Imagine reading a study that investigates the incidence of myocardial infarction among smokers hospitalized for acute chest pain. The authors report that only a minority of the patients received cardiovascular work-up with at least an ECG. They observe a low incidence of myocardial infarction and consequently conclude that myocardial infarction is infrequent in that at-risk population.
We could even take it a step further: imagine the same study, but now the authors do not even mention how many patients received cardiovascular work-up; nonetheless, their conclusion remains the same.
What would you think of the validity of such studies? Presumably, in many research fields, such studies would not survive peer review. Common sense would require us to ask the authors to clearly report how many patients received the necessary work-up to establish a diagnosis, and to adapt their conclusions about disease incidence accordingly.
This common sense does not seem to apply to the research field of influenza- or coronavirus disease (COVID-19)-associated pulmonary aspergillosis (IAPA or CAPA). Indeed, the extreme variation in reported incidences of these fungal superinfections in patients with severe viral pneumonia have partly been attributed to variations in diagnostic approaches (1). This is a major issue because mycological diagnosis of IAPA and CAPA relies heavily on BAL sampling, given the limited sensitivity of serum galactomannan. Consequently, if one does not actively look for IAPA or CAPA, one will not find it. Not only the depth of fungal work-up differs, as we noted that in many observational studies essential information for calculating the incidence is missing, namely the proportion of patients who received BAL sampling.
The diligence of a physician to find a disease should not have to define whether you have a disease or not. Therefore, to substantiate these issues, we investigated all studies found in PubMed using the search terms “(“Aspergill*”) AND (“Influenza” OR “COVID-19”)” that reported observational data on IAPA or CAPA published online between January 1, 2009 and October 1, 2023. We identified 51 studies that included more than 100 patients requiring critical care for severe influenza or COVID-19 and that used the original or modified AspICU criteria for aspergillosis on ICU (2, 3), the criteria by Verweij and colleagues for IAPA (4) or CAPA (5), the CAPA criteria of the European Confederation of Medical Mycology and the International Society for Human and Animal Mycology (6), or modifications based on these criteria. We excluded four studies that reported that no BAL samples were obtained. Two studies included both patients with influenza and patients with COVID-19; these were counted as four separate studies. Only proven, putative, or probable IAPA and CAPA were counted as cases.
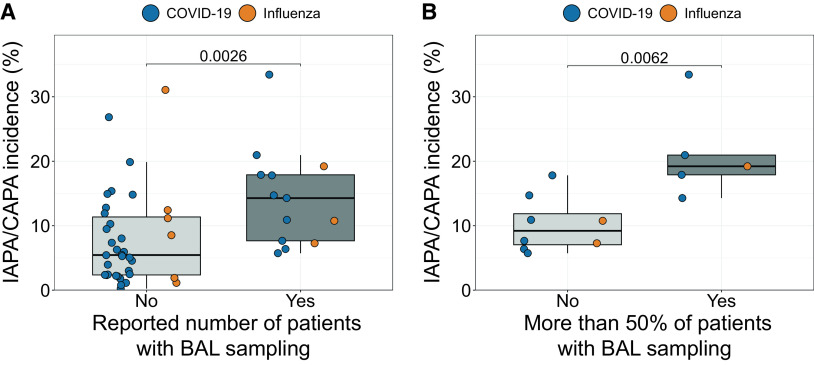
Strikingly, of the 49 included studies (with 22,381 inclusions and 1,592 IAPA or CAPA cases), only 27% (13 studies with 3,299 inclusions and 457 cases) reported the proportion of included patients who had BAL sampling performed during their ICU stay. IAPA and CAPA incidences were significantly higher in these studies than in studies that did not report this proportion (median incidence, 14% vs. 5%; P = 0.0026) (Figure 1A). Likewise, in studies reporting this proportion, those with high rates of sampled patients (arbitrarily defined as >50%) reported higher IAPA or CAPA incidences compared with studies with low rates (median incidence, 19% vs. 9%; P = 0.0062) (Figure 1B).
Figure 1.
(A) Boxplot showing differences in influenza-associated pulmonary aspergillosis (IAPA) or coronavirus disease (COVID-19)-associated pulmonary aspergillosis (CAPA) incidence between observational studies that did not (n = 36) versus those that did (n = 13) report the number of all included patients who received BAL sampling. Blue and orange dots represent studies investigating patients with COVID-19 and influenza, respectively. P value was calculated with Mann-Whitney U test. (B) Boxplot showing the differences in IAPA or CAPA incidence between studies with <50% versus studies with >50% of all included patients having received a BAL sampling. Only observational studies that unequivocally reported the number of all included patients in whom at least one BAL sampling was performed were included in this analysis. Blue and orange dots represent studies investigating patients with COVID-19 and influenza, respectively. P value was calculated with Mann-Whitney U test.
Our results demonstrate that mentioning the rates of BAL sampling is essential for reporting IAPA and CAPA incidences, and discussions should interpret the reported incidences in this context. Our results imply that IAPA and CAPA are often underdiagnosed in studies and that researchers report the wrong denominator by assuming that nonsampled patients are IAPA or CAPA negative. In extension, IAPA and CAPA are probably underdiagnosed in daily clinical practice as well. Indeed, our results show that the sampling itself is probably underperformed, contributing negatively to the detected incidences.
We advise having a low threshold to perform BAL sampling for culture and galactomannan testing. Importantly, instillation of a low volume of 2 × 20 ml saline bronchoscopically suffices for microbiological diagnostics and is usually safe (7). Ideally, every patient with influenza should receive immediate BAL sampling when admitted to an ICU, given that IAPA typically is already present upon admission, and given that the admission profiles of patients with influenza with or without IAPA are similar (3, 8). In patients with COVID-19 (and patients with influenza without IAPA upon admission), nonresolution or deterioration of the clinical picture should prompt BAL sampling, given that CAPA typically occurs later during ICU stay (1).
Our advice might seem to carry risk for overdiagnosing IAPA or CAPA instead of the present culture of underdiagnosis. Indeed, the current criteria for probable aspergillosis cannot always distinguish colonization from invasive disease, as both BAL culture- and non–culture-based tests are not 100% specific for invasive aspergillosis. Therefore, sampling more harbors the risk of treating more false-positive results (9). However, we have three good reasons to favor a more aggressive, bronchoscopy-based diagnostic strategy. First, a recently published, thorough case series of biopsied and autopsied patients with influenza or COVID-19 showed that a substantial number of IAPA and CAPA cases treated with systemic antifungals can be proven upon autopsy, making the debate on colonization or invasive disease less relevant (10, 11). Second, there is no good reason to justify the current mismatch between (extremely) low rates of BAL sampling and the substantial mortality rate of patients with severe influenza or COVID-19. Indeed, the negative impact of a missed and therefore untreated diagnosis is probably higher than that of a treated colonization that may or may not have developed in an airway-invasive infection in the absence of treatment. Third, BAL sampling should be preferred over nonbronchoscopic samples, as bronchoscopy allows us to detect invasive Aspergillus tracheobronchitis (4, 5).
In conclusion, we urge clinicians to actively look for aspergillosis using BAL sampling whenever feasible in patients with severe influenza or COVID-19, and we urge researchers who publish observational studies on IAPA or CAPA to include details on the rates of BAL sampling and testing and interpret their results in light of those diagnostic strategies.
Acknowledgments
Acknowledgment
The authors thank Hanne Moon Lauwers and Cato Jacobs from University Hospitals Leuven, Belgium for assisting in the data collection for this Viewpoint.
Footnotes
Supported by Fonds Wetenschappelijk Onderzoek grants 11M6922N and 11M6924N (S.F.).
Author Contributions: Conceptualization: S.F. and J.W.; interpretation: S.F., M.H., J.-P.G., P.E.V., and J.W.; writing – original draft: S.F.; writing – review & editing: S.F., M.H., J.-P.G., P.E.V., and J.W. All authors read and approved the final manuscript version.
Originally Published in Press as DOI: 10.1164/rccm.202310-1815VP on November 16, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7:1127–1140. doi: 10.1038/s41564-022-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. AspICU Study Investigators A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med . 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 3. Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Dutch-Belgian Mycosis study group Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med . 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 4. Verweij P, Rijnders B, Brüggemann R, Azoulay E, Bassetti M, Blot S, et al. International expert review of influenza-associated pulmonary aspergillosis in ICU patients and recommendations for a case definition. Intensive Care Med . 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verweij PE, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, Buil JB, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med . 2021;47:819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. European Confederation of Medical Mycology International Society for Human Animal Mycology; Asia Fungal Working Group; INFOCUS LATAM/ISHAM Working Group; ISHAM Pan Africa Mycology Working Group; European Society for Clinical Microbiology; Infectious Diseases Fungal Infection Study Group; ESCMID Study Group for Infections in Critically Ill Patients; Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy; Medical Mycology Society of Nigeria; Medical Mycology Society of China Medicine Education Association; Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology; Association of Medical Microbiology; Infectious Disease Canada. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis . 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med . 2008;177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 8. Vanderbeke L, Janssen NAF, Bergmans DCJJ, Bourgeois M, Buil JB, Debaveye Y, et al. Dutch-Belgian Mycosis Study Group Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive Care Med . 2021;47:674–686. doi: 10.1007/s00134-021-06431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clancy CJ, Nguyen MH. Coronavirus disease 2019-associated pulmonary aspergillosis: reframing the debate. Open Forum Infect Dis . 2022;9:ofac081. doi: 10.1093/ofid/ofac081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanderbeke L, Jacobs C, Feys S, Reséndiz-Sharpe A, Debaveye Y, Hermans G, et al. A pathology-based case series of influenza- and COVID-19-associated pulmonary aspergillosis: the proof is in the tissue. Am J Respir Crit Care Med . 2023;208:301–311. doi: 10.1164/rccm.202208-1570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albrich WC, Lamoth F. Viral-associated pulmonary aspergillosis: have we finally overcome the debate of colonization versus infection? Am J Respir Crit Care Med . 2023;208:230–231. doi: 10.1164/rccm.202306-1022ED. [DOI] [PMC free article] [PubMed] [Google Scholar]