Abstract
Background
Surgery is the cornerstone of the treatment of esophageal cancer (EC). This study is to evaluate the dietary habits and nutrition status in EC patients who underwent esophagectomy followed by esophageal reconstruction.
Methods
This retrospective study included patients with EC who underwent esophagectomy followed by esophageal reconstruction in the Department of Thoracic Surgery I of Peking University Cancer Hospital between February 2014 and December 2018. The primary outcomes were dietary habits and nutrition status. The secondary outcomes were gastrointestinal symptoms and quality of life (QoL).
Results
A total of 346 patients were included. At 30 months after the operation, 90.2% of the patients had recovered to regular dietary habits, 72.8% of patients had a restored frequency of preoperative regular food intake, 2.3% of the patients ate more than six times a day, and 0.6% had semi-liquid food because of bad teeth. The nutrition status remained stable after 6 months postoperatively and recovered slightly 1 year after the surgery. At 30 months after the operation, the most common gastrointestinal symptoms were reflux (38.4%), dysphagia (15.3%), hoarseness (11.8%), abdominal distension (6.6%), diarrhea (2.9%), and nausea and vomiting (2.3%). According to the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-OG 25 (EORTC QLQ-OG 25), the factors that affected the life quality of patients during follow-up were anxiety, reflux, and dietary limitations.
Conclusions
Most patients with EC who underwent esophageal reconstruction recovered to regular dietary habits and stable nutrition status, while some may still suffer from gastrointestinal symptoms, anxiety, and dietary limitations.
Keywords: Esophageal cancer (EC), esophageal reconstruction, nutrition, dietary habits, complications
Highlight box.
Key findings
• Most patients with esophageal cancer (EC) who underwent esophageal reconstruction might recover to regular dietary habits and stable nutrition status, while some may still suffer from gastrointestinal symptoms, anxiety, and dietary limitations.
What is known and what is new?
• Limited information is available about the changes in postoperative dietary habits of patients.
• Gastrointestinal symptoms after EC surgery gradually increased with oral feeding within 3 months after discharge, slowly relieving with the body’s adaptation and diet adjustment.
What is the implication, and what should change now?
• This article evaluates the dietary habits and nutrition status in EC patients who underwent esophagectomy followed by esophageal reconstruction. Objectively recording the changes in long-term dietary habits, nutrition, and symptoms of patients after gastric conduit substitution surgery can help us improve the operation and long-term postoperative nutritional management.
Introduction
Esophageal cancer (EC) is the 7th most common malignancy in the world, and China has a high incidence of EC, accounting for 50% of the world’s new cases (1,2). Surgery is the major treatment for EC, consisting of tumor resection and digestive tract reconstruction (3,4). Digestive tract reconstruction after resection directly influences nutrition, digestive tract symptoms, and quality of life (QoL) (5). With the development of comprehensive multidisciplinary treatments, particularly the addition of immunotherapy, the survival of patients has improved significantly with ensuring an optimal QoL during survival being essential.
Esophageal reconstruction using a gastric conduit after esophagectomy for EC has been the most important advance in EC management in the past two decades (6). In comparison with the whole stomach, gastric conduit has some advantages: (I) a thoracic gastric conduit does not interfere with cardiopulmonary function after eating; (II) the reduction of stomach volume and acid secretion area reduces the postoperative aspiration rate and acid reflux symptoms; (III) the severity of postoperative anastomotic leakage is alleviated; and (IV) postoperative stomach retention can be avoided (7). Nevertheless, normal physiological functions such as gastric storage, secretion, and emptying are weakened or even disappear after the operation, and the various symptoms experienced in the recovery stage will affect patients’ dietary habits to various extents (8). Still, considering the lack of long-term follow-up of postoperative QoL, limited information is available about the changes in postoperative dietary habits of patients.
Therefore, this study aimed to evaluate the dietary habits and nutrition status in EC patients who underwent esophagectomy followed by esophageal reconstruction using a gastric conduit. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1266/rc).
Methods
Study design and patients
This retrospective study included patients with EC who underwent esophagectomy followed by esophageal reconstruction in the Department of Thoracic Surgery I of Peking University Cancer Hospital between February 2014 and December 2018.
The inclusion criteria were (I) pathologically confirmed as esophageal or gastroesophageal junction malignancy; (II) underwent esophagectomy followed by esophageal reconstruction; (III) relapse-free status during the study; and (IV) survived longer than 30 months postoperatively. The exclusion criteria were (I) with other malignancies and under treatment; (II) comorbid with serious systemic diseases leading to difficulties in self-care; or (III) incomplete follow-up data.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Peking University Cancer Hospital (approve No. 2017YJZ32). Written informed consent was obtained from all patients.
Data collection and definition
All patients underwent esophageal reconstruction surgery and postoperative nutrition management, patient education, and follow-up (Appendix 1). The primary outcomes were long-term dietary habits and nutrition status. The secondary outcomes were gastrointestinal symptoms and QoL assessed by the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-OG 25 [EORTC QLQ-OG 25 (9)].
Evaluation
Dietary habits data
The dietary types and times of oral feeding were collected. The dietary types were divided into four types according to food properties, including liquid food, semi-liquid food, soft food, and common food (Table S1). According to the times of oral feeding, the feeding frequencies of the patients were divided into three times, four or five times, and six times or more per day.
Nutrition status evaluation data
The patient’s nutrition status was assessed according to their weight, recorded at 1, 3, 6, 12, and 30 months after the operation.
Gastrointestinal-related symptoms data
Symptoms including reflux, abdominal distension, diarrhea, nausea and vomiting, eating difficulties, and hoarseness were recorded. Patients’ treatment or remission was also recorded.
QoL assessment data
QoL was assessed based on the EORTC QLQ-OG 25. This scale effectively evaluates health-related QoL in patients with EC, esophageal gastric junction cancer (EGJC), and gastric cancer (GC). The scale consists of six symptoms, including dysphagia (three items), dietary limitations (four items), reflux (two items), swallowing pain (two items), pain and discomfort (two items), anxiety (two items), and 10 single items related to patients with upper gastrointestinal cancer who receive palliative treatment or potentially curative treatment or follow-up. Each item is divided into four levels, including never, a little, often, and frequent, which are assigned as 1, 2, 3, and 4, respectively. The higher the patient score is, the more serious the symptoms are.
Surgery
All included patients underwent curative surgery for EC. Subtotal esophagectomy was performed through the thorax or esophageal hiatus, and an esophageal reconstruction was used for esophagus substitution with cervical or intrathoracic anastomosis. The greater curvature was used in esophageal reconstruction tailoring, in which only the right gastroepiploic vascular arch remained. The width of the esophageal reconstruction was 3–4 cm. The esophageal reconstruction was lifted to the top of the chest or neck through the posterior mediastinum for end-to-side esophagogastrostomy (Videos 1,2).
Video 1.
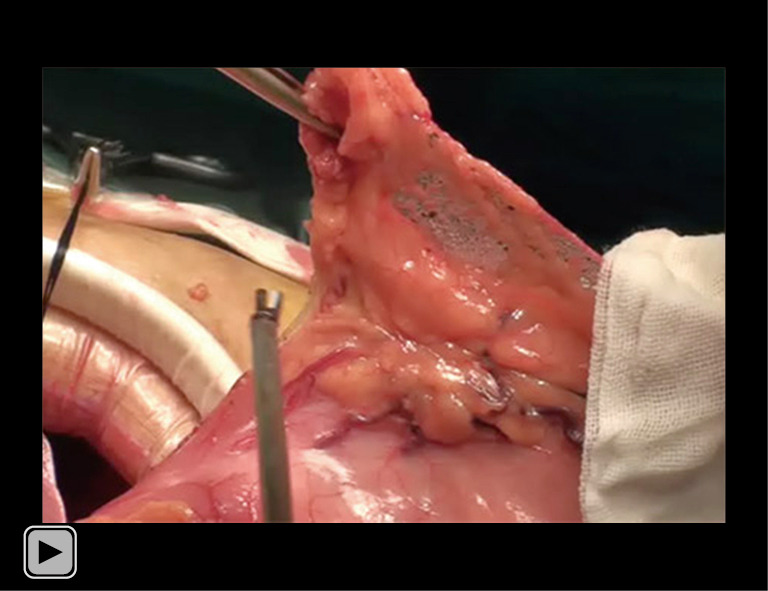
Tailoring of the esophageal reconstruction. The stomach was separated, where only the right gastroepiploic vessels remained under laparoscopy. The stomach was pulled out of the abdominal cavity, the tissue of the lesser curvature was released, and the stomach was tailored into the esophageal reconstruction with a 3–4 cm width along the greater curvature. The length of the esophageal reconstruction could reach 40 cm. The seromuscular layer was sutured intermittently, the cutting edge of the esophageal reconstruction was embedded, and the esophageal reconstruction was pulled from the esophageal bed in the posterior mediastinal to the neck. Cervical anastomosis: 90% of the patients had end-to-side esophagus-esophageal reconstruction anastomosis at the neck. A Johnson & Johnson 21- or 25-mm round stapler was used for anastomosis. After the completion of the anastomosis, it was not reinforced or embedded, and it was placed in the esophageal bed. The nasoduodenal nutrition tube and gastric tube were placed during anastomosis.
Video 2.
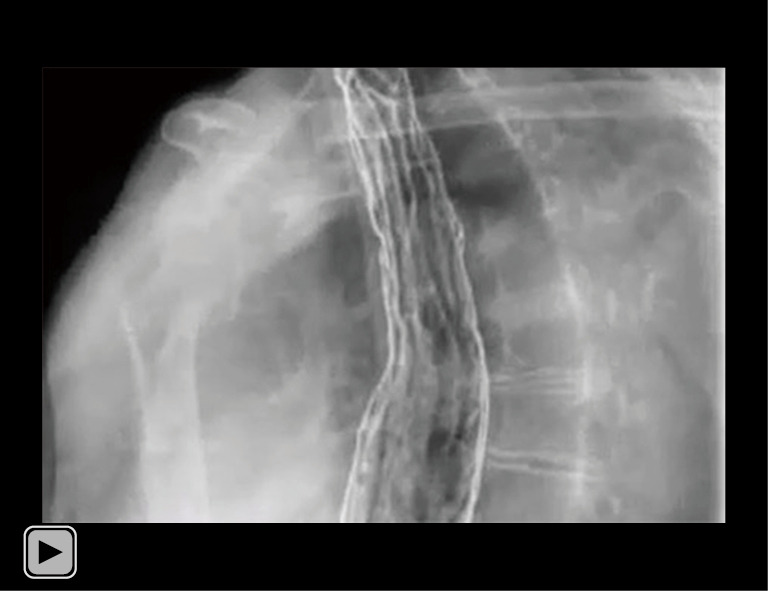
Postoperative radiography shows the esophageal reconstruction in the esophageal bed in the posterior mediastinal. The contrast medium passed through smoothly. No overflow or abnormal distribution of contrast medium was found. The shape of the duodenum was normal. No residual contrast medium was found.
Statistical analysis
SAS 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis. The categorical data were described as n (%) and analyzed using the Kruskal-Wallis H test. Means ± standard deviations were used to describe the continuous variables. A mixed effect model was used for the statistical analysis of repeated measurements to determine the statistically significant differences between different visits. Student’s t-test was conducted between different time points. Two-sided P values <0.05 were considered statistically significant.
Results
Finally, 346 patients were included in the study according to the inclusion and exclusion criteria (Figure 1). There were 263 (76.0%) males. The median age was 69 years (range, 39–78 years), and nearly half of the patients (n=169, 48.8%) were overweight or obese before the operation. The tumor was mainly located in the middle and lower thorax (n=300, 86.7%), and the pathological type was mainly squamous cell carcinoma (n=316, 91.3%). Most patients (n=321, 92.8%) were treated with minimally invasive surgery. The main surgical method was the McKeown operation (n=313, 90.5%; Table 1).
Figure 1.
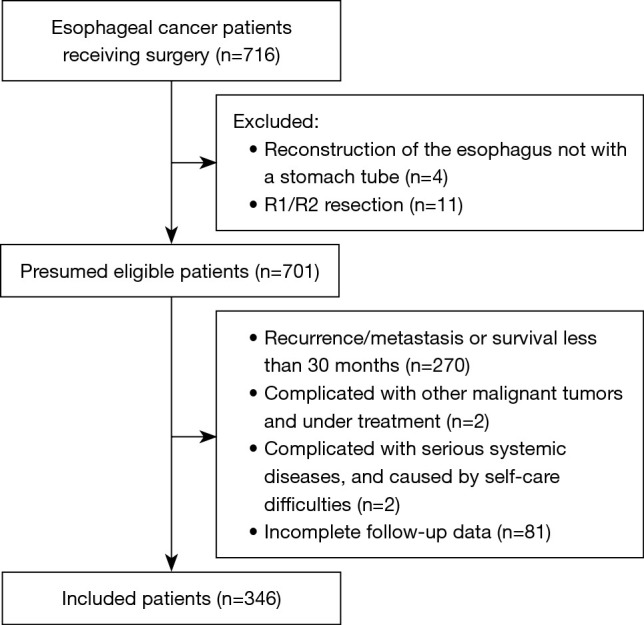
Patient flowchart.
Table 1. Characteristics of the patients.
| Variables | N (%) |
|---|---|
| Sex | |
| Male | 263 (76.0) |
| Female | 83 (24.0) |
| Age | |
| <65 years | 222 (64.2) |
| ≥65 years | 124 (35.8) |
| BMI | |
| ≤18.5 kg/m2 | 10 (2.9) |
| 18.6–23.9 kg/m2 | 167 (48.3) |
| ≥24 kg/m2 | 169 (48.8) |
| ECOG | |
| 0 | 303 (87.6) |
| 1 | 43 (12.4) |
| Tumor location | |
| Cervical | 8 (2.3) |
| Upper thoracic | 23 (6.7) |
| Middle thoracic | 169 (48.8) |
| Lower thoracic | 131 (37.9) |
| Gastroesophageal junction | 15 (4.3) |
| Pathologic type | |
| Squamous carcinoma | 316 (91.3) |
| Adenocarcinoma | 30 (8.7) |
| Surgical approach | |
| Open | 25 (7.2) |
| Minimally invasive esophagectomy | 321 (92.8) |
| Surgical procedure | |
| McKeown | 313 (90.5) |
| Ivor-Lewis | 18 (5.2) |
| THE | 15 (4.3) |
| Pathologic stage | |
| I | 114 (33.0) |
| II | 104 (30.1) |
| III | 118 (34.1) |
| IV | 10 (2.9) |
| Anastomotic position | |
| Cervical | 313 (90.5) |
| Thoracic | 18 (5.2) |
| Abdominal | 15 (4.3) |
| Clavien-Dindo grade | |
| 1–2 | 141 (40.8) |
| ≥3 | 60 (14.3) |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; THE, transhiatal esophagectomy.
Among patients, 36.1% did not recover from tube feeding to oral feeding within 1 month, and 99.4% of the patients had recovered to oral feeding 3 months after the surgery, among which 86.1% had regular food intake for more than thrice a day. One year after the operation, 80.6% of the patients had recovered to regular food. With the extension of follow-up time, patients of the whole group gradually recovered to daily regular food intake at the preoperative level. At 30 months after the operation, 90.2% of the patients had recovered to regular dietary habits, 72.8% of patients had a restored frequency of preoperative regular food intake, 2.3% of the patients ate more than six times a day, and 0.6% had semi-liquid food because of bad teeth (Table 2).
Table 2. Times of common food intake and major food types of EC patients after surgery.
| After surgery | Enteral feeding patients, n (%) | Patients at different feeding frequencies, n (%) | Patients with major food types, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3/day | 4–5/day | ≥6/day | Liquid | Semi-liquid | Soft | Common | |||
| 1 month | 125 (36.1) | 30 (8.7) | 123 (35.5) | 68 (19.7) | 2 (0.6) | 0 | 212 (61.3) | 7 (2.0) | |
| 3 months | 2 (0.6) | 46 (13.3) | 227 (65.6) | 71 (20.5) | 14 (4.0) | 3 (1.0) | 257 (74.3) | 70 (20.2) | |
| 6 months | 1 (0.3) | 115 (33.2) | 205 (59.2) | 25 (7.2) | 0 | 4 (1.2) | 142 (41.0) | 199 (57.5) | |
| 12 months | 0 | 196 (56.6) | 135 (39.0) | 15 (4.0) | 0 | 4 (1.2) | 63 (18.2) | 279 (80.6) | |
| ≥30 months | 0 | 252 (72.8) | 86 (24.9) | 8 (2.3) | 0 | 2 (0.6) | 32 (9.2) | 312 (90.2) | |
EC, esophageal cancer.
The patients’ weight showed a downward trend within half a year after the operation (P<0.001). The weight decreased the fastest in the first 3 months after the operation, with an average weight loss of 7% [95% confidence interval (CI): −7.3% to −6.5%], and then slowly downward to the lowest within 6 months after the operation, with an average weight loss of 10% (95% CI: −10.6% to −9.5%) compared with that at discharge. The nutrition status (weight) remained stable after 6 months postoperatively (P=0.924) and recovered slightly 1 year after the operation (P<0.001; Figure 2).
Figure 2.
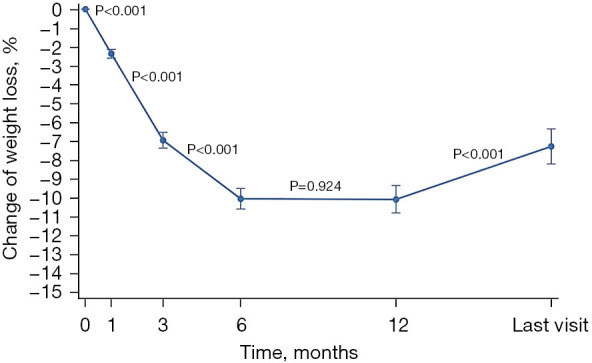
The trend of weight change in patients with EC after surgery. Body weight decreased rapidly 6 months after surgery (P<0.001), remained stable from 6 to 12 months (P=0.924), and gradually recovered 1 year after surgery (P<0.001). EC, esophageal cancer.
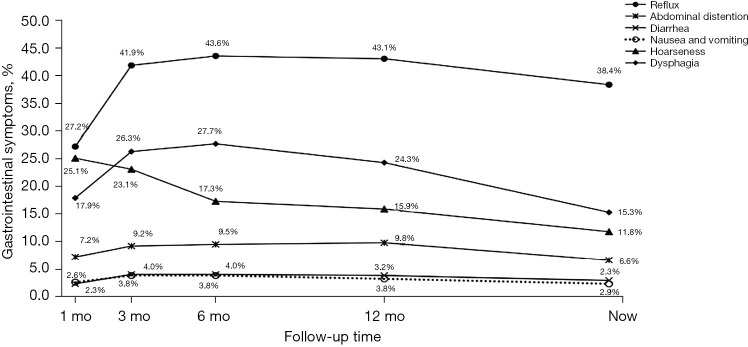
Gastrointestinal symptoms after EC surgery gradually increased with oral feeding within 3 months after discharge, slowly decreasing with the body’s adaptation and diet adjustment. Reflux was the most common symptom, with the highest incidence of 43.6% at 3–6 months after the operation. At 30 months, 38.4% of the patients still had reflux, but the degree of reflux in patients with gastric conduit was mild, and only 2.6% required medication. The incidence of dysphagia was the highest within half a year after the operation. As high as 27.7% of the patients had dysphagia, of which 8.4% needed dilation. Meanwhile, 25.1% of the patients had hoarseness 1 month after the operation. With the recovery of injured nerves, the proportion of hoarseness gradually decreased to 11.8%. Other postoperative gastrointestinal symptoms included abdominal distension, diarrhea, and nausea and vomiting, and their rates at 30 months postoperatively were 6.6%, 2.9%, and 2.3%, respectively (Figure 3).
Figure 3.
Trends in patients’ gastrointestinal symptoms. Three months after surgery, the incidence of reflux increased rapidly and remained high, while dysphagia increased rapidly and continued to decrease. The incidence of abdominal distension, diarrhea, nausea, and vomiting increased slightly and remained relatively low. mo, months.
The QoL of patients with EC was assessed 30 months after surgery using the EORTC QLQ-OG25 scale. Anxiety, followed by reflux and dietary limitations, were the long-term factors affecting the QoL of the patients (Table 3). Our analysis showed that the 6 symptoms in EORTC-OG25 questionnaire were not associated with the total complication rates postoperatively (see Tables 4,5). Further analysis showed that the proportions of dysphagia in patients with anastomotic fistula and non-anastomotic fistula were 13/21 (61.9%) and 52/325 (16.0%), respectively (P<0.01).
Table 3. The proportions of patients different symptoms and score based on EORTC QLQ-OG 25.
| Symptoms | EORTC QLQ-OG 25, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Reflux (two items) | 204 (59.0) | 16 (4.6) | 47 (13.6) | 44 (12.7) | 29 (8.4) | 2 (0.6) | 4 (1.2) | – |
| Swallowing pain (two items) | 307 (88.7) | 33 (9.5) | 6 (1.7) | – | – | – | – | – |
| Pain (two items) | 256 (74.0) | 60 (17.3) | 28 (8.1) | 2 (0.6) | – | – | – | – |
| Anxiety (two items) | 164 (47.4) | 98 (28.3) | 58 (16.8) | 23 (6.7) | 3 (0.9) | – | – | – |
| Dysphasia (three items) | – | 281 (81.2) | 55 (15.9) | 8 (2.3) | 2 (0.6) | – | – | – |
| Food limitation (four items) | – | – | 240 (69.4) | 49 (14.2) | 37 (10.7) | 15 (4.3) | 4 (1.2) | 1 (0.3) |
Reflux was consisted of two items, with score ranges from 2 to 8. Score 2 refers to no reflux and score 8 represents the most severe reflux. Proportions of patients with score 2, 3, 4, 5, 6, 7, and 8 were 59.0%, 4.6%, 13.6%, 12.7%, 8.4%, 0.6%, and 1.2%. The scores of other symptoms were similar to that of reflux. EORTC QLQ-OG 25, European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-OG 25.
Table 4. Complications of the patients after surgery.
| Complication | Complication group, n (%) | Non-complication group, n (%) |
|---|---|---|
| Pneumonia | 62 (17.9) | 284 (82.1) |
| Pleural effusion | 26 (7.5) | 320 (92.5) |
| Chylothorax | 9 (2.6) | 337 (97.4) |
| Anastomotic fistula | 21 (6.1) | 325 (93.9) |
| Incision Infection | 6 (1.7) | 340 (98.3) |
| Cardiovascular complications | 22 (6.4) | 324 (93.6) |
| Other | 16 (4.6) | 330 (95.4) |
Table 5. Relationship between six symptoms based on EORTC QLQ-OG 25 and complications.
| Symptom | Complication group (n=162) | Non-complication group (n=184) | P |
|---|---|---|---|
| Reflux | 70 (43.2) | 72 (39.1) | 0.44 |
| Swallowing pain | 19 (11.7) | 20 (10.9) | 0.80 |
| Pain | 48 (29.6) | 42 (22.8) | 0.15 |
| Anxiety | 90 (55.6) | 92 (50.0) | 0.30 |
| Dysphasia | 31 (19.1) | 34 (18.5) | 0.88 |
| Food limitation | 51 (31.5) | 55 (29.9) | 0.75 |
EORTC QLQ-OG 25, European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-OG 25.
Discussion
The results of this study indicate that most patients with EC who underwent esophageal reconstruction using gastric conduit might recover to regular dietary habits and stable nutrition status, while some may still suffer from gastrointestinal symptoms, anxiety, and dietary limitations. These results provide clinical guidance for the long-term outcomes and nursing of patients with EC and esophageal reconstruction.
EC surgery involves tumor resection and upper digestive tract reconstruction. Digestive tract reconstruction influences the patient’s eating habits, nutritional status, and QoL (10-13). The total stomach substitution for the esophagus remarkably affects the patients’ long-term nutritional status, gastrointestinal symptoms, and QoL (7,14). Recently, gastric conduit has gradually replaced the whole stomach as esophagus substitution in EC surgery. Nevertheless, limited research focused on the changes in dietary habits, nutritional status, and gastrointestinal symptoms of patients postoperatively. The present study was based on a single-center prospective database. The long-term follow-up results showed that more than 70% of patients could resume eating three meals daily and maintain a relatively stable weight 30 months postoperatively. Postoperative gastrointestinal symptoms gradually alleviated with time, but some symptoms remained for a long time.
Most Chinese people eat three meals a day, and carbohydrates are the main source of energy intake for each meal. In postoperative patients with EC, considering the loss of the upper esophagus and surrounding muscle groups that assist in swallowing, physiological changes such as gastric volume reduction (esophageal reconstruction), position change (lifting from the abdominal cavity to the thoracic cavity), and loss of innervation (disconnection of the vagus nerve) affect the types, amount, and patterns of eating (8,14). The changes in dietary habits and gastrointestinal symptoms during eating often cause the patients to have a “fear of eating” after discharge, resulting in an impaired nutritional status caused by insufficient energy intake (10,15,16). Therefore, for discharged patients with EC, the nutrition nurses would provide postoperative nutrition guidance, including enteral feeding nutrition supplements early after discharge, early oral feeding guidance, and the transition between the two.
At the authors’ hospital, it is suggested that the patients start oral feeding 2–4 weeks postoperatively based on the principle of “having more meals a day but less food at each”. The daily feeding frequency is gradually increased to 6–8 times, and the enteral tube feeding nutritional supplement gradually decreases with the increased amount. The transitional period from tube-feeding enteral nutrition to oral feeding was observed 1 month after the operation. At 3–6 months after the operation, 60% of the patients had oral feeding 4–5 times daily. Only a few patients still needed tube-feeding enteral nutrition. At 12 months after the operation, the number of patients who recovered to three meals a day gradually increased. At 30 months after the operation, the proportion exceeded 72%.
At the authors’ institution, it is also suggested that the patients start with soft food and gradually transit to regular food intake. In the early postoperative period of patients with EC, uncoordinated swallowing caused by the loss of neck swallowing auxiliary muscles easily cause symptoms such as dysphagia and even aspiration. Compared with liquid and semi-liquid food, soft food can effectively exercise swallowing function and avoid aspiration and anastomotic stenosis (17). This study suggests that the patient’s diet in the early stage after the operation mainly included soft food, gradually transitioning to regular food intake. More than 80% of the patients could resume regular dietary habits 1 year after the operation, and 90% of the patients could have regular dietary habits 30 months after the operation. Although esophagectomy with esophageal reconstruction substitution causes changes in the structure of the upper digestive tract, the strong adaptability of the human body can enable most patients to recover to their preoperative dietary habits. Currently, there are few research on physiological changes within the first 6 months after esophagectomy.
Weight and body mass index (BMI) can reflect the nutritional status of the patients, and monitoring their dynamic changes can help clinical nutrition specialists guide patients’ nutritional supplementation. All patients with EC experience weight loss caused by insufficient nutrition intake to varying degrees (18,19). Without proper nutrition education, weight loss will persist and even cause adverse events (18,19). In the present study, although the nutrition specialist nurse provided nutrition guidance to the patients after the operation, significant weight loss was still observed within 6 months postoperatively, with an average decrease of 10.0%. This condition was related to the changes in the patient’s dietary habits after digestive tract reconstruction, gastrointestinal symptoms, and inadequate oral intake caused by perioperative radiotherapy and chemotherapy. Six months after the operation, with the gradual recovery of physical function, self-adjustment and adaptation of diet, reduction of gastrointestinal symptoms, and completion of radiotherapy and chemotherapy, the patient’s weight gradually stabilized and then recovered to a certain extent at 1 year postoperatively.
Compared with whole stomach substitution for esophagus, gastric conduit in EC surgery greatly reduces serious gastrointestinal complications, but patients still have related gastrointestinal symptoms (7). In the present study, reflux and dysphagia were the most common symptoms, followed by hoarseness, abdominal distension, diarrhea, nausea, and vomiting. Through long-term follow-up, it was observed that most of the digestive tract symptoms of the patients peaked at 3-6 months after the operation. Then, with the change in patients’ perception and adaptation to the symptoms, these symptoms were alleviated within 6–12 months after the operation.
Part of the esophagus and cardia is resected during esophagectomy, and the gastric conduit is anastomosed with the remaining esophagus in the neck to reconstruct the upper digestive tract. These structural changes cause reflux symptoms (20). In the present study, the reflux rate was the highest at 3–6 months after the operation and reached 43.6%. After 6 months, some patients avoided or reduced the occurrence of reflux by adjusting the food properties, timing of eating, activities after eating, and rest position. Only 2.6% of the patients with severe reflux symptoms needed long-term medication to alleviate them.
In the early phase of oral feeding postoperatively, the patients seldom complained about dysphagia, which easily brought false impressions to the doctors and nurses. With the recovery of the patient’s life to normal status, the symptoms of dysphagia gradually increased within 3–6 months after the operation, reaching 27.7%. Still, only 8.4% of the patients needed esophageal dilatation, which might be related to the patient’s anxiety about the postoperative discomfort symptoms of the disease, the limited information on the relevant symptoms, and the inability to distinguish between discomfort in swallowing and dysphagia.
Hoarseness caused by recurrent laryngeal nerve injury during lymph node dissection is the most obvious within 3 months after the operation (21). At 3–6 months after the operation, the voices of some patients recovered to normal with the recovery of nerves or through measures such as autonomous voice training, drug therapy, and psychological rehabilitation guidance. Still, 11.8% of patients had long-term hoarseness caused by permanent nerve injury (22,23).
Cancer survivors urgently need to improve their QoL, particularly in the context of even better survival with improvements in treatments, and gastrointestinal symptoms directly affect their QoL (24). The results showed that the long-term QoL of patients is affected by anxiety (mainly the worry about future health), which emphasizes the importance of psychological counseling for patients with EC (25). The second factor is reflux and dietary restriction, which is similar to the results of Schandl et al. (26).
As a retrospective study on the long-term nutritional status, dietary habits, and digestive tract symptoms of postoperative patients with EC, this study has the following limitations. This study was a single-center study with biases due to local practice. The patients were all Chinese, whose physique and dietary habits differ from those in Europe and the United States. This study only focused on patients without recurrence after curative resection of EC and cannot reflect the possible effect of tumor recurrence, metastasis, and the relevant anti-tumor treatment. With the continuous progress of esophageal surgery and related disciplines, the “stomach substitution for esophagus” operation has continued to improve, and surgery still occupies the leading position in EC treatment. “Gastric conduit” has unique advantages, but the problems faced by patients after digestive tract reconstruction cannot be ignored. Objectively recording the changes in long-term dietary habits, nutrition, and symptoms of patients after gastric conduit substitution surgery can help us improve the operation.
Conclusions
In conclusion, most patients with EC who underwent esophageal reconstruction using gastric conduit might recover to regular dietary habits and stable nutrition status, while some may still suffer from gastrointestinal symptoms, anxiety, and dietary limitations. However, these findings still need further verification by multi-center, prospective, and intervention studies with large sample sizes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Peking University Cancer Hospital (approve No. 2017YJZ32). Written informed consent was obtained from all patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1266/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1266/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1266/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1266/coif). The authors have no conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:582-97. 10.1016/S2468-1253(20)30007-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Esophageal and Esophagogastric Junction Cancers. Version 3.2022. Fort Washington: National Comprehensive Cancer Network; 2022. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=patients&id=74
- 4.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. 10.1093/annonc/mdw329 [DOI] [PubMed] [Google Scholar]
- 5.Burrows WM. Gastrointestinal function and related problems following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:142-51. 10.1053/j.semtcvs.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Rove JY, Krupnick AS, Baciewicz FA, et al. Gastric conduit revision postesophagectomy: Management for a rare complication. J Thorac Cardiovasc Surg 2017;154:1450-8. 10.1016/j.jtcvs.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Yu D, Peng J, et al. Gastric-tube versus whole-stomach esophagectomy for esophageal cancer: A systematic review and meta-analysis. PLoS One 2017;12:e0173416 . 10.1371/journal.pone.0173416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginex P, Thom B, Jingeleski M, et al. Patterns of symptoms following surgery for esophageal cancer. Oncol Nurs Forum 2013;40:E101-7. 10.1188/13.ONF.E101-E107 [DOI] [PubMed] [Google Scholar]
- 9.Lagergren P, Fayers P, Conroy T, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer 2007;43:2066-73. 10.1016/j.ejca.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Baker M, Halliday V, Williams RN, et al. A systematic review of the nutritional consequences of esophagectomy. Clin Nutr 2016;35:987-94. 10.1016/j.clnu.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer AG, van Lanschot JJ, van Sandick JW, et al. Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol 2004;22:4202-8. 10.1200/JCO.2004.11.102 [DOI] [PubMed] [Google Scholar]
- 12.Mariette C, Markar S, Dabakuyo-Yonli TS, et al. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg 2020;271:1023-9. 10.1097/SLA.0000000000003559 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Yang X, Geng D, et al. The change of health-related quality of life after minimally invasive esophagectomy for esophageal cancer: a meta-analysis. World J Surg Oncol 2018;16:97 . 10.1186/s12957-018-1330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Wang P, Zhang X, et al. Gastric tube reconstruction prevents postoperative recurrence and metastasis of esophageal cancer. Oncol Lett 2016;11:2507-9. 10.3892/ol.2016.4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koizumi M, Hosoya Y, Dezaki K, et al. Postoperative weight loss does not resolve after esophagectomy despite normal serum ghrelin levels. Ann Thorac Surg 2011;91:1032-7. 10.1016/j.athoracsur.2010.11.072 [DOI] [PubMed] [Google Scholar]
- 16.Haverkort EB, Binnekade JM, de Haan RJ, et al. Suboptimal intake of nutrients after esophagectomy with gastric tube reconstruction. J Acad Nutr Diet 2012;112:1080-7. 10.1016/j.jand.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 17.Yuen MTY, Tsang RK, Wong IYH, et al. Long-term pharyngeal dysphagia after esophagectomy for esophageal cancer-an investigation using videofluoroscopic swallow studies. Dis Esophagus 2019. [DOI] [PubMed] [Google Scholar]
- 18.Shpata V, Prendushi X, Kreka M, et al. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch 2014;68:263-7. 10.5455/medarh.2014.68.263-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kight CE. Nutrition considerations in esophagectomy patients. Nutr Clin Pract 2008;23:521-8. 10.1177/0884533608323427 [DOI] [PubMed] [Google Scholar]
- 20.Mody R, Bolge SC, Kannan H, et al. Effects of gastroesophageal reflux disease on sleep and outcomes. Clin Gastroenterol Hepatol 2009;7:953-9. 10.1016/j.cgh.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am 2004;37:25-44, v. 10.1016/S0030-6665(03)00172-5 [DOI] [PubMed] [Google Scholar]
- 22.Wright CD, Zeitels SM. Recurrent laryngeal nerve injuries after esophagectomy. Thorac Surg Clin 2006;16:23-33, v. 10.1016/j.thorsurg.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 23.Scholtemeijer MG, Seesing MFJ, Brenkman HJF, et al. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis 2017;9:S868-78. 10.21037/jtd.2017.06.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghajanzadeh M, Safarpour F, Koohsari MR, et al. Functional outcome of gastrointestinal tract and quality of life after esophageal reconstruction of esophagus cancer. Saudi J Gastroenterol 2009;15:24-8. 10.4103/1319-3767.45050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Z, Fang Y, Liu C, et al. Early Interdisciplinary Supportive Care in Patients With Previously Untreated Metastatic Esophagogastric Cancer: A Phase III Randomized Controlled Trial. J Clin Oncol 2021;39:748-56. 10.1200/JCO.20.01254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schandl A, Lagergren J, Johar A, et al. Health-related quality of life 10 years after oesophageal cancer surgery. Eur J Cancer 2016;69:43-50. 10.1016/j.ejca.2016.09.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as