Abstract
The genome of the avian alphaherpesvirus infectious laryngotracheitis virus (ILTV) comprises ca. 155 kbp of which ca. one-third have been sequenced so far. To gain additional sequence information we analyzed two stretches of 15.5 and 1.9 kbp of the ILTV unique long (UL) genome region. The larger fragment contains homologs of the herpes simplex virus (HSV) UL23 (thymidine kinase) and UL22 (glycoprotein H) genes followed by five open reading frames (ORF) encoding putative proteins of 334 to 410 amino acids which exhibit no homology to any known herpesvirus protein. RNA analyses showed that these unique ILTV genes are indeed expressed. An origin of replication separates this cluster of unique genes from a conserved gene cluster consisting of the UL45, UL46, UL48, UL49, UL49.5, and UL50 homologs. The absence of UL47 from this position coincides with the localization of a UL47-homologous ORF within the unique short (US) region of the ILTV genome (M. Wild, S. Cook, and M. Cochran, Virus Genes 12:107–116, 1996). Within the second analyzed region the ILTV UL21 homolog was found adjacent to the UL44 gene. We thus identified five novel herpesvirus genes in ILTV and present evidence for a large internal inversion in the ILTV UL region, in contrast to the collinear genomes of other alphaherpesviruses. Interestingly, a similar inversion is also present in the porcine alphaherpesvirus pseudorabies virus.
Infectious laryngotracheitis is a contagious respiratory disease of chickens which causes severe losses in the poultry industry (1). The causative agent, infectious laryngotracheitis virus (ILTV) or gallid herpesvirus 1, was classified as a member of the subfamily Alphaherpesvirinae of the Herpesviridae (38). This classification correlates with the presence of latent ILTV in neurons of the central nervous system (52), which is a property of most alphaherpesviruses (39). In contrast to other alphaherpesviruses ILTV has a very narrow host range, which basically is restricted to chickens and chicken-derived cells (15, 42, 43). Phylogenetic studies revealed only a distant relationship between ILTV and the Simplexvirus and Varicellovirus genera of mammalian alphaherpesviruses as well as between ILTV and the avian Marek’s disease virus (MDV), a lymphotropic alphaherpesvirus (20, 33, 38). However, ILTV possesses a typical alphaherpesvirus type D genome, consisting of long (UL) and short (US) unique regions, the latter flanked by inverted repeat sequences (IR and TR) and present in two isomeric orientations (17, 27, 39). Partial sequence analyses of randomly cloned ILTV DNA fragments identified the presence of ILTV genes with significant homology to 21 genes of other alphaherpesviruses, but only a few of them appeared to be structurally related to genes of beta- or gammaherpesviruses (12).
More recent investigations resulted in complete sequences of conserved genes and gene clusters, demonstrating that the gene arrangement of ILTV is, at least in part, collinear to that found in the completely sequenced alphaherpesvirus genomes of herpes simplex virus type 1 (HSV-1) (31), varicella-zoster virus (VZV) (6), equine herpesvirus type 1 (EHV-1) (47), and bovine herpesvirus type 1 (BHV-1) (44). The 14-kbp US region of the ILTV genome was shown to contain six conserved alphaherpesvirus genes, including those for glycoproteins G, D, I, and E (51). Within the inverted repeat sequences flanking the ILTV US region an immediate-early gene whose predicted product exhibited homology to the ICP4 proteins of other alphaherpesviruses was localized (18). Close to the right terminus of the ILTV UL region homologs of the UL1 to UL5 genes were found (11), and near the left end of the genome the UL52, UL53, and UL54 genes were identified (19). These findings indicate that the UL region of the ILTV genome, like those of the genomes of VZV, EHV-1, BHV-1, and pseudorabies virus (PrV), is in opposite orientation to the prototypic isomer of the HSV-1 genome, which contains an invertible UL region (39).
Besides the conserved genes, several presumably ILTV-specific genes were identified. Among them is a unique open reading frame (ORF), UL0, which is located upstream from, and which partially overlaps, the 5′-terminal part of the UL1 gene (11). Also, in the US region of the ILTV genome three ORFs which are absent from the US regions of other alphaherpesviruses are located. One of them encodes the major viral glycoprotein gp60 of which no homolog has so far been identified in other herpesviruses (26, 51). Interestingly, the deduced product of another ILTV US ORF exhibits significant homologies to the UL47 protein, which is encoded within the UL regions of all other alphaherpesvirus genomes investigated so far (51).
Only limited information is available on the gene content of the central part of the ILTV UL region, which includes the DNA sequences of the UL44 (gC) gene (22) and a ca. 9-kbp segment extending from the UL23 (thymidine kinase) to the UL27 (gB) gene (13, 14). To gain additional sequence information, we analyzed two stretches of the ILTV UL region located adjacent to the known segments.
To this end, viral DNA of a pathogenic ILTV strain (obtained from D. Lütticken, Boxmeer, The Netherlands) was cloned in plasmids, as described previously (11). Terminal DNA sequences of cloned restriction fragments of the ILTV genome were determined (T7 sequencing kit; Pharmacia, Freiburg, Germany) and compared to available database sequences by using the Wisconsin sequence analysis package (GCG) (7). A 2.7-kbp BamHI-KpnI subfragment of a cloned 21-kbp KpnI fragment (pILT-K30; Fig. 1c) contained the UL44 gene, whereas an 11-kbp KpnI fragment (pILT-K23; Fig. 1c) was found to terminate within the UL26 ORF and, therefore, was predicted to also contain the UL23 gene. Both fragments were used to screen plasmid libraries of KpnI- and SalI-digested ILTV DNA by colony hybridization (41) to obtain duplicate and overlapping clones. Subfragments of the six resulting plasmids, pILT-S3 1 and 2, pILT-K23 1 and 2, and pILT-K30 1 and 2, were cloned as indicated in Fig. 1c and used to generate nested sets of deletion mutants (double-stranded nested deletion kit; Pharmacia). Complete sequences were obtained for at least two independent clones for each segment. Occasional sequence ambiguities were resolved by using 7-deaza sequencing mixes (Pharmacia). To determine the genomic arrangements and orientations of the subfragments, synthetic oligonucleotides (Gibco BRL, Eggenstein, Germany) were deduced from the fragment termini and used for sequencing the parental plasmids. DNA sequences were assembled and analyzed with the GCG software package (7) as described previously (11).
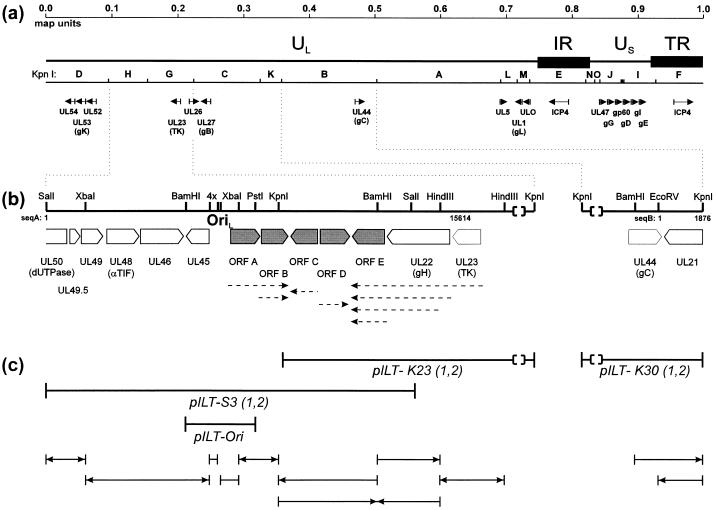
FIG. 1.
Genome structure of ILTV. (a) Diagram of the group D herpesviral genome of ILTV consisting of UL and US regions which are flanked by inverted repeat sequences (IR and TR). The locations of KpnI restriction fragments and characterized genes are indicated (see text). (b) Enlarged maps of sequenced genome regions A and B, with relevant restriction sites. ORFs are named according to their homologs in HSV-1. Unique ILTV genes (ORF A to E) are highlighted. Nucleotide numbers refer to the DNA sequences deposited in the GenBank (see text). Within sequence A an origin of DNA replication (Ori) and identified transcripts (dashed arrows) are indicated. (c) Illustration of the cloning and sequencing strategy. pILT-S3 1 and 2, -K23 1 and 2, and -K30 1 and 2 are independently cloned duplicates of viral DNA fragments. Plasmid pILT-Ori was used for functional characterization of the replication origin. Sequenced subclones are shown below, with arrows indicating the direction of nested deletion reactions. Parentheses indicate that DNA fragments are not plotted to scale.
The two resulting DNA sequences are 15,614 (sequence A) and 1,876 bp (sequence B) in length (Fig. 1b) with a G+C content of 47 and 43%, respectively. These values are in good agreement with the estimated overall G+C content of ILTV DNA of 45% (36). Sequence A overlaps the reverse strand of the ILTV UL23 sequence from nucleotide 15277 to 15614 (13), including a single base exchange. From nucleotide 1 to 340, sequence B is identical to the 3′-terminal part of the UL44 sequence (22).
Sequence A contains 12 ORFs of more than 115 codons (Fig. 1b). The locations and properties are summarized in Table 1. Most of them are preceded by putative transcriptional initiation signals matching the consensus sequence TATA(A/T)A(A/T) (4), with a maximum of one mismatch. In two cases, well-conserved TATA-box elements were found downstream from the first initiation codon (Table 1, asterisks), suggesting that transcription might initiate within the ORF and that translation starts at internal ATG codons. The locations of mRNA polyadenylation signals (AATAAA) followed by a GT-rich region after 20 to 30 nucleotides (50) indicate that many of the detected genes are probably expressed from 3′-coterminal transcripts (Table 1).
TABLE 1.
Properties of the identified ILTV genesa
| ORF | Location of:
|
Length of mRNA (kb) | Predicted protein
|
|||
|---|---|---|---|---|---|---|
| Codons | TATA box | Poly(A) signal | No. of amino acids | Mass (kDa) | ||
| UL50 | <1–1017 (r) | 1194–1200 (r) | ? | n.a. | >339 | >36.8 |
| UL49.5 | 1171–1521 | 1097–1113 | 2430–2435 | n.a. | 117 | 12.7 |
| UL49 | 1584–2381 | 1469–1475 | 2430–2435 | n.a. | 266 | 30.4 |
| UL48 | 2530–3717 | 2484–2490 | 5449–5454 | n.a. | 396 | 44.6 |
| UL46 | 3723–5393 | 3737–3743* | 5449–5454 | n.a. | 557 | 62.7 |
| UL45 | 5498–6340 (r) | 6402–6408 (r) | 5494–5499 (r) | n.a. | 281 | 30.5 |
| ORF A | 7113–8240 | — | 9310–9315 | 2.5 | 376 | 41.3 |
| ORF B | 8283–9302 | 8224–8230 | 9310–9315 | 1.2 | 340 | 38.1 |
| ORF C | 9363–10364 (r) | — | 9356–9361 (r) | 1.2 | 334 | 37.4 |
| ORF D | 10474–11595 | 10497–10503* | 11615–11620 | 1.5 | 374 | 41.5 |
| ORF E | 11673–12902 (r) | 13028–13034 (r) | 11644–11649 (r) | 1.6 | 410 | 45.1 |
| UL22 | 12963–15374 (r) | — | 11644–11649 (r) | 3.9 + 3.3 | 804 | 89.4 |
| UL21 | 238–1833 (r) | ? | 199–204 (r) | n.a. | 532 | 60.5 |
Nucleotide numbers of coding regions and transcription signals refer to GenBank sequence no. Y14300 (UL50-UL22) and Y14301 (UL21). Patterns located on the reverse DNA strand are marked by a parenthetical r. The absence of consensus elements is indicated by a dash, and question marks suggest that locations are outside of the analyzed genome regions. Putative promoter elements located downstream from the first initiation codons of the ORFs are marked by asterisks. The UL50 gene is not completely contained within the analyzed region. Sizes of transcripts as determined by Northern blotting are listed. The molecular masses of primary translation products were calculated from predicted amino acid sequences.
Database searches with the GCG programs Blast and Fasta revealed significant similarities between the products of the first six ILTV ORFs within sequence A and the UL50 to UL45 products of other alphaherpesviruses. Despite the overall moderate similarity (Table 2), the presence of short stretches of highly conserved amino acids within the particular proteins, as well as the similar gene arrangements, strongly indicates that the detected ILTV ORFs encode homologs of these herpesvirus genes.
TABLE 2.
Amino acid homologies of the predicted ILTV gene products to related alphaherpesvirus proteins
| ILTV gene | Homologya (% identical/% related) of gene product to related protein of:
|
||||||
|---|---|---|---|---|---|---|---|
| MDV | HVTb | PrV | EHV-1 | BHV-1 | VZV | HSV-1 | |
| UL50 | 29/41 | 27/36 | 25/34 | 24/36 | 26/34 | ||
| UL49.5 | 30/40 | 30/36 | 24/35 | 29/38 | 33/45 | 29/40 | |
| UL49 | 26/35 | 28/36 | 27/34 | 25/33 | 25/39 | ||
| UL48 | 27/38 | 25/37 | 26/37 | 27/38 | 25/37 | 30/42 | |
| UL46 | 23/33 | 26/37 | 23/33 | 23/35 | 24/31 | ||
| UL45 | 24/40 | 24/34 | — | 21/33 | — | — | 23/32 |
| UL22 | 21/35 | 24/39 | 23/34 | 23/37 | 24/34 | 25/37 | 22/33 |
| UL21 | 23/38 | 24/37 | 22/32 | 23/37 | 23/38 | ||
Homologies were determined by pairwise comparison with GCG program Gap. —, absence of the UL45 gene from seven alphaherpesvirus genomes; no entry, information not available.
HVT, herpesvirus of turkeys.
The UL50 gene encodes dUTPase (37). Only the 5′-terminal part of ILTV UL50 encoding the first two of six conserved motifs of herpesviral dUTPases (32) is included in the present sequence. The product of the second ORF is predicted to consist of 117 amino acids exhibiting characteristics of intrinsic membrane proteins, with two hydrophobic domains, one at the N terminus and one close to the C terminus, which could serve as signal and anchor sequences, respectively. The homologous UL49.5 proteins of PrV and BHV-1 were indeed shown to represent structural components of the viral envelope (21, 28). The deduced 266-, 396-, and 557-amino-acid proteins of the three following ORFs are homologous to the virion tegument proteins encoded by the UL49, UL48, and UL46 genes of alphaherpesviruses (5, 10, 35, 53). The UL48 gene product, in association with cellular transcription factors, transactivates immediate-early gene expression after virus infection, whereas the UL46- and UL47-derived proteins were shown to modulate this activity (for a recent review see reference 40). Remarkably, one component of this cluster of regulatory virion protein genes, UL47, is not present within this genomic region of ILTV. However, an ORF homologous to UL47 was identified in the US region (51), indicating a translocation of the gene from the UL to the US region. The predicted product of the next conserved ILTV ORF comprises 281 amino acids and has homology to the alphaherpesvirus UL45 proteins. Similar to homologous HSV-1, EHV-1, and MDV polypeptides (16, 31, 47), the predicted ILTV protein contains a large internal hydrophobic domain, correlating with the identification of the HSV-1 UL45 protein in virion envelopes (48). Interestingly, the UL45 ORF is not conserved in the genomes of VZV, BHV-1, and PrV (6, 30, 44).
Upstream from the UL45 gene five ORFs of 334 to 410 codons (ORF A to ORF E), whose deduced products exhibited no similarity with any known herpesviral protein, were detected. The lack of homology between them argues against the possibility of their creation by a duplication of viral gene sequences. Furthermore, analysis of the predicted proteins with GCG programs Peptidestructure and Motifs did not show any characteristic features or conserved sequence motifs pointing to a possible function for any of them. However, Northern blot analyses of RNA from ILTV-infected cells with strand-specific labeled cRNA probes derived from subcloned ILTV DNA fragments and nested deletion plasmids identified transcripts from each of the five ORFs (data not shown). These studies showed that the transcripts of ORF A and ORF B are 3′ coterminal, whereas ORF C and ORF D possess monocistronic mRNAs, and that ORF E is also transcribed 3′ coterminally with upstream genes (Fig. 1b). These findings agree with the locations of putative mRNA polyadenylation signals (Table 1).
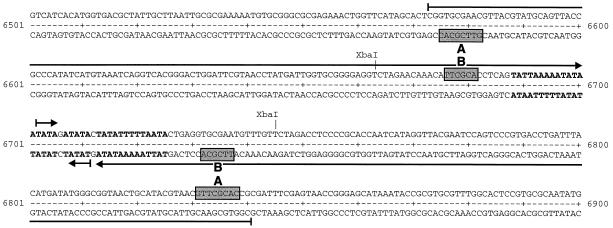
Between the conserved UL45 gene and unique ORF A, a presumably noncoding stretch of 268 bp (nucleotides 6573 to 6840) contained a nearly perfect palindromic structure with a central AT-rich region (Fig. 2). Similar features were found in different herpesvirus replication origins (29, 46). Furthermore, both arms of the detected palindrome contain the sequence motif GTTCGCAC, which was identified as a recognition domain of the HSV-1 origin-binding protein (Fig. 2) (25). In addition, two inverted copies of truncated motif TTCGCA are also present. The function of this ILTV origin was confirmed by autonomous replication of plasmids pILT-S3 and pILT-Ori (Fig. 1c) after transfection into chicken cells and superinfection with ILTV (data not shown).
FIG. 2.
Sequence of putative OriL. The sequence position (6501 to 6900) of sequence no. Y14300 is shown. Indicated by arrows are inverted repeat elements. Shaded boxes indicate the locations of origin-binding protein consensus recognition motifs (A) and truncated copies (B). The AT-rich region is indicated by boldface letters.
The deduced product of the 804-codon ILTV gene located immediately upstream of ORF E exhibits 21 to 25% amino acid identity with alphaherpesvirus gH proteins encoded by UL22 genes. Apparently, gH is structurally and functionally conserved within the entire herpesvirus family, forming a complex with gL (reviewed in reference 45). Recently, we identified a gene homologous to the gL gene (UL1) in ILTV (11). The predicted ILTV gH contains five putative N-glycosylation sites, a hydrophobic domain close to the C terminus, and a second hydrophobic sequence within the N-terminal region. Since this putative signal sequence lies approximately 30 residues downstream from the first initiation codon, it seems possible that translation of the ILTV gH protein initiates at the second in-frame ATG at codon 26. Remarkably, transcriptional analyses of the ILTV gH gene (Table 1) reproducibly showed two viral RNAs of 3.9 and 3.3 kb whose exact origin is yet unclear. The ILTV gH RNAs are part of a set of 3′-coterminal transcripts, which include the 1.2-kb ORF E transcript and the 5.3-kb mRNA of the UL23 gene (Fig. 1b). UL23, encoding thymidine kinase, was shown to be followed by the conserved UL24 to UL27 genes (13, 14).
In summary, sequence A encompasses two clusters of conserved genes which exhibit generally collinear arrangement with homologous genes of other alphaherpesviruses. However, the relative orientation of these clusters to each other is unique, in that the ILTV UL22 gene is not adjacent to the UL21 gene and UL45 is not preceded by UL44.
Since the UL44 gene encoding ILTV gC has already been characterized (22), we investigated a stretch of DNA downstream of this gene. The resulting sequence B of 1,876 bp contains a single 532-codon ORF (Fig. 1b), whose product is homologous to the alphaherpesvirus UL21 proteins, which in HSV-1 and PrV represent nonessential virion proteins (2, 8, 23). Although the overall identity of the deduced ILTV protein to its homologs in HSV-1, PrV, BHV-1, EHV-1, and VZV (6, 24, 31, 47, 49) amounts to only 22 to 24% (Table 2), multiple sequence alignments revealed a match to the consensus sequence in 63 positionally conserved residues (data not shown). ILTV UL21 is in the opposite orientation to UL44 (Fig. 1b). The termination codons of both genes are only 88 bp apart, and no unusual sequences indicative of a genomic rearrangement were found in between.
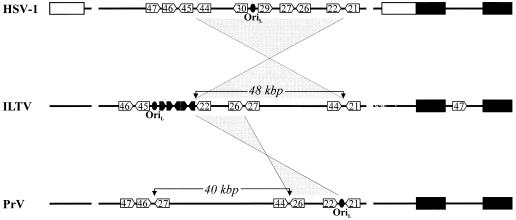
Taken together, our studies demonstrate the presence of a large inversion within the UL region of ILTV, a feature not found in the genomes of HSV-1, VZV, EHV-1, and BHV-1 (6, 31, 44, 47). The predicted inversion ranges from the UL22 (gH) gene to the UL44 (gC) gene (Fig. 3). Although the complete sequence of the inverted genome region is not yet known, restriction analyses of cosmid-cloned ILTV DNA fragments spanning this region revealed a distance of ca. 48 kbp between the 3′ ends of the UL22 and UL44 genes, a value which is in good agreement with the distances of the respective genes in HSV-1 (54 kbp; 31) and VZV (49 kbp; 6).
FIG. 3.
Comparative diagram of the genomes of HSV-1, ILTV, and PrV. Inverted repeat sequences flanking the US and, in HSV-1, also the UL genome regions are drawn as solid and open rectangles, respectively. Conserved ORFs are designated according to the nomenclature adopted for HSV-1 genes. The five unique ILTV ORFs, A, B, C, D, and E, are highlighted in black. Internal inversions within the UL regions are illustrated by shaded polygons. The depicted genomes are not drawn to scale, but approximate sizes in kilobase pairs of the inverted gene clusters in PrV and ILTV are indicated.
A large inversion of a part of the UL region has also been observed in the PrV genome (3, 9, 30). Compared to those of other alphaherpesviruses, the PrV genome contains an inversion of ca. 40 kbp encompassing the UL27 to UL44 ORFs (Fig. 3). Strikingly, the inverted sequences in both ILTV and PrV are bordered at one end by UL44. However, the opposite termini of the inversions differ between ILTV and PrV and are marked by the UL22 (gH) and UL27 (gB) genes, respectively.
Detailed phylogenetic analyses indicated that the alphaherpesvirus lineage leading to present-day ILTV branched first from the lineage leading to MDV and the mammalian alphaherpesviruses (33). In terms of evolutionary distance, ILTV is as distant from the avian MDV as from the mammalian alphaherpesviruses. This is also reflected in the amino acid homologies for several deduced proteins (Table 2). Thus, differences in genome organization and gene content could either reflect retention of primordial features in the ILTV lineage or could be the result of evolutionary adaptation after separation from the MDV and mammalian herpesvirus lineages. The presence of a cluster of ORFs unique to ILTV, which are absent not only from other alphaherpesviruses but also from beta- and gammaherpesviruses, indicates that the ORFs might have been acquired in the ILTV lineage. So far, the timescale for the divergence of alpha-, beta-, and gammaherpesviruses from a putative common ancestor is not clear (34).
Unique ORFs A to E are located at, or at least close to, one end of a large inversion within the ILTV genome, a feature not found in the genomes of most other alphaherpesviruses (Fig. 3). The hypothetical acquisition of these ORFs might thus be related to the inversion event. Interestingly, in the PrV genome a similar inversion is not associated with the presence of genes unique for PrV. Whether the presence of one common terminus in the PrV and ILTV inversions is purely coincidental or determined by a structural or functional feature in the respective genome regions remains to be determined.
Nucleotide sequence accession numbers.
The sequences obtained in this study have been assigned GenBank accession no. Y14300 and Y14301.
Acknowledgments
We thank C. Ehrlich for expert technical assistance.
This study was supported by a grant from Intervet Intl. B.V.
REFERENCES
- 1.Bagust T J, Johnson M A. Avian infectious laryngotracheitis: virus-host interactions in relation to prospects for eradication. Avian Pathol. 1995;24:373–391. doi: 10.1080/03079459508419079. [DOI] [PubMed] [Google Scholar]
- 2.Baines J D, Koyama A, Huang T, Roizman B. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J Virol. 1994;68:2929–2936. doi: 10.1128/jvi.68.5.2929-2936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Porat T, Veach R A, Ihara S. Localization of the regions of homology between the genomes of herpes simplex virus type 1 and pseudorabies virus. Virology. 1983;127:194–204. doi: 10.1016/0042-6822(83)90383-5. [DOI] [PubMed] [Google Scholar]
- 4.Breathnach R, Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- 5.Campbell M E M, Palfreyman J W, Preston C M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 6.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 7.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wind N, Wagenaar F, Pol J, Kimman T, Berns A. The pseudorabies virus homolog of the herpes simplex virus UL21 gene product is a capsid protein which is involved in capsid maturation. J Virol. 1992;66:7096–7103. doi: 10.1128/jvi.66.12.7096-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dezélée B, Bras F, Vende P, Simonet B, Nguyen X, Flamand A, Masse M J. The Bam HI fragment 9 of pseudorabies virus contains genes homologous to the UL24, UL25, UL26, and UL26.5 genes of herpes simplex virus type 1. Virus Res. 1996;42:27–39. doi: 10.1016/0168-1702(96)01293-2. [DOI] [PubMed] [Google Scholar]
- 10.Elliott G D, Meredith D M. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J Gen Virol. 1992;73:723–726. doi: 10.1099/0022-1317-73-3-723. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs W, Mettenleiter T C. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J Gen Virol. 1996;77:2221–2229. doi: 10.1099/0022-1317-77-9-2221. [DOI] [PubMed] [Google Scholar]
- 12.Griffin A M. Identification of 21 genes of infectious laryngotracheitis virus using random sequencing of genomic DNA. J Gen Virol. 1989;70:3085–3089. doi: 10.1099/0022-1317-70-11-3085. [DOI] [PubMed] [Google Scholar]
- 13.Griffin A M, Boursnell M E G. Analysis of the nucleotide sequence of DNA from the region of the thymidine kinase gene of infectious laryngotracheitis virus; potential evolutionary relationships between the herpesvirus subfamilies. J Gen Virol. 1990;71:841–850. doi: 10.1099/0022-1317-71-4-841. [DOI] [PubMed] [Google Scholar]
- 14.Griffin A M. The nucleotide sequence of the glycoprotein gB gene of infectious laryngotracheitis virus: analysis and evolutionary relationship to the homologous gene from other herpesviruses. J Gen Virol. 1991;72:393–398. doi: 10.1099/0022-1317-72-2-393. [DOI] [PubMed] [Google Scholar]
- 15.Hanson L E, Bagust T J. Laryngotracheitis. In: Calnek B W, editor. Diseases of poultry. 9th ed. Ames, Iowa: Iowa State University Press; 1991. pp. 485–495. [Google Scholar]
- 16.Ihara T, Kato A, Ueda S, Ishihama A, Hirai K. Comparison of the sequence of the secretory glycoprotein A (gA) in Md5 and BC-1 strains of Marek’s disease virus type 1. Virus Genes. 1989;3:127–140. doi: 10.1007/BF00125125. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M A, Prideaux C T, Kongsuwan K, Sheppard M, Fahey K J. Gallid herpesvirus 1 (infectious laryngotracheitis virus): cloning and physical maps of the SA-2 strain. Arch Virol. 1991;119:181–198. doi: 10.1007/BF01310669. [DOI] [PubMed] [Google Scholar]
- 18.Johnson M A, Tyack S G, Prideaux C T, Kongsuwan K, Sheppard M. Nucleotide sequence of infectious laryngotracheitis virus (gallid herpesvirus 1) ICP4 gene. Virus Res. 1995;35:193–204. doi: 10.1016/0168-1702(94)00096-u. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M A, Prideaux C T, Kongsuwan K, Tyack S G, Sheppard M. ICP27 immediate early gene, glycoprotein K (gK) and DNA helicase homologues of infectious laryngotracheitis virus (gallid herpesvirus 1) SA-2 strain. Arch Virol. 1995;140:623–634. doi: 10.1007/BF01309954. [DOI] [PubMed] [Google Scholar]
- 20.Johnson M A, Tyack S G. Molecular evolution of infectious laryngotracheitis virus (ILTV; gallid herpesvirus 1): an ancient example of the alphaherpesviridae? Vet Microbiol. 1995;46:221–231. doi: 10.1016/0378-1135(95)00086-p. [DOI] [PubMed] [Google Scholar]
- 21.Jöns A, Granzow H, Kuchling R, Mettenleiter T C. The UL49.5 gene of pseudorabies virus codes for an O-glycosylated structural protein of the viral envelope. J Virol. 1996;70:1237–1241. doi: 10.1128/jvi.70.2.1237-1241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingsley D H, Hazel J W, Keeler C L. Identification and characterization of the infectious laryngotracheitis virus glycoprotein C gene. Virology. 1994;203:336–343. doi: 10.1006/viro.1994.1492. [DOI] [PubMed] [Google Scholar]
- 23.Klupp B G, Kern H, Mettenleiter T C. The virulence-determining genomic BamHI fragment 4 of pseudorabies virus contains genes corresponding to the UL15 (partial), UL18, UL19, UL20, and UL21 genes of herpes simplex virus and a putative origin of replication. Virology. 1992;191:900–908. doi: 10.1016/0042-6822(92)90265-q. [DOI] [PubMed] [Google Scholar]
- 24.Klupp B G, Lomniczi B, Visser N, Fuchs W, Mettenleiter T C. Mutations affecting the UL21 gene contribute to avirulence of pseudorabies virus vaccine strain Bartha. Virology. 1995;212:466–473. doi: 10.1006/viro.1995.1504. [DOI] [PubMed] [Google Scholar]
- 25.Koff A, Tegtmeyer P. Characterization of major recognition sequences for a herpes simplex virus type 1 origin-binding protein. J Virol. 1988;62:4096–4103. doi: 10.1128/jvi.62.11.4096-4103.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kongsuwan K, Johnson M A, Prideaux C T, Sheppard M. Use of λgt11 and monoclonal antibodies to map the gene for the 60,000 dalton glycoprotein of infectious laryngotracheitis virus. Virus Genes. 1993;7:297–303. doi: 10.1007/BF01702590. [DOI] [PubMed] [Google Scholar]
- 27.Leib D A, Bradbury J M, Hart C A, McCarthy K. Genome isomerism in two alphaherpesviruses: herpesvirus saimiri-1 (herpesvirus tamarinus) and avian infectious laryngotracheitis virus. Arch Virol. 1987;93:287–294. doi: 10.1007/BF01310982. [DOI] [PubMed] [Google Scholar]
- 28.Liang X, Chow B, Raggo C, Babiuk L A. Bovine herpesvirus 1 UL49.5 homolog gene encodes a novel viral envelope protein that forms a disulfide-linked complex with a second virion structural protein. J Virol. 1996;70:1448–1454. doi: 10.1128/jvi.70.3.1448-1454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockshon D, Galloway D A. Sequence and structural requirements of a herpes simplex viral DNA replication origin. Mol Cell Biol. 1988;8:4018–4027. doi: 10.1128/mcb.8.10.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masse, M. J. Personal communication.
- 31.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the unique long region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch D J. Protein sequence comparisons show that the “pseudoproteases” encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990;18:4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGeoch D J, Cook S. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol. 1994;238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 34.McGeoch D J, Cook S, Dolan A, Jamieson F, Telford E. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 35.McKnight J L C, Pellett P E, Jenkins F J, Roizman B. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate α-trans-inducing factor-dependent activation of α genes. J Virol. 1987;61:992–1001. doi: 10.1128/jvi.61.4.992-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plummer G, Goodheart C R, Henson D, Bowling C P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology. 1969;39:134–137. doi: 10.1016/0042-6822(69)90355-9. [DOI] [PubMed] [Google Scholar]
- 37.Preston V G, Fisher F B. Identification of the herpesvirus type 1 gene encoding the dUTPase. Virology. 1984;138:58–68. doi: 10.1016/0042-6822(84)90147-8. [DOI] [PubMed] [Google Scholar]
- 38.Roizman B, Derosiers R, Fleckenstein B, Lopez C, Minson A C, Studdert M J. The family Herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 39.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2221–2230. [Google Scholar]
- 40.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Schnitzlein W M, Radzevicius J, Tripathy D N. Propagation of infectious laryngotracheitis virus in an avian liver cell line. Avian Dis. 1994;38:211–217. [PubMed] [Google Scholar]
- 43.Scholz E, Welniak E, Nyholm T, Guo P. An avian hepatoma cell line for the cultivation of infectious laryngotracheitis virus and for the expression of foreign genes with a mammalian promoter. J Virol Methods. 1993;43:273–286. doi: 10.1016/0166-0934(93)90146-i. [DOI] [PubMed] [Google Scholar]
- 44.Schwyzer M, Ackermann M. Molecular biology of ruminant herpesviruses. Vet Microbiol. 1996;53:17–29. doi: 10.1016/s0378-1135(96)01231-x. [DOI] [PubMed] [Google Scholar]
- 45.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–189. [Google Scholar]
- 46.Stow N D, Davison A J. Identification of a varicella-zoster virus origin of DNA replication and its activation by herpes simplex virus type 1 gene products. J Gen Virol. 1986;67:1613–1623. doi: 10.1099/0022-1317-67-8-1613. [DOI] [PubMed] [Google Scholar]
- 47.Telford E A, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 48.Visalli R J, Brandt C R. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29:167–178. doi: 10.1016/0168-1702(93)90057-t. [DOI] [PubMed] [Google Scholar]
- 49.Vlcek C, Benes V, Lu Z, Kutish G F, Paces V, Rock D, Letchworth G, Schwyzer M. Nucleotide sequence analysis of a 30-kb region of the bovine herpesvirus 1 genome which exhibits a colinear gene arrangement with the UL21 to UL4 genes of herpes simplex virus. Virology. 1995;210:100–108. doi: 10.1006/viro.1995.1321. [DOI] [PubMed] [Google Scholar]
- 50.Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990;15:277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- 51.Wild M A, Cook S, Cochran M. A genomic map of infectious laryngotracheitis virus and the sequence and organization of genes present in the unique short and flanking regions. Virus Genes. 1996;12:107–116. doi: 10.1007/BF00572949. [DOI] [PubMed] [Google Scholar]
- 52.Williams R A, Bennett M, Bradbury J M, Gaskell R M, Jones R C, Jordan F T W. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. J Gen Virol. 1992;73:2415–2420. doi: 10.1099/0022-1317-73-9-2415. [DOI] [PubMed] [Google Scholar]
- 53.Yanagida N, Yoshida S, Nazerian K, Lee L F. Nucleotide and predicted amino acid sequences of Marek’s disease virus homologues of herpes simplex virus major tegument proteins. J Gen Virol. 1993;74:1837–1845. doi: 10.1099/0022-1317-74-9-1837. [DOI] [PubMed] [Google Scholar]