Abstract
The aim of this study is to determine the factors affecting the CT attenuation of bone marrow, and its correlation with 18F-FDG uptake. The mean standardized uptake value (SUV) of vertebral bone marrow (Vertebral-SUV) and femoral bone marrow (Femoral-SUV) as well as CT number of bone marrow (BM-CT number) were measured in 243 patients who had undergone 18F-FDG PET/CT. The correlations among BM-CT number, Femoral-SUV, and Vertebral-SUV were investigated. The relationships of Femoral-SUV, Vertebral-SUV, and BM-CT number with blood parameters, age, blood sugar, and body weight were analyzed by correlation and multi-regression analyses. The Mann-Whitney U test and chi-square test and Binomial logistic analysis were used to examine the relationships between high BM-CT number (≥ 0 HU) and the above parameters. Significant correlations were observed between: BM-CT number and Femoral-SUV (r = 0.73, P < 0.01); Vertebral-SUV and Femoral-SUV (r = 0.78, P < 0.01); and BM-CT number and Vertebral-SUV (r = 0.52, P < 0.01). BM-CT number was correlated with patients’ age in both univariable (r = -0.27) and multivariable analyses (β = -0.20). Positive BM-CT number correlated with WBC in both univariable (P = 0.04) and multivariable (P < 0.01) analyses. Bone marrow glucose metabolism had a tendency to decrease with age, was increased in patients with elevated CRP. In conclusion, CT attenuation of bone marrow correlated well with bone marrow metabolism and also tended to decrease with age. High bone marrow attenuation (≥ 0 HU) could predict elevated serum WBC.
Keywords: PET, FDG, CT number, CT, Bone marrow
Introduction
On 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) image, physiological radio tracer uptake in the bone marrow (BM) is typically mild and diffuse, reflecting its low metabolic activity [1]. BM 18F-FDG accumulation has been reported to be influenced by age, serum white blood cell (WBC) count, and the C-reactive protein (CRP) level [2-6]. In examinations of malignant lesions such as bone metastasis, focal 18F-FDG uptake in the BM is considered to indicate abnormalities. However, in cases showing diffuse elevation of 18F-FDG accumulation at the BM, benign changes such as increased activity of the red marrow may be difficult to distinguish from metastasis or invasion of myeloproliferative disorders such as leukemia [1,7-9]. Moreover, BM uptake of 18F-FDG has been reported to be affected by radiotherapy, anticancer chemotherapy, and administration of hematopoietic cytokines such as granulocyte colony-stimulating factor (GCSF) and erythropoietin [10-14].
CT attenuation values reflect tissue composition and are usually expressed in Hounsfield units (HU), where the CT number of water is 0 HU. Measurement of the CT number is useful for speculating on components such as fat, calcification, and hematoma. The CT attenuation of the femoral BM has been reported to be elevated in anemia and various hematological disorders [15,16]. In the clinical settings, increments in the CT attenuation of BM which may indicate hyperactivity of the red marrow, are occasionally observed in patients with an inflammatory response or after GCSF administration. Decreased CT attenuation of BM has also been reported in patients with diseases such as aplastic anemia [15]. Hematopoietic elements, being the most metabolically active and densely populated cells in the BM, are expected to contribute to higher values on both PET and CT scans. Consequently, the activity of the BM and its uptake of 18F-FDG can potentially be deduced from the CT attenuation of BM. We sought to investigate that theory indirectly by correlating the peripheral indicators of BM cellularity with 18F-FDG positron emission tomography (PET)/CT. Thus, this study aimed to examine the factors affecting 18F-FDG uptake and CT attenuation of BM, including age, laboratory data, and anticancer chemotherapy-related factors. In addition, we investigated the relationship between 18F-FDG uptake and CT attenuation of BM. Understanding factors that influence 18F-FDG uptake and CT attenuation of BM may facilitate differentiation between malignancy and non-malignant BM conditions such as inflammation and anemia.
Materials and methods
Patient selection and data collection
This study was a retrospective analysis of 2,200 consecutive patients who underwent 18F-FDG PET/CT between January and December 2021 in our hospital. We enrolled patients whose blood data, including WBC count, hemoglobin (Hgb) level, and platelet (Plt) count, were obtained within one day of the PET/CT examination. We excluded patients with hematologic diseases, such as malignant lymphoma and bone metastasis, those aged under 20 years, those with a high blood sugar level (> 150 mg/dL), and those who had undergone radiotherapy at the measurement site or femoral replacement. Thus, we included 243 patients for analysis; 227 with malignancies, seven each with Takayasu aortitis and cardiac sarcoidosis, and one each with infective endocarditis and erythema multiforme. Of these 243 patients, 162 had no history of anticancer chemotherapy, 26 had received anticancer chemotherapy within 30 days of PET/CT examination, and 55 had received anticancer chemotherapy more than 30 days before (Figure 1).
Figure 1.
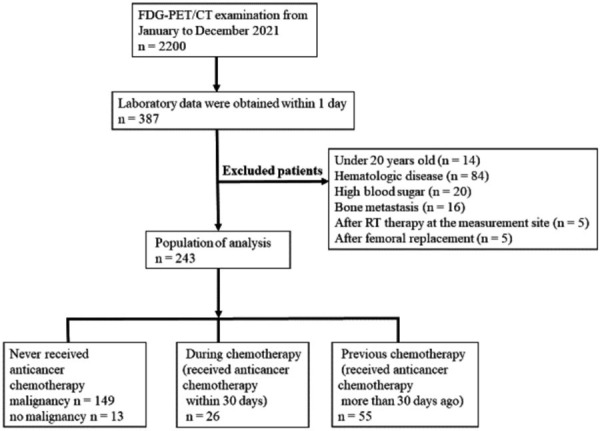
Flowchart of patient selection.
Patient characteristics including age, sex, surgery, and history of chemotherapy and radiotherapy, as well as blood cell counts including neutrophils, Plts, and lymphocytes were extracted from the patients’ medical records. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. Similarly, the platelet-to-lymphocyte ratio (PLR) was defined as the absolute Plt count divided by the absolute lymphocyte count. Among the 243 patients, neutrophil and lymphocyte counts were obtained in 218, and CRPs were obtained in 161.
PET/CT protocol
18F-FDG-PET/CT data were acquired using 128-slice CT with Biograph mCT (Siemens Healthcare, Erlangen, Germany). All patients fasted for at least four hours or skipped one meal before their examination. They were each injected with 4 MBq/kg of FDG, approximately one hour after which PET/CT examination was started. The acquisition of PET data started from the upper thigh to the head during shallow breathing. The acquisition time was 2-3 min per bed position, with 6-8 bed positions (each 21.8 cm). A 200 × 200 matrix was used for analysis. The PET data were reconstructed using ordered subset expectation maximization containing three iterations and 21 subsets with time of flight, point spread function, and a Gaussian filter of 3 mm full-width at half-maximum. Attenuation correction was performed based on the CT data derived from the CT scan acquired prior to the PET scan. Acquisition parameters for CT were as follows: tube voltage, 120 kVp; auto mAs (reference tube current, 80 mAs); rotation time, 0.5 s; matrix, 512 × 512; and reconstruction using a 3-mm slice thickness and 2-mm increments. CT data were acquired during expiratory breath holding.
Measurement
The standardized uptake value (SUV) of 18F-FDG for the BM (BM-SUV) and CT attenuation value were measured by using an elliptical region of interest (ROI) in an axial image of fused PET and CT images. The ROI was placed at the center of the L3-L5 vertebra using a scout tool, and the largest possible femoral BM without adjacent tissue was measured. The ROI of the femoral BM was placed on the caudal side of the greater trochanter where the cortical bone was not visually observed. The SUV of the femur and CT attenuation values were measured simultaneously using the same ROI. CT attenuation values were expressed as HU. The mean SUV of BM was averaged from the values obtained at L3, L4, and L5 (Vertebral-SUV) and from the values for bilateral femoral BM (Femoral-SUV), while the BM-CT number was determined as the mean of CT attenuation for bilateral femoral BM (Figure 2). Measurements were performed using the PACS software (EV Insite, PSP Corporation, Tokyo, Japan) by a board-certified radiologist with 20 years of experience.
Figure 2.
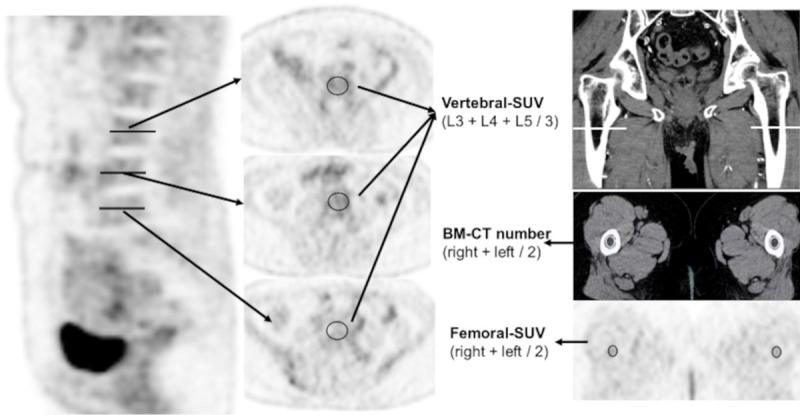
Placement of regions of interest (ROI). The regions of interest were placed at the center of the lumbar spine from L3-5, and the obtained standardized uptake values were averaged (Vertebral-SUV). The ROI for femoral bone marrow was placed on the caudal side of the greater trochanter, where the cortical bone was not visually observed in the same place in Femoral-SUV and BM-CT number.
Statistical analysis
The correlations among BM-CT number, Femoral-SUV, and Vertebral-SUV were investigated by Pearson’s correlation analysis. The relationships of Femoral-SUV, Vertebral-SUV, and BM-CT number with blood parameters (WBC, Plt counts, Hgb, CRP levels, NLR and PLR), patient age, blood sugar level, and body weight were also analyzed by Pearson’s correlation analysis for patients who had never received anticancer chemotherapy. Multi-regression analysis was performed with Femoral-SUV, Vertebral-SUV, BM-CT number as dependent variables. The independent variables were the factors for which significant correlations (P < 0.05) were observed in a single-correlation analysis.
The Mann-Whitney U test and chi-square test were used to examine the relationship between high BM-CT number (≥ 0 HU) and blood parameters (WBC and Plt counts and Hgb and CRP levels), patient age, sex, body weight, and blood sugar. Binomial logistic analysis was used to examine the factors that showed a significant correlation (P < 0.05) in the Mann-Whitney U test and chi-square test between patients with BM-CT number ≥ 0 HU and those with BM-CT number < 0 HU.
The Kruskal-Wallis H test and Mann-Whitney U test with Bonferroni correction were used to identify significant differences in Femoral-SUV, Vertebral-SUV, and BM-CT number among patients with malignancy who had never been treated by anticancer chemotherapy (n = 149), those who had received anticancer chemotherapy within 30 days before the PET/CT examination and had not received GCSF administration within 2 weeks previously (n = 23), and those who had received anticancer chemotherapy more than 30 days before the PET/CT examination (n = 55).
Vertebral-SUV, Femoral-SUV, and BM-CT number were evaluated by an intra-class correlation coefficient (ICC 1, 2). Statistical analysis was performed by SPSS version 28.0 (SPSS, Chicago, Ill, USA).
Results
In Pearson’s correlation analysis, a strong correlation was observed between BM-CT number and Femoral-SUV (r = 0.73, P < 0.01), as well as between Vertebral-SUV and Femoral-SUV (r = 0.78, P < 0.01), while a substantial correlation was observed between the BM-CT number and Vertebral-SUV (r = 0.52, P < 0.01) (Figure 3).
Figure 3.
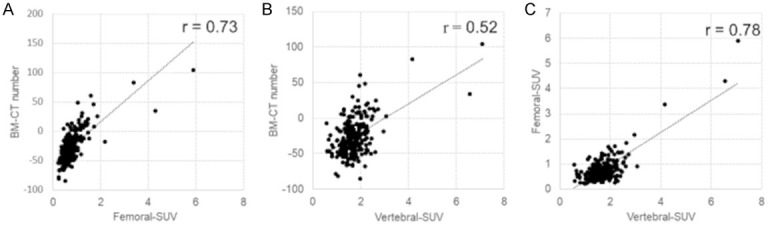
Correlations between Femoral-SUV and BM-CT number (A), Vertebral-SUV and BM-CT number (B), Vertebral-SUV and Femoral-SUV (C).
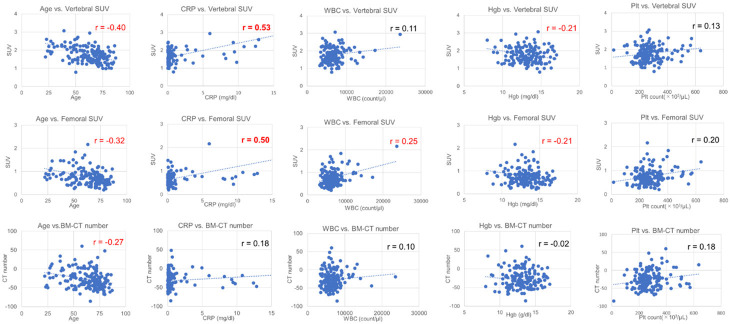
The results of the single-correlation and multivariable analyses of SUVs, BM-CT number, laboratory data, and patient age are shown in Table 1. A moderate correlation was observed between age and Vertebral-SUV (r = -0.40), while a weak correlation was observed between age and Femoral-SUV (r = -0.32), as well as between age and BM-CT number (r = -0.27). WBC count was weakly correlated with Femoral-SUV (r = 0.25), and Hgb level was weakly correlated with Femoral-SUV (r = -0.21) and Vertebral-SUV (r = -0.21). CRP level was moderately correlated with both Vertebral-SUV (r = 0.53) and Femoral-SUV (r = 0.50) (Figure 4).
Table 1.
Single correlation coefficients and standardized regression coefficients in multiple regression analysis of vertebral SUV, femoral SUV and BM-CT number, between age, blood sugar, body weight and blood parameters
| Variables | Univariate analysis Pearson’s coefficient (r) | Multivariable analysis Standardized regression coefficient (β) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Vertebral-SUV | Femoral-SUV | BM-CT number | Vertebral-SUV | Femoral-SUV | BM-CT number | ||
| R2 (adjusted) | 0.34 | 0.36 | 0.14 | ||||
| Age | -0.40** | -0.32** | -0.27** | -0.25** | -0.25** | -0.20** | |
| BS level | -0.04 | < 0.01 | -0.18 | ||||
| BW | 0.03 | -0.01 | 0.28** | 0.25** | |||
| WBC count | 0.11 | 0.25** | 0.10 | 0.18 | |||
| Hgb level | -0.21** | -0.21** | -0.02 | -0.03 | -0.13 | ||
| Plt count | 0.13 | 0.20* | 0.18* | -0.28* | 0.16* | ||
| CRP level | 0.53** | 0.50** | 0.18 | 0.46** | 0.49** | ||
| NLR | 0.02 | 0.09 | 0.11 | ||||
| PLR | 0.05 | 0.09 | 0.14 | ||||
SUV, standardized uptake value; BM, bone marrow; CT, computed tomography; BS, blood sugar; BW, body weight; WBC, white blood cell; Hgb, hemoglobin; Plt, platelet; CRP, C-reactive protein; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
P < 0.05.
P < 0.01.
Figure 4.
The relationships of Femoral-SUV, Vertebral-SUV, and BM-CT number with blood parameters. A moderate correlation was observed between age and Vertebral-SUV (r = -0.40), while weak correlations were observed between age and Femoral-SUV (r = -0.32) and between age and BM-CT number (r = -0.27). The WBC count was weakly correlated with Femoral-SUV (r = 0.25), and Hgb level was weakly correlated both with Femoral-SUV (r = -0.21) and Vertebral-SUV (r = -0.21). CRP level was moderately correlated with Vertebral-SUV (r = 0.53) and Femoral-SUV (r = 0.50).
Multi-regression analyses showed that Vertebral-SUV was negatively correlated with age (β = -0.25) and positively correlated with the CRP level (β = 0.46). Femoral-SUV was negatively correlated with age (β = -0.25) and Plt count (β = -0.28), and positively correlated with CRP level (β = 0.49). BM-CT number was negatively correlated with age (β = -020) and positively correlated with body weight (β = 0.25) (Table 1).
Significant differences in age (P = 0.01), body weight (P < 0.01), WBC count (P = 0.04), and CRP level (P = 0.04) were observed in the univariable analysis between the patients with BM-CT number ≥ 0 HU and < 0 HU; among these variables, only the WBC count (P < 0.01) showed significance in the multivariable analysis (Table 2).
Table 2.
Single and multi-regression analysis of the parameters associated with a high BM-CT attenuation value (≥ 0 HU)
| Variable | High BM-CT number (≥ 0 HU) | Low BM-CT number (< 0 HU) | P* (Univariable analysis) | P** (Multivariable analysis) |
|---|---|---|---|---|
| Age (years) | 59.5 (27-80) | 68 (22-87) | 0.01 | 0.82 |
| Sex (male/female) | 16/24 | 92/111 | 0.60 | |
| Blood sugar (mg/dL) | 102 (45-135) | 103 (76-149) | 0.71 | |
| Body weight (kg) | 64.5 (35.6-103.4) | 55.7 (30.8-114.1) | < 0.01 | 0.57 |
| WBC count (× 103/μL) | 6.35 (2.10-2.68) | 5.80 (1.80-1.72) | 0.04 | < 0.01 |
| Hgb (mg/dL) | 12.6 (8.3-16.7) | 12.9 (7.6-17.0) | 0.38 | |
| Plt count (× 103/μL) | 267.5 (28.0-636.0) | 240.0 (13.6-572.0) | 0.18 | |
| CRP (mg/dL) | 0.50 (0.03-21.67) | 0.17 (0.01-22.60) | 0.04 | 0.82 |
| NLR | 3.4 (0.9-45.5) | 2.4 (0.4-91) | 0.01 | 0.43 |
| PLR | 195.6 (61.3-854.5) | 165.5 (13.2-4000) | 0.08 |
Values are presented as median (range). CT, computed tomography; WBC, white blood cell; Hgb, hemoglobin; Plt, platelet; CRP, C-reactive protein; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Results of Mann-Whitney U and chi-square tests.
Results of binomial logistic analysis.
Significant differences in Vertebral-SUV were observed between patients who received anticancer chemotherapy more than 30 days previously and those who had never received anticancer chemotherapy (P < 0.01); however, no significant differences were observed in BM-CT number and Femoral-SUV among the three groups (Table 3; Figure 5).
Table 3.
Characteristics and statistical results of the patients grouped according to their chemotherapy history
| Variables | Groups | P value** | ||
|---|---|---|---|---|
|
| ||||
| A: No prior chemotherapy* | B: During chemotherapy* | C: Previous chemotherapy* | ||
| Vertebral-SUV | 1.69 (0.79-3.07) | 1.49 (0.69-2.53) | 1.40 (0.56-2.11) | 0.04 (A vs B) |
| < 0.01 (A vs C) | ||||
| 0.74 (B vs C) | ||||
| Femoral-SUV | 0.68 (0.21-1.85) | 0.70 (0.24-1.13) | 0.63 (0.24-1.63) | 0.76 |
| BM-CT number | -31.0 (-85.3-60.5) | -22.7 (-81.5-13.6) | -33.2 (-78.6-25.1) | 0.35 |
| Age (years) | 66 (22-87) | 69 (44-79) | 65.8 (24-86) | 0.41 |
| Male/female | 74/75 | 11/12 | 20/35 | 0.24*** |
| BS (mg/dL) | 103.1 (76-149) | 102.0 (45-147) | 103.4 (78-139) | 0.92 |
| BW (kg) | 59.3 (35.4-114.1) | 54.1 (40.8-85.5) | 56.5 (30.8-83.5) | 0.18 |
| WBC count (× 103/μL) | 6.20 (3.20-1.72) | 4.47 (1.80-11.10) | 4.78 (1.80-1.89) | < 0.01 (A vs B) |
| 0.04 (A vs C) | ||||
| 0.74 (B vs C) | ||||
| Hgb (mg/dL) | 13.2 (8.0-17.0) | 10.9 (7.8-15.0) | 12.4 (7.6-15.4) | < 0.01 (A vs B) |
| < 0.01 (A vs C) | ||||
| 0.03 (B vs C) | ||||
| Plt count (× 103/μL) | 255.7 (13.6-572.0) | 221.0 (58.0-444.0) | 214.3 (28.0-364.0) | < 0.01 (A vs C) |
| 0.01 (A vs B) | ||||
| 1.00 (B vs C) | ||||
| CRP (mg/dL) | 0.23 (0.01-12.9) | 0.15 (0.01-8.4) | 0.18 (0.02-4.68) | 0.44 |
Values are presented as median (range). SUV, standardized uptake value; BM, bone marrow; CT, computed tomography; BS, blood sugar; BW, body weight; WBC, white blood cell; Hgb, hemoglobin; Plt, platelet; CRP, C-reactive protein.
No prior chemotherapy: patients who had never received anticancer chemotherapy; during chemotherapy: patients who had received anticancer chemotherapy within 30 days previously; previous chemotherapy: patients who had received anticancer chemotherapy more than 30 days previously.
Results of the Kruskal-Wallis H test and Mann-Whitney U test with Bonferroni correction.
Results of the chi-square test.
Figure 5.
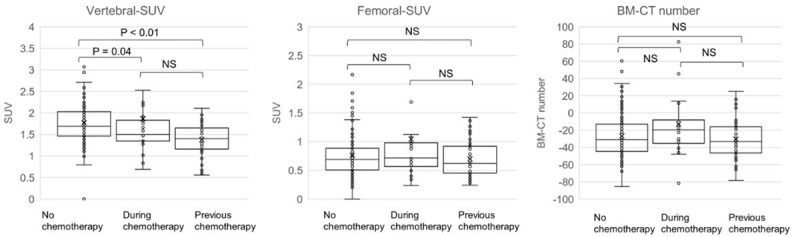
Comparison of SUV and CT number of bone marrow among patients with no anti-cancer chemotherapy, during chemotherapy and previous history of chemotherapy. Compared with patients who had never received anticancer chemotherapy, there were significant differences in Vertebral-SUV in patients who had received anticancer chemotherapy more than 30 days previously (P < 0.01) and in patients during chemotherapy with anticancer drugs (P = 0.04), but no significant differences were observed in the BM-CT number and Femoral-SUV among the three groups.
The ICC (1, 2) was 0.984, 0.987, 0.978 for the measurements of Vertebral-SUV, Femoral-SUV and BM-CT number respectively.
Discussion
The results of the present study showed that 18F-FDG uptake in the BM was correlated with age and the CRP level. BM-CT attenuation was also correlated with age and high BM-CT number (≥ 0 HU) was associated with an elevated WBC count. BM-CT attenuation was well correlated with BM-SUV; therefore, the BM-CT attenuation may be used as an indicator of BM-SUV. However, in the clinical setting, high BM-CT attenuation is also seen in BM edema due to liver, renal or heart failure; in such cases, the BM-CT attenuation may not be proportional to the BM-SUV.
The present study confirms previous findings that FDG uptake in bone marrow decreases with age; a trend observed in both healthy individuals and patients with various disorders [5,6]. The present study similarly demonstrated a decrease in BM-CT attenuation with age, suggesting a potential reduction in red marrow associated with aging.
Consistent with the findings of previous reports, a relatively strong correlation was observed between BM-SUV and CRP level, and a weak correlation was observed between BM-SUV and WBC count [7,8]. We speculated that the correlation between WBC count and BM-SUV would be higher in the present study compared to the previous studies due to a shorter interval between PET/CT examination and blood test (within 1 day vs. within 5 or 7 days). However, the correlation between WBC count and BM-SUV was low, and one possible reason for this is the small number of patients with abnormal WBC counts. The WBC count was correlated with BM-CT attenuation ≥ 0 HU (P < 0.01), therefore, high BM-CT attenuation may indicate an inflammatory response.
A weak correlation was observed between Hgb level and BM-SUV (r = 0.21) in single-correlation analysis, but no significant correlation was observed in the multivariable analysis. Several studies have reported mixed results regarding the association of Hgb level with BM-SUV [7-9]. One conference presentation suggested that anemic patients have higher BM-SUV than non-anemic patients [16]. On the other hand, previous studies have reported a significant association between intravascular CT attenuation and peripheral blood Hgb levels [17,18]. Furthermore, Maldjian et al. reported a correlation between high femoral BM-CT attenuation (> 20 HU) and anemia [19]. Therefore, an increased number of erythrocytes, the precursor cells of red blood cells, may elevate the BM-CT attenuation in anemic patients, but the results did not indicate such an elevation. Although BM-CT attenuation may increase in acute or subacute anemia, most of the anemic patients in our study had chronic anemia, which may explain why no significant correlation was found between anemia and BM-CT attenuation. High BM-CT attenuation (> 20 HU) was observed in 10 of 243 patients in this study. Among these 10 patients, seven were anemic (Hgb: < 11.6 in females, 13.7 in males), including three who were administered GCSF within 2 weeks, one with Takayasu arteritis, and one with infective endocarditis. However, the remaining three patients had no abnormal blood cell count or a history of GCSF administration within two weeks, and the reason why they had high BM-CT attenuation is unknown.
Almost no correlations were observed between Plt count and BM-SUV, Plt count and BM-CT attenuation. Moreover, no previous reports have described a significant correlation between Plt count and BM-SUV, which may be due to the long lifespan of Plts and the small number of megakaryocytes in the BM.
NLR and PLR are novel biomarkers for assessing inflammation and prognosis in various diseases [20,21]. However, neither NLR nor PLR significantly correlated with BM-SUV or BM-CT attenuation. Although both these indices and BM-SUV have been reported to be associated with patient prognosis, the low correlation between these indices and BM-SUV may be attributed to differences in the type of inflammation being reflected, or due to the heterogeneity of the patients included in the study [22-24].
Vertebral-SUV was higher in patients with no prior cancer chemotherapy than in those who had undergone or were currently undergoing cancer chemotherapy. However, Femoral-SUV did not differ significantly among the three groups. This may reflect the heterogeneity of BM distribution [25]. Chemotherapy has been shown to increase BM-SUV, and there are also reports of an increase in BM-SUV within 15 days post-chemotherapy [2,26]. Increased BM-SUV may depend on the effect on hematopoietic cells and due to BM regeneration. Increased BM-SUV in patients during chemotherapy is thought to be caused by the activation of hematopoietic cells, while the decreased BM-SUV after chemotherapy may be the result of a reduced population of normal hematopoietic cells. However, in the present study, the patients who had undergone chemotherapy showed significantly lower WBC counts than those who had not received chemotherapy, which may also be associated with the decreased BM-SUV. Moreover, the activation of inflammation by untreated or residual malignant lesions may have contributed to the increase in BM-SUV in patients with no prior chemotherapy, potentially accounting for the differences in BM-SUV [27,28].
Recent reports have shown that elevated BM-SUV in patients with malignant lesions correlated with clinical outcomes such as prognosis, disease progression, and distant metastasis [22-24]. In the present study, a strong correlation was demonstrated between BM-CT attenuation and BM-SUV. Future studies are needed to determine whether BM-CT attenuation is beneficial for predicting such important clinical outcomes.
A limitation of the current study was its single-center retrospective review conducted predominantly on patients with malignancy or with a history of various malignant lesions. Although it would have been preferable to obtain blood data on the same day as the PET/CT examination, in the present study, we opted to collect blood data within one day of the PET/CT examination, given the potential for selection bias.
Conclusions
BM glucose metabolism had a tendency to decrease with age, was increased in patients with elevated CRP, and was decreased in patients who had undergone chemotherapy. BM-CT attenuation correlated well with BM metabolism and also tended to decrease with age. High BM attenuation (≥ 0 HU) could predict elevated serum WBC.
Acknowledgements
This research was supported by grants from Nihon Medi-Physics Co., Ltd. (IICR-22048).
Disclosure of conflict of interest
None.
References
- 1.Kwee TC, de Klerk JMH, Nix M, Heggelman BGF, Dubois SV, Adams HJA. Benign bone conditions that may be FDGavid and mimic malignancy. Semin Nucl Med. 2017;47:322–351. doi: 10.1053/j.semnuclmed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Fan C, Hernandez-Pampaloni M, Houseni M, Chamroonrat W, Basu S, Kumar R, Dadparvar S, Torigian DA, Alavi A. Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography. Mol Imaging Biol. 2007;9:300–307. doi: 10.1007/s11307-007-0100-9. [DOI] [PubMed] [Google Scholar]
- 3.Shen G, Liang M, Su M, Kuang A. Physiological uptake of 18F-FDG in the vertebral bone marrow in healthy adults on PET/CT imaging. Acta Radiol. 2018;59:1487–1493. doi: 10.1177/0284185118762245. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Goto R, Okada K, Kinomura S, Fukuda H. A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann Nucl Med. 2009;23:643–649. doi: 10.1007/s12149-009-0286-9. [DOI] [PubMed] [Google Scholar]
- 5.Tsujikawa T, Oikawa H, Tasaki T, Hosono N, Tsuyoshi H, Rahman MGM, Yoshida Y, Yamauchi T, Kimura H, Okazawa H. Integrated [18F]FDG PET/MRI demonstrates the iron-related bone-marrow physiology. Sci Rep. 2020;10:13878. doi: 10.1038/s41598-020-70854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata Y, Kubota K, Yukihiro M, Ito K, Watanabe H, Shibuya H. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: measurements by PET/CT. Nucl Med Biol. 2006;33:999–1004. doi: 10.1016/j.nucmedbio.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Hapkido H, Hidayat B, Kartamihardja HS, Nugrahadi T. Differential diagnosis of diffuse bone marrow uptake on 18F-FDG PET/CT. Int J Clin Biomed Res. 2016;2:1–5. [Google Scholar]
- 8.Arimoto MK, Nakamoto Y, Nakatani K, Ishimori T, Yamashita K, Takaori-Kondo A, Togashi K. Increased bone marrow uptake of 18F-FDG in leukemia patients: preliminary findings. Springerplus. 2015;4:521. doi: 10.1186/s40064-015-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valls L, Badve C, Avril S, Herrmann K, Faulhaber P, O’Donnell J, Avril N. FDG-PET imaging in hematological malignancies. Blood Rev. 2016;30:317–331. doi: 10.1016/j.blre.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2:271–279. doi: 10.2174/157339406777934717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson M, Muirhead R, Jacobs C, Cooke R, Chu KY, Van den Heuvel F, Ng S, Virdee P, Strauss V, Hawkins M. Response of FDG avid pelvic bone marrow to concurrent chemoradiation for anal cancer. Radiother Oncol. 2020;143:19–23. doi: 10.1016/j.radonc.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casamassima F, Ruggiero C, Caramella D, Tinacci E, Villari N, Ruggiero M. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73:1677–1681. [PubMed] [Google Scholar]
- 13.Kazama T, Swanston N, Podoloff DA, Macapinlac HA. Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur J Nucl Med Mol Imaging. 2005;32:1406–1411. doi: 10.1007/s00259-005-1890-0. [DOI] [PubMed] [Google Scholar]
- 14.Plantade A, Montravers F, Selle F, Izrael V, Talbot JN. Diffusely increased F-18 FDG uptake in bone marrow in a patient with acute anemia and recent erythropoietin therapy. Clin Nucl Med. 2003;28:771–772. doi: 10.1097/01.rlu.0000082670.19100.83. [DOI] [PubMed] [Google Scholar]
- 15.Ishii S, Ohkawara H, Endo Y, Hara J, Hotsumi H, Yamakuni R, Sugawara S, Sekino H, Ito H. Evaluation of computed tomography attenuation value of proximal femoral marrow to diagnose and differentiate hematologic malignancies, myelofibrosis, and aplastic anemia. J Comput Assist Tomogr. 2021;45:912–918. doi: 10.1097/RCT.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 16.Habib P HN, Zhang J, Sarikaya I, Knopp M. Effects of anemia on bone marrow FDG uptake in PET imaging: impact on normal tissue SUV. J Nucl Med. 2007:62. [Google Scholar]
- 17.Lan H, Nishihara S, Nishitani H. Accuracy of computed tomography attenuation measurements for diagnosing anemia. Jpn J Radiol. 2010;28:53–57. doi: 10.1007/s11604-009-0385-5. [DOI] [PubMed] [Google Scholar]
- 18.Kamel EM, Rizzo E, Duchosal MA, Duran R, Goncalves-Matoso V, Schnyder P, Qanadli SD. Radiological profile of anemia on unenhanced MDCT of the thorax. Eur Radiol. 2008;18:1863–1868. doi: 10.1007/s00330-008-0950-9. [DOI] [PubMed] [Google Scholar]
- 19.Maldjian C, Curtis BR, Gatenby R, Milestone B, Revesz G. Clinical significance of increased density in the proximal femoral marrow detected by visual inspection on abdominopelvic postcontrast CT examination. J Comput Assist Tomogr. 1999;23:448–453. doi: 10.1097/00004728-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Park SH, Ahn H, Lee SM, Jang SJ. Predicting survival in patients with pancreatic cancer by integrating bone marrow FDG uptake and radiomic features of primary tumor in PET/CT. Cancers (Basel) 2021;13:3563. doi: 10.3390/cancers13143563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker CC, Lapi SE. Positron emission tomography imaging of macrophages in cancer. Cancers (Basel) 2021;13:1921. doi: 10.3390/cancers13081921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Choi JS, Lyu J, Lee SM. Prognostic significance of 18F-fluorodeoxyglucose uptake of bone marrow measured on positron emission tomography in patients with small cell lung cancer. Lung Cancer. 2018;118:41–47. doi: 10.1016/j.lungcan.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Kim SY, Han SW, Lee JE, Lee HJ, Heo NH, Lee SM. [18F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020;10:72. doi: 10.1186/s13550-020-00660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Na JO, Kang DY, Lee SY, Lee SM. Prognostic significance of FDG uptake of bone marrow on PET/CT in patients with non-small-cell lung cancer After curative surgical resection. Clin Lung Cancer. 2017;18:198–206. doi: 10.1016/j.cllc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Yagi M, Froelich J, Arentsen L, Shanley R, Ghebre R, Yee D, Hui S. Longitudinal FDG-PET revealed regional functional heterogeneity of bone marrow, site-dependent response to treatment and correlation with hematological parameters. J Cancer. 2015;6:531–537. doi: 10.7150/jca.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira M, Lasnon C, Nganoa C, Gac AC, Damaj G, Aide N. Comprehensive analysis of the influence of G-CSF on the biodistribution of 18F-FDG in lymphoma patients: insights for PET/CT scheduling. EJNMMI Res. 2019;9:79. doi: 10.1186/s13550-019-0546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimura K, Mabuchi S, Komura N, Yokoi E, Kozasa K, Sasano T, Kawano M, Matsumoto Y, Watabe T, Kodama M, Hashimoto K, Sawada K, Hatazawa J, Kimura T. Prognostic significance of bone marrow FDG uptake in patients with gynecological cancer. Sci Rep. 2021;11:2257. doi: 10.1038/s41598-021-81298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M, Chen Y, Liu J, Huang G. A predicting model of bone marrow malignant infiltration in 18F-FDG PET/CT images with increased diffuse bone marrow FDG uptake. J Cancer. 2018;9:1737–1744. doi: 10.7150/jca.24836. [DOI] [PMC free article] [PubMed] [Google Scholar]