Abstract
The herpesvirus saimiri (HVS) immediate-early gene product encoded by open reading frame (ORF) 57 shares limited amino acid homology with HSV-1 ICP27 and Epstein-Barr virus BMLF1, both regulatory proteins. The ORF 57 gene has been proposed to be spliced based on the genome sequence, and here we confirm the intron-exon structure of the gene. We also demonstrate that a cDNA construct of the ORF 57 gene product represses the transactivating capability of the ORF 50a gene product (which is produced from a spliced transcript), but activates that of ORF 50b (an unspliced transcript). Further analyses with cotransfection experiments show that ORF 57 can either activate or repress expression from a range of both early and late HVS promoters, depending on the target gene. These results indicate that repression of gene expression mediated by the ORF 57 gene product is dependent on the presence of an intron within the target gene encoding region. Furthermore, Northern blot analysis demonstrates that the levels of mRNA transcribed from genes not containing an intron are not significantly affected in the presence of the ORF 57 gene product. This suggests that it regulates gene expression through a posttranscriptional mechanism.
Herpesvirus saimiri (HVS) is a lymphotrophic rhadinovirus (gamma-2 herpesvirus) of squirrel monkeys (Saimiri sciureus), which persistently infects its natural host without causing any obvious disease. However, HVS infection of other species of New World primates results in fulminant polyclonal T-cell lymphomas and lymphoproliferative diseases (5). Analysis of the genome of HVS (strain A11) indicates it shares significant homology with the herpesviruses Epstein-Barr virus (EBV), bovine herpesvirus 4, and Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) (1, 3, 25). The genomes of EBV and HVS are generally colinear, in that homologous sequences are found in approximately equivalent locations and in the same relative orientation. However, conserved gene blocks are separated by unique genes respective to each virus (1).
Transcription in HVS is sequentially regulated during a lytic infection and occurs in three main temporal phases: immediate-early (IE), delayed-early (DE), and late (24). Two major IE transcripts have been identified in HVS encoded by an HindIII-G-IE gene (open reading frame [ORF] 14) and an IE 52-kDa protein gene (ORF 57) (18, 21). The IE 52-kDa protein has been mapped to the EcoRI-I/E fragments of HVS and is homologous to genes identified in all classes of herpesviruses, including the EBV transactivator encoded by BMLFI, ICP27 of herpes simplex virus, ORF 4 encoded by varicella-zoster virus (VZV), and UL69 in human cytomegalovirus (9, 11, 17, 19, 31).
ICP27 is a 63-kDa nuclear phosphoprotein which is essential for lytic virus replication (23). Analyses with temperature-sensitive and deletion mutants have shown that the protein is involved in the switch from early to late gene expression (12, 23). In addition, cotransfection experiments have demonstrated an activation or repression of reporter genes in the presence of ICP27 (4, 14, 22, 29). These transregulatory functions are independent of the target gene promoter sequences and appear to be mediated at the posttranscriptional level through 3′ end processing of the target gene, whereas repression of gene expression appears to correlate with the presence of introns (26). In addition, ICP27 contributes to the shutoff of host cell protein synthesis and contributes to a decrease in cellular mRNA levels during infection, because deletion mutant infections result in increased levels of cellular protein synthesis and mRNA levels compared to those in wild-type infections (6, 7). Furthermore, ICP27 has been shown to be involved in the reorganization of antigens associated with small nuclear ribonucleoprotein particles (10, 20, 28). In contrast, the EBV IE BMLF1 acts in trans by a posttranscriptional mechanism which is reporter gene dependent (9), and the VZV ORF 4 protein is a transcriptional activator which requires the presence of an upstream element within the promoter to mediate transcription (19).
Although the IE ORF 57 protein has been shown to activate chloramphenicol acetyltransferase (CAT) gene expression from heterologous promoter-CAT constructs in transient transfection studies, the role of the 52-kDa protein in a productive infection remains uncertain (17). In this paper, the effect of the ORF 57 gene product was assessed on the protein transactivators encoded by ORF 50, a homolog of the EBV R gene product (BRLFI) (16). ORF 50 produces two transcripts. The first is spliced containing a single intron and is detected at early times during the productive cycle, whereas the second is expressed later and is produced from a promoter within the second exon (30). In this paper, we show that the ORF 57 gene product represses the transactivation capability of the ORF 50a gene product (which is produced from a spliced transcript), but activates that of ORF 50b (an unspliced transcript). Furthermore, we demonstrate that ORF 57 can either activate or repress expression from a range of both early and late HVS promoters, depending on the presence of an intron within the target gene encoding region. We also present evidence that the ORF 57 gene product regulates gene expression through a posttranscriptional mechanism.
Mapping the initiation codon of ORF 57.
In order to create a full-length cDNA of ORF 57, the transcriptional start site of the gene was identified by 5′ rapid amplification of cDNA ends. Sequence analysis predicts the initiation codon to be at nucleotide 78291 and the splice donor and acceptor sites to be at 78309 and 78396 (of the published sequence), respectively (1). Total RNA was isolated from OMK-infected cells at 24 h. postinfection, and first-strand cDNA was reversed transcribed with Superscript II reverse transcriptase (Life Technologies) and an ORF 57 gene-specific antisense primer, 5′-CTG AGT AGG TAA GAA AAA CAG CCC TGT GGT. The first-strand cDNA was treated with terminal deoxynucleotidyl transferase (Boehringer Mannheim) in the presence of dATP, and second-strand synthesis was completed with an oligo(dT) primer (Life Technologies). PCR amplification was performed with a nested 3′ gene-specific primer (5′-GTA GTA TAA GCA CAA GTA GAG CTT TGG) and the 5′ oligo(dT) primer. The reaction, 30 cycles (1 min at 92°C, 1 min at 60°C, 1 min at 72°C) was performed with 4 U of Taq polymerase (Promega). The amplified 5′ cDNA was cycle sequenced by the fmol DNA sequencing system (Promega). Analysis of the sequence confirms the sequence prediction and illustrates that ORF 57 is a spliced gene, with its putative initiation codon at nucleotide 78291 (of the published sequence). The gene contains an intron of 87 bp, with splice donor and acceptor sites at nucleotides 78309 and 78396, respectively (data not shown).
Eukaryotic expression analysis of ORF 57.
In order to investigate whether the ORF 57 gene product has any regulatory activities on gene expression, a eukaryotic expression vector encoding ORF 57 was generated. Reverse transcription-PCR was performed to amplify a cDNA of the ORF 57 coding region. First-strand cDNA was reversed transcribed with Superscript II reverse transcriptase and an oligo(dT) primer. The ORF 57 cDNA was generated by PCR amplification with specific ORF 57 primers 5′-AAA CTG CAG AAC TGC CCA AAT GGA AGA TAT AAT TG and 5′-GCG GGA TCC CTG AGT AGG TAA GAA AAA CAG CCC TGT. These oligonucleotides incorporated PstI and BamHI restriction sites for convenient cloning of the PCR product. The reaction (30 cycles [1 min at 92°C, 1 min at 50°C, 2 min at 72°C]) was performed with 4 U of Pfu DNA polymerase (Stratagene); this product was inserted into the eukaryotic expression vector pBKRSV (Stratagene) to derive pRSVORF57. To determine the expression levels and subcellular localization of ORF 57, indirect immunofluorescence analysis of HVS-infected or transiently transfected cells was performed. Cells were fixed with 4% formaldehyde in phosphate-buffered saline (PBS), washed in PBS, and permeabilized in 0.5% Triton X-100 for 5 min. The cells were rinsed in PBS and blocked by preincubation with 1% (wt/vol) nonfat milk powder for 1 h at 37°C. A 1:100 dilution of anti-ORF 57 SB antibody (a gift from Rick Randall) was layered over the cells which were then incubated for 1 h at 37°C. Fluorescence-conjugated antimouse immunoglobulin (Dako) at a 1:50 dilution was added for 1 h at 37°C. After each incubation step, the cells were washed extensively with PBS. The immunofluorescence slides were observed with a Zeiss Axiovert 135TV inverted microscope with a Neofluar ×40 oil immersion lens. This revealed strong fluorescence of the nuclei of infected or pRSVORF57-transfected cells (data not shown), similar to previous observations with HVS-infected cells (21).
ORF 57 gene product represses the transactivating capability of ORF 50a but activates ORF 50b.
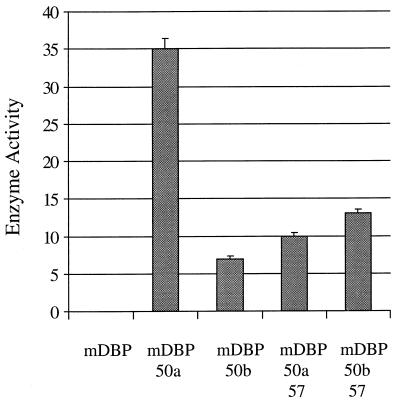
We were particularly interested in determining whether the ORF 57 gene product exerted any regulatory effect on another transcriptional activator encoded by HVS, ORF 50. This gene encodes two transcripts: the first is spliced and is detected at early times during the productive cycle, whereas the second is expressed later and is produced from a promoter within the second exon (30). Therefore, transfection studies were performed to assess the effect of the ORF 57 gene product on the transactivation capability of the ORF 50 gene products. The results of the cotransfection experiments of pAWCAT2 (ORF 6 promoter CAT expression vector) with ORF 50a or -b in the absence or presence of ORF 57 are shown in Fig. 1. The results indicate that in the presence of the ORF 57 gene product, the transactivation capability of ORF 50a is reduced but ORF 50b activity is slightly enhanced. Cotransfection experiments were also performed to assess the effect of the ORF 57 gene product on pAWCAT2 in the absence of ORF 50a or -b. The results indicate that ORF 57 exerted no effect on the DE promoter (data not shown). This suggests that the ORF 57 gene product has a regulatory effect on ORF 50a (a spliced transcript) and has a slight positive effect on ORF 50b (an unspliced transcript).
FIG. 1.
Response of transactivation capability of ORF 50a and ORF 50b to the ORF 57 gene product. OMK cells were transfected with 2 μg of pAWCAT2 and pAWHincII or pAWPstI in the absence or presence of pRSVORF57, respectively. Cells were harvested at 48 h posttransfection, and cell extracts were assayed for CAT activity as previously described. Percentages of acetylation were calculated by scintillation counting of the appropriate regions of the chromatography plate and are shown in a graphical format, and the variations between three replicated assays are indicated.
ORF 57 gene product regulates a range of HVS promoter constructs.
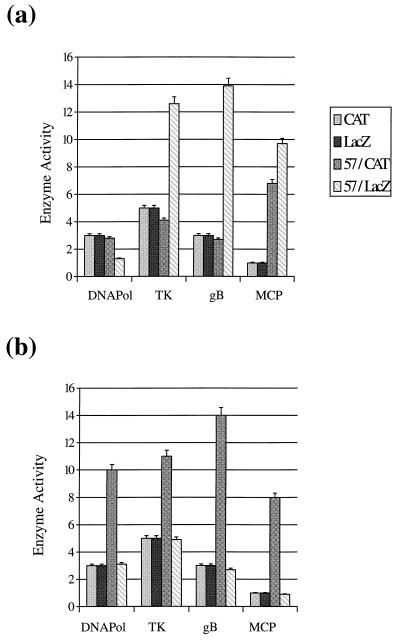
To further investigate the regulatory effects of ORF 57, transient expression studies were performed with a range of HVS promoters for the genes encoding DNA polymerase, thymidine kinase, major capsid protein, and glycoprotein B. The promoter sequence of each promoter was generated by PCR and ligated upstream of the CAT and β-Galactosidase (β-Gal) coding regions. The DNA polymerase promoter with primers 5′-CCC AAG CTT CTA GCA GAC TTA GGC TCT and 5′-GGG AAG CTT GTC AAG ACA GCA ACT CAG, thymidine kinase promoter with primers 5′-CCC AAG CTT GGT CTT GCA TTA GCT TGT CTA and 5′-GGG AAG CTT GAG ACA AGG AAG TGT TAG CAC, major capsid protein promoter with primers 5′-CCC AAG CTT TGC AAC TGA CCG TCT CTC AA and 5′-GGG AAG CTT GTG CGA GCT AAG TCT TCA AG, and glycoprotein B promoter with primers 5′-CCC AAG CTT GTT ACA TGA TGC GCA TGC TAG and 5′-GGG AAG CTT GGT TCT TCC CGC TCA ATT GC were used. These oligonucleotides incorporated HindIII restriction sites for convenient cloning of the PCR product. The reactions (30 cycles [1 min at 92°C, 1 min at 50°C, 2 min at 72°C]) were performed with 4 U of Pfu DNA polymerase (Stratagene). These fragments were inserted upstream of the CAT coding region in pCATBasic (Promega) to generate pDPCAT1, pTKCAT1, pMCPCAT1, and pgBCAT1 and upstream of the β-Gal coding region in pCMVβ (Clontech), previously digested with EcoRI and SmaI, and blunt ended with T4 polymerase (which removed the IE cytomegalovirus [CMV] promoter) to generate pDPLacZ1, pTKLacZ1, pMCPLacZ1, and pgBLacZ1, respectively. Cotransfection experiments were performed with each HVS promoter construct in the absence and presence of pRSVORF57, and the cell extracts were assayed for CAT and β-Gal activity, respectively (Fig. 2a). It can be determined from these results that the ORF 57 gene product transactivates the promoter β-Gal constructs to a much greater extent than the promoter-CAT constructs. This suggests that the ORF 57 gene product was functioning as a reporter gene-dependent regulator, because the promoter sequences in the CAT and β-Gal constructs were identical.
FIG. 2.
Response of HVS promoters to the ORF 57 gene product. HVS promoters from the DNA polymerase gene (DNAPol), thymidine kinase gene (TK), major capsid protein gene (MCP), and glycoprotein B (gB) were cloned upstream of the CAT and β-Gal coding regions. (a) The CAT constructs contained the small t antigen intron and SV40 poly(A) region, whereas the β-Gal constructs contained only the SV40 poly(A) signal. OMK cells were transfected with pDPCAT1, pTKCAT1, pMCPCAT1, pgBCAT1, pDPLacZ1, pTKLacZ1, pMCPLacZ1, and pgBLacZ1 in the absence or presence of pRSVORF57. (b) The CAT constructs contained only the SV40 poly(A) region, whereas the β-Gal constructs contained the small t antigen intron and the SV40 poly(A) signal. OMK cells were transfected with pDPCAT2, pTKCAT2, pMCPCAT2, pgBCAT2, pDPLacZ2, pTKLacZ2, pMCPLacZ2, and pgBLacZ2 in the absence or presence of pRSVORF57. Cells were harvested at 48 h posttransfection, and cell extracts were assayed for CAT and β-Gal activity. Percentages of CAT acetylation were calculated by scintillation counting of the appropriate regions of the chromatography plate. The activity of β-Gal was determined by the rate of production of ortho-nitrophenol from the substrate ortho-nitrophenyl galactoside. Results are shown in graphical format, and the variations between three replicated assays are indicated.
Sandri-Goldin and Mendoza (26) reported the regulatory activity of ICP27 is independent of the target gene sequence but due to the mRNA processing signals and the presence of introns 5′ or 3′ to the target coding sequences. Therefore, to examine the difference in ORF 57 regulation, the processing signals of the CAT and β-Gal constructs were investigated. Upon further examination, the promoter CAT constructs derived from pCATBasic contained the small t antigen intron and simian virus 40 (SV40) poly(A) region; however, the β-Gal constructs derived from pCMVβ contained only the SV40 poly(A) signal. These constructs were used to determine if ORF 57 transactivation was dependent on the reporter gene or the presence of the small t antigen intron. The mRNA processing signals of the CAT and β-Gal expression vectors were altered, in that the CAT constructs contained only the SV40 poly(A) region and the β-Gal constructs contained the small t antigen intron and SV40 poly(A) signal. Promoter CAT constructs which did not contain the small t antigen intron within the polyadenylation signals were generated by digestion of pDPLacZ1, pTKLacZ1, pMCPLacZ1, and pgBLacZ1 with NotI, which excised the β-Gal coding region, blunt ended with T4 polymerase, and ligated with the CAT coding region excised from pCM7 (Pharmacia) to derive pDPCAT2, pTKCAT2, pMCPCAT2, and pgBCAT2. Promoter β-Gal constructs containing the small t antigen intron and SV40 poly(A) region were generated by cloning the PCR promoter fragments upstream of the β-Gal coding region in pβgal-Basic (Clontech), previously digested with HindIII, to derive pDPLacZ2, pTKLacZ2, pMCPLacZ2, and pgBLacZ2. Cotransfection experiments were performed with each HVS promoter construct in the absence and presence of pRSVORF57, and the cell extracts were assayed for CAT and β-Gal activity, respectively (Fig. 2b). The results demonstrate that ORF 57 regulation is independent of the target reporter gene and is determined by the mRNA processing signals. Thus, repression of gene expression by the ORF 57 gene product is dependent on the presence of the small t antigen intron in the target gene sequence. This further indicates that ORF 57 repression of ORF 50a is due to the presence of the intron within its coding region.
ORF 57 gene product does not significantly affect mRNA levels.
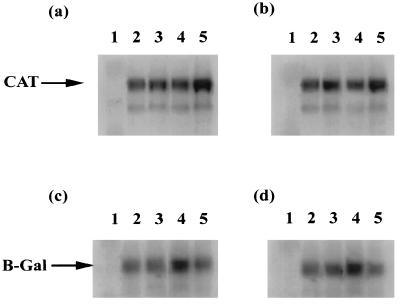
We have shown that the ORF 57 gene product increases the level of CAT and β-Gal activity from a range of HVS promoters with mRNA processing signals which do not contain the small t antigen intron. To ascertain whether this rise is due to an increase in the levels of CAT mRNA in the presence of the ORF 57 transactivator, Northern blot analysis was performed. Total RNA was isolated from OMK cells transfected with pTKCAT1;2, pTKLacZ1;2, pgBCAT1;2, or pgBLacZ1;2 in the absence or presence of pRSVORF57 and separated by electrophoresis on a 1% denaturing formaldehyde agarose gel. The RNA was transferred to Hybond-N membranes and hybridized with radiolabelled 32P-labelled random-primed probes specific for CAT and β-Gal coding sequences (Fig. 3). The results of the Northern blot analysis demonstrate that the increase in CAT and β-Gal activity was not correlated with a similar increase in CAT and β-Gal RNA levels in the presence of ORF 57. This suggests that the ORF 57 gene product acts posttranscriptionally to modulate gene expression.
FIG. 3.
Northern blot analysis of HVS promoter CAT and β-Gal constructs in response to the ORF 57 gene product. RNA was isolated from OMK cells transfected with the constructs pRSVORF57 (lane 1), pTKCAT1 (lane 2), pTKCAT2 (lane 3), pTKCAT1 and pRSVORF57 (lane 4), or pTKCAT2 and pRSVORF57 (lane 5) (a) plus pgBCAT (b), pTKLacZ (c), and pgBLacZ (d), separated by electrophoresis on a 1% denaturing formaldehyde agarose gel, blotted onto a nylon membrane, and hybridized with radiolabelled probes specific for the CAT and β-Gal coding regions.
In this report, we have demonstrated that the ORF 57 gene product represses the transactivating capability of the ORF 50a gene product but slightly activates the ORF 50b gene product. Therefore, the ORF 57 gene product may have a specific role in regulating the ORF 50 gene products during the virus replication cycle. We believe this repression by ORF 57 is linked to the presence of an intron within the coding region of ORF 50a. Furthermore, when the SV40 small t antigen intron was present 3′ to the CAT and β-Gal coding sequences, significantly lower expression of the reporter genes expressed from a range of HVS promoters was observed in the presence of ORF 57 compared to the levels of enzyme activity from constructs which contained only the SV40 poly(A) signal. This suggests that the repressor activity of the ORF 57 gene product is associated with the presence of an intron in the target gene coding region. Similar results have been demonstrated with HSV-1 ICP27; repression of CAT constructs by ICP27 correlated with the presence of introns 5′ or 3′ to the target gene coding region (26). Furthermore, HSV infection has been shown to inhibit host cell splicing, and ICP27 is required for this inhibition (6–8). At present, the effect of the ORF 57 gene product on host cell splicing has not been determined. Sequence analysis has shown that ORF 57 is highly conserved with other members of the ICP27 family at the C-terminal region of the gene. We believe the ORF 57 gene product contains a functional domain within the C terminus which is required for the repressor function of this protein. It has been demonstrated that the C-terminal domain of ICP27 must remain intact for the inhibitory effect (27, 28). This region contains a cysteine-histidine-rich region which resembles a single zinc finger-like motif or “zinc knuckle” which is conserved in all ICP27 homologs, including ORF 57 (histidine residue 383 and cysteine residues 387 and 392 in ORF 57). Similar motifs occur in a number of splicing factors (27). Further studies are being undertaken to determine if this domain is essential for the repressor activity of ORF 57.
The finding that the ORF 57 gene product has been shown to transactivate a range of HVS promoters but does not significantly increase the level of mRNA with respect to the level of CAT or β-Gal activity suggests a posttranscriptional mechanism. In addition, the effect of ORF 57 is independent of either the promoter which drives transcription or the temporal class of this promoter. However, we are unable at present to determine whether ORF 57 affects the mRNA processing, transport, or translational efficiency of the CAT and β-Gal mRNA. ICP27 appears to act posttranscriptionally by affecting mRNA processing, suggesting that ICP27 regulates the usage of poly(A) sites as a means of controlling gene expression (9, 12, 13). It has also been demonstrated that a bacterially expressed ICP27 fusion protein specifically binds to the 3′ ends of RNA, leading to accumulation and an increased half-life of the mRNAs (2). It is not known whether binding of ICP27 involves specific poly(A) signals, but its coding region does contain an RNA recognition sequence (15). The RNA binding motif (residues 138 and 152) is similar to an RGG box motif, and this is believed to be an RNA binding determinant (15). However, not all ICP27 homologs, including ORF 57, contain a homologous RGG box motif. Nevertheless, ORF 57 does encode arginine-rich amino or N termini, which may contain alternative RNA binding determinants. Deletion and mutational analyses of the N-terminal region of ORF 57 may help to clarify its role, if any, in RNA binding.
In summary, we have analyzed a regulatory protein encoded by HVS that can activate a range of HVS promoters, and this activation is independent of the target promoter sequences and occurs by a posttranscriptional mechanism. In addition, repression by this protein correlates with the presence of introns within the target gene sequence.
Acknowledgments
This work was supported in part by grants from the Yorkshire Cancer Research Campaign, Medical Research Council, and the Wellcome Trust.
We thank Rick Randall for providing the SB monoclonal antibody.
REFERENCES
- 1.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittman S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C R, Nakamura M S, Mosca J D, Hayward G S, Straus S E, Perera L P. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J Virol. 1995;69:7187–7195. doi: 10.1128/jvi.69.11.7187-7195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bublot M, Manet E, Lequarre A S, Albrecht J C, Nicholas J, Fleckenstein B, Pastoret P P, Thiry E. Genetic relationships between bovine herpesvirus 4 and the gamma-herpesviruses Epstein-Barr and herpesvirus saimiri. Virology. 1992;190:654–665. doi: 10.1016/0042-6822(92)90903-3. [DOI] [PubMed] [Google Scholar]
- 4.Everett R D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986;76:2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- 5.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–332. [Google Scholar]
- 6.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibbard M K, Sandri-Goldin R M. Arginine-rich regions succeeding the nuclear localization region of herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J Virol. 1995;69:4656–4667. doi: 10.1128/jvi.69.8.4656-4667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenney S, Holley-Guthrie E, Mar E-C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin T E, Barghusen S C, Leser G P, Spear P G. Redistribution of the nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987;105:2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;65:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 12.McGregor F, Phelan A, Dunlop J, Clements J B. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE61 in the early-late switch. J Virol. 1996;70:1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLauchlan J, Phelan A, Loney C, Sandri-Goldin R M, Clements J B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahon L, Schaffer P A. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990;64:3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mears W E, Rice S A. The RGG box of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholas J, Coles L S, Newman C, Honess R W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991;65:2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholas J, Gompels U A, Craxton M A, Honess R W. Conservation of sequence and function between the product of the 52-kilodalton immediate-early gene of herpesvirus saimiri and the BMLF1-encoded transcriptional effector (EB2) of the Epstein-Barr virus. J Virol. 1988;62:3250–3257. doi: 10.1128/jvi.62.9.3250-3257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholas J, Smith E P, Coles L S, Honess R W. Gene expression in cells infected with gammaherpesvirus saimiri: properties of transcripts from two immediate-early genes. Virology. 1990;179:189–200. doi: 10.1016/0042-6822(90)90288-3. [DOI] [PubMed] [Google Scholar]
- 19.Perera L P, Kaushal S, Kinchington P R, Mosca J D, Hayward G S, Straus S E. Varicella-zoster virus open reading frame 4 encodes a transcriptional activator that is functionally distinct from that of herpes simplex virus homolog ICP27. J Virol. 1994;68:2468–2477. doi: 10.1128/jvi.68.4.2468-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phelan A, Carmo-Fonseca M, McLauchlan J, Lamond A I, Clements J B. A herpes simplex virus type 1 immediate early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall R E, Newman C, Honess R W. A single major immediate-early virus gene product is synthesized in cells productively infected with herpesvirus saimiri. J Gen Virol. 1984;65:1215–1219. doi: 10.1099/0022-1317-65-7-1215. [DOI] [PubMed] [Google Scholar]
- 22.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus α protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice S A, Lam V, Knipe D M. The acidic amino-terminal region of the herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J Virol. 1993;67:1778–1787. doi: 10.1128/jvi.67.4.1778-1787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 11–68. [Google Scholar]
- 25.Russo J J, Bohenzhy R A, Chein M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequences of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 27.Sandri-Goldin R M, Hibbard M K. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70:108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandri-Goldin R M, Hibbard M K, Hardwicke M A. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69:6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekulovich R E, Leary K, Sandri-Goldin R M. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehouse A, Carr I M, Griffiths J C, Meredith D M. The herpesvirus saimiri ORF50 gene, encoding a major transcriptional activator homologous to the Epstein-Barr virus R protein, is transcribed from two distinct promoters of different temporal phases. J Virol. 1997;71:2550–2554. doi: 10.1128/jvi.71.3.2550-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3943–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]