virus (FGA/NA d1d) (B and C) were processed for TUNEL analysis. The TUNEL assay was performed with mouse brain sections as described in the instructions to an in situ cell death detection kit from Boehringer Mannheim Biochemicals. TUNEL-positive cells were observed by fluorescence. Cortical (A and B) and hippocampal (C) regions are shown. Magnification, ca. ×100.
TABLE 1.
Distribution of viral antigens and apoptosis within infected mouse brain sections
| Virus | Result for:
|
|||
|---|---|---|---|---|
| Cerebral cortex
|
Hippocampus
|
|||
| IFAa | TUNELb | IFA | TUNEL | |
| FGA/NA a5c | 1+c | 1+ | 1+ | 2+ |
| FGA/NA d1d | 2+ | 1+ | 2+ | 3+ |
| FGA/89 | 0 | 0 | 0 | 0 |
DEN virus-infected cells were detected by IFA with anti-E MAb 8C2 and graded as 0 (absent), 1+ (≤5 cells per section), 2+ (5 to 10 cells per section), and 3+ (>10 cells per section). Anti-poliovirus VP1 protein MAb C3 was used as a negative control.
Apoptosis was detected by TUNEL staining and graded as 0 (absent), 1+ (≤5 cells per section), 2+ (5 to 10 cells per section), and 3+ (>10 cells per section). The TUNEL reaction in the absence of terminal deoxynucleotidyl transferase enzyme was used as a negative control. Tissue sections pretreated with DNase I were used as a positive control.
Positive cells in 10 fields of three tissue sections were quantified.
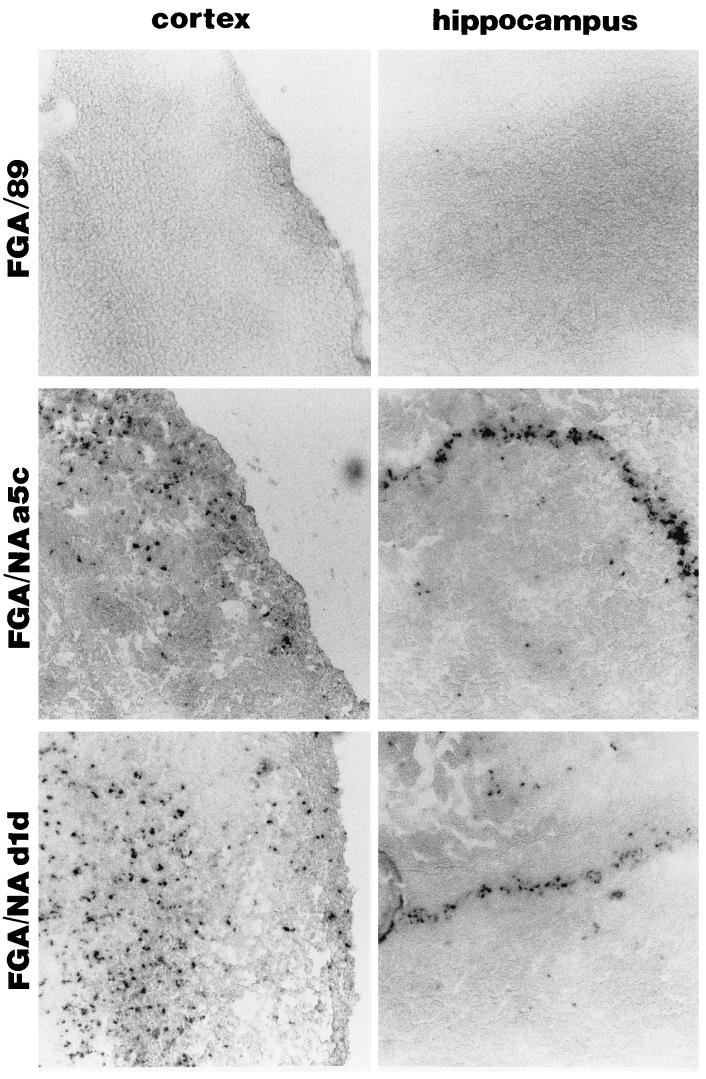
Brain tissue sections were assayed simultaneously for the presence of DEN antigens by indirect fluorescent-antibody assay (IFA) and for apoptosis by the TUNEL assay. Sections from mouse brains were treated with proteinase K to enhance antigenicity. The sections were incubated with a 1:100 dilution of anti-DEN virus protein E MAb 8C2 or a 1:300 dilution of anti-poliovirus VP1 protein MAb C3. The sections were incubated with a 1:20 dilution of biotin-conjugated goat anti-mouse serum (Sigma) and then with rhodamine isothiocyanate-conjugated streptavidin (10 μg/ml) (Boehringer Mannheim Biochemicals). The similarity of the distribution shown in Table 1 suggests that cells undergoing apoptosis were either the same cells infected with DEN virus or were located close to these cells. Only a small number of cells were double labeled by the TUNEL method in combination with IFA for DEN virus antigen (data not shown). TUNEL staining was stronger in the hippocampus than in the cerebral cortex infected with the two neurovirulent DEN virus variants (Table 1). The hippocampus is particularly vulnerable to apoptotic cell death (13). However, the number of TUNEL-positive cells was higher in the hippocampal region of mouse brains infected with FGA/NA d1d, and this correlated with a larger number of DEN virus-infected cells (Table 1). To more accurately determine the distribution of DEN virus in the cortical and hippocampal regions, RNA replication was investigated by in situ hybridization. This procedure was performed with a digoxigenin (DIG)-labeled antisense transcript complementary to the DEN virus genome. The DIG-labeled RNA hybrids were detected by enzyme-linked immunoassay (ELISA). Negative controls included mock-infected brain cryostat sections incubated with the DEN virus cRNA probe and sections of DEN virus-infected brains incubated with a DIG-labeled β-galactosidase cRNA probe (data not shown). Cortical and hippocampal regions tested positive for viral RNA of FGA/NA a5c and FGA/NA d1d, whereas no signal was observed in FGA/89-infected brain sections 9 days after infection (Fig. 3). The distribution of virus RNA-positive cells throughout the cerebral cortex and the CA1 and CA3 fields of the hippocampus suggests that cortical neurons and pyramidal neurons in the hippocampus could be the major target cells of DEN virus infection. This correlates with the neuronal cell specificity of DEN virus replication observed in mouse CNS (1, 5, 7, 16) and ex vivo (7, 16). Thus, apoptosis is one of the mechanisms of neuronal death triggered by viral replication in the cortical and hippocampal regions. Tissue injury in the CNS following DEN virus infection is likely associated with the ability of neurons in specific regions to undergo apoptotic cell death. Apoptosis was not significantly induced until 5 days after DEN virus production, suggesting it could be related to neuronal maturity. The extensive brain tissue injury may be a consequence of virus-triggered apoptosis in postmitotic neurons that cannot be regenerated (22–24, 29, 30). The loss of nonrenewable cells such as neurons may be especially critical in viral pathogenesis (24). Inhibition of apoptosis in the mature nervous system may be implicated in the protection of adult mice against fatal virus infection (9, 23). However, we cannot rule out the possibility that indirect mechanisms may also be involved in viral pathogenesis. The effects of stress hormones, toxic cytokines, reactive nitrogen species, or infiltrating mononuclear cells could account for CNS injury (38, 39).
FIG. 3.
Viral RNA detected by in situ hybridization in parasagittal sections of mouse brain harvested 9 days after inoculation. The DEN-1 cDNA, coding for proteins prM and E (residues 95 to 775 [6]) of FGA/NA d1d, was amplified by PCR and ligated into the mammalian expression vector pCI-neo (Promega) to generate the recombinant plasmid pCI/prM-E. Transcription with T3 RNA polymerase produced a DIG-labeled antisense transcript of 2,000 nucleotides complementary to the DEN virus genome. A negative control cRNA was generated by the same method with the β-galactosidase gene introduced into the plasmid pCI-neo (pCI/Lac-Z). To reduce the length of the DIG-labeled cRNA for in situ hybridization, the transcripts were treated by alkaline hydrolysis (26). In situ hybridization was performed as described previously (26). DIG-labeled RNA hybrids were detected by ELISA with an anti-DIG-alkaline phosphatase conjugate to catalyze a color reaction between X-phosphate solutions and nitroblue tetrazolium salt, according to the manufacturer’s instructions (Boehringer Mannheim Biochemicals). The tissue sections were incubated for 1 h in the dark and mounted on Vectabond-precoated slides (Vector Laboratories). Magnification, ca. ×64.
Previous studies have shown that mutations in the structural proteins may account for the increased virulence of neuroadapted flaviviruses (2, 10, 11, 19, 21, 31). This led us to determine the genetic origin of the differences between FGA/NA a5c, FGA/NA d1d, and the parental strain. Genomic RNA extracted from purified DEN virus was reverse transcribed and amplified by PCR. The nucleotide sequences of purified PCR products were determined with the Thermo Sequenase kit (Amersham) with a set of oligonucleotide primers (6). Complete sequencing of the 5′ noncoding regions and the C, prM, and E protein-coding genes of FGA/NA a5c and FGA/NA d1d showed that substitutions were essentially limited to the E protein. There were two substitutions in the E protein (i.e., Met196-Val and Thr276-Pro) of FGA/NA a5c and three substitutions in the E protein (i.e., Met196-Val, Val365-Ile, and Thr405-Ile) of FGA/NA d1d (the amino acid residues in protein E are numbered as referenced for DEN-1 virus [6]). The FGA/NA a5c RNA genome was not stabilized. The missense nucleotide which leads to the amino acid substitution Thr405-Ile was also detected in FGA/NA a5c. The heterogeneity of FGA/NA a5c RNA genome probably influences growth by limiting the spread of the virus in the CNS. Amino acid changes were found in the hinge region of the dimerization domain II (residues 196 and 276) and domain III (residue 365) (33, 37). The substitution at position 405 maps to a predicted amphipathic α-helix next to the membrane anchor stem region (10, 33, 37). The amino acid change in position 196 has already been described in other DEN-1 virus strains, and the substitutions at positions 276, 365, and 405 were identified in mouse-passaged or neutralization escape mutant flaviviruses (3, 14, 21, 27). Specific substitutions in protein E during tissue-specific adaptation may be sufficient to enable the human isolate of DEN virus to increase its growth in target tissues and its virulence potential. For example, changes in the envelope glycoprotein of the alphavirus Sindbis virus affect the speed of virus penetration, replication, maturation, and ability of the virus to induce tissue damage in mouse CNS (8, 22, 24, 40). The substitutions identified in protein E may change the binding affinity of the virus to receptors on neuronal cells or affect the entry of virus particles by altering the fusion-regulating structural change in the E protein (11, 18, 27). We examined whether DEN virus strains differed in their capacity to infect the murine neuroblastoma cell line Neuro 2a (7). Inoculation with 200 FFU per cell was required to infect 50% of Neuro 2a cell monolayers with FGA/NA a5c or FGA/NA d1d, suggesting that the infectivity of neuroadapted DEN virus variants was similar in a neuronal cell line to that of FGA/89 (7). Thus, substitutions in protein E of FGA/NA a5c or FGA/NA d1d did not affect the efficiency of infection of neuronal cells in vitro.
We examined whether DEN virus strains differed in their capacity to induce apoptosis in Neuro 2a cells (7). Forty hours after infection, DNA of Neuro 2a cells infected with mouse-neurovirulent DEN virus variants showed the typical internucleosome-sized DNA fragment pattern seen with apoptotic DNA degradation (Fig. 4A). Cells were infected at a multiplicity of infection (MOI) range of 100 to 400 FFU per cell to compare the abilities of DEN virus strains to induce apoptosis as previously described (7) (Fig. 4B). Thirty hours after infection, the proportion of FGA/NA a5c- or FGA/NA d1d-infected Neuro 2a cells with apoptotic nuclei was at least 30% for each MOI tested, whereas only 15% of FGA/89-infected Neuro 2a cells had an apoptotic morphology at the highest MOI tested. Thus, cellular damage and apoptotic DNA fragmentation were more pronounced after infection with mouse-neurovirulent DEN virus variants than with the parental strain. The higher cytotoxicity of mouse-neurovirulent DEN virus variants to Neuro 2a cells was not due to greater production of infectious virus than the parental strain 25 h after infection, as judged by virus titers (data not shown) (7).
FIG. 4.
DEN virus-induced apoptosis in Neuro 2a cells. (A) Oligonucleosomal DNA laddering in DEN virus-infected Neuro 2a cells. Fragmented DNA was extracted from Neuro 2a cells 40 h after mock infection (M.I.) or infection with the indicated DEN virus (MOI of 400 FFU/cell) as previously described (7). To detect cleaved DNA by endonucleases, free 3′-OH DNA termini were labeled with DIG-labeled ddUTP in the presence of terminal deoxynucleotidyl transferase. DIG-labeled DNA samples were subjected to electrophoresis in a 1.8% agarose gel and transferred to Hybond-N nylon membrane, and the immunodetection protocol described in the DIG DNA labeling and detection kit (Boehringer Mannheim Biochemicals) was then used. Results from ethidium bromide staining (left) and immunodetection (right) of low-molecular-weight DNA are shown. (B) Detection of DEN virus-infected cells in the apoptotic state 30 h postinfection. Paraformaldehyde-fixed cells were permeabilized with Triton X-100, and viral antigens were visualized by an immunofluorescence assay with anti-DEN virus hyperimmune mouse ascites fluid. The proportion of DEN virus-infected cells with chromatin condensation was determined by propidium iodide staining as previously described (7).
In this paper, we showed that intracerebral inoculation of newborn mice with mouse-neurovirulent DEN virus caused morphological alterations consistent with apoptosis in neurons. This may account for the tissue damage resulting from DEN virus infection of the CNS (7). Apoptotic cell death in DEN virus-infected mice occurred in the regions of the brain that had high levels of DEN virus replication. The higher efficiency of mouse-neurovirulent DEN virus strains to induce apoptosis in mouse neuroblastoma cells may be related to their capacity to replicate in neuronal cells (17). It is possible that substitutions in protein E might account for increased virulence, but the mechanism for this is unknown (10). Substitutions that increase the stability of the E oligomers would affect cell membrane permeability, releasing apoptosis mediators such as Ca2+ from stores into the endoplasmic reticulum to the nucleus (7). However, there may also be changes in the regions of the DEN virus genome of the neurovirulent variants that were not sequenced. Additional substitutions in the 3′-untranslated region and in nonstructural proteins could affect neurovirulence (32, 36). Thus, a full-length infectious cDNA for DEN-1 with the identified substitutions is needed to define their role in virus morphogenesis and in the pathophysiology of the host in the response to DEN virus infection (2, 19, 31).
Acknowledgments
We thank Michelle W. Wien and Felix A. Rey for helpful discussions. We thank T. Couderc and R. Putnak for providing antipoliovirus and anti-dengue virus MAbs.
This research was supported by a grant from CNRS Interdisciplinaire de Recherche Environnement Vie et Société, program 95N82/0134.
REFERENCES
- 1.Bhamarapravati N, Halstead S B, Sookavachana P, Boonyapaknavik V. Studies on dengue virus infection. 1. Immunofluorescence localization of virus in mouse tissue. Arch Pathol. 1964;77:538–543. [PubMed] [Google Scholar]
- 2.Chen W, Kawano H, Men R, Clark D, Lai C-J. Construction of intertypic chimeric dengue viruses exhibiting type 3 antigenicity and neurovirulence for mice. J Virol. 1995;69:5186–5190. doi: 10.1128/jvi.69.8.5186-5190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu M C, O’Rourke E J, Trent D W. Genetic relatedness among structural protein genes of dengue virus strains. J Gen Virol. 1989;70:1701–1712. doi: 10.1099/0022-1317-70-7-1701. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D K, Duarte E A, Moya A, Elena S F, Domingo E, Holland J. Genetic bottlenecks and population passages cause profound fitness differences in RNA viruses. J Virol. 1993;67:222–228. doi: 10.1128/jvi.67.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craighead J E, Sather G E, Hammon W M, Dammin G J. Pathology of dengue virus infection in mice. Arch Pathol. 1966;81:232–239. [Google Scholar]
- 6.Desprès P, Frenkiel M-P, Deubel V. Differences between cell membrane fusion activities of two dengue type-1 isolates reflect modifications of viral structure. Virology. 1993;196:209–216. doi: 10.1006/viro.1993.1469. [DOI] [PubMed] [Google Scholar]
- 7.Desprès P, Flamand M, Ceccaldi P-E, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dropulic L K, Hardwick J M, Griffin D E. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J Virol. 1997;71:6100–6105. doi: 10.1128/jvi.71.8.6100-6105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farlie P G, Dringen R, Rees S M, Kannourakis G, Bernard O. Bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gritsun T S, Holmes E C, Gould E A. Analysis of flavivirus envelope proteins reveals variable domains that reflect their antigenicity and may determine their pathogenesis. Virus Res. 1995;35:307–321. doi: 10.1016/0168-1702(94)00090-y. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa H, Yoshida M, Shiosaka T, Fujita S, Kobayashi Y. Mutations in the envelope protein of Japanese encephalitis virus affect entry into cultured cells and virulence in mice. Virology. 1992;191:158–165. doi: 10.1016/0042-6822(92)90177-q. [DOI] [PubMed] [Google Scholar]
- 12.Henchal E A, Putnak R J. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heron A, Pollard H, Dessi F, Moreau J, Lasbennes F, Ben-Ari Y, Charriaut-Marlangue C. Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem. 1993;61:1973–1976. doi: 10.1111/j.1471-4159.1993.tb09843.x. [DOI] [PubMed] [Google Scholar]
- 14.Holzmann H, Stiasny K, Ecker M, Kunz C, Heinz F. Characterization of monoclonal antibody-escape mutants of tick-borne encephalitis virus with reduced neuroinvasiveness in mice. J Gen Virol. 1997;78:31–37. doi: 10.1099/0022-1317-78-1-31. [DOI] [PubMed] [Google Scholar]
- 15.Hotta S. Experimental studies on dengue. I. Isolation, identification and modification of the virus. J Infect Dis. 1952;90:1–9. doi: 10.1093/infdis/90.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Imbert J L, Guevara P, Ramos-Castañeda J, Ramos C, Sotelo J. Dengue virus infects mouse cultured neurons but not astrocytes. J Med Virol. 1994;42:228–233. doi: 10.1002/jmv.1890420304. [DOI] [PubMed] [Google Scholar]
- 17.Jelachich M L, Lipton H L. Theiler’s murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W R, Lowe A, Higgs S, Reid H, Gould E A. Single amino acid codon changes detected in Louping ill virus antibody-resistant mutants with reduced neurovirulence. J Gen Virol. 1993;74:931–935. doi: 10.1099/0022-1317-74-5-931. [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Rostapshov V, Rosen L, Lai C-J. Genetic determinants of dengue type 4 virus neurovirulence for mice. J Virol. 1993;67:6567–6575. doi: 10.1128/jvi.67.11.6567-6575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;6:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 21.Lee E, Weir R C, Dalgarno L. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology. 1997;232:281–290. doi: 10.1006/viro.1997.8570. [DOI] [PubMed] [Google Scholar]
- 22.Levine B, Griffin D E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67:6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marianneau P, Cardona A, Eldman L, Deubel V, Desprès P. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMinn P C, Dalgarno L, Weir R C. A comparison of the spread of Murray Valley encephalitis virus of high or low neuroinvasiveness in the tissues of Swiss mice after peripheral inoculation. Virology. 1996;220:414–423. doi: 10.1006/viro.1996.0329. [DOI] [PubMed] [Google Scholar]
- 27.McMinn P C, Weir R C, Dalgarno L. A mouse-attenuated envelope protein variant of Murray Valley encephalitis virus with altered fusion activity. J Gen Virol. 1996;77:2085–2088. doi: 10.1099/0022-1317-77-9-2085. [DOI] [PubMed] [Google Scholar]
- 28.Nicotera P, Ankarcrona M, Bonfoco E, Orrenius S, Lipton S A. Neuronal apoptosis versus necrosis induced by glutamate or free radicals. Apoptosis. 1996;1:5–10. [Google Scholar]
- 29.Ogata A, Nagashima K, Hall W W, Ichikawa M, Kimura-Kuroda J, Yasui K. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J Virol. 1991;65:880–886. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pekosz A, Philipps J, Pleasure D, Merry D, Gonzalez-Scarano F. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J Virol. 1996;70:5329–5335. doi: 10.1128/jvi.70.8.5329-5335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pletnev A G, Bray M, Lai C-J. Chimeric tick-borne encephalitis and dengue type 4 viruses: effect of substitutions on neurovirulence in mice. J Virol. 1993;67:4956–4963. doi: 10.1128/jvi.67.8.4956-4963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proustki V, Gaunt M W, Gould E A, Holmes E C. Secondary structure of the 3′ untranslated region of yellow fever virus: implications for virulence, attenuation and vaccine development. J Gen Virol. 1997;78:1543–1549. doi: 10.1099/0022-1317-78-7-1543. [DOI] [PubMed] [Google Scholar]
- 33.Rey F, Heinz F X, Mandl C, Kunz C, Harrison S C. The envelope glycoprotein from tick-borne encephalitis virus at 2Å resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 34.Rink A, Fung K-M, Trojanowski J Q, Lee V M-Y, Neugebauer E, McIntosh T K. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 35.Sabin A B. Recent advances in our knowledge of dengue and sandfly fever. Am J Trop Med. 1952;1:30–50. doi: 10.4269/ajtmh.1955.4.198. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger J J, Chapman S, Nestorowicz A, Rice C M, Ginocchio T E, Chambers T J. Replication of yellow fever virus in the mouse central nervous system: comparison of neuroadapted and non-neuroadapted virus and partial sequence analysis of the neuroadapted strain. J Gen Virol. 1996;77:1277–1285. doi: 10.1099/0022-1317-77-6-1277. [DOI] [PubMed] [Google Scholar]
- 37.Stiasny K, Allison S L, Marchler-Bauer A, Kunz C, Heinz F X. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J Virol. 1996;70:8142–8147. doi: 10.1128/jvi.70.11.8142-8147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trgovcich J, Ryman K, Extrom P, Eldridge J C, Aronson J F, Johnston R E. Sindbis virus infection of neonatal mice results in a severe stress response. Virology. 1997;227:234–238. doi: 10.1006/viro.1996.8289. [DOI] [PubMed] [Google Scholar]
- 39.Tucker P, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker P C, Lee S H, Bui N, Martinie D, Griffin D E. Amino acid changes in the Sindbis virus E2 glycoprotein that increase neurovirulence improve entry into neuroblastoma cells. J Virol. 1997;71:6106–6112. doi: 10.1128/jvi.71.8.6106-6112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]