Abstract
Approved therapies for the treatment of patients with pulmonary arterial hypertension (PAH) mediate pulmonary vascular vasodilatation by targeting distinct biological pathways. International guidelines recommend that patients with an inadequate response to dual therapy with a phosphodiesterase type‐5 inhibitor (PDE5i) and endothelin receptor antagonist (ERA), are recommended to either intensify oral therapy by adding a selective prostacyclin receptor (IP) agonist (selexipag), or switching from PDE5i to a soluble guanylate‐cyclase stimulator (sGCS; riociguat). The clinical equipoise between these therapeutic choices provides the opportunity for evaluation of individualized therapeutic effects. Traditionally, invasive/hospital‐based investigations are required to comprehensively assess disease severity and demonstrate treatment benefits. Regulatory‐approved, minimally invasive monitors enable equivalent measurements to be obtained while patients are at home. In this 2 × 2 randomized crossover trial, patients with PAH established on guideline‐recommended dual therapy and implanted with CardioMEMS™ (a wireless pulmonary artery sensor) and ConfirmRx™ (an insertable cardiac rhythm monitor), will receive ERA + sGCS, or PDEi + ERA + IP agonist. The study will evaluate clinical efficacy via established clinical investigations and remote monitoring technologies, with remote data relayed through regulatory‐approved online clinical portals. The primary aim will be the change in right ventricular systolic volume measured by magnetic resonance imaging (MRI) from baseline to maximal tolerated dose with each therapy. Using data from MRI and other outcomes, including hemodynamics, physical activity, physiological measurements, quality of life, and side effect reporting, we will determine whether remote technology facilitates early evaluation of clinical efficacy, and investigate intra‐patient efficacy of the two treatment approaches.
Keywords: oral prostacyclin‐receptor agonist, remote monitoring, soluble guanylate‐cyclase stimulator, targeted therapy
INTRODUCTION
Pulmonary arterial hypertension (PAH) represents a spectrum of disease that may be idiopathic or associated with genetic mutations, connective tissue disease, congenital heart disease, or drug/toxin exposure. 1 At a cellular level, disease is driven by remodeling and constriction of the small pulmonary arteries, which can lead to right‐sided heart failure and premature death. 1 Currently available targeted therapies for this progressive disease mediate pulmonary vascular vasodilatation by acting on one of three pathways—the endothelin pathway via endothelin receptor antagonists (ERA), the nitric oxide (NO) pathway via phosphodiesterase type‐5 inhibitors (PDE5i) or soluble guanylate cyclase stimulators (sGCS), or the prostacyclin pathways via prostacyclin analogs and prostacyclin receptor (IP) agonists. 1 , 2 Despite the range of therapies available, drug choice is empirical and based on a hospital‐based risk stratification that matches the number of vasodilator agents to disease severity. 2
Approved therapies targeting the endothelin, NO, or prostacyclin pathways have been shown to provide improvements in pulmonary vascular hemodynamics and 6‐min walk test (6MWT) in phase 2/3 studies. 1 , 3 , 4 , 5 , 6 , 7 Evidence shows that time to clinical worsening is further improved in patients established on dual therapy with an ERA and a PDE5i, when compared with monotherapy with either agent. 2 , 8 In line with European guidelines, 2 for patients established on dual oral therapy (PDE5i/ERA) with an inadequate treatment response, NHS England's National Commissioning Policy permits the addition of the selective IP receptor agonist selexipag, or switching of PDE5i for the sGCS riociguat. 9 , 10 There is clinical equipoise between these two approaches (i.e., triple therapy with selexipag + PDE5i + ERA and dual therapy with riociguat + ERA).
Selexipag, an IP receptor agonist, improves the time‐to‐clinical‐worsening in patients on a range of background therapy regimens (including patients on no therapy [20.4%]; ERA or PDE5i monotherapy [47.1%]; and ERA/PDE5i dual therapy [32.5%]). 4 However, data suggest that initiating triple therapy (PDE5i/ERA/IP receptor agonist) compared with dual therapy (PDE5i/ERA) in newly diagnosed treatment naïve patients offers no significant improvement in snapshot hemodynamic measurements or exercise capacity. 11 Additionally, switching PDE5i for another drug targeting the NO pathway (riociguat, an sGCS) improves disease severity as assessed by World Health Organization (WHO) functional class and 6MWT distance, and reduces clinical worsening events compared with continuing PDE5i therapy. 12 In all regulatory approval studies, significant side effects and therapeutic non‐adherence were observed. 4 , 6 , 12 , 13 , 14 , 15 , 16 , 17 , 18
In clinical practice, response to therapy and/or assessment of disease progression is made by assessing pressure and flow during invasive right heart catheterization, by monitoring the downstream effect on right heart structure and function, and by evaluating exercise capacity. European Society of Cardiology (ESC) guidelines recommend invasive assessment of cardiopulmonary hemodynamics for disease diagnosis, assessment of severity, and to inform treatment decisions (therapeutic change and transplant). 2 Other recommended means of guiding treatment decisions include regular measurement of exercise capacity by 6MWT, disease‐specific risk scoring, assessment of right heart strain by N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), and/or noninvasive imaging, with magnetic resonance imaging (MRI) acknowledged to be more accurate than echocardiography. 2
The currently established standard for phase 2 studies of PAH therapies includes the assessment of invasive hemodynamics and the 6MWT. 1 However, recent studies have demonstrated that noninvasive endpoints, such as right ventricular ejection fraction (RVEF) and right ventricular stroke volume (RVSV) measured by MRI, are repeatable, and detect treatment change in a manner similar to invasive catheterization and NTpro‐BNP, thereby establishing MRI as a robust, objective, noninvasive assessment of treatment response in patients with PAH. 19 , 20
Despite these advances, there remains a need for invasive/hospital‐based investigations to assess disease severity and demonstrate therapeutic benefit, and there are currently no means for early assessment of clinical efficacy in patients with PAH. This limits experimental medicine and drug development studies and prevents personalized medicine in clinical practice. Development of innovative approaches to monitor PAH outcomes is essential for a number of reasons including poor prognosis among patients with PAH, reduced quality of life, side effect profile of approved therapies, nonuniform drug response among patients, high cost of PAH‐specific therapies (£5–120k/medication/patient/year), and emerging therapies with proven benefit in preclinical studies. 9 , 19 , 20 , 21 , 22
In patients with PAH, cardiopulmonary hemodynamics are closely associated with clinical outcomes, 7 and are affected by both diseases worsening and increase or withdrawal of therapy. 23 , 24 The development of minimally invasive technology that provides remote, daily measurement of cardiopulmonary hemodynamic parameters and physical activity may provide more comprehensive coverage of the effects of treatment on patients' daily functioning, allowing insight into the intervening periods between scheduled hospital visits. 25 Remote monitoring may provide benefits to both patients and care teams by allowing remote monitoring of efficacy following a treatment change. This may permit a personalized management approach, with the care team able to optimize therapy remotely—balancing therapeutic efficacy with side effects in each individual patient. Indeed, in patients with heart failure, remote, hemodynamic‐guided therapy has been demonstrated to reduce heart failure hospitalization, 26 , 27 and these studies have led to regulatory approval of pulmonary artery pressure (PAP) monitors. Furthermore, studies of patients with PAH implanted with a PAP monitor and an insertable cardiac monitor (ICM) demonstrated that clinically indicated therapeutic changes altered physiological parameters associated with mortality, indicating that early, remote assessment of clinical efficacy may be achieved using these devices. 28
CardioMEMS™ HF System (Abbott) is a wireless, PAP monitor implanted at the time of right heart catheterization to provide remote measurement of cardiopulmonary hemodynamics. CardioMEMS is approved for routine clinical practice in the USA and Europe, and to date over 30,000 of these monitors have been implanted. The Confirm Rx™ ICM (Abbott) is a minimally invasive regulatory‐approved cardiac monitor, implanted in a clinical setting for patients who experience unexplained symptoms, such as dizziness; palpitations, chest pain, syncope, shortness of breath, as well as for patients who are at risk for cardiac arrhythmias. Over 40,000 Confirm RX™ have been implanted and the device is in routine clinical use in the United Kingdom.
Here, we detail the protocol for a study in which patients with PAH, established on guideline‐recommended dual oral therapy and evaluated as intermediate‐low risk, 29 will be implanted with CardioMEMS and ICM devices. Following implantation, patients will enter a 2 × 2 crossover study in which PDEi will be replaced with sGCS (ERA + sGCS), or an IP receptor agonist will be added to PDEi and ERA (PDEi + ERA + IP receptor agonist). Data obtained from remote monitoring will be compared with that from established clinical investigations undertaken at baseline and maximal therapy on each drug. The crossover design of this study, which will incorporate structured up‐titration of these drugs, is aimed at evaluating the capacity of implantable technology for early evaluation of the clinical efficacy of these drugs. A crossover study is a logical study designed to investigate the intra‐patient efficacy of these treatment options and increase the power to detect clinical efficacy. Additionally, the study will provide insight into the capacity of remote monitoring technology to facilitate trials that are not currently possible due to the requirement for repeated, hospital‐based invasive/imaging procedures.
METHODS
Study design
This open‐label, phase 4, multicentre, randomized 2 × 2 crossover study (NCT05825417) in patients with PAH established on dual therapy (PDE5i/ERA) will evaluate the effects via clinical investigations, patient‐reported outcomes and remote cardiac monitoring of two therapeutic strategies—adding an oral drug targeting the prostacyclin pathway (selexipag; PDEi + ERA + IP receptor agonist) and switching of PDE5i to an sGCS (riociguat; ERA + sGCS). Using the 2 × 2 crossover trial design, patients will receive both therapies, but the sequence will be randomly assigned with washout phases between therapies, and assessments of response to each therapy to be performed. 30 The study protocol was approved by the NHS Health Research Authority (IRAS PROJECT ID 325120, REC Reference 23/NE/0067). A tabulated summary of all visits and assessments is provided in Supporting Information: Table S1.
Objectives and endpoints
The main aims of this study will be to assess the individual difference in effect between treatment escalation with selexipag (PDEi + ERA + IP receptor agonist) or riociguat (riociguat; ERA + sGCS) on RV stroke volume (flow) as measured by cardiac MRI in patients with intermediate‐low risk PAH, and to determine whether remote monitoring devices can be used to provide an early assessment of clinical efficacy of drug therapies for PAH.
The primary endpoint will be change in RVSV (flow) measured by MRI from baseline to 12 weeks for each therapeutic strategy. Change in RVSV provides a robust, objective assessment of clinical efficacy and will represent a clinically meaningful change in physiology. 31 ESC guidelines recommend follow‐up at 3–6 months after a change in therapy. Titration protocols mimic standard clinical care and expected time‐to‐improvement. 2
Secondary endpoints for each therapeutic strategy include change from baseline to 12 weeks in hemodynamics (total peripheral resistance [TPR], mean pulmonary artery pressure [mPAP], cardiac output [CO], cardiac index, stroke volume [SV], heart rate [HR]), 6MWT, NTpro‐BNP, MRI parameters (RVEF, right ventricular end‐systolic volume [RVESV], right ventricular end‐diastolic volume [RVEDV], RVSV (volume), left ventricular ejection fraction [LVEF], left ventricular end‐systolic volume [LVESV], left ventricular end‐diastolic volume [LVEDV], and LVSV flow), quality of life (EmPHasis‐10), medication compliance (PHoenix PRO) and side effects, depression and anxiety symptoms (Generalized Anxiety Disorder‐[GAD]‐2/7 and Patient Health Questionnaire [PHQ]‐2/9), WHO functional class, and activity levels as measured with a Garmin Venu2 smartwatch. A full list of outcome measures is provided in Table 1.
Table 1.
Outcome measures.
| Hospital‐based measures | Remotely monitored measures | |
|---|---|---|
| Primary outcomes | Change in RVSV (flow) measured by MRI | |
| Secondary outcomes | Haemodynamics:
|
Confirm Rx:
|
Abbreviations: 6MWT, 6‐min walk test; CO, cardiac output; GAD, Generalized Anxiety Disorder; HR, heart rate; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; mPAP, mean pulmonary artery pressure; PHQ, Patient Health Questionnaire; PRO, patient‐reported outcome; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume; RVSV, right ventricular stroke volume; SV, stroke volume; TPR, total peripheral resistance.
Established clinical study endpoint measures at maximal therapy will be compared to changes in remotely monitored parameters measured at 4 and 8 weeks to determine if the implanted devices can detect structured changes in the clinical therapy, thereby facilitating early assessment of clinical efficacy. Remotely monitored parameters to be correlated with maximal therapy assessments, measured as absolute change from baseline and area under the curve to 4 and 8 weeks on each therapy, include hemodynamics (mPAP, CO, cardiac index, TPR, day HR, night HR, and HR reserve), activity (minutes per day), 6MWT, and PRO.
The analysis will also be performed to determine if changes in established and remotely monitored parameters (primary and secondary endpoints) can detect individual patient‐level therapy effects, thereby determining the utility of remote monitoring for personalized treatment plans.
Study population
The study aims to recruit 40 patients with PAH, established on PDE5i and ERA, through the UK National Pulmonary Hypertension Clinical Studies Network (UNIPHY)—a collaboration of UK centers commissioned for the treatment of PAH, providing access to all patients within the UK currently receiving targeted therapy for PAH (>5000). 32 , 33 Suitable patients will be identified from existing patient lists by local teams and invited to screening via clinical and research teams.
Eligible patients meeting the inclusion and exclusion criteria (Table 2) will be established on PDE5i/ERA dual therapy and will meet NHS England's National Commissioning and ESC guideline criteria for initiation of IP receptor agonist or sGCS. 2 , 9 , 10 While it is expected the majority of patients recruited will have intermediate‐low risk PAH, patients with intermediate‐high risk PAH who decline intravenous therapy will also be considered.
Table 2.
Summary of eligibility criteria.
Inclusion criteria:
|
Exclusion criteria:
|
Abbreviations: eGFR, estimated glomerular filtration rate; ERA, endothelin‐1 receptor antagonist; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase‐5 inhibitor.
Device implantation
Eligible patients will be implanted with CardioMEMS and Confirm Rx ICM devices using standard techniques and remote monitoring data collected using regulatory‐approved online portals. 28
Treatment
Patients will be randomized 1:1 to one of two treatment sequences (Figure 1), with comparisons to be made using patient‐level data within the two treatment arms. Randomization will be done by authorized staff at study sites using a concealed randomization system via the Zeesta electronic case report form (eCRF—www.zeesta.ai/) portal. A block randomization stratified by site with a block size of four will be employed.
Figure 1.
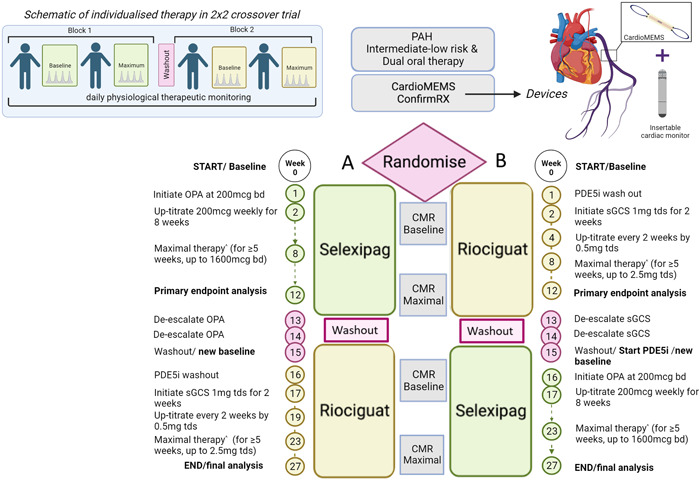
Dose escalation and de‐escalation protocol. Bd, twice daily; CMR, cardiac magnetic resonance imaging; ERA, endothelin receptor antagonist; OPA, oral IP‐receptor agonist; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase type‐5 inhibitor; sGCS, soluble guanylate‐cyclase stimulator; tds, three times daily; WHO FC, World Health Organization Functional Class.
As per standard practice, the treatment schedules will include a minimum PDE5i washout period of 24–48 h (depending on PDE5i) before initiation of riociguat 12 ; in addition, riociguat and selexipag will be titrated according to established dose‐adjustment schemes to maximum doses of 2.5 mg three times per day, and 1600 μg twice daily, respectively as tolerated (Figure 1).
In brief, patients in Arm A will initiate treatment with selexipag for uptitration to maximal therapy. Before crossover, patients will undergo selexipag dose de‐escalation and washout, followed by PDE5i washout. Patients will then initiate treatment with riociguat for uptitration to maximal therapy. Patients in Arm B will have an initial PDE5i washout period before commencing treatment with riociguat. This will be followed by de‐escalation and washout, and initiation of treatment with PDE5i before crossover to selexipag treatment. In both arms, the primary endpoint evaluation will be undertaken following a minimum intended duration of 5 weeks on the maximal tolerated dose of each therapy. The dose escalation and de‐escalation protocol are shown in Supporting Information: Table S2.
Clinical assessments
Patients will undergo clinical assessments at baseline before receiving study drug treatment, and at Weeks 12 and 27 of the treatment schedule (Figure 1); these will include hemodynamics, 6MWT, MRI, and NT‐proBNP assessments.
MRI analysis will be provided by a study‐appointed core lab using certified clinicians; scans will be deidentified and analyzed in random order independent of patient and time point. Analysis of primary and secondary endpoints will be undertaken in a blinded manner by an independent statistical team in accordance with a pre‐specified statistical analysis plan.
Remote monitoring
Physiological parameters to be monitored will include TPR, mPAP, CO, SV, and HR (Table 1). Patients will be given instructions on how to take readings at the time of implantation. In addition, remote detection of changes in physical activity levels will be measured by Garmin Venu2 smartwatch, and the 6‐min walk test (6MWT) performed at home.
Patient‐reported outcomes
To date, no published randomized controlled trials in PAH have undertaken PAH‐specific PRO instruments as secondary endpoints (Figure 2). The current study aims to understand patients' attitudes about PAH medications and the impact of the study medication on quality of life, as well as explore attitudes about the use of remote technologies.
Figure 2.
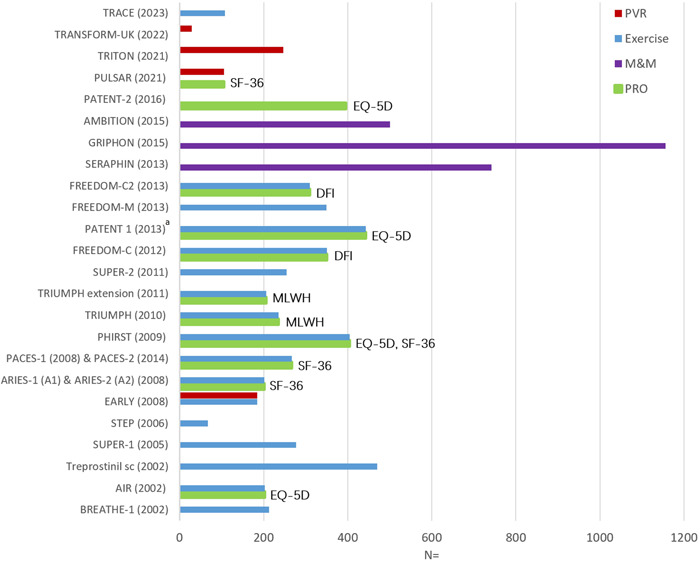
Primary outcomes in landmark PAH randomized controlled trials with patient‐reported outcomes included as secondary endpoints. DFI, dyspnea fatigue index; EQ‐5D, EuroQoL five‐dimension; MLWH, Minnesota Living with Heart Failure questionnaire; M&M, morbidity and mortality; PAH, pulmonary arterial hypertension; PRO, patient‐reported outcome; PVR, pulmonary vascular resistance; SF‐36, Short‐Form 36; Exercise endpoint inclusive of 6‐min walk distance and actigraphy; aLiving with Pulmonary Hypertension (LPH) questionnaire was undertaken in PATENT‐1; however, this was considered exploratory.
Quality of life outcomes will be assessed weekly using the validated EmPHasis‐10 questionnaire. 34 Validated questionnaires will be used twice monthly to screen for anxiety (GAD‐2) 35 and depression (PHQ‐2) symptoms. 36
Patients will also be asked to record, on a weekly basis, any side effects of the study medications and to track dose–response changes that are observed. Data will be collected on patients' attitudes toward their PAH medications and patient‐reported medication compliance (PHoenix PRO questionnaire; Supporting Information: Figure S1). All participants will be invited to co‐enroll in the COHORT‐digital study, 37 which enables PRO reporting through a mobile application called Atom5™ (Figure 3). If participants decline to co‐enroll for digital PRO reporting, data for these outcomes will be collected using a 10‐item questionnaire via telephone communication into the eCRF.
Figure 3.
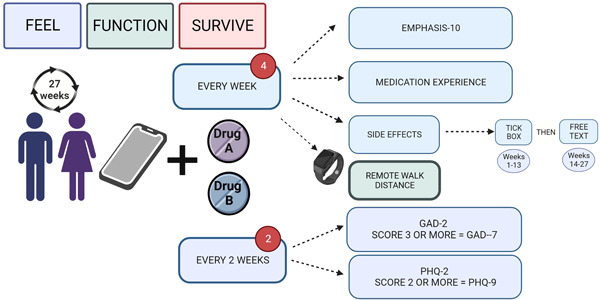
Patient‐reported outcomes in the PHoenix study. A, Arm A = riociguat/selexipag and B, Arm B = selexipag/riociguat GAD, Generalized Anxiety Disorder questionnaire; PHQ, Patient Health Questionnaire.
Additionally, patients will also be asked to provide their insight to help understand attitudes regarding remote monitoring, clinical care, and health outcomes at enrollment and study completion.
Safety
Adverse events (AEs) will be monitored over the duration of the study period; AEs that are definitely or possibly related to the device or the insertion procedure should be considered device‐related (adverse device effect [ADE]).
Statistical analyses
Sample size calculation
The sample size was chosen to ensure adequate power to detect differences in the clinical efficacy of the two treatment approaches in population‐level analyses and to have adequate power to evaluate the ability of implantable/remote technology to provide early evaluation of such clinical efficacy. For comparing the clinical efficacy of the two treatment approaches, we have used published RVSV data reporting a minimal clinically important difference of 12 mL and within‐patient standard deviation (SD) of 16.5 mL. 38 The current study will be powered using a standardized effect (SE) of 12/16.5 = 0.73. Assuming 1:1 randomization of the participants to the two treatment sequences, 40 participants will provide 90% power in the population‐level clinical efficacy analysis for a 5% two‐sided type‐I error rate, with SE of 0.73. This is below the SE previously reported for the pulmonary vascular resistance (PVR; SE = 353.4/219.0 = 1.61), 3 and the RVEF (SE = 9.12/7.39 = 1.23) 20 and comparable to that observed for the 6MWT (SE = 36.0/46.7 = 0.77). 6 Therefore, the study will be well‐powered for population‐level analyses of these additional important outcomes.
The sample size of 40 also provides good power for assessing whether changes in remote physiological measures from baseline to 4 and 8 weeks (mPAP, CO, HR, and heart rhythm) are correlated with changes in clinical measures from baseline to 12 weeks. The sample size of 40 patients provides 90% power, at a two‐sided 5% type‐I error rate, for correlations greater than 0.5, which would represent those of clinical interest.
The study is not powered for formal mediation analysis, so this will be considered exploratory. No formal multiple‐testing correction will be applied.
Statistical analysis plan
All statistical analysis plans (SAPs) will be drafted early in the study and finalized before the analysis of unblinded data.
The primary clinical efficacy analysis will use a linear mixed effects model with the dependent variable being the change in RVSV (flow) from baseline to 12 weeks. “Baseline” and “12 weeks” refer to the time points within each treatment period. Each participant will contribute up to two observations if they complete both treatment periods. A random effect for each participant will be included together with the within‐treatment period baseline RVSV (flow) measurement, a fixed period effect, and treatment (selexipag or riociguat) allocated during the treatment period. This model will be used to estimate the mean difference between the two treatment approaches (ERA + sGCS and PDEi + ERA + IP receptor agonist), together with a 95% confidence interval and a p value using a Wald test.
Similar methods will be applied to the analysis of secondary efficacy outcomes. Treatment effect heterogeneity between subgroups will be assessed by including treatment‐by‐subgroup interaction terms in regression models. Main analyses will use complete data only, but multiple imputation will be used for missing data in sensitivity analyses. No adjustments will be made for multiple statistical tests. All efficacy analyses will follow the intention to treat principle (i.e., analysis according to randomized treatment, regardless of treatment compliance). Safety data (adverse events and side effects) will be summarized in relation to treatment being received at the onset of the event, and the study period (pretreatment period 1, treatment period 1, washout period, treatment period 2, posttreatment period 2); no formal statistical comparisons will be applied.
To analyze whether changes in RVSV (flow) may be explained by remotely monitored physiological parameters, we will test whether there is a significant correlation between the change between baseline and Week 4/Week 8 physiological parameters and the change between baseline and Week 12 RVSV (flow) outcome. We will also adopt a mediation analysis approach to investigate what proportion of the change between baseline and Week 12 RVSV (flow) is explained by the change between baseline and Week 4/Week 8 physiological parameters using the mediation package in R. Secondary endpoints will be analyzed using appropriate regression models.
DISCUSSION
Patients with PAH receiving dual combination treatment (PDE5i + ERA) who are stratified as being at intermediate‐low risk 39 are recommended to intensify therapy via the addition of the IP receptor agonist, selexipag, or to switch from a PDE5i to the sGCS, riociguat. 9 , 10 , 29 There is clinical equipoise between triple therapy with selexipag + PDE5i + ERA and dual therapy with riociguat + ERA. Conducting head‐to‐head clinical trials to compare treatment strategies in patients with PAH is challenging due to the current necessity for repeated, hospital‐based invasive/imaging procedures to evaluate treatment efficacy. Remote hemodynamic and cardiac monitoring may provide a means for early, minimally invasive evaluation of clinical efficacy and early identification of clinical worsening in patients with PAH, which may facilitate study designs evaluating dose–response, time‐to‐effect and head‐to‐head comparison. This study is designed to assess the individualized effect of selexipag and riociguat on RV stroke volume as measured by cardiac MRI, and to determine whether remote monitoring devices can be used to provide an early assessment of the clinical efficacy of drug therapies for PAH. This hybrid drug‐device regulatory‐approved design represents the first evaluation of an sGCS and IP agonist.
This blinded analysis of objective MRI measures provides an efficient, robust, and effective clinical study structure. As the primary endpoint is objective with blinded analysis, patients and clinicians will not be blinded to the sequence of drug allocation and up‐titration.
In this study, data will be relayed daily from regulatory‐approved, minimally invasive monitors to care teams through secure online clinical portals, with the aim of facilitating early, individual‐level, remote evaluation of treatment effects. This study will offer the potential to build on existing evidence showing that remotely monitored parameters may be used at diagnosis to categorize patients with PAH as low, intermediate, or high risk. 24 In addition to offering the potential for early evaluation of therapies, the use of remotely monitored outcomes may provide a broader picture of the effects of treatment on patients' daily functioning. 25 Remote patient monitoring may also facilitate more patient‐centric research, and improve study recruitment and retention, which are key issues for research into a rare disease such as PAH. 21 , 25 Additionally, patients with PAH are typically prescribed combination therapies, which can make it challenging to power trials to demonstrate the effectiveness of novel therapies. 20 , 21
In addition to capturing data on established physiological and biochemical markers of clinical risk, this study will provide valuable insight into patient‐reported quality of life and mental health outcomes, as well as explore side effects experienced during therapeutic up‐titration and withdrawal of therapy.
AUTHOR CONTRIBUTIONS
The study was designed by AR with representatives from the UK Pulmonary Hypertension Network (CC, GC, RC, LH, DK, JL, AR, JS, MT, and SJW), the clinical research fellow (FV), and the clinical trial manager (JD). All the authors contributed to the development of the manuscript and approved the final version of the article.
CONFLICT OF INTEREST STATEMENT
Dr Frances Varian: MRC clinical fellow, travel and conference funding from Janssen Ltd. Dr Jennifer Dick, Mr Christian Battersby, Mr Stefan Roman, Miss Jenna Ablott, Dr Lisa Watson, Mrs Sarah Bizmahfooz, and Dr Hamza Zafar: none declared. Dr Gerry Colgan: no direct conflicts, has undertaken consultancy work & honoraria for Janssen Ltd, Bayer Ltd, MSD. Received research funding from Janssen Ltd. Dr John Cannon: support to attend conferences from Janssen and been paid for advisory boards by Janssen and Ferrer. Jay Suntharalingam and Dr Jim Lordan: none declared. Professor Luke Howard: I have received honoraria for advisory boards, steering committees, and speaking from Janssen. My department has received research funding support from Janssen. I have received personal support for travel, accommodation, and registration at international meetings. I have received honoraria for advisory boards and speaking from MSD. I have received honoraria for advisory boards from Endotronix. Colm McCabe: none declared. Dr John Wort: I have received honoraria from Janssen, MSD, Ferrer, and Acceleron, research grants from Janssen and Ferrer and travel and accommodation grants from Janssen. Laura Price and Dr Colin Church: none declared. Dr Neil Hamilton: Honoraria from MSD and Janssen, travel and accommodation grants from Janssen, participation on advisory boards for Bayer, MSD, Janssen, and Vifor, and is a board member on the NHS Specialist respiratory clinical reference group. Dr Abdul Hameed: none declared. Dr Judith Hurdman, Dr Iain Armstrong and Dr Charlie Elliot: none declared. Prof Robin Condliffe: No COI. Received honoraria for speaking and advisory boards from Janssen and MS. Prof Martin Wilkins: support from NIHR for clinical research facility and biomedical research center infrastructure support BHF center support (RE/18/4/34215), consulting fees for MorphogenIX, Janssen and Janssen, Kinaset, Chiesi, Aerami, BenevolentAI, Novartis, and VIVS, participation on data safety monitoring board for Acceleron and GSK. Associate Professor Alastair Webb: none declared. Dr David Adlam: none declared. Professor Ray L Benza: steering and adjudication committees ABBOTT. Professor Kazem Rahimi: receives grants from the Oxford Martin School and the British Heart Foundation. He is an associate editor of Heart and a specialty editor of PLOS Medicine. And he is a cofounder of Zeesta and sits on the advisory board of Medtronic. Dr Moha Shojaei, Dr Nan Lin, Prof James Wason, Dr Alasdair McIntosh, Prof Alex McConnachie, and Dr Jennifer Middleton: none declared. Dr Roger Thompson: I have received honoraria, travel support, and grant funding from Janssen. Prof David Kiely: Support and grants received from NIHR Sheffield Biomedical Research Centre, Janssen Pharmaceuticals; additional grants from Ferrer; consulting fees, honoraria payments, and supports for attending meetings received from Janssen Pharmaceuticals, Ferrer, Altavant, MSD, and united Therapeutics, participants on advisory boards with Janssen and MSD; members of clinical reference group for specialist respiratory medicine (NHS England) and lead of UK national audit of pulmonary hypertension. Dr Mark Toshner: funding from NIHR Cambridge BRC, NIHR HTA; consulting fees from MorphogenIX and Jansen; participation on data safety monitoring board/advisory board with ComCov and FluCov. Dr Alex Rothman: research funding: Wellcome Trust Clinical Research Career Development Fellowship (206632/Z/17/Z), Medical Research Council (UK) Experimental Medicine Award (MR/W026279/1), NIHR Biomedical Research Center Sheffield, Contribution in kind: Medtronic, Abbott, Endotronix, Novartis, Janssen. Research support and consulting: NXT Biomedical, Endotronix, SoniVie, Neptune, Gradient.
ETHICS STATEMENT
IRAS project ID: 325120.
REC reference: 23/NE/0067.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank Aisling O'Keeffe, PhD, and Allison Kirsop, PhD, of Scientific Writers Ltd., for providing medical writing support. The study is funded by an MRC Experimental Medicine Project award MR/W026279/1, Wellcome Trust Clinical Research Career Development Fellowship (AR: 206632/Z/17/Z; AS: 205188/Z/16/Z; and PDM: 214567/Z/18/Z), BHF Intermediate Fellowship (AART: FS/18/13/33281), and National Institute for Health Research (JMSW: NIHR301614). Devices are provided by Abbott. AR is grateful to Richard Hughes, whose generous philanthropic support has helped to make this work possible. In addition to survey information gathered from Pulmonary Hypertension Association United Kingdom (PHA UK), five patients of diverse age, ethnicity, and gender with experience in research were involved in the design of the Patient‐Reported Outcome Measures. The patient information sheet was submitted to a generic online advisory panel via the NIHR Clinical Research and Innovation Office, and amendments were made following feedback. This lay summary has been reviewed by a patient from PHA UK volunteering to collaborate on the steering committee. PPIE work is planned throughout the trial, including the steering committee, focussing on patient information and updates. Recruitment for PPIE is ongoing. If you are a patient or member of the public reading this and would like to be involved, then please contact the corresponding author, Dr Rothman at a.rothman@sheffield.ac.uk.
Varian F, Dick J, Battersby C, Roman S, Ablott J, Watson L, Binmahfooz S, Zafar H, Colgan G, Cannon J, Suntharalingam J, Lordan J, Howard L, McCabe C, Wort J, Price L, Church C, Hamilton N, Armstrong I, Hameed A, Hurdman J, Elliot C, Condliffe R, Wilkins M, Webb A, Adlam D, Benza RL, Rahimi K, Shojaei‐Shahrokhabadi M, Lin NX, Wason JMS, McIntosh A, McConnachie A, Middleton JT, Thompson R, Kiely DG, Toshner M, Rothman A. Pulmonary Hypertension: intensification and Personalization of Combination Rx (PHoenix): a phase IV randomized trial for the evaluation of dose‐response and clinical efficacy of riociguat and selexipag using implanted technologies. Pulm Circ. 2024;14:e12337. 10.1002/pul2.12337
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analyzed as this is a methodological description of a study.
REFERENCES
- 1. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. [DOI] [PubMed] [Google Scholar]
- 3. Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N, Degano B, Bonderman D, Kurzyna M, Efficace M, Giorgino R, Lang IM. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40(4):874–880. [DOI] [PubMed] [Google Scholar]
- 4. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. [DOI] [PubMed] [Google Scholar]
- 5. Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, Ewert R, Weimann G, Grimminger F. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792–799. [DOI] [PubMed] [Google Scholar]
- 6. Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. [DOI] [PubMed] [Google Scholar]
- 7. Weatherald J, Boucly A, Chemla D, Savale L, Peng M, Jevnikar M, Jaïs X, Taniguchi Y, O'Connell C, Parent F, Sattler C, Hervé P, Simonneau G, Montani D, Humbert M, Adir Y, Sitbon O. Prognostic value of follow‐up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation. 2018;137(7):693–704. [DOI] [PubMed] [Google Scholar]
- 8. Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk‐Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JHN, Langley J, Rubin LJ. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. [DOI] [PubMed] [Google Scholar]
- 9. NHS England . Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (adults). Published December 2018. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2018/12/Selexipag-for-treating-pulmonary-arterial-hypertension-adults.pdf. Accessed June 2023.
- 10. NHS England . Clinical Commissioning Policy: Riociguat for pulmonary arterial hypertension. Published February 2017. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/06/ccp-riociguat-pulmonary-arterial-hypertension.pdf. Accessed June 2023.
- 11. Chin KM, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, Hoeper MM, Martin N, Mathai SC, McLaughlin VV, Perchenet L, Poch D, Saggar R, Simonneau G, Galiè N. Three‐ versus two‐drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78(14):1393–1403. [DOI] [PubMed] [Google Scholar]
- 12. Hoeper MM, Al‐Hiti H, Benza RL, Chang SA, Corris PA, Gibbs JSR, Grünig E, Jansa P, Klinger JR, Langleben D, McLaughlin VV, Meyer GMB, Ota‐Arakaki J, Peacock AJ, Pulido T, Rosenkranz S, Vizza CD, Vonk‐Noordegraaf A, White RJ, Chang M, Kleinjung F, Meier C, Paraschin K, Ghofrani HA, Simonneau G, Olschewski H, Delcroix M, Andrade‐Lima M, de Amorim Corrêac R, Figueiredo Campos F, Ota Arakaki J, Meyer G, De Souza R, Langleben D, Al‐Hiti H, Jansa P, Mellemkjær S, Bauer F, Montani D, Simonneau G, Drömann D, Ghofrani HA, Grünig E, Halank M, Held M, Hoeper M, Klose H, Kneidinger N, Leuchte H, Opitz C, Rosenkranz S, Wilkens H, Wirtz H, Karvounis H, Pitsiou G, Orfanos S, D'Alto M, Ghio S, Vizza C, Vitulo P, Nakayama T, Maki H, Tatebe S, de los Rios Ibarra M, Pulido T, Van Dijk A, Vonk‐Noordegraaf A, Roleder T, Castro G, Loureiro M, Robalo‐Martins S, Barberá J, Lázaro M, Perez‐Penate G, Román A, Cheng CC, Hsu CH, Hsu HH, Atahan E, Mogulkoc Bishop N, Okumus N, Onen Z, Chang HJ, Chang SA, Lee JS, Kim HK, Coghlan J, Corris P, Church A, Condliffe R, Gibbs J, Peacock A, Wort S, Allen R, Allen S, Awdish R, Benza R, DeSouza S, Feldman J, Johri S, Klinger J, Layish D, McConnell J, McLaughlin V, Migliore C, Rahaghi F, Rischard F, Robbins I, Satterwhite L, Shah T, Sulica R, White R. Switching to riociguat versus maintenance therapy with phosphodiesterase‐5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open‐label, randomised controlled trial. Lancet Respir Med. 2021;9(6):573–584. [DOI] [PubMed] [Google Scholar]
- 13. Ghofrani HA, Grimminger F, Grünig E, Huang Y, Jansa P, Jing ZC, Kilpatrick D, Langleben D, Rosenkranz S, Menezes F, Fritsch A, Nikkho S, Humbert M. Predictors of long‐term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT‐2 open‐label, randomised, long‐term extension trial. Lancet Respir Med. 2016;4(5):361–371. [DOI] [PubMed] [Google Scholar]
- 14. Rubin LJ, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh A, Langleben D, Fritsch A, Menezes F, Davie N, Ghofrani HA. Riociguat for the treatment of pulmonary arterial hypertension: a long‐term extension study (PATENT‐2). Eur Respir J. 2015;45(5):1303–1313. [DOI] [PubMed] [Google Scholar]
- 15. Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. [DOI] [PubMed] [Google Scholar]
- 16. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. [DOI] [PubMed] [Google Scholar]
- 17. Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double‐blind, placebo‐controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. [DOI] [PubMed] [Google Scholar]
- 18. Galiè N, Rubin L, Hoeper M, Jansa P, Al‐Hiti H, Meyer G, Chiossi E, Kusic‐Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double‐blind, randomised controlled trial. Lancet. 2008;371(9630):2093–2100. [DOI] [PubMed] [Google Scholar]
- 19. Kiely DG, Channick R, Flores D, Galiè N, Marcus JT, Peacock A, Rosenkranz S, Tawakol A, Torbicki A, Noordegraaf AV, Wetherill G, Swift AJ. Comparison of standardized treatment effect sizes for invasive and non‐invasive endpoints in pulmonary arterial hypertension: insights from the REPAIR study. J Am Coll Cardiol. 2021;77(18_Suppl_1):S1672. [Google Scholar]
- 20. Swift AJ, Wilson F, Cogliano M, Kendall L, Alandejani F, Alabed S, Hughes P, Shahin Y, Saunders L, Oram C, Capener D, Rothman A, Garg P, Johns C, Austin M, Macdonald A, Pickworth J, Hickey P, Condliffe R, Cahn A, Lawrie A, Wild JM, Kiely DG. Repeatability and sensitivity to change of non‐invasive end points in PAH: the RESPIRE study. Thorax. 2021;76(10):1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weatherald J, Boucly A, Peters A, Montani D, Prasad K, Psotka MA, Zannad F, Gomberg‐Maitland M, McLaughlin V, Simonneau G, Humbert M. The evolving landscape of pulmonary arterial hypertension clinical trials. Lancet. 2022;400(10366):1884–1898. [DOI] [PubMed] [Google Scholar]
- 22. NHS England . Commissioning Policy: Targeted therapies for use in pulmonary hypertension in adults. Published July 2015. Available from: https://www.england.nhs.uk/wp-content/uploads/2018/07/Targeted-therapies-for-use-in-pulmonary-hypertension-in-adults.pdf. Accessed June 2023.
- 23. Middleton JT, Binmahfooz S, Zafar H, Patel J, Giri D, Battersby C, Toshner M, Reddy A, Lewis R, Condliffe R, Elliot C, Hameed A, Charalampopoulos A, Thompson A, Kiely DG, Rothman AM. Abstract 13553: remote monitoring of the haemodynamic response to clinically indicated therapeutic escalation and clinical worsening events in patients with pulmonary arterial hypertension. Circulation. 2022;146(Suppl_1):A13553. [Google Scholar]
- 24. Middleton JT, Binmahfooz S, Zafar H, Patel J, Neelam Naganathan DG, Battersby C, Toshner M, Reddy A, Lewis R, Swift AJ, Condliffe R, Elliot C, Hameed A, Charalampopoulos A, Thompson A, Kiely DG, Rothman AM. Abstract 13527: the development of a remote pulmonary arterial hypertension risk score utilising remote monitoring technology to monitor parameters associated with mortality in pulmonary arterial hypertension. Circulation. 2022;146(Suppl_1):A13527. [Google Scholar]
- 25. Clinical Trials Transformation Initiative (CTTI) . Digital Health Trials: Developing novel endpoints generated by digital health technology for use in clinical trials. Available from: https://ctti-clinicaltrials.org/wp-content/uploads/2022/03/CTTI-Digital-Health-Trials-Novel-Endpoint-Acceptance-Recommendations.pdf. Accessed June 2023.
- 26. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453–461. [DOI] [PubMed] [Google Scholar]
- 27. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB, Costanzo MR. Haemodynamic‐guided management of heart failure (GUIDE‐HF): a randomised controlled trial. Lancet. 2021;398(10304):991–1001. [DOI] [PubMed] [Google Scholar]
- 28. Middleton JT, Binmahfooz S, Zafar H, et al. Remote evaluation of risk and physiological response to therapeutic escalation and clinical worsening in patients with pulmonary hypertension. medRxiv. Preprint posted online April 28, 2023. 10.1101/2023.04.27.23289153 [DOI]
- 29. Rosenkranz S, Delcroix M, Giannakoulas G, Hoeper MM, Kovacs G, Humbert M. The ‘Ten Commandments' of the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2023;44(10):792–793. [DOI] [PubMed] [Google Scholar]
- 30. Kane PB, Bittlinger M, Kimmelman J. Individualized therapy trials: navigating patient care, research goals and ethics. Nat Med. 2021;27(10):1679–1686. [DOI] [PubMed] [Google Scholar]
- 31. Alabed S, Shahin Y, Garg P, Alandejani F, Johns CS, Lewis RA, Condliffe R, Wild JM, Kiely DG, Swift AJ. Cardiac‐MRI predicts clinical worsening and mortality in pulmonary arterial hypertension. JACC Cardiovasc Imaging. 2021;14(5):931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pulmonary Hypertension Association UK (PHA UK) . Pulmonary hypertension specialist centres. Available from: https://www.phauk.org/treatment-for-pulmonary-hypertension/pulmonary-hypertension-specialist-centres/. Accessed July 2023.
- 33. NHS Digital . National Pulmonary Hypertension Audit, 13th Annual Report. Published 19 January 2023. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-pulmonary-hypertension-audit/13th-annual-report. Accessed June 2023.
- 34. Lewis RA, Armstrong I, Bergbaum C, Brewis MJ, Cannon J, Charalampopoulos A, Church AC, Coghlan JG, Davies RJ, Dimopoulos K, Elliot C, Gibbs JSR, Gin‐Sing W, Haji G, Hameed AG, Howard LS, Johnson MK, Kempny A, Kiely DG, Lo Giudice F, McCabe C, Peacock AJ, Peleyeju O, Pepke‐Zaba J, Polwarth G, Price L, Sabroe I, Schreiber BE, Sheares K, Taboada D, Thompson AAR, Toshner MR, Wanjiku I, Wort SJ, Yorke J, Condliffe R. EmPHasis‐10 health‐related quality of life score predicts outcomes in patients with idiopathic and connective tissue disease‐associated pulmonary arterial hypertension: results from a UK multicentre study. Eur Respir J. 2021;57(2):2000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. [DOI] [PubMed] [Google Scholar]
- 36. Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 37. National Cohort Study of Idopathic and Heritable PAH . Available from: https://www.ipahcohort.com/. Accessed July 2023.
- 38. van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KMJ, Bronzwaer JGF, Heymans MW, Boonstra A, Postmus PE, Westerhof N, Vonk Noordegraaf A. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139(5):1003–1009. [DOI] [PubMed] [Google Scholar]
- 39. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, Staehler G, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Park DH, Ewert R, Kaemmerer H, Kabitz HJ, Skowasch D, Behr J, Milger K, Halank M, Wilkens H, Seyfarth HJ, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H, Claussen M, Lange TJ, Rosenkranz S. COMPERA 2.0: a refined four‐stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2022;60(1):2102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed as this is a methodological description of a study.


