ABSTRACT
Drug fever is an adverse drug reaction accompanied by a febrile response and is a common problem among clinicians, hence an updated knowledge of drug fever is important. A consensus regarding the definition of drug fever is lacking. Thus, descriptions of drug fever in previous literature are often inconsistent. In this narrative review, we summarized various features of drug fever, including its definition, epidemiology, risk factors, clinical presentation, diagnosis, treatment and prognosis, based on the earliest literature. Recent advances in information technology have encouraged researchers to use pharmacovigilance databases for clinical and pharmacological research. We outlined how a pharmacovigilance database, along with recently developed research methods, could be used to research drug fever.
Keywords: drug fever, drug adverse reactions, narrative review
INTRODUCTION
Fever is a condition in which patients manifest elevated body temperatures, and a body temperature of above 37.3°C is usually considered abnormal [1]. Although the precise mechanism is yet to be elucidated, the human body temperature is regulated by a thermoregulatory center located in the anterior hypothalamus [2]. When exogenous pyrogens, such as bacteria or viruses, enter the human body, they are phagocytosed by lymphocytes and activated. Activated lymphocytes release cytokines, such as interleukin-1, that stimulate the thermoregulatory center, and the stimulated thermoregulatory center raises the “set point” of the body temperature. The human body responds to elevated body temperature (e.g., by shivering); thus, the patient manifests with an elevated body temperature.
Heat stroke also accompanies elevated body temperature but the “set point” of the body temperature is not changed. In this situation, excessive heat increases body temperature beyond the thermoregulatory center. This condition is called “hyperthermia” and is distinguished from fever. In adverse drug reactions (ADRs), both fever and hyperthermia can occur, depending on the type of culprit drug.
Drug fever is an adverse reaction that accompanies fever. Drug fever is a common problem for clinicians because drugs are used for treatment, prevention, and even diagnosis. As all clinicians may prescribe drugs that can cause drug fever and treat patients who present with drug fever, clinicians must be familiar with the diagnosis and treatment of drug fever.
The main pathophysiology of drug fever is hypersensitivity to drugs, such as an immune response. Among the four classical classifications of allergic reactions [3], drug fever is classified as a type III reaction [4]. This finding originated from the observation of patients with serum sickness, which is considered an entity similar to drug fever. The pathophysiology of serum sickness was speculated to be a type III reaction because the patients had decreased serum C3 and C4 levels, and skin biopsy revealed immune deposits in the blood vessels of some patients.
Although previous narrative reviews of drug fever outlined the diagnosis and treatment of drug fever [5], several new agents have emerged in the past decade. Some of the diseases are known to cause drug fevers. In addition, a recent study on drug fever using a pharmacovigilance database, along with several methods, such as the proportional reported ratio, provided new insights into drug fever [6].
In this seminar article, we provide an updated review of the clinical aspects, such as epidemiology, diagnosis, and treatment of drug fever.
METHOD
We searched Pubmed and Embase on November 26, 2022. We used the terms “drug fever” and “drug-induced fever.” We included all English-language review articles and cohort studies without any restrictions on publication year. For case reports, we included articles published after 2010 because a previous narrative review [5] summarized case reports published between 1950 and 2009. We checked the references in the included articles and assessed their relevance. In addition, we checked the articles that cited our included articles in the Citation Chaser [7]. Google Scholar was used for articles that were not indexed in the citation chaser.
DEFINITION
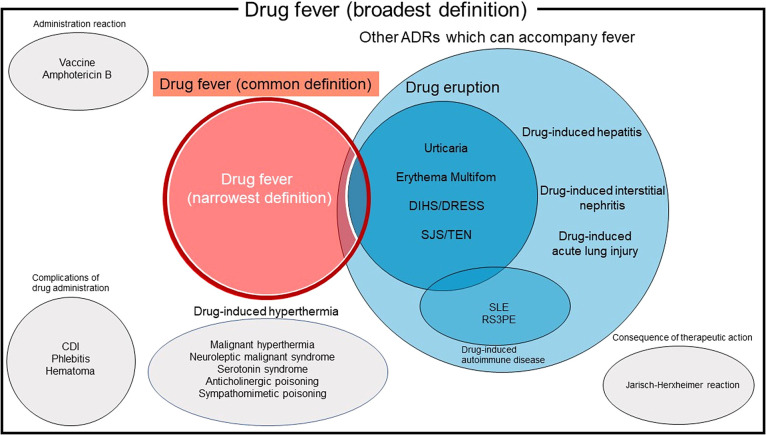
To date, there is no unified definition for drug fever. Many specific types of ADRs accompany febrile reactions (Fig. 1); however, there is a disagreement among researchers regarding which diseases should be considered part of drug fever.
Fig. 1 . Venn diagram schema of the definition of drug fever.
Drug fever in this review is highlighted in red and circled by a dark red line. Drug fever by the narrowest definition excludes skin manifestation and is circled by a white line.
Abbreviations: ADRs, adverse drug reaction; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal syndrome; DIHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms; SLE, systemic lupus erythematosus; RS3PE, remitting seronegative symmetrical synovitis with pitting edema; CDI, Clostridioides difficile infection.
The broadest definition of drug fever includes complications of drug administration, such as phlebitis, hematoma, chemical meningitis, Clostridioides difficile infection, drug-induced hyperthermia (malignant hyperthermia, neuroleptic malignant syndrome, serotonin syndrome, anticholinergic poisoning, and sympathomimetic poisoning), administration reactions (e.g., amphotericin B or vaccine-related reactions), and consequences of therapeutic action (Jarisch-Herxheimer reaction) [8]. However, we have excluded these entities from this review.
In this review, we define drug fever as a febrile response that fulfills all the following criteria: i) initiation after drug administration; ii) resolution within 72 h after discontinuation of the drug without specific therapy; iii) no other cause identified by history, physical examination, laboratory, or imaging investigation; and iv) no relapse of fever 72 h after defervescence. Some researchers have excluded febrile ADR with skin manifestation [6, 9]; however, this definition may be too strict. We included ADR with fever and skin rash in drug fever, but severe forms of drug eruption, such as Stevens-Johnson syndrome and drug-induced hypersensitivity syndrome, were excluded. Although other febrile ADRs, such as drug-induced hepatitis, drug-induced interstitial nephritis, and drug-induced acute lung injury, can be included in the above definition, it is impossible to distinguish these entities from drug fever.
EPIDEMIOLOGY
Compared to ADR, few reports have described the incidence of drug fever. A national health insurance claims data-based study in Korea estimated that the annual incidence of drug fever is approximately 0.001% [10]. An old single-center retrospective cohort study reported that among 1,000 consecutive patients treated in a general internal medicine ward, 42 (4.2%) developed some type of ADR, and two (0.2%) had fever and rash [11]. A three-year prospective single-center cohort study reported that among the 7,765 included patients, 122 (1.6%) experienced major antibiotic-induced disease and 9 (0.1%) had fever [12]. We found no available data on the incidence of drug fever in a population receiving multiple types of drugs. Single-center retrospective cohort studies revealed that 0.7–13.1% of patients who received antibiotics developed drug fever [16–18]. Retrospective cohort studies revealed that 2.8–8.7% of patients who received anti-neoplastic agents developed drug fever [19, 20]. Recent studies based on pharmacovigilance data have reported that drug fever in ADR is 2–10% [13–15].
Drug fever is an important differential diagnosis among patients admitted to investigating the cause of the fever. Japanese single-center retrospective cohort studies reported that drug fever accounted for 1.8–5.7% of all causes of fever in patients who were admitted for investigating the causes of fever [21, 22].
Drug fever is an important differential diagnosis of fever of unknown origin. Fever of unknown origin was defined as a temperature of ≥38.3°C for at least 3 weeks without a definite diagnosis despite 3 inpatient days or at least three outpatient visits [23]. A few single-center retrospective cohort studies have reported that drug fever accounts for approximately 2% of cases with fever of unknown origin [24–26].
It is commonly believed that drug fever is a common cause of nosocomial fever yielding about 10% [23, 27, 28]. However, this number may have been misquoted to include all types of ADR, not just drug fever [11]. A prospective cohort study of nosocomial fever in Thailand reported that among 86 patients with nosocomial fever, only 3 (3.5%) were diagnosed with drug fever [29].
RISK FACTORS
Risk factors for drug fever have not yet been determined. A previous narrative review discussed the risk factors for drug fever using an old study [5]. However, the cited study was a case series of drug fever [30]. The study had no comparator and, thus, could not estimate the quantitative risk. Previous narrative reviews reported that older age and women are risk factors for drug fever [27, 31, 32]. However, this description may have been misquoted to include all types of ADR, not just drug fever [11]. No studies have investigated the risk factors of drug fever in a population receiving multiple types of drugs.
There are few reports on the risk factors of drug fever induced by specific drugs. A single-center retrospective cohort study revealed that age was negatively associated with carboplatin-associated drug fever (adjusted odds ratio, 0.126; 95% confidence interval, 0.025–0.628) [19]. In other words, younger patients are more likely to develop drug fever. Patient sex was not investigated because the study was conducted in a hospital with female patients. Other factors, such as body mass index, allergy history, disease type or stage, and carboplatin history, were not associated with carboplatin-induced drug fever. Another single-center retrospective cohort study revealed that female sex (adjusted odds ratio, 3.162; 95% confidence interval, 1.264–7.914) and concomitant use of clarithromycin (adjusted odds ratio, 4.834; 95% confidence interval, 2.165–10.794) were associated with furazolidone-induced drug fever [33]. Age was not associated with furazolidone-induced drug fever. However, these findings are related to specific agents and have no assurance of generalizability.
CLINICAL PRESENTATION
Time Between Initiation of the Culprit Drug and Development of Fever
A case series of drug fever due to various drugs reported that the median time between the initiation of the culprit drug and the development of fever was 2–8 days [6, 30]. However, it is well known that the time interval can vary widely depending on the type of drug [6, 30]. For example, the time interval for antibiotics is 1–5 weeks (most commonly within 1–2 weeks) [16, 18, 30, 34–40]. The time interval between the administration of anti-neoplastic agents is 3–4 days [20].
The time interval for drug fever may be shortened if patients have previously received the same class of agents. A single-center retrospective cohort study reported that the median time interval of piperacillin-induced drug fever in patients without a history of administration of some kind of β-lactams was 22.5 days, whereas it was 13 days in patients with a history of administration of some kind of β-lactams [16].
Fever Pattern
No specific patterns were observed for drug fever. This knowledge is common in old textbooks and recent literature [6, 8, 30]. Similar to other febrile illnesses, such as infectious diseases, drug fever can develop into various fever patterns [1]. Therefore, fever patterns cannot be used as diagnostic indicators of drug fever.
Associated Symptoms of Drug Fever
Bradycardia is the most common symptom of drug fever. The pulse rate increases in proportion to high body temperature in many infectious diseases, which is known as Liebermeister’s rule [41]. The pulse rate at 102 degrees Fahrenheit (around 39) is estimated to be 110 beats per minute, from which the pulse rate increases by 10 beats per minute for every 1 degree Fahrenheit (approximately 0.55°C) increase in body temperature. However, this correlation between fever and pulse rate has not been observed in a few diseases. A constant or decreasing pulse rate with an elevated body temperature is referred to as pulse-temperature dissociation (deficit), Faget’s sign, or, most commonly, relative bradycardia [42]. It is defined as a pulse rate lower than that expected from the body temperature [42]. In some case series of antibiotic-induced drug fever, the incidence of relative bradycardia in drug fever was reported to be 83–100% [37, 38]. Another case series reported that the incidence of relative bradycardia in patients with drug fever was 11%; however, the authors used a different definition for relative bradycardia (pulse rate 100/min or lower during fever) [30]. Based on these results, relative bradycardia in the correct definition may be a common symptom of drug fever. Although the diagnostic accuracy of relative bradycardia for drug fever in febrile patients has not been fully investigated, the absence of relative bradycardia may help rule out drug fever.
Some clinicians believe that one of the characteristic findings of drug fever is the “inappropriate sense of well-being” of the patient despite the fever [35]. A case series of antibiotics-induced fever reported that “reduced general condition” was observed in 3 out of 11 patients with drug fever. However, the definition of “reduced general condition” was not described in detail in their research. Whether an “inappropriate sense of well-being” can help to diagnose drug fever remains to be determined.
Skin manifestations are a common symptom of drug fever. Although the reported incidence is highly variable according to the type of causative drug, it is reportedly 15–31% [16, 30, 34, 36–38]. The most common type of skin rash in drug fever is maculopapular rash, which is also called morbilliform (which means ‘measles-like’) or exanthematous rash [43]. Patients with maculopapular rashes typically exhibit symmetrical rashes consisting of red macules and papules on the entire body. Urticaria is a possible type of skin rash associated with drug fever.
Shaking chills, defined as chills accompanied by shaking of the generalized body (rigor) despite wearing a thick blanket, are strongly associated with bacteremia [44]. However, shaking chills can also occur in drug fevers, yielding 36–51% [30, 36]. Therefore, the presence of shaking chills does not exclude a diagnosis of drug fever.
Other symptoms such as nausea, headache, and arthralgia are known to cause drug fever [30].
Laboratory Findings
Although there are no specific laboratory findings for drug fever, some laboratory findings have been observed in patients with drug fever.
Eosinophilia is the most common laboratory finding associated with fever. The reported incidence of eosinophilia and its definition vary greatly among studies, yielding 0.9–25% [6, 16, 30, 34, 38–40]. Some authors define eosinophilia as eosinophils 300 or greater [30], 500 or greater [16], or 5% or greater [39]. Other studies have not reported a definition of eosinophilia [6, 34, 38].
Leukopenia is also associated with drug fever. Although the definition is not always clear, the reported incidence of leukopenia is 0.9–90% [6, 16, 36].
Elevated C-reactive proteinlevels are also observed in drug fever [36, 40]. A case series of antibiotics-induced drug fever reported that C-reactive protein levels increase in 10 out of 11 cases of drug fever, with a median value of 5 mg/dL (range:3.2–22.9 mg/dL) [36].
Procalcitonin, a precursor peptide of calcitonin, is a predictive marker of bacteremia [45]. A case series of antibiotics-induced drug fever reported that among patients with drug fever and procalcitonin examined, it was lower than 0.25 ng/dL in 10 out of 11 patients [38].
Other laboratory findings included elevated liver enzyme levels, renal failure, or anemia [39, 46].
DRUGS ASSOCIATED WITH DRUG FEVER
Numerous drugs have been associated with fever (Table 1). Clinicians should be aware that some of these drugs are not only prescribed in hospitals or clinics but may also be included as one of the ingredients of over-the-counter drugs (e.g., ibuprofen is included in many over-the-counter painkillers). Since the publication of a previous review [5], several new drugs have been reported to cause drug fever. These include acitretin [47], bendamustine [48], celecoxib [49], dalteparin [50], dexmedetomidine [51], doxycycline[52], enoxaparin [53, 54], ertapenem [55], favipiravir [56–59], imipenem/cilastatin [60], mesalamine [61], olanzapine [62, 63], pantoprazole [64, 65], propofol [66], S-carboxymethyl-L-cysteine [67], sorafenib[68], teicoplanin [69], tigecycline[70], and vildagliptin [71].
Table 1. Drugs associated with drug fever (adopted from Patel et al. [5]).
Drugs reported after 2010 are highlighted in bold font, while those listed in the review by Patel et al. are written in standard font.
| Category | Drug |
|---|---|
| Antimicrobials | Penicillins: Ampicillin, carbenicillin, cloxacillin, mezlocillin, nafcillin, oxacillin, penicillin, piperacillin, staphcillin, ticarcillin Cephalosporins: Cefazolin, cefotaxime, ceftazidime, cephalexin, cephalothin Other antimicrobials: Acyclovir, amphotericin B, aureomycin, declomycin, doxycycline, ertapenem, erythromycin, furadantin, imipenem/cilastatin, isoniazid, minocycline, nitrofurantonin, novobiocin, rifampicin, streptomycin, teicoplanin, terramycin, tetracycline, tigecycline, trimethoprim-sulfamethoxazole, vancomycin |
| Anti-neoplastic agents | Bendamustine, bleomycin, chlorambucil, cisplatin, cytosine arabinoside, daunorubicin, hydroxyurea, interferon, L-asparaginase, mercaptopurine, procarbazine, streptozocin,vincristine |
| Cardiovasuclar agents | Clofibrate, dalteparin, diltiazem, dobutamine, enoxaparine, furosemide, heparin, hydrochlorothiazide, methyldopa, oxprenolol, procainamide, quinidine and quinine, triameterene |
| Immunosuppressants | Azathioprine, everolimus, mycophenolate mofetil, sirolimus |
| NSAIDs | Celecoxib, ibuprofen, naproxen, tolmetin |
| Sympathomimetic and hallucinogenic agents | Amphetamine, lysergic acid, 3,4-methylene dioxymethamphetamine |
| Anticonvulsants | Carbamazepine, phenytoin |
| Antidepressants | Doxepin, nomifensine |
| Others | Acitretin, allopurinol, cimetidine, dexmedetomidine, favipiravir, folate, iodide, mebendazole, methalamine, metoclopramide, olanzapine, pantoprazole, piperazine adipate, propofol, propylthiouracil, prostaglandin E2, ritodrine, sorafenib, sulfasalazine, theophylline, thyroxine, vildagliptin |
DIAGNOSIS
An essential point in the diagnosis of drug fever is suspected. There are two main situations in which drug fever should be considered as a differential diagnosis. One is new-onset fever, in which patients develop a fever that is afebrile for more than 48 h [72]. The other is prolonged fever, where patients’ fever persists for more than 72 h despite appropriate treatment for febrile illnesses [73]. Clinicians should be aware that there are no single signs or symptoms that can diagnose or exclude drug fever (e.g., the absence of a cutaneous region cannot be a reason for excluding drug fever).
When clinicians obtain a history of fever from a patient, care must be taken to include all medications, including those prescribed by other doctors or OTC drugs, within one month. A single administration of a drug can induce drug fever [64, 74], and hence a history of the used-as-needed drug should be obtained.
There is no method to provide a definitive diagnosis of drug fever when a patient is febrile. A tentative diagnosis of drug fever should be established after excluding other critical diseases. Therefore, the evaluation of patients with suspected drug fever should include a thorough history and physical examination to explore the focus of the fever. If laboratory testing or imaging studies are performed, clinicians should be cautious again that no single finding can diagnose or exclude drug fever (e.g., the absence of eosinophilia cannot be a reason for excluding drug fever). If clinicians are confident of a tentative diagnosis of drug fever after a fever workup, they should stop the drug that is considered the culprit. Determining the culprit drug may be difficult when various drugs are administered orally or parenterally. In such cases, stopping the drugs individually every two to three days, beginning with the drug suspected to be the culprit, may be reasonable [8]. If the diagnosis is correct, the fever will usually alleviate within 24–48 h, and the diagnosis will be confirmed [6, 18, 30, 34–39].
If a patient’s fever persists for more than 72 h after discontinuing the presumed culprit drug, four possibilities should be considered.
i) Wrong diagnosis. The patient may have diseases completely different from ADR, such as abscesses, collagenous disease, or malignant disease. Clinicians should rule out other diseases before tentatively diagnosing drug fever. However, if a patient’s fever persists after drug discontinuation, clinicians should consider tentative diagnoses other than ADR.
ii) Complication from other forms of drug events. Patients with a more severe form of ADR, such as drug-induced interstitial nephritis, may have a fever longer than 72 h after discontinuation of the drug [75]. Clinicians should cautiously evaluate patients for organ-specific symptoms other than fever, such as liver injury, renal failure, and rashes. If a patient experiences severe organ failure, clinicians should consider administering corticosteroids [76, 77].
iii) Wrong drug. Although the diagnosis of drug fever is correct, the fever will not resolve if the clinician’s assumption about the causative drug is incorrect. If clinicians maintain a tentative diagnosis of drug fever after reviewing the patient’s history and physical examination, they should consider discontinuing other drugs.
iv) Rare cases of prolonged drug fever. A case series of antibiotic-induced fever reported that 36% of patients with drug fever had a fever for >3 days after discontinuing the drug [35]. One patient had a persistent fever for as long as 7 days. However, this was a rare case. Clinicians should seek other possibilities for prolonged fever rather than adhere to the first tentative diagnosis and optimistically observe patients.
After the patient is defervesced, physicians should consider whether the suspected drug is truly the culprit. Criteria for assessing causality in individual cases were developed by the Council for International Organizations of Medical Sciences (CIOMS) [78]. These include:
• Positive rechallenge (discussed in detail below)
• Causality established in previous studies
• Time to onset of plausibility
• Symptom resolution after stopping the drug (dechallenge positive)
Lack of confounding risk factors
• Amount and duration of exposure consistent or plausible with the cause and effect
• Corroborating the accuracy of the case history
• Comedication is unlikely to play a significant role
• Lack of alternative explanations
Not all the criteria must be met to prove causality. The priority of each criterion may depend on the case and can be determined by the clinicians. A more objective scale with attributed weights for each item is available for drug-induced liver injury (CIOMS scale) [79]. However, this has not yet been validated for drug fever. Scales that can be used for ADRs include the Naranjo scale [80], World Health Organization global introspection method [81], and Karg and Lasagna’s method [82]. Among these, the Naranjo scale seems to be the most frequently used in previous reports on drug fever [6]. The Naranjo scale consists of 10 questions answered as “yes”, “no”, or “do not know”. A different value (from 1 to 2) was assigned to each answer. The probability of a drug adverse event can be estimated as “doubtful,” “possible,” “probable,” or “definite” depending on the total score. The drug is likely the cause if the result is “possible,” “probable,” or “definite” [6].
The reappearance of fever with drug rechallenge can be one of the components of the diagnosis of drug fever and assessment of causality [6]. If the causative drugs are re-administered, the patient will develop a fever shortly (usually within a day); thus, clinicians can confirm the diagnosis [39]. Drug rechallenge has long been controversial because it may be harmful to patients, and some reviews recommend extreme caution if clinicians decide to perform it [5, 8, 9, 83]. A previous review discouraged clinicians from utilizing this tool [5]. A case series reported that one of nine patients with drug rechallenge developed myocardial infarction during rechallenge with quinidine sulfate [30]. However, a more recent case series of 167 patients with drug fever reported that 38% of the patients received drug rechallenge, and no patients experienced serious adverse events [6]. Because drug rechallenge involves some risks, it may not be necessary if discontinuation of the possible causative drug does not affect the patient’s outcome. However, withholding all drugs that are suspected to cause drug fever may harm the patient, especially if the possible causative drugs are the key drugs of treatment (e.g., anti-neoplastic drugs). If the key drugs of treatment are possible causative drugs, clinicians should consider drug rechallenge and identify the causative drug.
The drug-induced lymphocyte stimulation test (DLST), also called the lymphocyte transformation test, is a reliable test for determining the culprit drug in drug hypersensitivity reactions [84]. Although no studies have investigated the diagnostic significance of DLST only in patients with drug fever, DLST positivity may help to determine the culprit drug in drug fever. However, clinicians must be cautious that the DLST has limited sensitivity, and the negative result of the DLST does not deny the possibility that the testing drug is the culprit. Clinicians should perform the DLST only in cases in which it is difficult to identify the culprit drug from the clinical course.
TREATMENT
If clinicians strongly suspect drug fever, they usually discontinue the suspected culprit drug to confirm the diagnosis. Drug discontinuation is both a diagnostic and treatment procedure. However, no specific treatments were necessary.
PROGNOSIS
The prognosis of drug fever is usually favorable. A recent case series on drug fever reported that 154 of 167 (97%) patients recovered without sequelae [6]. They reported that one patient died of myocardial infarction, but this was not related to ADR. An older case series reported that 6 of 148 patients (4%) died, and drug fever was one of the contributing factors [30]. However, according to this narrow definition, some of these cases were not cases of drug fever. One case was neuroleptic malignant syndrome induced by haloperidol [85] and two cases were bleomycin-induced fulminant hyperpyrexia [86, 87], which rarely occurs when bleomycin is administered to febrile patients with lymphoma and is considered to be one of the infusion reactions [88].
Therefore, the mortality rate of drug fever in the narrow definition is considered very low.
RESEARCH METHODOLOGY
The cited articles in case series or cohort studies were mostly based on chart reviews (Table 2). One study used the pharmacovigilance database of France [6].
Table 2. List of previous studies of drug fever.
| Study | Definition of drug fever | Causative drug | Study period | Number of cases | Data |
|---|---|---|---|---|---|
| Foster 1963 | (1) Fever followed the administration of the antibiotic in question, and disappeared soon after it was discontinued. (2) Fever was not accounted for other causes | Antibiotics | 1952–1963 | 25 | Chart review |
| Young 1982 | (1) temperatures were taken and recorded at least four times each day during the febrile period and after its resolution; (2) no infection or other cause of fever was detected from results of physical examination, appropriate cultures, and other studies (x rays, scans, etc.); (3) there was no underlying condition that might itself have caused a febrile state; (4) fever coincided temporally with the administration of the offending drug; (5) fever disappeared promptly, within <96 hr and usually <48 hr after discontinuation of the drug without other therapeutic measures; and (6) the temperature remained normal thereafter for at least three and usually more than five days. | Various drugs | 1975–1980 | 12 | Chart review |
| LeRoy 1986 | A temperature greater than or equal to 100.4°F for which there was no cause other than administration of the drug, which disappeared within 48 hours of discontinuing the drug, and which did not return for at least 72 hours | Various drugs | 1984–1985 | 36 | Chart review |
| Mackowiak 1987 | Fever coinciding the administration of a drug and disappearing after discontinuation of the drug when no other cause for the fever could be ascertained after a careful physical examination and appropriate laboratory study | Various drugs | Chart review; 1959–1986 Case report review: 1966–1986 |
148 | Chart review and case report review |
| Mikasa 1989 | Fever without other systemic symptoms followed the administration of the antibiotic and lasted for 2 days or more, and disappeared soon after it was discontinued. | Antibiotics | Jan 1986–Dec 1986 | 22 | Chart review |
| Oizumi 1989 | (1) A fever of 37.5°C or above which lasted for more than two days during treatment with an antibiotic ; (2) The fever was associated neither with other clinical manifestations nor with laboratory findings suggestive of an infectious exacerbation ; (3) The fever could not be ascribed to any other measures that were instituted for the management of infections ; and (4) The fever subsided immediately after cessation of a suspected antibiotic (“dechallange”). | Antibiotics | Not reported | 56 | Chart review |
| Kuwabara 1990 | Fever without skin manifestation followed the administration of the antibiotic and disappeared soon after it was discontinued.Fever was not accounted for other causes | Antibiotics | 1983–1988 | 8 | Chart review |
| Vodovar 2012 | (1) an oral or rectal body temperature above 38°C; (2) the absence of other causes of fever as determined by physical examination and appropriate biological and microbiological tests (i.e. absence of any infection); (3) the absence of any underlying condition causing fever by itself; (4) the absence of skin reactions; (5) the coincidence of fever onset with drug administration; (6) disappearance of the fever within 72 h following drug discontinuation without any other intervention; (7) no recurrence of fever within at least 72 h after normalization of temperature; and 8)- exclusion of other differential diagnoses for hyperthermia including antipsychotic malignant syndrome, serum sickness-like reactions, serotonin syndrome, and malignant hyperthermia. | Various drugs | 1986–2007 | 167 | French national pharmacovigilance database |
| Fang 2016 | body temperature reduced rapidly after taking suspected drugs was stopped and rose when the same drug was used again. Otherwise, it is considered as drug fever when the patients suffered from allergic reaction (with or without rashes) in combination with any one of the following conditions: (1) for patients with infectious fever, the body temperature reduced when antibiotics were used but rose again in continue medication; (2) after the treatment of antibiotics, body temperature became higher and cannot be explained by original infection and other reasons for the other normal conditions; and (3) patients with non-febrile illnesses suffered from fever after dosing that cannot be explained by secondary infections | Antituberculous drugs | Apr 2006–Mar 2013 | 94 | Chart review |
| Ogawara 2016 | Not reported | Anti- neoplastic drugs | Apr 2004–Mar 2007, or Apr 2007–Dec 2008. | 88 | Chart review |
| Yaita 2016 | was defined by clinical characteristics that met all of the following criteria: (1) an axial temperature above 37.5°C (in Japan, an oral or rectal temperature is generally not measured); (2) no other origin of fever can be detected by detailed ID consultation (including appropriate imaging tests and microbiological tests); (3) any underlying febrile illness, the improvement of which can be confirmed by the ID physician; and (4) after the discontinuation of drugs, the fever is alleviated. | Antibiotics | Apr 2014–May 2015 | 16 | Chart review |
| Peng 2017 | Unexplained fever due to other reasons, recurrence of fever after re-medication | Antibiotics | Feb 2011–Feb 2014 | 20 | Chart review |
| Labbus 2018 | If all of the following criteria were fulfilled: (1) central (ear) body temperature >38.0°C measured on two occasions; (2) intravenous antibiotic treatment for > 3 days; (3) exclusion of infectious or other non-infectious causes of fever; and (4) defervescence after discontinuation of antibiotic treatment. | Antibiotics | 2014–2017 | 11 | Chart review |
| Zhang 2021 | Hypersensitivity was classified according to the US National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v3.0 | Carboplatin | Jan 2017–Dec 2018 | 27 | Chart review |
| Zhang 2022 | Fever had been caused by the medication if it cleared rapidly after the suspected drug was discontinued | Furazolidone | Jul 2018–Sep 2018. | 45 | Chart review |
The pharmacovigilance database is a database of individual ADR reports [89]. Reports are typically collected using spontaneous reporting systems. The World Health Organization has an international database called Vigibase [90], but some countries have national databases such as the Food and Drug Administration Adverse Event Reporting System (the United States) [91], Canada Vigilance Adverse Reaction Online Database [92], French Pharmacovigilance Database, and Japanese Adverse Drug Event Report database [93].
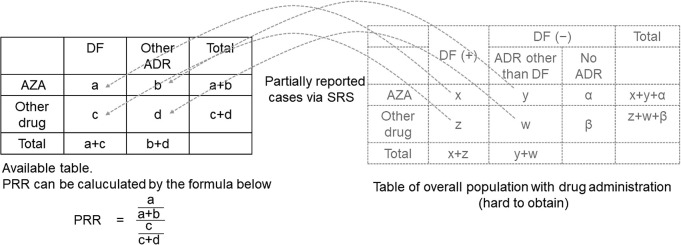
Researchers can make assumptions about the association between a certain drug and ADR by analyzing these databases. For example, if researchers want to determine whether there is an association between azathioprine and drug fever, the best way is to observe the overall population after drug administration and create a two-by-two contingency table. However, it is difficult to obtain this data. Instead, they can create a two-by-two contingency table to display the number of cases with azathioprine and other drug administration and with drug fever and other ADRs from the database (Fig. 2). The association between azathioprine use and drug fever can be discussed by calculating the proportional reporting ratio [94]. The proportional reporting ratio is a method of disproportionality analysis and is often used to analyze spontaneous ADR databases [95].
Fig. 2 . An example of two-by-two contingency table in disproportionality analysis.
To calculate the risk of azathioprine (AZA)-induced drug fever and compare the risk with other drugs, data of the whole population with AZA and other drug administrations are necessary; however, such data are unavailable. However, the number of spontaneously reported cases of AZA and other drug administrations with drug fever and ADR other than drug fever is available in the pharmacovigilance database. Researchers can create a two-by-two contingency table from these data. The proportional reporting ratio (PRR) can be calculated from the table using the formula. If the PRR exceeds a certain value, AZA and drug fever may occur.
Notably, when analyzing drug fever using these databases, researchers should be cautious about the inherent limitations of pharmacovigilance databases based on spontaneous reports. First, ADRs tend to be under-reported in spontaneous reporting systems and are easily affected by external factors such as media coverage [96]. Thus, the signals in these databases are vulnerable to reporting biases. Second, these databases lack information on the total number of people receiving the drug and therefore cannot calculate the risk ratio of ADRs. Researchers cannot draw any conclusions regarding the risk or prevalence of ADRs from studies using these databases. Third, the definition of drug fever in these databases may not be accurate. When researchers create a two-by-two contingency table, cases with ADR of interest and cases with other ADR must be separated according to the researchers’ definition. ADRs are usually classified using the Medical Dictionary for Regulatory Activities (medDRA) [97]. Drug fever was included in medDRA. However, other classifications such as drug-induced hepatitis or drug-induced renal disease may be included in the definition of drug fever. Disease classification was performed using an ADR reporter. The definition of drug fever used by reporters is often unknown. Precise clinical information, such as laboratory or imaging findings, was not available. In the above example, when separating cases with drug fever from cases with other ADR, there is no guarantee that the cases identified as drug fever in the database use the same definition as the researchers’ definition of drug fever.
To overcome these limitations, the validation of diagnostic codes is necessary. In addition, confirmation studies must be performed after detecting the signal using pharmacovigilance databases. Researchers should keep in mind that signal detection is not necessarily a side effect of the drug. We present two examples of validation study types. The first is the use of post-market surveillance for new drugs. In Japan, all patients administered new drugs were surveyed. Researchers can create a two-by-two contingency table by setting the reporters’ diagnosis of ADR as the reference standard, and the diagnostic code extracted from the administrative claims data as the comparator. Diagnostic algorithms based on administrative claims data can also be used for comparison. The sensitivities and specificities of the diagnostic codes and algorithms were calculated from the tables. Researchers should be cautious when using this method for frequent ADR. Therefore, it may not be suitable for the treatment of drug-induced fever. The second involves the use of pharmacovigilance databases. Researchers extract the cases classified as “drug fever” from a certain pharmacovigilance database and obtain relevant clinical information. If there are too many reports classified as “drug fever,” researchers can select the reports randomly (about 100 reports). Using clinical information, the researchers assessed whether the extracted cases could be truly classified as drug fever based on their definition. There will be true positives (cases classified as drug fever in medDRA and the researcher’s definition) and false positives (cases classified as drug fever in medDRA but not in the researcher’s definition). The positive predictive value can be calculated from these numbers, and researchers can determine whether the medDRA classification of drug fever is appropriate. The most important thing is the confirmation study of the association between drugs and fever. To confirm the presumption or signal of drug fever, establishment of a definition of drug fever is warranted.
CONCLUSIONS AND FUTURE DIRECTIONS
Drugs are essential in clinical practice. All clinicians can treat patients with drug fever and should be familiar with its diagnosis and treatment of drug fever. Clinicians should always consider drug fever in the differential diagnosis when treating patients with fever.
Although the accumulation of knowledge over the last decade has led to a better understanding of the diagnosis and treatment of drug fever, some areas remain unresolved. First, there is a disagreement among researchers regarding the definition of drug fever. When researchers use databases to study drug-related fever, diagnostic codes or algorithms must be validated unless they review the medical records of all patients directly. Second, there is a lack of means to estimate the pre-test probability of drug fever before drug discontinuation in patients with fever. Clinical signs such as relative bradycardia should be verified and validated to help differentiate drug fever from other febrile illnesses. Third, although rechallenge is the gold standard for diagnosing drug-induced fever, its safety has only been confirmed in a few studies. Large population studies are necessary to confirm the safety of this treatment.
As new drugs are introduced into the market, the types of drugs causing drug fever are likely to become more diverse, and it is necessary to closely monitor information about drug fever.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
CONFLICT OF INTEREST
The authors have nothing to declare.
REFERENCES
- 1.Musher DM, Fainstein V, Young EJ, Pruett TL. Fever patterns. Their lack of clinical significance. Arch Intern Med 1979;139:1225–1228. [DOI] [PubMed] [Google Scholar]
- 2.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol 2008;22:627–644. [DOI] [PubMed] [Google Scholar]
- 3.Gell PGH, Coombs RRA, Others. Clinical aspects of immunology. Clinical Aspects of Immunology Published Online First: 1963. https://www.cabdirect.org/cabdirect/abstract/19632705010
- 4.Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med 1984;311:1407–1413. [DOI] [PubMed] [Google Scholar]
- 5.Patel RA, Gallagher JC. Drug fever. Pharmacotherapy 2010;30:57–69. [DOI] [PubMed] [Google Scholar]
- 6.Vodovar D, LeBeller C, Mégarbane B, Lillo-Le-Louet A, Hanslik T. Drug Fever: a descriptive cohort study from the French national pharmacovigilance database. Drug Saf 2012;35:759–767. [DOI] [PubMed] [Google Scholar]
- 7.Haddaway NR, Grainger MJ, Gray CT. citationchaser: an R package for forward and backward citations chasing in academic searching. 2021. doi: 10.5281/zenodo.4543513 [DOI] [PubMed] [Google Scholar]
- 8.Cluff LE, Johnson JE. Drug Fever. In: drug fever. 1964. 149–194. [Google Scholar]
- 9.Johnson DH, Cunha BA. Drug fever. Infect Dis Clin North Am 1996;10:85–91. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Ye Y-M, Lee S. Epidemiology of drug hypersensitivity reactions using 6-year national health insurance claim data from Korea. Int J Clin Pharm 2018;40:1359–1371. [DOI] [PubMed] [Google Scholar]
- 11.Burnum JF. Letter: Preventability of adverse drug reactions. Ann Intern Med 1976;85:80–81. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell JR, Cluff LE. Adverse reactions to antimicrobial agents. JAMA 1974;230:77–80. [PubMed] [Google Scholar]
- 13.Ogar CK, Abiola A, Yuah D, Ibrahim A, Oreagba IA, Amadi EC, et al. A Retrospective Review of Serious Adverse Drug Reaction Reports in the Nigerian VigiFlow Database from September 2004 to December 2016. Pharmaceut Med 2019;33:145–157. [DOI] [PubMed] [Google Scholar]
- 14.Bigi C, Tuccori M, Bocci G. Healthcare professionals and pharmacovigilance of pediatric adverse drug reactions: a 5-year analysis of Adverse Events Reporting System Database of the Food and Drug Administration. Minerva Pediatr 2022;74:272–280. [DOI] [PubMed] [Google Scholar]
- 15.Uwai Y, Nabekura T. Analysis of adverse drug events in patients with bipolar disorders using the Japanese Adverse Drug Event Report database. Pharmazie 2022;77:255–261. [DOI] [PubMed] [Google Scholar]
- 16.Oizumi K, Onuma K, Watanabe A, Motomiya M. Clinical study of drug fever induced by parenteral administration of antibiotics. Tohoku J Exp Med 1989;159:45–56. [DOI] [PubMed] [Google Scholar]
- 17.Yoshii T, Takenono I, Otsuka Y, Nakasuji K, Hirano F, Motoji S, et al. Clinical observation on drug fever induced by parenterally administered antibiotics in oral and maxillofacial surgery. J Oral Ther Pharmacol 1991;10:43–50. [Google Scholar]
- 18.Mikasa M, Sawaki M, Konishi M, Kunimatsu M, Fujimura M, Narita N. Fever induced by antibacterial drugs. Chemotherapy 1990;38:21–25. [Google Scholar]
- 19.Zhang X, Zhao M, Zheng C. Drug fever induced by carboplatin-based regimens: Higher incidence in a women’s hospital. Taiwan J Obstet Gynecol 2021;60:882–887. [DOI] [PubMed] [Google Scholar]
- 20.Ogawara D, Fukuda M, Nakamura Y, Ueno S. Fever during cancer chemotherapy: Analysis of 1,016 chemotherapy cycles. J Clin Oncol 5 2011;29.https://dialog.proquest.com/professional/docview/1013571971?accountid=187153 [Google Scholar]
- 21.Goto M, Koyama H, Takahashi O, Fukui T. A retrospective review of 226 hospitalized patients with fever. Intern Med 2007;46:17–22. [DOI] [PubMed] [Google Scholar]
- 22.Ryuko H, Otsuka F. A comprehensive analysis of 174 febrile patients admitted to Okayama University Hospital. Acta Med Okayama 2013;67:227–237. [DOI] [PubMed] [Google Scholar]
- 23.Haidar G, Singh N. Fever of Unknown Origin. N Engl J Med 2022;386:463–477. [DOI] [PubMed] [Google Scholar]
- 24.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine 1961;40:1–30. [DOI] [PubMed] [Google Scholar]
- 25.Zenone T. Fever of unknown origin in adults: evaluation of 144 cases in a non-university hospital. Scand J Infect Dis 2006;38:632–638. [DOI] [PubMed] [Google Scholar]
- 26.Iikuni Y, Okada J, Kondo H, Kashiwazaki S. Current fever of unknown origin 1982–1992. Intern Med 1994;33:67–73. [DOI] [PubMed] [Google Scholar]
- 27.Roush MK, Nelson KM. Understanding drug-induced febrile reactions. Am Pharm 1993;NS33:39–42. [DOI] [PubMed] [Google Scholar]
- 28.Cunha BA.Drug fever. The importance of recognition. Postgrad Med 1986;80:123–129. [DOI] [PubMed] [Google Scholar]
- 29.Dankul P, Karaketklang K, Jitmuang A. Nosocomial Fever in General Medical Wards: A Prospective Cohort Study of Clinical Characteristics and Outcomes. Infect Drug Resist 2021;14:3873–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackowiak PA, LeMaistre CF. Drug fever: a critical appraisal of conventional concepts. An analysis of 51 episodes in two Dallas hospitals and 97 episodes reported in the English literature. Ann Intern Med 1987;106:728–733. [DOI] [PubMed] [Google Scholar]
- 31.Lipsky BA, Hirschmann JV. Drug fever. JAMA 1981;245:851–854. [PubMed] [Google Scholar]
- 32.Tisdale JE, Miller DA. Drug-induced Diseases: Prevention, Detection, and Management. American Society of Health-System Pharmacists 2005. [Google Scholar]
- 33.Zhang J, Rong C, Yan C, Chen J, Yang W, Yu L, Dai H. Risk factors of furazolidone-associated fever. PLoS One 2022;17:e0266763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang Y, Xiao H, Tang S, Liang L, Sha W, Fang Y. Clinical features and treatment of drug fever caused by anti-tuberculosis drugs. Clin Respir J 2016;10:449–454. [DOI] [PubMed] [Google Scholar]
- 35.Foster FP, Beard RW. Fever from antibiotics: some lessons drawn from 25 cases. Med Clin North Am 1963;47:532–539. [PubMed] [Google Scholar]
- 36.Labbus K, Junkmann JK, Perka C, Trampuz A, Renz N. Antibiotic-induced fever in orthopaedic patients-a diagnostic challenge. Int Orthop 2018;42:1775–1781. [DOI] [PubMed] [Google Scholar]
- 37.Peng W-X, Lin Y, Shi X-J.. Retrospective analysis about 20 cases of drug fever induced by antibiotics. CLINICAL MEDICATION JOURNAL 2017;15:16–18. [Google Scholar]
- 38.Yaita K, Sakai Y, Masunaga K, Watanabe H. A Retrospective Analysis of Drug Fever Diagnosed during Infectious Disease Consultation. Intern Med 2016;55:605–608. [DOI] [PubMed] [Google Scholar]
- 39.Young EJ, Fainstein V, Musher DM. Drug-induced fever: cases seen in the evaluation of unexplained fever in a general hospital population. Rev Infect Dis 1982;4:69–77. [DOI] [PubMed] [Google Scholar]
- 40.Kuwabara H, Masuda J, Kanamaru M, Goto K, Uehara M, Uehara M. Clinical study on drug fever without drug eruption. Iryo 1990;44:430–435. [Google Scholar]
- 41.Seneta E, Seif FJ, Liebermeister H, Dietz K. Carl Liebermeister (1833–1901): a pioneer of the investigation and treatment of fever and the developer of a statistical test. J Med Biogr 2004;12:215–221. [DOI] [PubMed] [Google Scholar]
- 42.Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect 2000;6:633–634. [DOI] [PubMed] [Google Scholar]
- 43.Gerson D, Sriganeshan V, Alexis JB. Cutaneous drug eruptions: a 5-year experience. J Am Acad Dermatol 2008;59:995–999. [DOI] [PubMed] [Google Scholar]
- 44.Tokuda Y, Miyasato H, Stein GH, Kishaba T. The degree of chills for risk of bacteremia in acute febrile illness. Am J Med 2005;118:1417. [DOI] [PubMed] [Google Scholar]
- 45.Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld ABJ. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015;21:474–481. [DOI] [PubMed] [Google Scholar]
- 46.Harris LF, Holdsambeck HK. Drug fever—surprisingly common and costly. Ala Med 1986;56:19–22. [PubMed] [Google Scholar]
- 47.Rob F, Fialová J, Brejchová M, Džambová M, Sečníková Z, Zelenková D, et al. Drug fever as an adverse effect of acitretin in complicated psoriasis patient. Dermatol Ther 2015;28:366–368. [DOI] [PubMed] [Google Scholar]
- 48.Barbarroja-Escudero J, Sanchez-Gonzalez M-J, Antolin-Amerigo D, Rodriguez-Rodriguez M, Alvarez-Mon M. Hypersensitivity reactions and drug fever by bendamustine: a case report of three patients. Allergol Int 2015;64:109–111. [DOI] [PubMed] [Google Scholar]
- 49.Xiao J, Jia S-J, Wu C-F.. Celecoxib-induced drug fever: A rare case report and literature review. J Clin Pharm Ther 2022;47:402–406. [DOI] [PubMed] [Google Scholar]
- 50.Wackernagel D, Obaya S, Nydert P. Dalteparin-sodium induced drug fever in a neonate. BMJ Case Rep 2016;2016. 10.1136/bcr-2016-217621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schurr JW, Ambrosi L, Lastra JL, McLaughlin KC, Hacobian G, Szumita PM. Fever Associated With Dexmedetomidine in Adult Acute Care Patients: A Systematic Review of the Literature. J Clin Pharmacol 2021;61:848–856. [DOI] [PubMed] [Google Scholar]
- 52.Yuan H-L, Lu N-W, Xie H, Zheng Y-Y, Wang Q-H.. Doxycycline-induced drug fever: a case report. Infect Dis 2016;48:844–846. [DOI] [PubMed] [Google Scholar]
- 53.Ng QX, Seng C, Ho CYX, Yeo W-S.. Enoxaparin: A cause of postoperative fever? Med Hypotheses 2018;121:47–48. [DOI] [PubMed] [Google Scholar]
- 54.Gosnell H, Stein A, Vanegas Acosta DE. Postoperative fever secondary to enoxaparin usage with pork allergy. BMJ Case Rep 2022;15:e246904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naschitz JE. Drug fever induced by ertapenem. QJM 2011;104:730–731. [DOI] [PubMed] [Google Scholar]
- 56.Takoi H, Togashi Y, Fujimori D, Kaizuka H, Otsuki S, Wada T, et al. Favipiravir-induced fever in coronavirus disease 2019: A report of two cases. Int J Infect Dis 2020;101:188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurita T, Ishida K, Muranaka E, Sasazawa H, Mito H, Yano Y, et al. A Favipiravir-induced Fever in a Patient with COVID-19. Intern Med 2020;59:2951–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tawara J, Uehara T, Sakao S, Igari H, Taniguchi T, Kasai H, et al. Drug Fever Due to Favipiravir Administration for the Treatment of a COVID-19 Patient. Intern Med 2021;60:1115–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murai Y, Kawasuji H, Takegoshi Y, Kaneda M, Kimoto K, Ueno A, et al. A case of COVID-19 diagnosed with favipiravir-induced drug fever based on a positive drug-induced lymphocyte stimulation test. Int J Infect Dis 2021;106:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Wang Q, Wang S, Zhang Y, Wang Z. Unusual Drug Fever Caused by Imipenem/Cilastatin and a Review of Literature. Heart Surg Forum 2019;22:E119–E123. [DOI] [PubMed] [Google Scholar]
- 61.Slim R, Amara J, Nasnas R, Honein K, Jaoude JB, Yaghi C, et al. Isolated fever induced by mesalamine treatment. World J Gastroenterol 2013;19:1147–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C-H, Chen Y-Y.. A Case of Olanzapine-Induced Fever. Psychopharmacol Bull 2017;47:45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Georges A, Fitz-Gerald MJ. Reconsidering Olanzapine as a Possible Culprit for Drug Fever, defying ‘Incomplete Neuromalignant Syndrome’. J La State Med Soc 2016;168:123–124. [PubMed] [Google Scholar]
- 64.Schiller D, Maieron A, Schöfl R, Donnerer J. Drug fever due to a single dose of pantoprazole. Pharmacology 2014;94:78–79. [DOI] [PubMed] [Google Scholar]
- 65.Melo N, Policarpo S, Dias M, Almeida J. Pantoprazole: An Unusual Suspect in a Patient with Fever. Eur J Case Rep Intern Med 2021;8:002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yatabe T, Yamashita K, Yokoyama M. Drug fever caused by propofol in the intensive care unit. J Anesth 2015;29:786–789. [DOI] [PubMed] [Google Scholar]
- 67.Hatakeyama M, Fukunaga A, Shimizu H, Oka M, Horikawa T, Nishigori C. Drug fever due to S-carboxymethyl-L-cystein: demonstration of a causative agent with patch tests. J Dermatol 2012;39:555–556. [DOI] [PubMed] [Google Scholar]
- 68.Cui T, Diao X, Chen X, Huang S, Sun J. A case report: delayed high fever and maculopapules during Sorafenib treatment of ectopic hepatocellular carcinoma. BMC Cancer 2016;16:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ochi H, Wada K, Okada H, Kohara N, Fujita T, Toda K, et al. The persistence of drug-induced fever by teicoplanin—a case report. Int J Clin Pharmacol Ther 2011;49:339–343. [DOI] [PubMed] [Google Scholar]
- 70.Shao Q-Q, Qin L, Ruan G-R, Chen R-X, Luan Z-J, Ma X-J.. Tigecycline-induced Drug Fever and Leukemoid Reaction: A Case Report. Medicine 2015;94:e1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anno T, Kaneto H, Kawasaki F, Shigemoto R, Aoyama Y, Kaku K, et al. Drug fever and acute inflammation from hypercytokinemia triggered by dipeptidyl peptidase-4 inhibitor vildagliptin. J Diabetes Investig 2019;10:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konerding K, Moffet HL. New episodes of fever in hospitalized children. Am J Dis Child 1970;120:515–519. [DOI] [PubMed] [Google Scholar]
- 73.O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 2008;36:1330–1349. [DOI] [PubMed] [Google Scholar]
- 74.Mori F, Fili L, Barni S, Sarti L, Pucci N, Parronchi P, et al. Drug fever after a single dose of amoxicillin-clavulanic acid. J Allergy Clin Immunol Pract 2016;4:533–534.e1. [DOI] [PubMed] [Google Scholar]
- 75.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int 2001;60:804–817. [DOI] [PubMed] [Google Scholar]
- 76.Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010;6:461–470. [DOI] [PubMed] [Google Scholar]
- 77.Hama N, Abe R, Gibson A, Phillips EJ. Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis. J Allergy Clin Immunol Pract 2022;10:1155–1167.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CIOMS VI Working Group Report: Management of Safety Information from Clinical Trials, CIOMS, 2005. [Google Scholar]
- 79.Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–1330. [DOI] [PubMed] [Google Scholar]
- 80.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–245. [DOI] [PubMed] [Google Scholar]
- 81.World Health Organization. The use of the WHO-UMC system for standardised case causality assessment. WHO Collaborating Centre for International Drug Monitoring (Uppsala Monitoring Centre, UMC), Database. Chichester: John Wiley & Sons, Ltd; 2000. http://who-umc.org/Graphics/24734.pdf. (accessed 3 Mar 2023).
- 82.Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther 1977;21:247–254. [DOI] [PubMed] [Google Scholar]
- 83.Kumar KL, Reuler JB. Drug fever. West J Med 1986;144:753–755. [PMC free article] [PubMed] [Google Scholar]
- 84.Srinoulprasert Y, Rerkpattanapipat T, Sompornrattanaphan M, Wongsa C, Kanistanon D. Clinical value of in vitro tests for the management of severe drug hypersensitivity reactions. Asia Pac Allergy 2020;10:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greenblatt DJ, Gross PL, Harris J, Shader RI, Ciraulo DA. Fatal hyperthermia following haloperidol therapy of sedative-hypnotic withdrawal. J Clin Psychiatry 1978;39:673–675. [PubMed] [Google Scholar]
- 86.Carter JJ, McLaughlin ML, Bern MM. Bleomycin-induced fatal hyperpyrexia. Am J Med 1983;74:523–525. [DOI] [PubMed] [Google Scholar]
- 87.Rosenfelt F, Palmer J, Weinstein I, Rosenbloom B. A fatal hyperpyrexial response to bleomycin following prior therapy: a case report and literature review. Yale J Biol Med 1982;55:529–531. [PMC free article] [PubMed] [Google Scholar]
- 88.Leung WH, Lau JY, Chan TK, Kumana CR. Fulminant hyperpyrexia induced by bleomycin. Postgrad Med J 1989;65:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bihan K, Lebrun-Vignes B, Funck-Brentano C, Salem J-E.. Uses of pharmacovigilance databases: An overview. Therapie 2020;75:591–598. [DOI] [PubMed] [Google Scholar]
- 90.Uppsala Monitoring Centre. VigiBase. https://who-umc.org/vigibase/ (accessed 26 Jan 2023).
- 91.FDA Adverse Event Reporting System. https://open.fda.gov/data/faers/ (accessed 26 Jan 2023).
- 92.Health Canada. Canada Vigilance Adverse Reaction Online Database. 2009. https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database.html (accessed 26 Jan 2023).
- 93.Japanese Adverse Drug Event Report database. https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0003.html (accessed 26 Jan 2023).
- 94.Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 2011;72:905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noguchi Y, Ueno A, Otsubo M, Katsuno H, Sugita I, Kanematsu Y, et al. A simple method for exploring adverse drug events in patients with different primary diseases using spontaneous reporting system. BMC Bioinformatics 2018;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf 2006;29:385–396. [DOI] [PubMed] [Google Scholar]
- 97.Brown EG, Wood L. Coding of Data–MedDRA and other Medical Technologies. In: Clinical Data Management. Chichester, UK: John Wiley & Sons, Ltd 2003. 177–205. [Google Scholar]