ABSTRACT
Objective
Little is known about how polycystic ovary syndrome (PCOS) is linked to irritable bowel syndrome (IBS). This study aimed to review the existing literature regarding the association between PCOS or its symptoms and complications with IBS.
Methods
In this review, studies that investigated the proposed cross‐link between features of PCOS and IBS were included. This review collectively focused on recent findings on the mechanism and novel insight regarding the association between IBS and PCOS in future clinical practice. An electronic search of PubMed, Scopus, Epistemonikos, Cochrane Library and Google Scholar was performed. We did not restrict the study setting and publication date.
Results
The existing evidence has not completely answered the question of whether there is an association between PCOS and IBS and vice versa. Six case–control studies (793 women with PCOS and 547 women in the control group) directly assessed the association between PCOS and IBS. The prevalence of IBS among women with PCOS in these studies has ranged from 10% to 52% compared with 5%–50% in control groups. Evidence suggested the common pathways may have contributed to the interaction between IBS and PCOS, including metabolic syndrome, sex hormone fluctuation, dysregulation of neurotransmitters, psychological problems and environmental and lifestyle factors. To date, it is still ambiguous which of the mentioned components largely contributes to the pathogenesis of both.
Conclusion
Although limited evidence has shown a higher prevalence of IBS in women with PCOS, there are several potential, direct and common indirect pathways contributing to the development of both IBS and PCOS.
Keywords: irritable bowel syndrome, metabolic syndrome, polycystic ovary syndrome, review
Although limited evidence has shown a higher incidence of IBS in women with PCOS, there are several potential direct and common indirect pathways contributing to the development of both IBS and PCOS.
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common reproductive disorder around the world. PCOS is mainly characterised by hyperandrogenism and anovulation [1, 2]. This endocrine disorder is linked to some short‐ and long‐term complications, including cardio‐metabolic, obstetric, oncology and psychological complications [3, 4]. It has been proposed that gastrointestinal dysbiosis can play a role in the pathophysiology of PCOS [5]. PCOS patients are more prone to experience gastrointestinal (GI) disturbances such as irritable bowel syndrome (IBS) [6, 7]. A recent meta‐analysis showed that the risk of IBS in women with PCOS is two times higher in the than control group [8].
IBS is one of the most prevalent functional bowel disorders, and its prevalence varies widely in different countries according to diagnostic criteria [9]. The Rome IV diagnostic criteria defined IBS as recurrent abdominal pain that is associated with a change in bowel habits or defecation. Disordered bowel habits are typically present (i.e. constipation, diarrhoea or a mix of constipation and diarrhoea), as are symptoms of abdominal bloating/distension. The symptoms started 6 months prior to the diagnosis, and they should have persisted for 3 months at this point [10]. Gender differences in IBS symptom severity are influenced by female sex hormones [11]. Indeed, the impact of the fluctuation of sex hormones which appears in relation to pregnancy, menstrual cycle or menopausal states on gastrointestinal disorders was addressed previously [11, 12].
Genetic factors, in combination with epigenetic, environmental and peripheral factors, contribute to the development of IBS [13]. The increased prevalence of IBS among females with PCOS [6, 14] could be explained in several ways including the fluctuation of sex hormones. In the light of this fact, IBS is highly correlated with metabolic syndrome (METs) [15]. Similarly, women with PCOS are at high risk of developing METs [16]. Moreover, it has been reliably demonstrated that there is a link between psychological morbidity and symptoms of both IBS and PCOS [17].
Due to the lack of evidence, it is unclear whether it is PCOS in women that make them susceptible to the increased risk of developing IBS or vice versa. Hence, this review aimed to summarise the key evidence that supports the putative association between PCOS or its symptoms and complications with IBS. It is hoped that this study could shed light on the direction of future studies.
2. Methods
This narrative review aimed to determine the association between PCOS and IBS and the common pathway between them. We searched PubMed, Scopus, Epistemonikos, Cochrane Library: Cochrane Reviews and Google Scholar for all kinds of studies showing the link between PCOS and IBS till December 2023. In this narrative review, human and animal studies (clinical trials, review and observational studies) investigating the association between clinical (anthropometric, reproductive and metabolic factors) and biochemical characteristic features of PCOS and IBS were included. Also, we excluded case reports, commentaries, editorials and letters to the editor. We did not restrict the study setting and publication date; however, there was a restriction in this study regarding the English language. The search was performed around the key terms, including polycystic ovary syndrome, PCOS, Stein‐Leventhal Syndrome, Sclerocystic Ovarian Degeneration, Sclerocystic Ovary Syndrome, Ovarian Syndrome Polycystic, Irritable Bowel Syndromes, Colon Irritable, Irritable Colon, Colitis, Mucous Colitis, Gastrointestinal Diseases, Colonic Diseases, Colonic Diseases, Functional, Intestinal Diseases.
We identified additional studies through a manual search of the bibliographic references of relevant articles and existing reviews. Articles that met the inclusion criteria were carefully read and, when appropriate, further articles retrieved from their references were also reviewed with the aim to include other critical studies that might have been missed in the initial search. Qualitative studies, which directly assessed the association between PCOS and IBS, were performed utilising the Newcastle–Ottawa Scale for case–control studies. This scale was used to assess the selection, comparability and exposure domains [18]. In this study, scores <3, between 3 and 5 and >6 were considered as low, moderate and high‐quality studies.
3. Overview of the Relationship Between PCOS and IBS
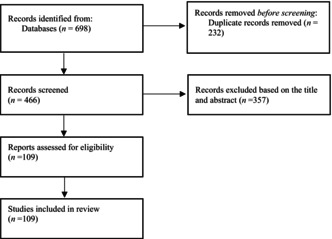
Figure 1 shows a flow chart of the included studies. So far, according to our knowledge, there are six case–control published articles that have directly investigated the association between PCOS and IBS. Overall, these studies included 793 women with PCOS and 547 women in the control group (Table 1). All of these studies had moderate to high‐quality score. In two studies, the prevalence of IBS was similar in women with PCOS compared with the controls [6, 7]. The results of Kałużna et al.'s study showed that the prevalence of IBS symptoms in patients with PCOS was not different from that in the control group. In addition, hyperandrogenism and obesity in patients with PCOS had no effect on the occurrence of IBS symptoms, and hormonal, anthropometric and chemical differences were not seen between IBS‐PCOS and non‐IBS‐PCOS patients. But in this study, the prevalence of metabolic syndrome and depression was higher in IBS‐PCOS than in non‐IBS‐PCOS patients [6].
FIGURE 1.
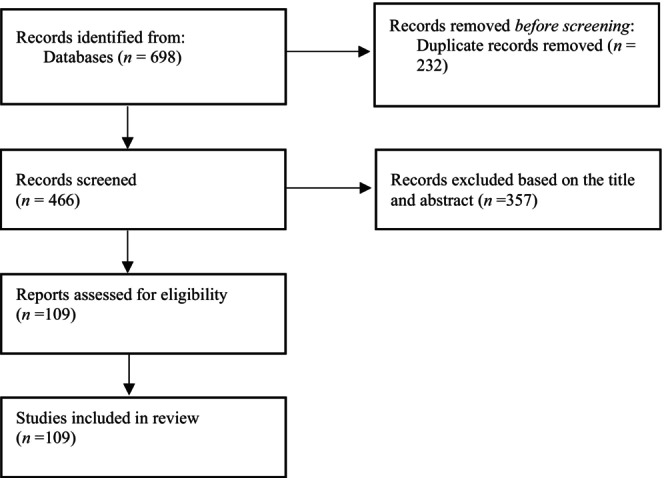
Flow chart of included studies.
TABLE 1.
Characteristics of included studies related the PCOS and IBS.
| Author, year, reference | Study design | Participants | PCOS diagnosis criteria | IBS diagnosis criteria | Findings | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | Total | ||||||
| Dursun et al. (2018) [19] | Case–control |
Patients with PCOS (n = 54) Controls (n = 53) |
Revised 2003 Rotterdam criteria | Rome III criteria | Prevalence of IBS was 39% in PCOS patients vs 19% in control | 2 | 1 | 2 | 5 |
| Mathur et al. (2010) [20] | Case–control |
Patients with PCOS (n = 36) Controls (n = 29) |
NIH 1990 | Bowel‐related questions | Prevalence of IBS was 41.7% in PCOS patients vs 10.3% in control | 4 | — | 2 | 6 |
| Kałużna et al. (2022) [6] | Case–control |
Patients with PCOS (n = 133) Controls (n = 72) |
ESHRE guideline | Rome IV criteria | Prevalence of IBS was 24% (32/133) in PCOS patients vs. 21% in control (15/72) (p = 0.60) | 3 | 2 | 3 | 8 |
| Bazarganipour et al. (2020) [14] | Case–control |
Patients with PCOS (n = 101) Controls (n = 100) |
Rotterdam diagnostic criteria | Rome III criteria | IBS symptoms were higher in PCOS (20.7%) than control group (11%) (p = 0.05). | 3 | — | 3 | 6 |
| Tseng et al. (2020) [17] | Case–control |
Patients with PCOS (n = 431) Controls (n = 259) |
Rotterdam diagnostic criteria | Rome III criteria | Women with PCOS were more likely to have IBS (10.7% vs. 5.8%, p = 0.029) and obesity (29% vs. 4%, p < 0.001) than healthy volunteers. Mixed‐type IBS (IBS‐M) was the most common subtype (74%) among patients with PCOS and IBS. | 3 | — | 3 | 6 |
| Naziye Gürkan et al. (2022) [7] | Case–control | Women with PCOS (n = 38) and control group (n = 34) | Rotterdam Criteria | Roma IV | IBS prevalence was similar in PCOS (52%) and the control group (50%). | 3 | — | 2 | 5 |
Abbreviations: ESHRE, European Society of Human Reproduction and Embryology; IBS, irritable bowel syndrome; NIH, National Institutes of Health; PCOS, polycystic ovary syndrome.
In the study by Gürkan, Mehmet, and Gürbüz [7] the prevalence of IBS was similar in PCOS and control groups. In addition in the IBS‐PCOS group, fasting insulin (FI) and luteinizing hormone (LH) were significantly lower than in the non‐IBS‐PCOS group (p < 0.05), but there was no statistically significant association between IBS‐PCOS and non‐IBS‐PCOS in terms of gastrointestinal symptoms.
In the study by Tseng et al. [17] women with PCOS were more likely to have IBS. In addition, in women with PCOS and IBS, sleep difficulties and psychiatric morbidities were more prevalent compared to PCOS patients without IBS, but anthropometric, metabolic and hormonal profiles were similar between IBS‐PCOS and non‐IBS‐PCOS patients.
Bazarganipour et al. [14] in their study, in addition to showing that the prevalence of IBS is higher in patients with PCOS than that in the control group, stated that the quality of life in patients who had both IBS and PCOS at the same time was lower than in other groups.
4. Potential Pathways That Link PCOS and IBS
Figure 2 depicts the potential cross‐link between IBS and PCOS.
FIGURE 2.
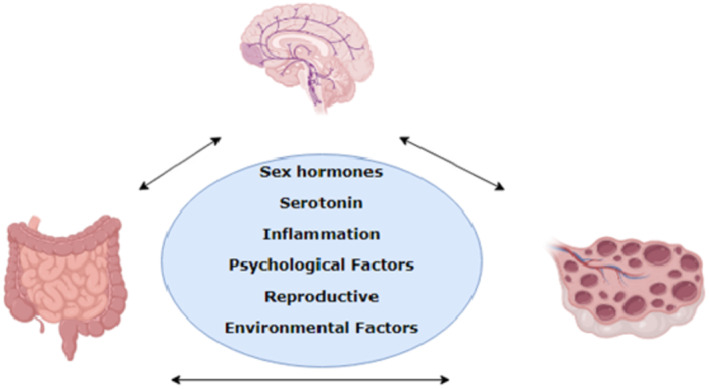
Cross‐link between IBS and PCOS.
4.1. Sex Hormone Alterations
The link between sex hormones and IBS has gained growing interest over the last decade. Evidence emphasises that women are more susceptible to IBS than men [21]. Houghton et al. [22] reported that testosterone levels are inversely associated with IBS symptomatology. Although the main role of sex hormones is in human reproduction, their role in other organs has been demonstrated. The role of sex hormones in GI tract motility function has been evidenced in the recent literature [23, 24, 25]. This observation suggests that if hormonal changes occur throughout the female's life (pregnancy, menopause, etc.), these alterations could affect the GI function [23]. Generally, hormonal changes during the hormonal transition phase of menopause might be relevant to gastrointestinal health and IBS. Interestingly, Ruigomez et al. [26] in their study observed that postmenopausal women who are under hormone therapy are at increased risk of IBS similar to premenopausal women. It is also well known that the fluctuation of sex hormones during the menstrual cycle could affect the severity of symptoms of IBS [24], which highlights the important role of sex hormones in the activation of the pathological pathways of IBS.
Kim et al. [27] in their study among young males demonstrated that testosterone and sex hormone‐binding globulin (SHBG) levels in patients with IBS were higher than in the control group, which reflects the differing status of sex hormones in patients with IBS. It is surprising that sex hormones can affect not only the susceptibility to IBS, but also pain perception. Evidence has indicated that oestrogen receptors (OR) and androgen receptors (AR) commonly act as central nervous system (CNS) stimulants and inhibition, respectively, hence variations in sex hormone levels are able to alter the symptoms of IBS [21, 28]. Previously, the results of a meta‐analysis demonstrated that the symptoms of IBS among women with and without IBS were commonly reported in menses rather than in other phases of the menstrual cycle [11]. Furthermore, other reviews concluded that oestrogen and progesterone withdrawal around menopause and menses might contribute to increased symptoms of GI [29]. The lower levels of progesterone during menses, is thought to contribute to greater somatic pain [29].
Gynaecological complaints are more commonly reported by women with IBS [30]. A population‐based study revealed that women with dysmenorrhoea are more prone to IBS symptoms [31]. Oestrogen plays an important role in visceral afferent, autonomic nervous system and pain pathways [32]. Far less research has investigated the fluctuation of hormones and IBS in women with PCOS. Apart from this, ovarian hormones could also contribute to physiological responses and coping behaviour against stress [33]. It is recommended that in women with PCOS, more attention should be paid to the history of menstrual cycle irregularity [34]. To sum up, these findings indicated that pathophysiological changes in sex hormone levels may play a role in the development of IBS in women with PCOS.
4.2. Serotonin Dysfunction
Neurotransmitter alteration could be considered another factor suggested to be contributing to the development of both PCOS and IBS. It has been reported that serotonin dysregulation is involved in the pathophysiology of PCOS [35]. Similarly, evidence has been shown that the release of serotonin is disturbed in patients with IBS [36]. The evidence revealed that the decrease in intestinal serotonin leads to weakness in the intestinal lining, which inevitably results in constipation and an increase in serotonin levels within the gut [37]. Another hypothesis in this context implies the deficiency of the serotonin transporter‐serotonin reuptake transporter (SERT) enterocytes in IBS patients [38]. Indeed, serotonin, which is regulated by SERT, could influence gut distension, motility and visceral sensitivity [39]. So, any disturbance in serotonin levels could alter the development of IBS. However, animal studies demonstrated that gut dysbiosis can result in insulin resistance in the PCOS mouse model [40].
What's more, oral contraceptive (OCs) are the first‐line treatment option in the management of PCOS. A recent study has shown that in women who use OCs, global brain serotonin four receptor binding was 9%–12% lower than in women not using this agent; it is speculated that the reduction in ovarian hormones could result in a reduction in 5‐HT4R gene expression [41]. So, this is one possible cause of increased risk of IBS in women with PCOS.
Today, researchers are exploring novel pharmacological approaches such as 5‐HT3 receptor antagonists and 5‐HT4 receptor agonists for IBS management [42]. Furthermore, scientists reported the positive effect of α‐lactalbumin maintaining high levels of serotonin in the management of PCOS [43].
4.3. Inflammatory Factors
Inflammation is a component that greatly contributes to the pathogenesis of both PCOS and IBS. Mucosal inflammation and neuroinflammation are more likely involved in the pathophysiology of IBS [44]. In addition, similar findings showed the role of high‐sensitive C‐reactive protein in both PCOS [45, 46] and IBS [47]. With PCOS, this inflammation marker is associated with obesity [45] and cardiovascular disease [46].
Recently, Parker, O'Brien, and Hawrelak [5] in a narrative review attempted to present the role of gastrointestinal symbiosis and revealed that lipopolysaccharide (LPS) and LPS‐binding protein (LPS‐BP) might be involved in the pathogenesis of PCOS. Dlugosz et al. [48] emphasised the important role of LPS in the development of IBS. Inflammation could alter the SERT, which in turn decreases serotonin levels and acts as a trigger for IBS [39].
Moreover, inflammation (inflammation induced by diet, adipose tissue and chronic low‐grade inflammation) and oxidative stress could result in insulin resistance and ovarian dysfunction in women with PCOS [49]. Also, in IBS, inflammation and oxidative stress, as well as stress‐modulating pathways, can be related to the development of gastrointestinal symptoms of IBS. Generally, the main disorder that definitively contributes to the pathology of IBS may be two‐way communication errors of the gut–brain axis [50].
4.4. Gut–Brain Alteration
The role of the gut–brain axis in the pathogenesis of IBS and d PCOS has drawn much attention recently [51, 52, 53, 54]. Gut microbiome alteration in women with PCOS might be closely linked to insulin resistance, sex hormone levels, and immune change function and inflammation [21, 55]. Furthermore, gut microbiome by mediating the systemic low‐grade inflammation and insulin resistance in women with PCOS contributes to the development of PCOS [56]. In fact, gut microbiota plays an important role in the incidence, progression and phenotype of PCOS [57, 58].
Gut microbial symbiosis may be responsible for neuroendocrine alteration in women with PCOS [53, 59]. Similarly, the gut–brain alteration was also observed in patients with IBS. Evidence suggests that gut inflammation, cytokine response, and the gut microbiome contribute to such gut‐to‐brain changes in IBS [51, 60]. Therefore, when it comes to the gut–brain axis it is hypothesised that both IBS and PCOS share a common pathway.
Recently, scientists demonstrated the positive effect of bacteriotherpeutics like probiotics, synbiotics and faecal microbiota transplant (FMT) in PCOS and IBS [61, 62, 63].
4.5. Metabolic Disturbances
Numerous studies have been postulated to explain the role of metabolic parameters in the pathogenesis of both PCOS and IBS. It is well documented that women with PCOS are more likely to suffer from METs [64]. The results of a review on humans supported these findings and demonstrated that insulin resistance, obesity, dyslipidaemia and hyperandrogenism contributing factors to the metabolic syndrome in PCOS [65]. Although some researchers have supported the association between IBS and METs [15, 66], others have found no association between IBS and METs [67]. Subsequent observational studies on the relationship between Mets and IBS and PCOS are needed.
There is an established link between IBS and prediabetes/diabetes [68] as well as PCOS and diabetes [69]. Furthermore, co‐incidence of nonalcoholic fatty liver disease with both IBS and PCOS [70, 71] has been supported recently. The recognition of the mechanisms behind these observations is unclear.
Obesity is a possible common comorbidity of PCOS and IBS [72, 73]. As the aforementioned evidence seems to support the role of obesity in the pathogenesis of IBS and PCOS, it should be expected that any weight reduction would have a beneficial effect on IBS and PCOS. It is also reported that inflammation, physical inactivity, microbiota, diet and psychological factors could mediate the association between obesity and IBS [74, 75].
Collectively, metabolic abnormality not only acts as a mechanical effect but also plays the role of risk factor for the close link between PCOS and IBS. Further studies are needed to deepen our understanding in this regard.
4.6. Psychological Stress
One of the key elements for developing PCOS and IBS is psychological stress. Stress could act as a trigger for IBS via activation of the neuro‐endocrine‐immune pathways and following it gut–brain axis and microbiota‐gut‐brain axis [13, 76]. Roohafza et al. suggested a high prevalence of mental disorders like stress, anxiety and depression in subjects with IBS [77]. In IBS, impairment in the brain–gut pathway results in psychological manifestations of the disease. A systematic review revealed that individuals with IBS highly suffer from depression and anxiety. It is said that both PCOS and IBS are stress‐sensitive disorders. The stress‐induced alteration could adversely affect the bacterial composition of the GI tract [78].
Furthermore, omen who use OCs are at risk for depressive symptoms [79, 80], which can be altered for the development of IBS. Conversely, there is also evidence which demonstrated that using OC was not associated with mood disorders [81]. Nevertheless, as adolescent females start on OCs at an increasingly young age, (12) their brains are more susceptible to the potential impact of exogenous hormones as they go through critical stages of brain maturation (13). Mood disorders like depression and anxiety were associated with an increased risk of developing IBS [82].
Evidence also reported that the elevated cortisol/dehydroepiandostrerone ratio after waking up was observed among individuals with IBS compared to the non‐IBS ones [83]. This highlights the effect of long‐term stress. Like IBS, stress is also an important component of PCOS [84]. A meta‐analysis among women with PCOS has shown higher levels of cortisol as a potential stress marker in the pathogenesis of PCOS [85]. Other animal models demonstrated that long‐term stress in rat models could induce the PCOS phenotype [86].
4.7. Reproductive Disturbances
It has been proposed that in women with IBS the odds of conception might be decreased due to the putative mechanism of oxidative stress [87]. Furthermore, women with IBS are more prone to fertility problems including miscarriage and ectopic pregnancy [88, 89]. The result of another study among 9,096,788 deliveries demonstrates that women with IBS have a higher risk of developing adverse pregnancy outcomes [90].
Similar to women with IBS, women with PCOS are at risk of infertility, especially ovulatory infertility. Difficulty in conceiving is a common reproductive problem of women with PCOS [91]. In addition, it is associated with an increased risk of pregnancy complications, such as abortion, gestational diabetes and preeclampsia [92, 93]. On the contrary, women with PCOS are more prone to suffer from being overweight and obese; these factors themselves cause the decreased possibility of fertility with impaired ovulation, quality of oocyte and embryo implantation. In addition, if women with PCOS use assisted reproductive technology (ART), the rates of implantation, pregnancy and live birth will decrease, whereas, the risk of miscarriage will increase [94, 95].
The prevalence of obesity and body fat percentage is higher in women with PCOS and IBS than in women with PCOS alone, [20] and obesity/overweight simultaneously increases the risk of sub‐fecundity, infertility, miscarriage, poor ART outcome and decreases live birth rate in the former [96].
The prevalence of insulin resistance, impaired glucose intolerance (IGT) and diabetes mellitus is higher in women with PCOS than in healthy women which can induce the reproductive traits of PCOS [97, 98].
4.8. Environmental and Lifestyle Factors
There is more evidence that shows that environmental toxins have a significant impact on reproduction as well as gastrointestinal disturbances [99, 100]. Advanced glycation end products (AGEs) are examples of environmental factors in the development or progression of PCOS and IBS [101]. Prepared fast food and cooking food at very high temperatures can increase the AGEs, which have an adverse effect on the pathophysiology of PCOS and IBS [102]. The literature showed that AGEs are associated positively with insulin resistance, testosterone and anti‐Müllerian hormone levels [102, 103]. In addition, in animals fed enriched AGE diets showed hormonal and metabolic disorders and an accumulation of AGEs in the ovarian tissue and [104]. On the contrary, the low AGE diet seemed to have a beneficial effect on oxidative stress in PCOS [105]. The literature showed that most dietary receptors of AGEs accumulate in the ileum and colon [106]. These AGEs by reducing enzymatic antioxidant pathways and increasing the level of inflammatory cytokines can reduce the first‐line antioxidant defence and stimulate the inflammatory response in the gastrointestinal tract [107].
Endocrine disrupting chemicals (EDCs) are other environmental toxins in the environment, food, personal care products and manufactured products that affect the reproductive and health system and interfere with hormones that are responsible for homeostasis, reproduction and developmental process [108]. The results of a study by Eleni Kandaraki [109] showed the levels of bisphenol A (BPA), the most common chemical produced worldwide and one of the most widely studied EDC, were significantly higher in the PCOS group than in the controls. The main role of BPA in the pathogenesis of PCOS is still not well understood, but there are many reports in regard to the effect of these EDSs on ovarian steroidogenesis [110].
Diet as a main component of lifestyle plays role in both pathophysiology as well as treatment of IBS [111] and PCOS [112], so diet therapy plays an important role in controlling and improving symptoms of IBS and PCOS [113]. Evidence has demonstrated that lifestyle modification is the cornerstone for the management of IBS and PCOS [113, 114, 115].
For example, the result of a systematic review showed that the Dietary Approaches to Stop Hypertension (DASH) is most effective in insulin resistance in PCOS [113], which is in line with the results of previous studies in patients with type 2 diabetes [116]. In IBS patients, the general recommendation is consuming a regular diet, exercising, physical activity, drinking enough water, avoiding spicy and fatty foods and following a low FODMAP diet [117].
Stress as a lifestyle factor plays an important role in the development and severity of IBS symptoms [76]. It can be due to the effect of stress on the brain–gut interactions through the change in the activity of the hypothalamic–pituitary–adrenal (HPA) axis and of the autonomic nervous (ANS), metabolic and immune systems [118]. In addition, stress stimulates the sympathetic nervous system and releases ACTH and cortisol, which affect gut function [76].
Like IBS, in the PCOS population also stress is an important component [84]. The main role of catecholamine in response to stress in the brain can be the main cause of mental and metabolic disorders in PCOS [119].
4.9. Limitation and Strength
The main strength of this review lies in presenting the factors that are common in the pathological pathways of PCOS and IBS. This review sets the background for future biological studies that aim to clear understanding the aetiology of the coincidence of PCOS–IBS. This review mainly is limited by observational studies that used different criteria for diagnosis and does not fully considered some confounders. What's, there is a lack of knowledge regarding the association between different phenotypes of PCOS and IBS. To better understand these, future studies with a large sample size are recommended. Furthermore, in this study we did not include grey literature.
4.10. Conclusion
This study aimed to shed light on the crosstalk between PCOS and IBS. The existing evidence has not completely answered the question of an association between PCOS and IBS and vice versa, and a few studies have shown a higher prevalence of IBS in women with PCOS. Despite that, several common potential pathways directly and indirectly may contribute to the interaction between IBS and PCOS, including alteration in sex hormones or gut–brain, dysregulation of neurotransmitters and inflammatory factors, metabolic or reproductive disturbances, and psychological, environmental and lifestyle factors.
Author Contributions
Marzieh Saei Ghare Naz: Designed and directed the project; writing of the manuscript; methodology; review and edit the manuscript. Vida Ghasemi: Investigation (equal); writing – original draft (equal); writing – review and editing (equal). Shabahang Amirshekari: Methodology (equal); writing – original draft (equal); writing – review and editing (equal). Fahimeh Ramezani Tehrani: Designed and directed the project; supervised the work; writing of the manuscript; methodology; and review and edit the manuscript.
Ethics Statement
The study was approved by the ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
Consent
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
We thanks ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
Funding: This study funded by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant number: 1‐43009224).
Data Availability Statement
Not applicable.
References
- 1. Wolf W. M., Wattick R. A., Kinkade O. N., and Olfert M. D., “Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity,” International Journal of Environmental Research and Public Health 15, no. 11 (2018): 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teede H. J., Misso M. L., Costello M. F., et al., “Recommendations from the International Evidence‐Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome,” Human Reproduction 33, no. 9 (2018): 1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert E. W. and Tay C. T., “Comorbidities and Complications of Polycystic Ovary Syndrome: An Overview of Systematic Reviews,” Clinical Endocrinology 89, no. 6 (2018): 683–699. [DOI] [PubMed] [Google Scholar]
- 4. Palomba S., Santagni S., Falbo A., and La Sala G. B., “Complications and Challenges Associated With Polycystic Ovary Syndrome: Current Perspectives,” International Journal of Women's Health 7 (2015): 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parker J., O'Brien C., and Hawrelak J., “A Narrative Review of the Role of Gastrointestinal Dysbiosis in the Pathogenesis of Polycystic Ovary Syndrome,” Obstetrics & Gynecology Science 65, no. 1 (2022): 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kałużna M., Kompf P., Wachowiak‐Ochmańska K., et al., “Are Patients With Polycystic Ovary Syndrome more Prone to Irritable Bowel Syndrome?” Endocrine Connections 11, no. 4 (2022): e210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gürkan N., Mehmet A., and Gürbüz T., “The Relationship between Polycystic Ovary Syndrome and Irritable Bowel Syndrome,” Journal of Health Sciences and Medicine 5, no. 5 (2022): 1220–1224. [Google Scholar]
- 8. Wei Z., Chen Z., Xiao W., and Wu G., “A Systematic Review and Meta‐Analysis of the Correlation between Polycystic Ovary Syndrome and Irritable Bowel Syndrome,” Gynecological Endocrinology 39, no. 1 (2023): 2239933. [DOI] [PubMed] [Google Scholar]
- 9. Oka P., Parr H., Barberio B., Black C. J., Savarino E. V., and Ford A. C., “Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta‐Analysis,” The Lancet Gastroenterology & Hepatology 5, no. 10 (2020): 908–917. [DOI] [PubMed] [Google Scholar]
- 10. Lacy B. E. and Patel N. K., “Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome,” Journal of Clinical Medicine 6, no. 11 (2017): 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adeyemo M., Spiegel B., and Chang L., “Meta‐Analysis: Do Irritable Bowel Syndrome Symptoms Vary between Men and Women?” Alimentary Pharmacology & Therapeutics 32, no. 6 (2010): 738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulak A., Taché Y., and Larauche M., “Sex Hormones in the Modulation of Irritable Bowel Syndrome,” World Journal of Gastroenterology 20, no. 10 (2014): 2433–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahurkar‐Joshi S. and Chang L., “Epigenetic Mechanisms in Irritable Bowel Syndrome,” Frontiers in Psychiatry 11 (2020): 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bazarganipour F., Taghavi S.‐A., Asemi Z., et al., “The Impact of Irritable Bowel Syndrome on Health‐Related Quality of Life in Women With Polycystic Ovary Syndrome,” Health and Quality of Life Outcomes 18, no. 1 (2020): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo Y., Niu K., Momma H., et al., “Irritable Bowel Syndrome Is Positively Related to Metabolic Syndrome: A Population‐Based Cross‐Sectional Study,” PLoS One 9, no. 11 (2014): e112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ali A. T., “Polycystic Ovary Syndrome and Metabolic Syndrome,” Ceská Gynekologie 80, no. 4 (2015): 279–289. [PubMed] [Google Scholar]
- 17. Tseng P. H., Chiu H. M., Tu C. H., Wu M. S., Ho H. N., and Chen M. J., “Obesity Exacerbates Irritable Bowel Syndrome‐Related Sleep and Psychiatric Disorders in Women With Polycystic Ovary Syndrome,” Front Endocrinol (Lausanne). 12 (2021): 779456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G., Shea B., O'Connell D., et al., The Newcastle Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta‐Analyses (Ottawa: Ottawa Hospital Research Institute, 2011). [Google Scholar]
- 19. Dursun H., Uyanıkoglu H., Uyanıkoglu A., and Sabuncu T., “Incidences of Irritable Bowel Syndrome and its Subtypes in Patients With Polycystic Ovary Syndrome,” Journal of Clinical and Analytical Medicine 9, no. 4 (2018): 329–332. [Google Scholar]
- 20. Mathur R., Ko A., Hwang L. J., Low K., Azziz R., and Pimentel M., “Polycystic Ovary Syndrome is Associated With an Increased Prevalence of Irritable Bowel Syndrome,” Digestive Diseases and Sciences 55 (2010): 1085–1089. [DOI] [PubMed] [Google Scholar]
- 21. Kim Y. S. and Kim N., “Sex‐Gender Differences in Irritable Bowel Syndrome,” Journal of Neurogastroenterology and Motility 24, no. 4 (2018): 544–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houghton L. A., Jackson N. A., Whorwell P. J., and Morris J., “Do Male Sex Hormones Protect from Irritable Bowel Syndrome?” The American Journal of Gastroenterology 95, no. 9 (2000): 2296–2300. [DOI] [PubMed] [Google Scholar]
- 23. Alqudah M., Al‐Shboul O., Al Dwairi A., Al‐U'Datt D. G., and Alqudah A., “Progesterone Inhibitory Role on Gastrointestinal Motility,” Physiological Research 71, no. 2 (2022): 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roisinblit K. C., “Irritable Bowel Syndrome in Women,” Journal of Midwifery & Women's Health 58, no. 1 (2013): 15–24. [DOI] [PubMed] [Google Scholar]
- 25. Hogan A. M., Collins D., Baird A. W., and Winter D. C., “Estrogen and its Role in Gastrointestinal Health and Disease,” International Journal of Colorectal Disease 24, no. 12 (2009): 1367–1375. [DOI] [PubMed] [Google Scholar]
- 26. Ruigómez A., García Rodríguez L. A., Johansson S., and Wallander M.‐A., “Is Hormone Replacement Therapy Associated With an Increased Risk of Irritable Bowel Syndrome?” Maturitas 44, no. 2 (2003): 133–140. [DOI] [PubMed] [Google Scholar]
- 27. Kim B. J., Rhee P.‐L., Park J. H., et al., “Male Sex Hormones May Influence the Symptoms of Irritable Bowel Syndrome in Young Men,” Digestion 78, no. 2–3 (2008): 88–92. [DOI] [PubMed] [Google Scholar]
- 28. Aloisi A. M., Bachiocco V., Costantino A., et al., “Cross‐Sex Hormone Administration Changes Pain in Transsexual Women and Men,” Pain 132 (2007): S60–S67. [DOI] [PubMed] [Google Scholar]
- 29. Heitkemper M. M. and Chang L., “Do Fluctuations in Ovarian Hormones Affect Gastrointestinal Symptoms in Women With Irritable Bowel Syndrome?” Gender Medicine 6 (2009): 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prior A., Wilson K., Whorwell P. J., and Faragher E. B., “Irritable Bowel Syndrome in the Gynecological Clinic. Survey of 798 New Referrals,” Digestive Diseases and Sciences 34, no. 12 (1989): 1820–1824. [DOI] [PubMed] [Google Scholar]
- 31. Olafsdottir L. B., Gudjonsson H., Jonsdottir H. H., Björnsson E., and Thjodleifsson B., “Natural History of Irritable Bowel Syndrome in Women and Dysmenorrhea: A 10‐Year Follow‐Up Study,” Gastroenterology Research and Practice 2012 (2012): 534204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ouyang A. and Wrzos H. F., “Contribution of Gender to Pathophysiology and Clinical Presentation of IBS: Should Management Be Different in Women?” Official Journal of the American College of Gastroenterology| ACG. 101 (2006): S602–S609. [DOI] [PubMed] [Google Scholar]
- 33. Ter Horst G. J., Wichmann R., Gerrits M., Westenbroek C., and Lin Y., “Sex Differences in Stress Responses: Focus on Ovarian Hormones,” Physiology & Behavior 97, no. 2 (2009): 239–249. [DOI] [PubMed] [Google Scholar]
- 34. Chandar A. K., “Diagnosis and Treatment of Irritable Bowel Syndrome With Predominant Constipation in the Primary‐Care Setting: Focus on Linaclotide,” International Journal of General Medicine 10 (2017): 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaudhari N., Dawalbhakta M., and Nampoothiri L., “GnRH Dysregulation in Polycystic Ovarian Syndrome (PCOS) Is a Manifestation of an Altered Neurotransmitter Profile,” Reproductive Biology and Endocrinology: RB&E 16, no. 1 (2018): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saha L., “Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence‐Based Medicine,” World Journal of Gastroenterology 20, no. 22 (2014): 6759–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manocha M. and Khan W. I., “Serotonin and GI Disorders: An Update on Clinical and Experimental Studies,” Clinical and Translational Gastroenterology 3, no. 4 (2012): e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruta K. and Bhasin K., “The Role of Serotonin and Diet in the Prevalence of Irritable Bowel Syndrome: A Systematic Review,” Translational Medicine Communications 6 (2021): 1–9. [Google Scholar]
- 39. Vahora I. S., Tsouklidis N., Kumar R., Soni R., and Khan S., “How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome,” Cureus 12, no. 8 (2020): e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y. L., Zhou W. W., Wu S., et al., “Intestinal Flora Is a Key Factor in Insulin Resistance and Contributes to the Development of Polycystic Ovary Syndrome,” Endocrinology 162 (2021): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsen S., Köhler‐Forsberg K., Dam V., et al., “Oral Contraceptives and the Serotonin 4 Receptor: A Molecular Brain Imaging Study in Healthy Women,” Acta Psychiatrica Scandinavica 142, no. 4 (2020): 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gros M., Gros B., Mesonero J. E., and Latorre E., “Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management,” Journal of Clinical Medicine 10, no. 15 (2021): 3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardinale V., Lepore E., Basciani S., et al., “Positive Effects of α‐Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome,” Nutrients 14, no. 15 (2022): 3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng Q. X., Soh A. Y. S., Loke W., Lim D. Y., and Yeo W.‐S., “The Role of Inflammation in Irritable Bowel Syndrome (IBS),” Journal of Inflammation Research 11 (2018): 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ganie M. A., Hassan S., Nisar S., et al., “High‐Sensitivity C‐Reactive Protein (hs‐CRP) Levels and its Relationship With Components of Polycystic Ovary Syndrome in Indian Adolescent Women With Polycystic Ovary Syndrome (PCOS),” Gynecological Endocrinology 30, no. 11 (2014): 781–784. [DOI] [PubMed] [Google Scholar]
- 46. Boulman N., Levy Y., Leiba R., et al., “Increased C‐Reactive Protein Levels in the Polycystic Ovary Syndrome: A Marker of Cardiovascular Disease,” The Journal of Clinical Endocrinology & Metabolism. 89, no. 5 (2004): 2160–2165. [DOI] [PubMed] [Google Scholar]
- 47. Hod K., Ringel‐Kulka T., Martin C. F., Maharshak N., and Ringel Y., “High Sensitive C‐Reactive Protein as a Marker for Inflammation in Irritable Bowel Syndrome,” Journal of Clinical Gastroenterology 50, no. 3 (2016): 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dlugosz A., Nowak P., D'Amato M., et al., “Increased Serum Levels of Lipopolysaccharide and Antiflagellin Antibodies in Patients With Diarrhea‐Predominant Irritable Bowel Syndrome,” Neurogastroenterology and Motility 27, no. 12 (2015): 1747–1754. [DOI] [PubMed] [Google Scholar]
- 49. González F., “Inflammation in Polycystic Ovary Syndrome: Underpinning of Insulin Resistance and Ovarian Dysfunction,” Steroids 77, no. 4 (2012): 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balmus I.‐M., Ciobica A., Cojocariu R., Luca A.‐C., and Gorgan L., “Irritable Bowel Syndrome and Neurological Deficiencies: Is there a Relationship? The Possible Relevance of the Oxidative Stress Status,” Medicina 56, no. 4 (2020): 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holtmann G. J., Ford A. C., and Talley N. J., “Pathophysiology of Irritable Bowel Syndrome,” The Lancet Gastroenterology & Hepatology 1, no. 2 (2016): 133–146. [DOI] [PubMed] [Google Scholar]
- 52. Ozgen Saydam B. and Yildiz B. O., “Polycystic Ovary Syndrome and Brain: An Update on Structural and Functional Studies,” The Journal of Clinical Endocrinology & Metabolism. 106, no. 2 (2021): e430–e441. [DOI] [PubMed] [Google Scholar]
- 53. Liang Z., Di N., Li L., and Yang D., “Gut Microbiota Alterations Reveal Potential Gut–Brain Axis Changes in Polycystic Ovary Syndrome,” Journal of Endocrinological Investigation 44, no. 8 (2021): 1727–1737. [DOI] [PubMed] [Google Scholar]
- 54. Chong P. P., Chin V. K., Looi C. Y., Wong W. F., Madhavan P., and Yong V. C., “The Microbiome and Irritable Bowel Syndrome–A Review on the Pathophysiology, Current Research and Future Therapy,” Frontiers in Microbiology 10 (2019): 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giampaolino P., Foreste V., Di Filippo C., et al., “Microbiome and PCOS: State‐of‐Art and Future Aspects,” International Journal of Molecular Sciences 22, no. 4 (2021): 2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He F.‐f. and Li Y.‐m., “Role of Gut Microbiota in the Development of Insulin Resistance and the Mechanism Underlying Polycystic Ovary Syndrome: A Review,” Journal of Ovarian Research 13, no. 1 (2020): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corrie L., Awasthi A., Kaur J., et al., “Interplay of Gut Microbiota in Polycystic Ovarian Syndrome: Role of Gut Microbiota, Mechanistic Pathways and Potential Treatment Strategies,” Pharmaceuticals 16, no. 2 (2023): 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang L., Zhou J., Gober H.‐J., et al., “Alterations in the Intestinal Microbiome Associated With PCOS Affect the Clinical Phenotype,” Biomedicine & Pharmacotherapy 133 (2021): 110958. [DOI] [PubMed] [Google Scholar]
- 59. Saydam B. O. and Yildiz B. O., “Gut‐Brain Axis and Metabolism in Polycystic Ovary Syndrome,” Current Pharmaceutical Design 22, no. 36 (2016): 5572–5587. [DOI] [PubMed] [Google Scholar]
- 60. Liebregts T., Adam B., Bredack C., et al., “Immune Activation in Patients With Irritable Bowel Syndrome,” Gastroenterology 132, no. 3 (2007): 913–920. [DOI] [PubMed] [Google Scholar]
- 61. Corrie L., Gulati M., Vishwas S., et al., “Combination Therapy of Curcumin and Fecal Microbiota Transplant: Potential Treatment of Polycystic Ovarian Syndrome,” Medical Hypotheses 154 (2021): 110644. [DOI] [PubMed] [Google Scholar]
- 62. El‐Salhy M., Patcharatrakul T., and Gonlachanvit S., “Fecal Microbiota Transplantation for Irritable Bowel Syndrome: An Intervention for the 21st Century,” World Journal of Gastroenterology 27, no. 22 (2021): 2921–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumar L. S., Pugalenthi L. S., Ahmad M., Reddy S., Barkhane Z., and Elmadi J., “Probiotics in Irritable Bowel Syndrome: A Review of Their Therapeutic Role,” Cureus 14, no. 4 (2022): e24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lim S. S., Kakoly N. S., Tan J. W. J., Fitzgerald G., and Bahri Khomami M., “Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review, Meta‐Analysis and Meta‐Regression,” Obesity Reviews 20, no. 2 (2019): 339–352. [DOI] [PubMed] [Google Scholar]
- 65. Chen W. and Pang Y., “Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites,” Metabolites 11, no. 12 (2021): 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee S. H., Kim K. N., Kim K. M., and Joo N. S., “Irritable Bowel Syndrome May be Associated With Elevated Alanine Aminotransferase and Metabolic Syndrome,” Yonsei Medical Journal 57, no. 1 (2016): 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Javadekar N. S., Oka G. A., Joshi A. S., Vaste P., Tamane S., and Lawate P. S., “Prevalence of Irritable Bowel Syndrome and Metabolic Syndrome Among Young Adults in an Annual Health Check‐up Setting,” JGH Open: An Open Access Journal of Gastroenterology and Hepatology 5, no. 10 (2021): 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gulcan E., Taser F., Toker A., Korkmaz U., and Alcelik A., “Increased Frequency of Prediabetes in Patients With Irritable Bowel Syndrome,” The American Journal of the Medical Sciences 338, no. 2 (2009): 116–119. [DOI] [PubMed] [Google Scholar]
- 69. Livadas S., Anagnostis P., Bosdou J. K., Bantouna D., and Paparodis R., “Polycystic Ovary Syndrome and Type 2 Diabetes Mellitus: A State‐of‐the‐Art Review,” World Journal of Diabetes 13, no. 1 (2022): 5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Purssell H., Whorwell P. J., Athwal V. S., and Vasant D. H., “Non‐alcoholic Fatty Liver Disease in Irritable Bowel Syndrome: More than a Coincidence?” World Journal of Hepatology 13, no. 12 (2021): 1816–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vassilatou E., “Nonalcoholic Fatty Liver Disease and Polycystic Ovary Syndrome,” World Journal of Gastroenterology 20, no. 26 (2014): 8351–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sadik R., Björnsson E., and Simren M., “The Relationship between Symptoms, Body Mass Index, Gastrointestinal Transit and Stool Frequency in Patients With Irritable Bowel Syndrome,” European Journal of Gastroenterology & Hepatology 22, no. 1 (2010): 102–108. [DOI] [PubMed] [Google Scholar]
- 73. Barber T. M., Hanson P., Weickert M. O., and Franks S., “Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies,” Clinical Medicine Insights Reproductive Health 13 (2019): 1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pugliese G., Muscogiuri G., Barrea L., Laudisio D., Savastano S., and Colao A., “Irritable Bowel Syndrome: A New Therapeutic Target when Treating Obesity?” Hormones (Athens, Greece) 18, no. 4 (2019): 395–399. [DOI] [PubMed] [Google Scholar]
- 75. Lee C. G., Lee J. K., Kang Y.‐S., et al., “Visceral Abdominal Obesity Is Associated With an Increased Risk of Irritable Bowel Syndrome,” The American Journal of Gastroenterology 110, no. 2 (2015): 310–319. [DOI] [PubMed] [Google Scholar]
- 76. Qin H.‐Y., Cheng C.‐W., Tang X.‐D., and Bian Z.‐X., “Impact of Psychological Stress on Irritable Bowel Syndrome,” World Journal of Gastroenterology 20, no. 39 (2014): 14126–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Roohafza H., Bidaki E. Z., Hasanzadeh‐Keshteli A., Daghaghzade H., Afshar H., and Adibi P., “Anxiety, Depression and Distress Among Irritable Bowel Syndrome and their Subtypes: An Epidemiological Population Based Study,” Advanced Biomedical Research 5 (2016): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Raskov H., Burcharth J., Pommergaard H.‐C., and Rosenberg J., “Irritable Bowel Syndrome, the Microbiota and the Gut‐Brain Axis,” Gut Microbes 7, no. 5 (2016): 365–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anderl C., Li G., and Chen F. S., “Oral Contraceptive Use in Adolescence Predicts Lasting Vulnerability to Depression in Adulthood,” Journal of Child Psychology and Psychiatry 61, no. 2 (2020): 148–156. [DOI] [PubMed] [Google Scholar]
- 80. Oinonen K. A. and Mazmanian D., “To What Extent Do Oral Contraceptives Influence Mood and Affect?” Journal of Affective Disorders 70, no. 3 (2002): 229–240. [DOI] [PubMed] [Google Scholar]
- 81. de Wit A. E., de Vries Y. A., de Boer M. K., et al., “Hormonal Contraceptive Use and Depressive Symptoms: Systematic Review and Network Meta‐Analysis of Randomised Trials,” BJPsych Open 7, no. 4 (2021): e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Simpson C. A., Mu A., Haslam N., Schwartz O. S., and Simmons J. G., “Feeling Down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome,” Journal of Affective Disorders 266 (2020): 429–446. [DOI] [PubMed] [Google Scholar]
- 83. Sugaya N., Izawa S., Saito K., Shirotsuki K., Nomura S., and Shimada H., “Effect of Prolonged Stress on the Adrenal Hormones of Individuals With Irritable Bowel Syndrome,” BioPsychoSocial Medicine 9, no. 1 (2015): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Papalou O. and Diamanti‐Kandarakis E., “The Role of Stress in PCOS,” Expert Review of Endocrinology & Metabolism 12, no. 1 (2017): 87–95. [DOI] [PubMed] [Google Scholar]
- 85. Benjamin J. J., Kuppusamy M., Koshy T., Kalburgi Narayana M., and Ramaswamy P., “Cortisol and Polycystic Ovarian Syndrome–A Systematic Search and Meta‐Analysis of Case‐Control Studies,” Gynecological Endocrinology 37, no. 11 (2021): 961–967. [DOI] [PubMed] [Google Scholar]
- 86. Divyashree S. and Yajurvedi H., “Long‐Term Chronic Stress Exposure Induces PCO Phenotype in Rat,” Reproduction 152, no. 6 (2016): 765–774. [DOI] [PubMed] [Google Scholar]
- 87. Anton C., Ciobica A., Doroftei B., et al., “A Review of the Complex Relationship between Irritable Bowel Syndrome and Infertility,” Medicina (Kaunas, Lithuania) 56, no. 11 (2020): 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khashan A. S., Quigley E. M., McNamee R., McCarthy F. P., Shanahan F., and Kenny L. C., “Increased Risk of Miscarriage and Ectopic Pregnancy Among Women With Irritable Bowel Syndrome,” Clinical Gastroenterology and Hepatology 10, no. 8 (2012): 902–909. [DOI] [PubMed] [Google Scholar]
- 89. Talavera J. I., Parrill A. M., Elsayad C., Fogel J., Riggs J. C., and Peng B., “The Association between Ectopic Pregnancy and Inflammatory Bowel Disease, Irritable Bowel Syndrome, and Celiac Disease: A Systematic Review,” The Journal of Obstetrics and Gynaecology Research 47, no. 5 (2021): 1601–1609. [DOI] [PubMed] [Google Scholar]
- 90. Alnoman A., Badeghiesh A. M., Baghlaf H. A., and Dahan M. H., “Pregnancy, Delivery, and Neonatal Outcomes Among Women With Irritable Bowel Syndrome (IBS) an Evaluation of over 9 Million Deliveries,” The Journal of Maternal‐Fetal & Neonatal Medicine 35, no. 25 (2022): 5935–5942. [DOI] [PubMed] [Google Scholar]
- 91. McDonnell R. and Hart R. J., “Pregnancy‐Related Outcomes for Women With Polycystic Ovary Syndrome,” Women's Health 13, no. 3 (2017): 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Maliqueo M., Lara H. E., Sánchez F., Echiburú B., Crisosto N., and Sir‐Petermann T., “Placental Steroidogenesis in Pregnant Women With Polycystic Ovary Syndrome,” European Journal of Obstetrics, Gynecology, and Reproductive Biology 166, no. 2 (2013): 151–155. [DOI] [PubMed] [Google Scholar]
- 93. Morgante G., Massaro M., Di Sabatino A., Cappelli V., and De Leo V., “Therapeutic Approach for Metabolic Disorders and Infertility in Women With PCOS,” Gynecological Endocrinology 34, no. 1 (2018): 4–9. [DOI] [PubMed] [Google Scholar]
- 94. Luke B., Brown M. B., Stern J. E., et al., “Female Obesity Adversely Affects Assisted Reproductive Technology (ART) Pregnancy and Live Birth Rates,” Human Reproduction 26, no. 1 (2011): 245–252. [DOI] [PubMed] [Google Scholar]
- 95. Jungheim E. S. and Moley K. H., “Current Knowledge of obesity's Effects in the Pre‐ and Periconceptional Periods and Avenues for Future Research,” American Journal of Obstetrics and Gynecology 203, no. 6 (2010): 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Amiri M. and Tehrani F. R., “Potential Adverse Effects of Female and Male Obesity on Fertility: A Narrative Review,” International Journal of Endocrinology and Metabolism 18, no. 3 (2020): e101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moghetti P. and Tosi F., “Insulin Resistance and PCOS: Chicken or Egg?” Journal of Endocrinological Investigation 44, no. 2 (2021): 233–244. [DOI] [PubMed] [Google Scholar]
- 98. Lee H., Oh J.‐Y., Sung Y.‐A., Chung H., and Cho W. Y., “The Prevalence and Risk Factors for Glucose Intolerance in Young Korean Women With Polycystic Ovary Syndrome,” Endocrine 36, no. 2 (2009): 326–332. [DOI] [PubMed] [Google Scholar]
- 99. Merkin S. S., Phy J. L., Sites C. K., and Yang D., “Environmental Determinants of Polycystic Ovary Syndrome,” Fertility and Sterility 106, no. 1 (2016): 16–24. [DOI] [PubMed] [Google Scholar]
- 100. Okafor P. N., Dahlen A., Youssef M., et al., “Environmental Pollutants Are Associated With Irritable Bowel Syndrome in a Commercially Insured Cohort of California Residents,” Clinical Gastroenterology and Hepatology 21 (2022): 1617–1626.e9. [DOI] [PubMed] [Google Scholar]
- 101. Rutkowska A. Z. and Diamanti‐Kandarakis E., “Polycystic Ovary Syndrome and Environmental Toxins,” Fertility and Sterility 106, no. 4 (2016): 948–958. [DOI] [PubMed] [Google Scholar]
- 102. Palimeri S., Palioura E., and Diamanti‐Kandarakis E., “Current Perspectives on the Health Risks Associated With the Consumption of Advanced Glycation End Products: Recommendations for Dietary Management,” Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 8 (2015): 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Diamanti‐Kandarakis E., Piouka A., Livadas S., et al., “Anti‐Mullerian Hormone Is Associated With Advanced Glycosylated End Products in Lean Women With Polycystic Ovary Syndrome,” European Journal of Endocrinology 160, no. 5 (2009): 847–853. [DOI] [PubMed] [Google Scholar]
- 104. Diamanti‐Kandarakis E., Piperi C., Korkolopoulou P., et al., “Accumulation of Dietary Glycotoxins in the Reproductive System of Normal Female Rats,” Journal of Molecular Medicine 85 (2007): 1413–1420. [DOI] [PubMed] [Google Scholar]
- 105. Tantalaki E., Piperi C., Livadas S., et al., “Impact of Dietary Modification of Advanced Glycation End Products (AGEs) on the Hormonal and Metabolic Profile of Women With Polycystic Ovary Syndrome (PCOS),” Hormones 13, no. 1 (2014): 65–73. [DOI] [PubMed] [Google Scholar]
- 106. Tessier F. J., Niquet‐Léridon C., Jacolot P., et al., “Quantitative Assessment of Organ Distribution of Dietary Protein‐Bound 13C‐Labeled Nɛ‐Carboxymethyllysine After a Chronic Oral Exposure in Mice,” Molecular Nutrition & Food Research 60, no. 11 (2016): 2446–2456. [DOI] [PubMed] [Google Scholar]
- 107. Nie C., Li Y., Qian H., Ying H., and Wang L., “Advanced Glycation End Products in Food and Their Effects on Intestinal Tract,” Critical Reviews in Food Science and Nutrition 62, no. 11 (2022): 3103–3115. [DOI] [PubMed] [Google Scholar]
- 108. Ghosh A., Tripathy A., and Ghosh D., Impact of Endocrine Disrupting Chemicals (EDCs) on Reproductive Health of Human. (Paper presented at: Proceedings of the Zoological Society 2022)2022.
- 109. Kandaraki E., Chatzigeorgiou A., Livadas S., et al., “Endocrine Disruptors and Polycystic Ovary Syndrome (PCOS): Elevated Serum Levels of Bisphenol A in Women With PCOS,” The Journal of Clinical Endocrinology & Metabolism 96, no. 3 (2011): E480–E484. [DOI] [PubMed] [Google Scholar]
- 110. Srnovršnik T., Virant‐Klun I., and Pinter B., “Polycystic Ovary Syndrome and Endocrine Disruptors (Bisphenols, Parabens, and Triclosan)—A Systematic Review,” Lifestyles 13, no. 1 (2023): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. El‐Salhy M., Hatlebakk J. G., and Hausken T., “Diet in Irritable Bowel Syndrome (IBS): Interaction With Gut Microbiota and Gut Hormones,” Nutrients 11, no. 8 (2019): 1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Diamanti‐Kandarakis E., Christakou C., and Marinakis E., “Phenotypes and Enviromental Factors: Their Influence in PCOS,” Current Pharmaceutical Design 18, no. 3 (2012): 270–282. [DOI] [PubMed] [Google Scholar]
- 113. Aly J. M. and Decherney A. H., “Lifestyle Modifications in PCOS,” Clinical Obstetrics and Gynecology 64, no. 1 (2021): 83–89. [DOI] [PubMed] [Google Scholar]
- 114. Camilleri M., “Management Options for Irritable Bowel Syndrome,” Mayo Clinic Proceedings 93, no. 12 (2018): 1858–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Adriani A., Ribaldone D. G., Astegiano M., Durazzo M., Saracco G. M., and Pellicano R., “Irritable Bowel Syndrome: The Clinical Approach,” Panminerva Medica 60, no. 4 (2018): 213–222. [DOI] [PubMed] [Google Scholar]
- 116. Chiavaroli L., Viguiliouk E., Nishi S. K., et al., “DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta‐Analyses,” Nutrients 11, no. 2 (2019): 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cozma‐Petruţ A., Loghin F., Miere D., and Dumitraşcu D. L., “Diet in Irritable Bowel Syndrome: What to Recommend, Not What to Forbid to Patients!” World Journal of Gastroenterology 23, no. 21 (2017): 3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Konturek P. C., Brzozowski T., and Konturek S., “Stress and the Gut: Pathophysiology, Clinical Consequences, Diagnostic Approach and Treatment Options,” Journal of Physiology and Pharmacology 62, no. 6 (2011): 591–599. [PubMed] [Google Scholar]
- 119. Gaillet S., Lachuer J., Malaval F., Assenmacher I., and Szafarczyk A., “The Involvement of Noradrenergic Ascending Pathways in the Stress‐Induced Activation of ACTH and Corticosterone Secretions Is Dependent on the Nature of Stressors,” Experimental Brain Research 87 (1991): 173–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


