Abstract
Background
Thoracic epidural analgesia (TEA) and liposomal bupivacaine (LB) are two methods used for postoperative pain control after thoracic surgery. Some studies have compared LB to standard bupivacaine. However, data comparing the outcomes of LB to TEA after minimally invasive lung resection is limited. Therefore, the objective of our study was to compare postoperative pain, opioid usage, and outcomes between patients who received TEA vs. LB.
Methods
We conducted a retrospective chart review of patients who underwent minimally invasive lung resections over an 8-month period. Intraoperatively, patients received either LB under direct vision or a TEA. Pain scores were obtained in the post-anesthesia care unit (PACU) and at 12, 24, and 48 hours postoperatively. Morphine milligram equivalents (MMEs) were calculated at 24 and 48 hours postoperatively. Postoperative outcomes were then compared between groups.
Results
In total, 391 patients underwent minimally invasive lung resection: 236 (60%) wedge resections, 51 (13%) segmentectomies, and 104 (27%) lobectomies. Of these, 326 (83%) received LB intraoperatively. Fewer patients in the LB group experienced postoperative complications (18% vs. 34%, P=0.004). LB patients also had lower median pain scores at 24 (P=0.03) and 48 hours (P=0.001) postoperatively. There was no difference in MMEs at 24 hours (P=0.49). However, at 48 hours, patients who received LB required less narcotics (P=0.02). Median hospital length of stay (LOS) was significantly shorter in patients who received LB (2 vs. 4 days, P<0.001). On multivariable analysis, increasing age, postoperative complications, and use of TEA were independently associated with a longer hospital LOS.
Conclusions
Compared to TEA, LB intercostal block placed under direct vision reduced morphine use 48 hours after thoracic surgery. It was also associated with fewer postoperative complications and shorter median hospital LOS. LB is a good alternative to TEA for pain management after minimally invasive lung resection.
Keywords: Liposomal bupivacaine (LB), epidural, postoperative pain after thoracic surgery
Highlight box.
Key findings
• Compared to thoracic epidural analgesia (TEA), direct witness of liposomal bupivacaine (LB) intercostal block reduced morphine use 48 hours postoperatively and decreased hospital length of stay.
What is known and what is new?
• Intercostal nerve blocks have been compared to LB, and LB has been compared to standard bupivacaine. However, comparisons between LB and TEA after minimally invasive lung resections are limited.
• Our work adds to literature by not only comparing narcotic use 24–48 hours postoperatively between those who received LB vs. TEA, but we also specifically describe witnessed subpleural injection.
What is the implication and what should change now?
• LB intercostal blocks placed under direct vision intraoperatively are a good alternative to TEA and result in decreased postoperative opioid use. This is important given the ongoing discussions addressing opioid use postoperatively. LB should be considered when possible.
Introduction
Thoracic epidural analgesia (TEA) with continuous infusion of bupivacaine was the gold standard for postoperative pain control in thoracic surgery for several decades (1). While it is an effective pain management strategy, TEA has been associated with higher rates of hypotension, urinary retention, peripheral paresthesia, subarachnoid puncture, and local anesthetic toxicity. Technical challenges may increase failure rate and result in catheter migration or need for repositioning (2,3).
Liposomal bupivacaine (LB) (Exparel®, Pacira Pharmaceuticals Inc., Parsippany, NJ, USA) is an extended-release form indicated for local administration. It allows for slower drug diffusion for up to 72 hours and provides adequate local analgesia postoperatively (4).
Though some studies, including two randomized trials, have compared intercostal injections of LB to those of standard bupivacaine after thoracic surgery (5-8), data comparing outcomes of LB and TEA use in patients undergoing minimally invasive lung resection is limited. Furthermore, few studies have included the quality assurance of witnessing the subpleural delivery of the drug.
The objective of our study was to compare the use of intercostal injections of LB under direct vision at time of thoracoscopy to TEA. Our primary outcome was postoperative opioid consumption. Our secondary outcomes were postoperative pain, adverse events, and hospital length of stay (LOS). We hypothesized equivalency in these outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1405/rc).
Methods
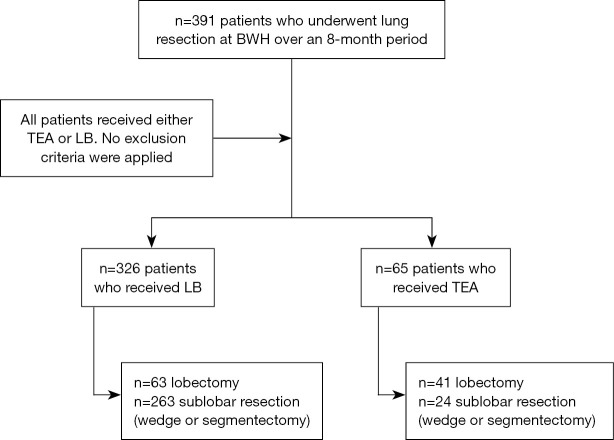
This is a retrospective study of prospectively collected clinical data. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval at our institution, Brigham and Women’s Hospital, was obtained (protocol #2014P002478). Informed consent for this retrospective analysis was waived. All patients undergoing minimally invasive lung resections over an 8-month period at Brigham and Women’s Hospital were included (Figure 1). The operating surgeon and anesthesiologist chose the method of postoperative analgesia based on the possibility of conversion to a thoracotomy, which was more likely if the following factors were present: 10 cm or larger tumor, central location of tumor, previous surgery on the same side or if the patient was on home oxygen pre-operatively. These patients were more likely to receive an epidural. Demographics, preoperative pulmonary function tests, perioperative data, and pre-existing conditions were collected. Morbidity was graded based on the Clavien-Dindo classification (9) (Table S1).
Figure 1.
Flow diagram of patients included in this study. BWH, Brigham and Women’s Hospital; TEA, thoracic epidural analgesia; LB, liposomal bupivacaine.
Surgical technique
Surgical incisions were the same for all patients undergoing minimally invasive lung resection: two incisions 2 cm in length in two different interspaces, and one utility incision of 4 cm without rib spreading. We considered a thoracotomy to be an incision that is 6 cm or longer or if rib spreading was involved.
Postoperative analgesia
Patients receiving LB were injected by the thoracic surgeon (attendings and/or fellows) prior to the end of surgery and placement of chest tube. Twenty mL vials of 1.3% LB diluted with 20 mL of 0.25% bupivacaine hydrochloride (HCl) were utilized. An eighteen-gauge needle was advanced percutaneously with an oscillating motion down to the pleura where the 2–5 mL bolus of diluted solution was injected from the third to the 10th intercostal spaces under direct vision of the thoracoscope. These injections were subpleural and raised a pleural weal beneath the intercostal nerve. For each incision, injections were made an interspace above and an interspace below, always posterior to the incision and between the incision and the thoracic spine. Direct vision by thoracoscope confirmed delivery of the drug to the subpleural depth and avoided missing an interspace. An additional 3 mL of the same solution was then injected in each of the port sites. Maximum doses were calculated by the anesthesiologist.
For patients receiving TEA, standard practice was to insert epidural multiport catheters preoperatively by the anesthesiologist at thoracic vertebral interspace numbers 6–7 (±1 level) and test-dose with 3 mL of 2% lidocaine with epinephrine (1:200,000). Blockade was initiated during the last hour of the operation with boluses of 10–15 mL of 0.0625–0.125% bupivacaine HCl followed by a continuous infusion of the same at 4–6 mL/h.
Patients in both groups received rescue analgesia with narcotic and non-narcotic medications as needed based on pain scores. Patients who scored above 7/10 on the standard visual analog pain scale received narcotics. These included morphine, fentanyl, hydromorphone, oxycodone, and tramadol. Adjunct non-narcotic agents included ketorolac, gabapentin, ibuprofen, and acetaminophen. Morphine milligram equivalents (MMEs) were calculated by transforming the daily dose of each pain medication into standardized units using validated conversion factors (9,10). Intraoperative narcotic administration was not factored into this calculation. The MMEs were summed for each patient at 24 and 48 hours postoperatively.
Postoperative pain assessment
Postoperative pain was assessed regularly by the nurse using the visual analog scale for pain, where one indicates ‘no pain’ and ten indicates the ‘worst pain possible’. Scores were collected in the post-anesthesia care unit (PACU) and at 12, 24, and 48 hours postoperatively. Patients who were discharged before postoperative day 2 were excluded from the 48-hour analysis.
Statistical analysis
Two-tailed Fisher’s exact or chi-square tests were used to compare categorical variables and Mann-Whitney or Kruskal-Wallis tests were used for continuous variables. A multivariable linear regression was created to assess the dependence of hospital LOS on age, postoperative complications, the use of TEA and preoperative forced expiratory volume in 1 second (FEV1%) (preoperative test, predicted by age, sex, and body composition). Statistical analysis was performed using R, version 4.2.1 (June 2022). A P value of <0.05 was considered statistically significant.
Results
In total, 391 patients were included, and 144 were males (36.8%). Operations performed were 236 (60%) wedge resections, 51 (13%) segmentectomies, and 104 (27%) lobectomies. A total of 326 (83%) patients received LB in the operating room (Figure 1). Table 1 shows the characteristics of the cohort stratified by pain management strategy. The median age at surgery for the whole cohort was 68 years (range, 26–89 years). The median age of patients who received TEA was slightly higher (72 years; range, 51–88 years) compared to those who received LB (66 years; range, 26–88 years) (P=0.001). TEA patients had lower median FEV1% (80%; range, 65–96%) compared to LB patients (88%; range, 23–157%) (P=0.004). Three hundred and thirty-two patients (85%) underwent resection for malignant causes: 245 patients with lung primary and 87 with pulmonary metastasis from an extra-thoracic malignancy. Sixty patients (92%) in the TEA group underwent resection for a malignant lesion compared to 272 patients (83%) in the LB group. Other demographic and clinical characteristics were similar between groups.
Table 1. Characteristics and outcomes of patients undergoing minimally invasive lung resections by postoperative pain strategy.
| Variables | LB (n=326) | TEA (n=65) | P value |
|---|---|---|---|
| Gender (female) | 209 (64.1) | 38 (58.5) | 0.40 |
| Charlson comorbidity index | 5±3 | 5±2 | 0.20 |
| ASA score | 0.25 | ||
| 2: mild systemic disease | 8 (2.5) | 3 (4.6) | |
| 3: severe systemic disease | 308 (94.5) | 62 (95.4) | |
| 4: incapacitating systemic disease | 10 (3.1) | 0 (0.0) | |
| Age at surgery (years) | 66 [26–88] | 72 [51–88] | 0.001 |
| FEV1 (% of predicted) | 88 [23–157] | 80 [65–96] | 0.004 |
| FEV1 ≥80% predicted | 227 (69.6) | 33 (50.8) | 0.004 |
| FEV1 50–79% predicted | 88 (27.0) | 23 (35.4) | 0.18 |
| FEV1 <50% predicted | 11 (3.4) | 9 (13.8) | 0.002 |
| Surgery type | <0.001 | ||
| Lobectomy | 63 (19.3) | 41 (63.1) | |
| Sublobar resection | 263 (80.7) | 24 (36.9) | |
| Indication | 0.086 | ||
| Malignancy | 272 (83.4) | 60 (92.3) | |
| Benign disease | 54 (16.6) | 5 (7.7) | |
| Patients with adverse events† | 57 (17.5) | 22 (33.8) | 0.004 |
| Grade II | 49 (15.0) | 20 (30.8) | 0.004 |
| Grade III | 16 (4.9) | 4 (6.2) | 0.76 |
| Grade IV | 5 (1.5) | 3 (4.6) | 0.13 |
| Perioperative mortality | 1 (0.3) | 0 (0.0) | >0.99 |
| Pain scores | |||
| PACU | 5 [0–10] | 6 [0–10] | 0.107 |
| 12 hours | 3 [0–10] | 4 [0–10] | 0.38 |
| 24 hours | 4 [0–10] | 5 [0–10] | 0.03 |
| 48 hours‡ | 2 [0–8] | 3 [0–10] | 0.001 |
| MME | |||
| 24 hours | 45 [0–440] | 38 [0–165] | 0.41 |
| 48 hours‡ | 20 [0–244] | 30 [0–143] | 0.03 |
| Non-narcotic analgesic adjuncts | |||
| Ketorolac | 178 (54.6) | 33 (50.8) | 0.59 |
| Gabapentin | 184 (56.4) | 34 (52.3) | 0.59 |
| Acetaminophen | 325 (99.7) | 64 (98.5) | 0.31 |
| Ibuprofen | 153 (46.9) | 26 (40.0) | 0.34 |
| Hospital LOS (days) | 2 [1–18] | 4 [1–22] | <0.001 |
Data are presented as n (%), mean ± SD, or median [range]. †, includes patients who experienced more than one adverse event; ‡, for patients with hospital LOS ≥2 days (n=323). Sixty-eight patients were excluded from the LB group and one patient from TEA group. LB, liposomal bupivacaine; TEA, thoracic epidural analgesia; ASA, American Society of Anesthesiologists; FEV1, forced expiratory volume in 1 second; PACU, post-anesthesia care unit; MME, morphine milligram equivalent; LOS, length of stay; SD, standard deviation.
Pain scores
Sixty-nine patients were discharged on postoperative day 1, 68 of whom had received LB. These patients were therefore excluded from the 48-hour pain score analysis. There was no difference in the pain scores in the PACU (P=0.107) or at 12 hours postoperatively (P=0.38) between groups (Table 1). At 24 hours postoperatively, TEA patients reported a median score of 5 (range, 0–10) compared to 4 (range, 0–10) reported by LB patients (P=0.03). At 48 hours postoperatively, TEA patients reported a median pain score of 3 (range, 0–10) compared to 2 (range, 0–8) reported by LB patients (P=0.001).
Opioid consumption and non-narcotic analgesics
The median MME at 24 hours of patients who received LB was 45 (range, 0–440) compared to the median MME of 38 (range, 0–165) for patients who received TEA (P=0.41). At 48 hours, the median MME for LB patients was 20 (range, 0–244) compared to the median MME of 30 (range, 0–143) for TEA patients (P=0.03; Table 1).
Use of non-narcotic analgesic adjuncts was similar between groups (Table 1). Acetaminophen was the most frequent drug administered. All patients except for two required at least one non-narcotic analgesic. Of these, 63 (16%) were treated with only one drug, 124 (32%) were treated with two drugs, 122 (31%) were treated with three drugs, and 80 (20%) used all four non-narcotic analgesic adjuncts. This was not different between groups.
Conversions
There were two conversions to thoracotomy (0.01%), and both patients had received TEA. One patient was undergoing a segmentectomy for a malignant lesion and was converted to a thoracotomy given dense adhesions. The second patient was undergoing a wedge resection and was converted to a thoracotomy because the surgeon was unable to safely palpate the lesion thoracoscopically.
Influence of preoperative FEV1
A possible confounder of the outcomes was the increased number of patients with a preoperative FEV1 <50% selected for the TEA group (14% vs. 3% of the LB group, P=0.002; Table 1). We performed subgroup analysis comparing perioperative outcomes between patients with similar FEV1% predicted who received LB or TEA. The results of these subgroup analyses are summarized in Table 2. FEV1% was trichotomized into patients with ≥80%, 50–79%, and <50% to reflect mild, moderate, and severe disease respectively. Among the 260 patients with FEV1 ≥80% of predicted, those who received LB had shorter median hospital LOS (2 vs. 4 days, P<0.001) and fewer complications (15% vs. 33% complication rate, P=0.01) than those who received TEA. Among the 111 patients with FEV1 50–79% of predicted, patients who received LB had shorter median hospital LOS (2 vs. 4 days, P<0.001) but similar complication rates (17% vs. 22%, P=0.56) compared to those who received TEA. Among the 20 patients with FEV1 <50% of predicted, no association was observed between pain management strategy (LB or TEA) and hospital LOS or complication rate. We also noted that TEA was chosen preoperatively for most patients treated with lobectomy and severely depressed lung function (FEV1 <50% predicted) due to the preference of the attending surgeon.
Table 2. Selected perioperative outcomes by postoperative pain strategy in subgroups of patients with (A) preoperative FEV1 ≥80%, (B) 50–79%, and (C) <50% of predicted value.
| FEV1 groupings | Outcomes of interest | LB | TEA | P value |
|---|---|---|---|---|
| A. FEV1 ≥80% of predicted | n=227 | n=33 | ||
| Hospital LOS (days) | 2 [1–16] | 4 [1–14] | <0.001 | |
| Patients with complication(s) | 34 (15.0) | 11 (33.3) | 0.01 | |
| Surgery type | <0.001 | |||
| Lobectomy | 44 (19.4) | 20 (60.6) | ||
| Sublobar resection | 183 (80.6) | 13 (39.4) | ||
| B. FEV1 50–79% of predicted | n=88 | n=23 | ||
| Hospital LOS (days) | 2 [1–18] | 4 [2–9] | <0.001 | |
| Patients with complication(s) | 15 (17.0) | 5 (21.7) | 0.56 | |
| Surgery type | <0.001 | |||
| Lobectomy | 18 (20.5) | 17 (73.9) | ||
| Sublobar resection | 70 (79.5) | 6 (26.1) | ||
| C. FEV1 <50% of predicted | n=11 | n=9 | ||
| Hospital LOS (days) | 7 [1–18] | 6 [5–22] | 0.94 | |
| Patients with complication(s) | 8 (72.7) | 6 (66.7) | >0.99 | |
| Surgery type | 0.13 | |||
| Lobectomy | 1 (9.1) | 4 (44.4) | ||
| Sublobar resection | 10 (90.9) | 5 (55.6) |
Data are presented as median [range] or n (%). FEV1, forced expiratory volume in 1 second; LB, liposomal bupivacaine; TEA, thoracic epidural analgesia; LOS, length of stay.
Hospital LOS
Median hospital LOS was significantly shorter in patients who received LB (2 vs. 4 days, P<0.001; Table 1). On multivariable analysis, increasing age [β, 0.02; 95% confidence interval (CI): 0.00 to 0.04; P=0.03], incidence of one or more postoperative complications (β, 3.87; 95% CI: 3.29 to 4.44; P<0.001), and TEA (β, 1.19; 95% CI: 0.57 to 1.81; P<0.001) were independently associated with a longer hospital stay. FEV1% predicted was inversely related to hospital LOS (β, −0.02; 95% CI: −0.03 to −0.01; P<0.001; Table 3). Average length for epidurals was 3 days (range, 1–13 days). Average length of chest tube duration was 2 days (range, 1–13 days).
Table 3. Multivariable linear regression modeling hospital LOS (in days) based on incidence of postoperative complication(s), postoperative pain strategy, age, and preoperative FEV1% of predicted.
| Variables | Minimally invasive lung resections (n=391) | ||
|---|---|---|---|
| Coefficient | 95% CI | P value | |
| Postoperative complication(s) | 3.87 | 3.29 to 4.44 | <0.001 |
| TEA | 1.19 | 0.57 to 1.81 | <0.001 |
| Age | 0.02† | 0.00 to 0.04 | 0.03 |
| Preoperative FEV1% predicted | −0.02† | −0.03 to −0.01 | <0.001 |
†, change in LOS expected after a corresponding increase of one in the independent variable. LOS, length of stay; FEV1, forced expiratory volume in 1 second; CI, confidence interval; TEA, thoracic epidural analgesia.
Postoperative complications
Overall, 79 (20%) patients developed at least one postoperative complication: 22 TEA patients (34%) and 57 LB patients (18%) (P=0.004). In the TEA cohort, 20 patients experienced at least one grade II event, four patients grade III, three patients grade IV, and there were no grade V events. In the LB group, 49 patients experienced a grade II event, 16 patients grade III, five patients grade IV, and one patient experienced a grade V event. Breakdown of the complications is shown in Table 4.
Table 4. Breakdown of complications between the LB and TEA group.
| Classification of complications† | LB group (n=326) | TEA group (n=65) |
|---|---|---|
| Grade II | ||
| Atrial fibrillation | 9 | 5 |
| Pneumonia | 7 | 1 |
| DVT | 0 | 1 |
| Delirium | 7 | 3 |
| UTI | 11 | 7 |
| Prolonged air leaks | 14 | 8 |
| Chyle leak | 1 | 0 |
| Empyema | 1 | 0 |
| Cellulitis | 1 | 0 |
| Grade III | ||
| Prolonged air leak | 5 | 1 |
| Pneumothorax | 3 | 1 |
| Hemothorax | 4 | 1 |
| Copious secretions | 1 | 2 |
| Empyema | 2 | 0 |
| Bronchopleural fistula | 1 | 1 |
| Grade IV | ||
| Respiratory failure | 2 | 1 |
| Pneumonia | 1 | 2 |
| Atrial fibrillation | 0 | 1 |
| Hypovolemic shock | 1 | 0 |
| Stroke | 1 | 0 |
| Grade V | ||
| Sepsis | 1 | 0 |
Data are presented as n. †, grade of complication is based on Clavien-Dindo classification. This breakdown includes patients who experienced more than one grade of a complication. LB, liposomal bupivacaine; TEA, thoracic epidural analgesia; DVT, deep vein thrombosis; UTI, urinary tract infection.
In the entire cohort, 14 patients (3.6%) developed hypotension, which was defined as an unexpected mean arterial pressure <70 mmHg that required medications or fluids. All 14 patients had undergone a wedge resection, and one patient had received TEA. All responded appropriately with fluid boluses, and additional medications were not required. A bolus was defined as a certain volume of fluid, usually 250–1,000 mL, given over a short period of time (i.e., 15–30 minutes). There were no documented cases of urinary retention. A comparison of complication rates between the LB and TEA groups is listed in Table 1. More patients who received TEA experienced grade II complications (20, 31%) compared to the LB group (49, 15%) (P=0.004). There were no differences in the rates of grades III and IV complications between the two groups.
Discussion
Our findings demonstrate that direct witness of LB intercostal block reduced MMEs 48 hours postoperatively. It was associated with fewer postoperative pulmonary and cardiovascular complications, shorter LOS, and lower subjective pain scores.
Between 2015 and 2019, four studies comparing TEA and LB in thoracic surgery have reported mixed results. In a study of 45 VATS lobectomies, Sztain et al. reported shorter hospital LOS in patients with LB but an opioid reduction in patients who received TEA for pain control. They did not find any differences in pain scores (10). In their study of VATS lung resections, Medina et al. reported lower pain scores and decreased use of opioids in the LB group, without an impact on hospital LOS (11). In a 2015 study of open and minimally invasive lung resections, Rice et al. found no difference in pain or opioid consumption, but reduced hospital LOS in patients with LB (12). Also in 2015, Khalil et al. reported decreased pain, reduced pulmonary complications, and decreased LOS for patients undergoing thoracotomies for thoracic surgery with the use of LB. They did not find any differences in opioid consumption (13). The conflicting data among these four studies is likely the result of multiple factors including patient populations, operative procedures, and study design.
None of the previous studies had thoracoscopically witnessed intercostal injection with raising of a pleural weal. To our knowledge, there are no studies in thoracic surgery comparing blind vs. visualized injection of intercostal blocks. However, a study done on cadavers demonstrated that ultrasound-guided injection resulted in higher accuracy of the blocks and lower volume of solution used (14). Visualization during injection is effective, and this aligns with our technique for administering intercostal blocks.
An interesting finding in our study was that pain scores were significantly lower with LB at 24 and 48 hours. We did not find a difference in the PACU or at 12 hours. This finding is like those demonstrated by Dunham and colleagues, who found that thoracic surgery patients who received intercostal nerve block with LB vs. those with TEA reported decreased MME consumption at 48 hours. However, there was no difference in MME consumption at 24 hours (15). Early mobilization could have affected increased discomfort, as hypothesized by Dominguez et al. (16). Another possible explanation is that the LB did not reach full therapeutic effect within the first 12 hours.
Early postoperative mobilization decreases the risk for venous thromboembolism, improves pulmonary function, and decreases pulmonary complications (17). This may help explain the reduction in cardiovascular and pulmonary events in the LB group. Even though specific pulmonary complications were not statistically different, there was a trend toward higher rates of those events in patients receiving an epidural. This may have contributed to an increase in adverse events and, as evidenced in the multivariable analysis, a surrogate for longer LOS.
In our cohort, LB was associated with a shorter LOS and fewer complication than TEA in patients with FEV1 ≥80% of predicted. Data associating FEV1 with LOS is sparse in thoracic surgery, but it has been shown to predict LOS in cardiac surgery patients. A study of over 2,241 patients undergoing coronary artery bypass grafting or valve surgery demonstrated that patients with FEV1 <80% predicted had LOS that was 1–3 days longer than that of patients with FEV1 >80% (18). Our analysis suggests that pain management strategy may also predict LOS in patients with FEV1 ≥80%. In addition to FEV1, TEA was also a strong predictor of longer hospital LOS in our multivariable model. This may be explained by reluctance to remove the catheter given the upfront procedural risks and desire to maximize pain relief. This, in turn, may have resulted in delayed patient mobilization (19). Additionally, epidural catheters have been associated with urinary retention and hypotension, which can further prolong hospital course. However, these factors were unlikely to contribute to the findings in our cohort given no documented urinary retentions and only one documented hypotension in a patient who had received an epidural.
With the increase in the opioid epidemic in the United States, there has been an emphasis on concurrent use of non-opioid medications to reduce narcotic use (20,21). Our data shows that opioid consumption was significantly lower in patients who received LB at 48 hours postoperatively, but not at 24 hours. These results are consistent with those of Parascandola et al., in which they also saw a benefit of reduced opioid consumption after 24 hours of LB use (22). These results contrast with those of Kelley et al., in which they found reduced opioid consumption at 24 hours but not beyond that (23). It is important to mention that the doses of LB and method of administration differed between these studies and from our doses and technique. This could be influencing some of the findings. However, optimal doses can differ among institutions. In addition, interesting is that despite differing LB doses, our analysis contributes to the larger body of literature that supports the use of LB to reduce postoperative opioid consumption (24,25).
Limitations
This study has several limitations. First, this was a single, academic institution experience, and results may not be generalizable. Second, this is not a randomized controlled trial. Therefore, there is some lack of standardization as is expected from an observational study like ours. For instance, surgeons’ discretion of postoperative pain management may have contributed to selection bias. However, pain is a highly subjective measure, and we used the visual analog scale that is commonly used across the country in various disciplines. We also enforced similar methods for the entire cohort regarding when to administer non-narcotic vs. narcotic medications.
Conclusions
Injection of LB under direct witness decreases hospital LOS and contributes to good pain control at discharge. LB may be a good alternative to TEA for postoperative pain control in patients undergoing minimally invasive lung resections. Further investigations are warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the John D. Mitchell Fellowship to Anupama Singh.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval at Brigham and Women’s Hospital was obtained (protocol #2014P002478). Informed consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1405/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1405/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1405/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1405/coif). A.S. is personally funded by the John D. Mitchell Fellowship. R.B. has research grants and clinical trials support from MedGenome, Roche, Verastem, Genentech, Merck, Bicycles therapeutics, Serum, Intuitive, Siemens, NIH and DOD. Additionally, he has four patents through BWH (no royalties to date) and equity in a new start-up company, Navigation Sciences. These disclosures or conflicts of interest are not relevant to this manuscript. The other authors have no conflicts of interest to declare.
References
- 1.Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. 10.1213/01.ane.0000333274.63501.ff [DOI] [PubMed] [Google Scholar]
- 2.Koehler RP, Keenan RJ. Management of postthoracotomy pain: acute and chronic. Thorac Surg Clin 2006;16:287-97. 10.1016/j.thorsurg.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 3.Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312-21. 10.1053/j.jvca.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 4.Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol 2014;28:15-27. 10.1016/j.bpa.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Rayaz H, Bravos ED, Gottschalk A. The role of liposomal bupivacaine in thoracic surgery. J Thorac Dis 2019;11:S1163-8. 10.21037/jtd.2019.04.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121 . 10.1002/14651858.CD009121.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CY, Robinson DA, Johnson CA, Jr, et al. A Randomized Controlled Trial of Liposomal Bupivacaine Parasternal Intercostal Block for Sternotomy. Ann Thorac Surg 2019;107:128-34. 10.1016/j.athoracsur.2018.06.081 [DOI] [PubMed] [Google Scholar]
- 8.Weksler B, Sullivan JL, Schumacher LY. Randomized trial of bupivacaine with epinephrine versus bupivacaine liposome suspension in patients undergoing minimally invasive lung resection. J Thorac Cardiovasc Surg 2021;161:1652-61. 10.1016/j.jtcvs.2020.01.112 [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sztain JF, Gabriel RA, Said ET. Thoracic Epidurals are Associated With Decreased Opioid Consumption Compared to Surgical Infiltration of Liposomal Bupivacaine Following Video-Assisted Thoracoscopic Surgery for Lobectomy: A Retrospective Cohort Analysis. J Cardiothorac Vasc Anesth 2019;33:694-8. 10.1053/j.jvca.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 11.Medina M, Foiles SR, Francois M, et al. Comparison of cost and outcomes in patients receiving thoracic epidural versus liposomal bupivacaine for video-assisted thoracoscopic pulmonary resection. Am J Surg 2019;217:520-4. 10.1016/j.amjsurg.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Rice DC, Cata JP, Mena GE, et al. Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg 2015;99:1953-60. 10.1016/j.athoracsur.2015.02.074 [DOI] [PubMed] [Google Scholar]
- 13.Khalil KG, Boutrous ML, Irani AD, et al. Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. Ann Thorac Surg 2015;100:2013-8. 10.1016/j.athoracsur.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 14.Bhatia A, Gofeld M, Ganapathy S, et al. Comparison of anatomic landmarks and ultrasound guidance for intercostal nerve injections in cadavers. Reg Anesth Pain Med 2013;38:503-7. 10.1097/AAP.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 15.Dunham WC, Lombard FW, Edwards DA, et al. Effect of Regional Analgesia Techniques on Opioid Consumption and Length of Stay After Thoracic Surgery. Semin Cardiothorac Vasc Anesth 2021;25:310-23. 10.1177/1089253220949434 [DOI] [PubMed] [Google Scholar]
- 16.Dominguez DA, Ely S, Bach C, et al. Impact of intercostal nerve blocks using liposomal versus standard bupivacaine on length of stay in minimally invasive thoracic surgery patients. J Thorac Dis 2018;10:6873-9. 10.21037/jtd.2018.10.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneda H, Saito Y, Okamoto M, et al. Early postoperative mobilization with walking at 4 hours after lobectomy in lung cancer patients. Gen Thorac Cardiovasc Surg 2007;55:493-8. 10.1007/s11748-007-0169-8 [DOI] [PubMed] [Google Scholar]
- 18.McAllister DA, Wild SH, MacLay JD, et al. Forced expiratory volume in one second predicts length of stay and in-hospital mortality in patients undergoing cardiac surgery: a retrospective cohort study. PLoS One 2013;8:e64565 . 10.1371/journal.pone.0064565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joelsson-Alm E, Nyman CR, Lindholm C, et al. Perioperative bladder distension: a prospective study. Scand J Urol Nephrol 2009;43:58-62. 10.1080/00365590802299122 [DOI] [PubMed] [Google Scholar]
- 20.Kaye AD, Jones MR, Kaye AM, et al. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1. Pain Physician 2017;20:S93-S109. [PubMed] [Google Scholar]
- 21.Kaye AD, Jones MR, Kaye AM, et al. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse (Part 2). Pain Physician 2017;20:S111-33. [PubMed] [Google Scholar]
- 22.Parascandola SA, Ibañez J, Keir G, et al. Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resection†. Interact Cardiovasc Thorac Surg 2017;24:925-30. 10.1093/icvts/ivx044 [DOI] [PubMed] [Google Scholar]
- 23.Kelley TM, Jr, Bailey DW, Sparks P, et al. Intercostal Nerve Blockade with Exparel® Results in Lower Opioid Usage during the First 24 Hours after Video-Assisted Thorascopic Surgery. Am Surg 2018;84:1433-8. [PubMed] [Google Scholar]
- 24.Dasta J, Ramamoorthy S, Patou G, et al. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin 2012;28:1609-15. 10.1185/03007995.2012.721760 [DOI] [PubMed] [Google Scholar]
- 25.Malik O, Kaye AD, Kaye A, et al. Emerging roles of liposomal bupivacaine in anesthesia practice. J Anaesthesiol Clin Pharmacol 2017;33:151-6. 10.4103/joacp.JOACP_375_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as