Highlights
-
•
Cervical cancer is one of the leading causes of cancer burden in Kenya.
-
•
Two thirds of cervical cancer cases in Kenya are diagnosed in advanced stages.
-
•
Cost of care and quality of clinical evaluation were main predictors of advanced stage at diagnosis.
-
•
Decentralization, clear diagnostic and referral algorithms and innovative financing can reduce burden of advanced cervical cancer in Kenya.
Keywords: Cervical cancer, Factors, Late diagnosis, Case control study, Kenya
Abstract
Background
Cervical cancer is the leading cause of cancer mortality among women in Kenya. Two thirds of cervical cancer cases in Kenya are diagnosed in advanced stages. We aimed to identify factors associated with late diagnosis of cervical cancer, to guide policy interventions.
Methods
An unmatched case control study (ratio 1:2) was conducted among women aged ≥ 18 years with cervical cancer at Kenyatta National and Moi Teaching and Referral Hospitals. We defined a case as patients with International Federation of Gynecology and Obstetrics (FIGO) stage ≥ 2A and controls as those with stage ≤ 1B. A structured questionnaire was used to document exposure variables. We calculated adjusted odds ratio (aOR) to identify any associations.
Results
We enrolled 192 participants (64 cases, 128 controls). Mean age 39.2 (±9.3) years, 145 (76 %) were married, 77 (40 %) had primary level education, 168 (88 %) had their first pregnancy ≤ 24 years of age, 85 (44 %) were > para 3 and 150 (78 %) used contraceptives. Late diagnosis of cervical cancer was associated with cost of travel to cancer centres > USD 6.1 (aOR 6.43 95% CI [1.30, 31.72]), age > 50 years (aOR 4.71; 95% CI [1.18, 18.80]), anxiety over cost of cancer care (aOR 5.6; 95% CI [1.05, 32.72]) and ultrasound examination during evaluation of symptoms (aOR 4.89; 95% CI [1.07–22.42]). Previous treatment for gynecological infections (aOR 0.10; 95% CI [0.02, 0.47]) was protective against late diagnosis.
Conclusion
Cost of seeking care and the quality of the diagnostic process were important factors in this study. Decentralization of care, innovative health financing solutions and clear diagnostic and referral algorithms for women presenting with gynecological symptoms could reduce late-stage diagnosis in Kenya.
1. Introduction
Cervical cancer is easily detectable and curable in its early stages (World Health Organization, 2023). Cervical cancer patients commonly present with the abnormal vaginal bleeding, abnormal vaginal discharge, or pain during sexual intercourse. However, patients may present with other signs and symptoms that are associated with advanced stage of disease (Pereira and Garey, 2020). The highest incidence of cervical cancer is in Sub-Saharan Africa (SSA), with incidence of over 20 cases per 100,000 women in majority of these countries (Zhang et al., 2021). Cervical cancer is also one of the leading causes of cancer mortality in SSA, and average five-year survival is approximately 33–35 % (Sengayi-Muchengeti et al., 2020, IARC , 2023, Kassa et al., 2023). Cervical cancer has two very effective control strategies; vaccination against the human papillomavirus (HPV), and screening followed by treatment of pre-cancerous lesions (Canfell, 2019). Population-level effectiveness of these interventions is evidenced by reduction in cervical cancer incidence in most high-income settings over the last two decades, attributed to scaling of screening and vaccination programs (World Health Organization, 2023, Wilailak et al., 2021). Unfortunately, the same has not been replicated in most low and middle-income settings (LMIC), which are characterised by low uptake of vaccination and screening, as well as late diagnosis and lack of access to treatment (Bruni et al., 2021, Lemp et al., 2020, Denny et al., 2017). To mobilize global action towards cervical cancer control, the World Health Organization (WHO) launched the Global Strategy for Elimination of Cervical Cancer as a Public Health Problem (WHO, 2020). The strategy has set targets for tracking progress by 2030, covering vaccination, screening, and treatment for both pre-cancerous lesions and invasive cancer (WHO, 2020). Implementation of this strategy is estimated to reduce cervical cancer mortality in LMIC by 34 % by 2030, and 92 % by 2070 (Canfell et al., 2020).
A recent systematic review and meta-analysis identified education level and place of residence as factors associated with late-stage presentation among cervical cancer patients (Tekalign and Teshome, 2022). However, drivers of late diagnosis may differ in various contexts. For instance, in Ethiopia, income level and level of awareness on cervical cancer screening, diagnosis and treatment were the factors identified to drive late diagnosis (Zeleke et al., 2021). Friebel-Klingner et al (2021) identified marital status and seeking care at traditional healers as important factors in Botswana (Friebel-Klingner et al., 2021). In Uganda, financial difficulties, and pre-referral diagnosis of cancer at primary care were associated with advanced stage at diagnosis (Mwaka et al., 2016). In Ghana, only previous screening history was a predictor of late-stage diagnosis (Dunyo et al., 2018). Therefore, countries need to identify unique drivers of late-stage diagnosis of cervical cancer, conduct a root-cause analysis of identified factors, and implement evidence-based strategies adapted to the local context. All the studies highlighted here were cross-sectional in nature, therefore could have been affected by temporal bias (Olsen et al., 2010). In the systematic review and metanalysis of prevalence and factors associated with late diagnosis of cervical cancer mentioned above, only two of the 25 studies that were eligible were cohort studies; all the other were cross-sectional (Tekalign and Teshome, 2022). Majority of case control studies conducted have been to identify factors associated with cervical cancer, not specifically late diagnosis. We hypothesize that there are some factors that make some women be diagnosed with cervical cancer at early stage than others and identifying them would guide public health authorities in improving early diagnosis programs. The elimination strategy recognizes the role of enhancing cervical cancer diagnosis and treatment through implementation of management guidelines, establishment of referral pathways, and expansion of pathology, surgical, radiotherapy, chemotherapy, and palliative care services (WHO, 2020). Therefore, identification of the drivers of late diagnosis would guide interventions to enable realization of the third ‘90′ of the elimination strategy 2030 goals. A case-control study provides an opportunity to identify associations of potential public health benefit quickly and cost-effectively (Susser et al., 2006, Tenny et al., 2023).
Cervical cancer is the second most frequent occurring cancer among women in Kenya and is the leading cause of cancer related deaths in women of reproductive age (Sung et al., 2021). Despite various policy interventions in the last decade, early detection remains low and five-year survival is approximately 44 % (Sengayi-Muchengeti et al., 2020, Ng’Ang’A et al.,2018, Mwenda et al., 2022). Identifying unique drivers of late diagnosis of cervical cancer late in Kenya would guide targeted solutions in a precision-public health approach (Weeramanthri et al., 2018). We set out to assess the factors associated with advanced stage at diagnosis of cervical cancer at two main national referral hospitals in Kenya.
2. Methods
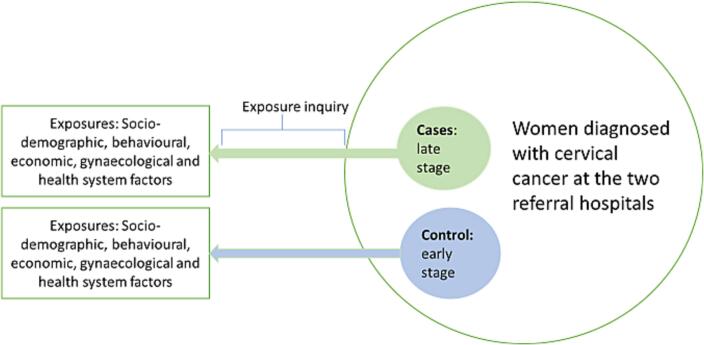
We conducted an unmatched hospital-based case control study with a ratio of 1:2 for cases and controls in May 2017 to December 2017. The study was conducted in Kenyatta National Hospital (KNH) and Moi Teaching & Referral Hospital (MTRH) which are national referral hospitals in Kenya. These facilities have designated comprehensive cancer centres that offer patient education, screening services, diagnosis, management including palliative care. These cancer centres have the diagnostic capacity and expertise within the specialized cervical cancer clinics that run daily screening, diagnosis, and treatment planning for cervical cancer. The study population was women diagnosed with cervical cancer attending and receiving care at KNH and MTRH. The study focused on women diagnosed within the previous three months to minimize recall bias. In case control studies, cases and controls are supposed to be derived from the same population (women diagnosed with cervical cancer in this instance) (Setia, 2016). To answer our study question, cases were women with late-stage cervical cancer at the two national referral hospitals, while controls were women diagnosed with early-stage cervical cancer. Fig. 1 shows the schematic representation of the study design.
Fig. 1.
Schematic representation of the study design.
2.1. Case definition
A case was defined as patient diagnosed with cervical cancer within the previous three months with International Federation of Gynecology and Obstetrics (FIGO) stage 2A and above at diagnosis attending the gynecological clinic in KNH or MTRH (Bhatla et al., 2019). A control was defined as patient diagnosed with cervical cancer within the last three months with FIGO staging 1A1, 1A2, 1B1 and 1B2 at diagnosis attending the gynecological clinic in KNH or MTRH.
2.2. Sample size determination
Based on the Fleiss method (Centre for Clinical Research, 2023), we needed a minimum sample size of 192 (64 cases and 128 controls) to detect odds of lack of formal education among cases of at least 2.5 times compared with controls, based on the assumptions of prevalence of the lack of formal education among controls of 33 % (Mwaka et al., 2016), alpha of 0.05 and power of 80 %.
2.3. Selection of cases and controls
Cases were selected based on confirmed histopathological results and the FIGO stage. Respondents who met the case definition were selected from cervical cancer patients attending weekly gynecology oncology clinics at the two hospitals. In both KNH and MTRH, the average number of cervical cancer cases seen per week was 20. To achieve a sample size of 33 cases per hospital within the study period, four cases had to be enrolled weekly. On every clinic day, we reviewed files for the patients to be seen on that particular clinic day and a list of all patients meeting the case definition was compiled. The list was used as the daily sampling frame from which cases were selected using systematic random sampling. The interval was calculated by dividing the total number of cases in the sampling frame (20) by the number to be enrolled on each clinic day, which was four. We selected every fifth patient on the list. A random starting point was selected between one and five by simple random sampling. Any case that declined to participate was replaced with the next case on the list. To achieve the desired sample size of 132 controls, all cervical cancer patients who met the control definition in both hospitals during the study period (15th May 2017 to 20th December 2017) were enrolled into the study. Recruitment of controls was conducted from different service areas that included the gynecologic oncology clinic, cancer centre, screening centres, mother–child clinics, and family planning clinics. Enrolling controls from the same facilities ensured that the both cases and controls were similar in most aspects, other than the hypothesized drivers of late-stage diagnosis.
2.4. Data collection and management
A structured questionnaire was used to collect data on the following variables: biographical information, socio-demographic information, clinical and gynecological information, patient related factors (behavioural and economic), and health care provider factors. For wealth status, we used the Principal Components Analysis (PCA) to generate a wealth index for the respondents based on a set of household characteristics and asset ownership (Measuring Equity with Nationally Representative Wealth Quintiles, 2023). The conceptual characteristics were:
a) Possession of specific household items: television, radio, computer, refrigerator, telephone (mobile or other), automatic washing machine, water heater, microwave/oven, air conditioning, central heating, video, or DVD player.
b) Household has a domestic servant, running water, running hot water, sanitation (toilet), earth floor (any room), house is rented or owned, electricity.
Based on this wealth index, we determined wealth quintiles in which each respondent fell into by dividing the indices into equal quintiles; a fifth (20 %) of the respondents. Table 1 summarizes the cut-off points for the wealth index into the quintiles. Data from questionnaires were entered, cleaned, and analyzed using Epi Info 7 (CDC, Atlanta, GA, USA). The outcome of interest was stage at diagnosis of cervical cancer among the study respondents. Descriptive statistics were calculated in form of proportions for categorical variables and mean for continuous variables. Bivariate analysis was carried out to evaluate the association between potential exposure factors and late-stage at diagnosis of cervical cancer. Odds Ratio was used as a measure of association, its 95 % confidence interval (CI) and chi-square test were used as test of significance whereby factors with p-value of ≤ 0.05 and/or CI excluding the null value were considered significant. All factors with a p-value of 0.2 and below at bivariate analysis were included into multiple logistic regression model. The model was developed by backward elimination, dropping the least significant independent variable until all the remaining exposure variables were significant (p-value ≤ 0.05).
Table 1.
Cut-off points used for grouping the wealth index into wealth quintiles.
| Wealth Quintiles |
Wealth Score |
|
|---|---|---|
| Minimum | Maximum | |
| Poor | −3.695198 | −2.10337 |
| Second poor | −2.026607 | −0.96371 |
| Middle | −0.9601527 | −0.23797 |
| Second rich | −0.1721852 | 2.452282 |
| Rich | 2.512264 | 6.211455 |
2.5. Ethical considerations
The aim and procedures of the study was explained to participants who then gave written consent prior to their voluntary participation in the study. Confidentiality was observed and maintained by ensuring identifying data was encrypted and data was stored in password protected tablets and computers. Protocol approval was obtained from the Moi University Institutional Review and Ethical Committee (IREC, Number 1844) and permission to conduct the study secured from the administration of KNH and MTRH.
3. Results
Out of 192 patients enrolled in the study, the mean age was 39.2 (9.3) years. Table 2 shows the distribution of socio-demographic variables in the study population. Fifty-eight percent of the participants (111/192) were above the age 40 years. Approximately 61.0 % (117/192) of the participants belonged to middle wealth quintile or less. Two-thirds (67.2 % [129/192]) of the women were enrolled into the National Health Insurance Fund (NHIF); however, 29.7 % (57/192) had no health insurance. Majority (75.5 %[145/192]) of the patients were married. More than half of the participants (51.6 % [99/192]) had attained at least secondary education (minimum of 12 years of formal schooling). A higher proportion of controls had tertiary education (31.3 %) compared to cases (6.3 %).
Table 2.
Socio demographic characteristics of cervical cancer patients in Kenyatta National Hospital and Moi Teaching & Referral Hospital, Kenya, 2017.
| Variable | Case (n = 64) | Control (n = 128) | Total (n = 192) |
|---|---|---|---|
| Patients age groups | |||
| 18–29 | 0 (0) | 14 (10.9) | 14 (7.3) |
| 30–39 | 12 (18.8) | 53 (41.4) | 65 (33.9) |
| 40–49 | 23 (35.9) | 47 (36.7) | 70 (36.5) |
| ≥50 | 29 (45.3) | 14 (10.9) | 43 (22.4) |
| Wealth quintiles | |||
| Poor | 2 (3.1) | 37 (28.9) | 39 (20.3) |
| Second poor | 12 (18.8) | 26 (20.3) | 38 (19.8) |
| Middle | 10 (15.6) | 30 (23.4) | 40 (20.8) |
| Second rich | 15 (23.4) | 22 (17.2) | 37 (19.3) |
| Rich | 25 (39.1) | 13 (10.2) | 38 (19.8) |
| Marital status | |||
| Never married | 5 (7.8) | 17 (13.3) | 22 (11.5) |
| Married | 45 (70.3) | 100 (78.1) | 145 (75.5) |
| Formerly married | 14 (21.9) | 11 (8.6) | 25 (13) |
| Residence by county | |||
| Uasin Gishu | 7 (10.9) | 40 (31.3) | 47 (24.5) |
| Nairobi | 6 (9.4) | 33 (25.8) | 39 (20.3) |
| Kiambu | 4 (6.3) | 9 (7) | 13 (6.8) |
| Bungoma | 6 (9.4) | 5 (3.9) | 11 (5.7) |
| Elgeyo-Marakwet | 3 (4.7) | 5 (3.9) | 8 (4.2) |
| Trans-Nzoia | 2 (3.1) | 4 (3.1) | 6 (3.1) |
| Kajiado | 0 (0) | 6 (4.7) | 6 (3.1) |
| Others | 39 (61) | 26 (20.3) | 65 (33.3) |
| Patient education level (highest level achieved) | |||
| No formal schooling | 12 (18.8) | 4 (3.1) | 16 (8.3) |
| Primary school | 30 (46.9) | 47 (36.7) | 77 (40.1) |
| Secondary school | 18 (28.1) | 37 (28.9) | 55 (28.7) |
| Tertiary | 4 (6.3) | 40 (31.3) | 44 (22.9) |
| Health insurance cover | |||
| No health insurance | 10 (15.6) | 47 (36.7) | 57 (29.7) |
| Governmental health insurance (NHIF) | 53 (82.8) | 76 (59.4) | 129 (67.2) |
| Governmental + private health insurance | 1 (1.6) | 5 (3.9) | 6 (3.1) |
Table 3 shows the prevalence of various cervical cancer risk factors among cases and controls. We found significant differences between cases and controls in the following variables: parity higher than 3 (64.1 % among cases vs 34.4 % among controls; p < 0.001), age at first intercourse of less than 16 years (35.9 % among cases vs 14.8 % among controls; p = 0.001) and history of being ever screened for cervical cancer (42.2 % among cases vs 73.5 % among controls; p < 0.001). However, there was no difference in HIV positive status (25 % among cases vs. 19.5 % among controls; p = 0.382), age at first pregnancy of less than 25 years (92.2 % among cases vs. 85.2 % among controls; p = 0.167) and history of contraceptive use (76.6 % among cases vs. 78.9 % among controls; p = 0.717).
Table 3.
Reproductive health profile of cervical cancer patients at Kenyatta National Hospital and Moi Teaching & Referral Hospital, Kenya, 2017.
| Variable | Case (n = 64) | Control (n = 128) | Total (n = 192) |
|---|---|---|---|
| Patients age group at first pregnancy | |||
| ≤24 | 59 (92.2) | 109 (85.2) | 168 (87.5) |
| 25–29 | 2 (3.1) | 17 (13.3) | 19 (9.9) |
| 30–45 | 3 (4.7) | 2 (1.6) | 5 (2.6) |
| Parity with full term pregnancies | |||
| para 0 | 0 (0) | 1 (0.8) | 1 (0.5) |
| para 1 | 2 (3.1) | 8 (6.3) | 10 (5.2) |
| para 2 | 8 (12.5) | 35 (27.3) | 43 (22.4) |
| para 3 | 13 (20.3) | 40 (31.3) | 53 (27.6) |
| > para 3 | 41 (64.1) | 44 (34.4) | 85 (44.3) |
| Use of contraceptives | |||
| Yes | 49 (76.6) | 101 (78.9) | 150 (78.1) |
| No | 15 (23.4) | 27 (21.1) | 42 (21.9) |
| HIV status | |||
| Positive | 16 (25.0) | 25 (19.5) | 41 (21.4) |
| Negative | 44 (68.8) | 100 (78.1) | 144 (75) |
| Unknown | 4 (6.3) | 3 (2.3) | 7 (3.7) |
| Age at first sexual intercourse | |||
| < 16 years | 23 (35.9) | 19 (14.8) | 42 (21.9) |
| 17–20 years | 34 (53.1) | 86 (67.2) | 120 (62.5) |
| > 21 years | 7 (10.9) | 23 (18) | 30 (15.6) |
| History of being screened | |||
| VIA/VILLI | 8 (12.5) | 101 (78.9) | 109 (56.8) |
| VIA/VILLI and Pap Smear | 19 (29.7) | 10 (7.8) | 29 (15.1) |
| No | 37 (57.8) | 14 (10.9) | 51 (26.6) |
| Pap Smear | 0 (0) | 3 (2.3) | 3 (1.6) |
At bivariate level, late diagnosis of cervical cancer was associated with financial challenges to accessing care (cOR 13.6 CI = 2.9, 63.3), not having digital vaginal examination during the first contact with a health care provider (cOR 11.2 CI = 3.6, 34.5), not having been previously screened for cervical cancer (cOR 11.2 CI = 4.7, 26.4),) having received no medications/referral after diagnosis of other gynecological infections (cOR 9.0 CI = 4.0, 20.1), having no formal education (cOR 7.2 CI = 2.1, 24.3), age > 50 years (cOR 6.7 CI = 3.0, 15.1) and abnormal vaginal bleeding as first presenting symptom (cOR 4.4 CI = 2.2, 8.8). Conversely, seeking professional advice within three months after noticing the first symptom (cOR 0.3 CI = 0.1, 0.5), and having tertiary education (cOR 0.1 CI = 0.0, 0.5) were protective against cervical cancer diagnosis in advanced stage (Table 4).
Table 4.
Bivariate analysis of factors associated with late diagnosis of cervical cancer patients in Kenyatta National Hospital and Moi Teaching & Referral Hospital Kenya, 2017.
| Exposure | Case (col %) | Control (col %) | MH Odds Ratio |
95 % CI |
p-value | |
|---|---|---|---|---|---|---|
| lower limit | upper limit | |||||
| Catastrophic health expenditure on planned treatment | ||||||
| Yes | 62 (96.9) | 89 (69.5) | 13.6 | 2.9 | 63.3 | <0.00 |
| No | 2 (3.1) | 39 (30.5) | Ref | |||
| Cost to travel to the treatment center above average (USD 6) | ||||||
| Yes | 38 (59.4) | 20 (15.6) | 7.9 | 4.0 | 15.7 | <0.00 |
| No | 26 (40.6) | 108 (84.4) | Ref | |||
| Vaginal Exam | ||||||
| Yes | 20 (31.3) | 5 (3.9) | 11.2 | 3.6 | 34.5 | <0.00 |
| No | 44 (68.8) | 123 (96.1) | Ref | |||
| Previously screened for cervical cancer | ||||||
| Yes | 37 (57.8) | 14 (10.9) | 11.2 | 4.7 | 26.4 | <0.00 |
| No | 27 (42.2) | 114 (89.1) | Ref | |||
| Medications for previous gynecological infections | ||||||
| Yes | 51 (79.7) | 39 (30.5) | 9 | 4 | 20.1 | <0.00 |
| No | 13 (20.3) | 89 (69.5) | Ref | |||
| Formal schooling | ||||||
| Yes | 12 (18.8) | 4 (3.1) | 7.2 | 2.1 | 24.3 | <0.00 |
| No | 52 (81.3) | 124 (96.9) | Ref | |||
| Patients age groups | ||||||
| <40 | 12 (18.8) | 67 (52.3) | Ref | |||
| 40–50 | 23 (35.9) | 47 (36.7) | 2.7 | 1.2 | 6 | 0.01 |
| >50 | 29 (45.3) | 14 (10.9) | 11.6 | 4.8 | 28 | <0.00 |
| Patients Age > 50 | ||||||
| Yes | 29 (45.3) | 14 (10.9) | 6.7 | 3 | 15.1 | <0.00 |
| No | 35 (54.7) | 114 (89.1) | Ref | |||
| Wealth quintiles | ||||||
| Poor | 2 (3.1) | 37 (28.9) | Ref | |||
| Second poor | 12 (18.8) | 26 (20.3) | 8.5 | 1.8 | 41.4 | 0.01 |
| Middle | 10 (15.6) | 30 (23.4) | 6.2 | 1.3 | 30.3 | 0.03 |
| Second rich | 15 (23.4) | 22 (17.2) | 12.6 | 2.6 | 60.4 | <0.00 |
| Rich | 25 (39.1) | 13 (10.2) | 35.6 | 7.4 | 171.5 | <0.00 |
| Wealth Quintile – Rich | ||||||
| Yes | 25 (39.1) | 13 (10.2) | 5.7 | 2.5 | 12.8 | <0.00 |
| No | 39 (60.9) | 115 (89.8) | Ref | |||
| This illness caused personal/ family financial difficulties | ||||||
| Yes | 56 (87.5) | 71 (55.5) | 5.6 | 2.4 | 13.4 | <0.00 |
| No | 8 (12.5) | 57 (44.5) | Ref | |||
| No prior knowledge on cervical cancer | ||||||
| Yes | 28 (43.8) | 19 (14.8) | 4.5 | 2.1 | 9.3 | <0.00 |
| No | 36 (56.3) | 109 (85.2) | Ref | |||
| First presenting symptom was abnormal vaginal bleeding | ||||||
| Yes | 33 (51.6) | 25 (19.5) | 4.4 | 2.2 | 8.8 | <0.00 |
| No | 31 (48.4) | 103 (80.5) | Ref | |||
| Visit to a traditional healer at least 3 times | ||||||
| Yes | 6 (9.4) | 3 (2.3) | 4.3 | 1 | 18.2 | 0.03 |
| No | 58 (90.6) | 125 (97.7) | Ref | |||
| Self-reported feeling that the treatment is futile or useless | ||||||
| Yes | 6 (9.4) | 3 (2.3) | 4.3 | 1 | 18.2 | 0.03 |
| No | 58 (90.6) | 125 (97.7) | Ref | |||
| Patient experienced long waiting times for investigations and/or treatment | ||||||
| Yes | 27 (42.2) | 19 (14.8) | 4.2 | 2 | 8.7 | <0.00 |
| No | 37 (57.8) | 109 (85.2) | Ref | |||
| Patient was apprehensive/ anxious about the cost of investigations and treatment | ||||||
| Yes | 37 (57.8) | 32 (25.0) | 4.1 | 2.1 | 8.1 | <0.00 |
| No | 27 (42.2) | 96 (75.0) | Ref | |||
| Patient is illiterate | ||||||
| Yes | 14 (21.9) | 9 (7.0) | 3.7 | 1.5 | 9.3 | <0.00 |
| No | 50 (78.1) | 119 (93.0) | Ref | |||
| Apprehension about possible outcome of test result contributes in not getting tested early | ||||||
| Yes | 14 (21.9) | 9 (7.0) | 3.7 | 1.5 | 9.3 | <0.00 |
| No | 50 (78.1) | 119 (93.0) | Ref | |||
| Patient waited for longer than 3 months before seeking medical advice | ||||||
| Yes | 46 (71.9) | 53 (41.4) | 3.6 | 1.8 | 7.1 | <0.00 |
| No | 18 (28.1) | 75 (58.6) | Ref | |||
| There was minimal support from the patient’s friends/family | ||||||
| Yes | 26 (40.6) | 21 (16.4) | 3.5 | 1.7 | 7.1 | <0.00 |
| No | 38 (59.4) | 107 (83.6) | Ref | |||
| Patient’s choice of facility of initial consultation was a private health facility | ||||||
| Yes | 12 (18.8) | 8 (6.3) | 3.5 | 1.3 | 9.2 | 0.01 |
| No | 52 (81.3) | 120 (93.8) | Ref | |||
| Parity > 3 | ||||||
| Yes | 41 (64.1) | 44 (34.4) | 3.4 | 1.8 | 6.6 | <0.00 |
| No | 23 (35.9) | 84 (65.6) | Ref | |||
| Patient has Governmental health insurance (NHIF) | ||||||
| Yes | 53 (82.8) | 76 (59.4) | 3.3 | 1.5 | 7.1 | <0.00 |
| No | 11 (17.2) | 52 (40.6) | Ref | |||
| Perception of ‘am not at risk’ contribute in not getting tested | ||||||
| Yes | 54 (84.4) | 120 (93.8) | 0.4 | 0.1 | 1 | 0.04 |
| No | 10 (15.6) | 8 (6.3) | Ref | |||
| I readily go to the doctor when am sick or not feeling well | ||||||
| Yes | 42 (65.6) | 111 (86.7) | 0.3 | 0.1 | 0.6 | <0.00 |
| No | 22 (34.4) | 17 (13.3) | Ref | |||
| Patient sought professional advice within less than 3 month after first symptoms | ||||||
| Yes | 18 (28.1) | 75 (58.6) | 0.3 | 0.1 | 0.5 | <0.00 |
| No | 46 (71.9) | 53 (41.4) | Ref | |||
| Do you know what cervical cancer is? | ||||||
| Yes | 36 (56.3) | 109 (85.2) | 0.2 | 0.1 | 0.5 | <0.00 |
| No | 28 (43.8) | 19 (14.8) | Ref | |||
| Patient had > 16 years of formal education | ||||||
| Yes | 4 (6.3) | 40 (31.3) | 0.1 | 0 | 0.5 | <0.00 |
| No | 60 (93.8) | 88 (68.8) | Ref | |||
After controlling for confounding, we found that the odds of cost of travel to referral hospitals of above USD 6.1 was six times as high among cases compared with controls (aOR 6.43 CI = 1.30, 31.72). The odds of reporting anxiety or apprehension over cost of diagnostic evaluation and treatment was six times as high among cases compared with controls (aOR 5.86 CI = 1.05, 32.72). We also found that the odds of having undergone an ultrasound examination during evaluation of presenting gynaecological symptoms was five times as high among cases compared with controls (aOR 4.89 CI = 1.07, 22.24). Lastly, the odds of being above the age of 50 years were five times as high among cases compared with controls (aOR 4.71 CI = 1.18, 18.80). Conversely, the odds of previous treatment or referral for gynecological infections were 90 % lower among cases compared with controls (aOR 0.10 CI = 0.02, 0.42) (Table 5).
Table 5.
Multivariate analysis of factors associated with late diagnosis of cervical cancer patients in Kenyatta National Hospital and Moi Teaching & Referral Hospital Kenya, 2017.
| Exposure | Odds Ratio | P-value | 95 % CI |
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Cost to travel to the cancer treatment center | ||||
| Less of equal to average fare (USD 6.1) | 1.00 | Ref | ||
| Above average fare (USD 6.1) | 6.43 | 0.022 | 1.30 | 31.72 |
| Apprehension/anxiety over cost of cervical cancer diagnosis and/or treatment | ||||
| No | 1.00 | Ref | ||
| Yes | 5.86 | 0.04 | 1.05 | 32.72 |
| Obtain ultrasound scans during a past clinical evaluation? | ||||
| No | 1.00 | Ref | ||
| Yes | 4.89 | 0.04 | 1.07 | 22.42 |
| Age > 50 years | ||||
| No | 1.00 | Ref | ||
| Yes | 4.71 | 0.03 | 1.18 | 18.80 |
| Gynecological consultation/treatment for gynaecological infections in the past? | ||||
| No | 1.00 | Ref | ||
| Yes | 0.10 | 0.004 | 0.02 | 0.47 |
4. Discussion
This study sought to identify any modifiable drivers of late-stage diagnosis of cervical cancer in Kenya, which could be potentially amenable to public health and policy interventions. We found that cost of seeking care, patient anxiety about the cost of the clinical evaluation process, diagnostic approach after presenting with general gynecological symptoms and age above 50 years were significantly associated with late-stage diagnosis. History of receiving treatment or referral for other gynecological conditions was protective against diagnosis with advanced cervical cancer.
Majority of studies that have investigated predictors of late-stage cervical cancer diagnosis have been cross-sectional. A few cohort studies have examined possibly-related factors, though none was primarily focusing on stage at diagnosis as the main outcome. Osok and colleagues conducted a retrospective cohort study to assess factors associated with cervical cancer survival; they noted treatment type and completion status as main determinants of survival (Osok et al., 2018). Another retrospective cohort focusing on time to death found age above 50 years, late-stage at diagnosis, receiving no treatment and living with HIV as key factors (Seifu et al., 2022). The only prospective cohort was conducted among a HIV-positive and negative population with cervical cancer and found that patients who received cancer-directed therapies had a lower two-year mortality (Glasmeyer et al., 2022). Majority of the other studies discussed below are cross-sectional in nature.
Concerns and anxiety over the cost of diagnosis and treatment for cervical cancer could dissuade women with early symptoms from seeking care, hence finally being diagnosed already in advanced stages. Mwaka and colleagues found financial difficulties and precautious care-seeking attitudes to be key determinants of stage at diagnosis in a cross-sectional study conducted in Northern Uganda (Mwaka et al., 2016). A mixed methods cross-sectional study in Cote d’Ivoire also identified lacking health insurance as a barrier to early diagnosis of cervical cancer (Plaisy et al., 2023).
In our study, the nature of the patient-health system interaction prior to their diagnosis was also a significant factor. One reason may be the cost of transport to access diagnosis and management at tertiary level health facilities. Cancer diagnosis and treatment is a very specialized service, mostly situated within large urban centers. Women of low socio-economic status may not execute referrals promptly even when referred from primary care early enough. The Ugandan study found that being referred from primary care was associated with late-stage diagnosis, implying that either women present at primary care in already advanced stage, or more likely, delay after referral to tertiary facilities (Mwaka et al., 2016). A systematic review by Allahqoli and colleagues identified service availability and accessibility as important determinants of delayed diagnosis (Allahqoli et al., 2022).
We also found having had an ultra-sound examination as part of evaluation of initial presenting symptoms was associated with late diagnosis. We postulate that patients presenting with atypical pelvic symptoms or even chronic vaginal discharge may be presumed to have other diagnoses like pelvic inflammatory disease. Any time lost in investigating alternative diagnoses could then be a contributor to late diagnosis. In addition, an ultrasound examination reported as normal may give the woman a false reassurance, even when they have persistent symptoms. A study by Nebiyu and colleagues found that having first contact in primary care facilities and being seen at four or more facilities prior to diagnosis as determinants of being diagnosed in advanced stages (Dereje et al., 2020). These multiple health facility visits are likely due to a low index of suspicion when presenting with non-specific symptoms. The study by Nebiyu et al also reported waiting for additional symptoms before seeking care as a factor; this may be relevant for women who are reassured after evaluation for gynaecological complaints, without being offered a cervical cancer early detection service. Seeking alternative medicine before presenting at health facilities was also identified as an important factor in late diagnosis of cervical cancer in a cross-sectional study in north-western Tanzania (Mlange et al., 2016).
We also found that previous gynecological consultation or referral was associated with early diagnosis of cervical cancer. An early referral for gynaecological consultation, even when the referring clinician is not considering cervical cancer as part of their differential diagnosis, could increase the likelihood of the women being diagnosed promptly. A study among immigrants who had settled in Canada found that having had a gynecological consultation within the previous three years was associated with early diagnosis of cervical cancer (Voruganti et al., 2016). Age above 50 years was also associated with late-stage diagnosis in our study. This may be explained by the fact that common early symptoms for cervical cancer like vaginal bleeding in the pre-menopausal period may be construed as normal biological processes. By the time the women seeks care or undergoes evaluation after menopause, the disease is already at an advanced stage. This could then lead to shorter time to death for women above 50 years identified by Benyam and colleagues, as well as Roza et al, in their survival analysis (Kassa et al., 2023, Seifu et al., 2022).
Other factors that we failed to show associations in our study have been reported by other studies. In a study in Iran, Behnamfar and Azadehrah reported low education, low socio-economic status and having no history of screening as being predictors of late-stage diagnosis (Behnamfar and Azadehrah, 2015). Dunyo et al in Ghana and Plaisy in Cote d’Ivoire also identified previous screening as an important determinant of stage at diagnosis (Plaisy et al., 2023, Dunyo et al., 2018). Although previous screening was not significantly associated with early diagnosis in our study, we found higher prevalence of being ever-screened in the past among controls compared with cases. Studies in Kenya among women living with HIV where screening is more stablished have a found a lower uptake compared with our study (Kemper et al., 2022, Kangethe et al., 2022). The higher prevalence of having been ever-screened in comparison with previous studies could be attributed to screening service points being an entry point for majority of the controls in our study. Having comorbidities has also been reported in some studies (Kassa et al., 2023, Park et al., 2017). Living with HIV has been reported to have different effects on cervical cancer diagnosis. While Benyam et al as well as Roza et al found HIV infection to be associated with poor cervical cancer survival (Kassa et al., 2023, Seifu et al., 2022), Plaisy et al found that being HIV uninfected was a barrier to early diagnosis of cervical cancer (Plaisy et al., 2023). Both of these findings may be plausible; HIV is known to contribute to faster progression of cervical cancer, hence the association with poor outcomes (Stelzle et al., 2021). However, on the contrary, cervical cancer screening programs, especially in sub-Saharan Africa are more stablished among women living with HIV, increasing their chances of women living with HIV being detected early (Tapera et al., 2021, Wanyenze et al., 2017, Perez-Guzman et al., 2020, Nyambe and Lubeya, 2021).
Our findings are subject to several limitations. First the respondents may not have been representative of the general Kenyan population because it was conducted in hospitals. This hospital-based study involved a selected population of women who had reached the hospital. The characteristics of women who may have cervical cancer but who did come to the study hospital remain unknown. Despite this limitation, our study also has particular strengths. First, we adopted a case-control design, which enabled us to have more robust associations. As noted earlier, majority of the studies that have examined this topic have been cross-sectional, with limited potential inferentially. Therefore, the factors identified in this study have the potential to reduce late-stage diagnosis of cervical cancer if they are translated to policy and practice interventions. Second, we recruited our study population from the two largest national referral hospital that were offering cancer treatment then. This reduced the possibility of identifying factors only unique to one hospital. The two study sites are located more than 300 km apart; therefore, their catchment populations may not have been similar and therefore we were able to capture cases with a varied socio-demographic profile.
5. Conclusion
Cost of seeking diagnostic evaluation and/or treatment, age as well as approach adopted during initial evaluation of presenting symptoms contribute to late diagnosis of cervical cancer in Kenya. We recommended targeted awareness creation on early warning symptoms for cervical cancer to improve health seeking behavior especially in women of reproductive age. Further, innovative health financing, decentralization of cancer treatment services and clearly defined diagnostic and referral algorithms can aid in early diagnosis of cervical cancer. Further studies should evaluate efficacy of various individual, health system and policy interventions in reducing late diagnosis and improving outcomes.
CRediT authorship contribution statement
Valerian Mwenda: Writing – original draft, Writing – review & editing. Martin Mwangi: Conceptualization, Investigation, Data curation, Writing – review & editing. Gladwell Gathecha: Supervision, Project administration, Writing – review & editing. Joseph Kibachio: Supervision, Project administration, Writing – review & editing. Robert Too: Validation, Methodology, Writing – review & editing. Zeinab Gura: Supervision, Project administration, Writing – review & editing. Marleen Temmerman: Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the administration of MTRH, KNH, and the multi-disciplinary oncology teams at the two hospitals for their support during the study. We would also like to thank the data collection and analysis team. Lastly, we thank the Kenya Ministry of Health and the US Centers for Disease Control and Prevention for financial support for the study.
References
- Allahqoli L., Dehdari T., Rahmani A., Fallahi A., Gharacheh M., Hajinasab N., Salehiniya H., Alkatout I. Delayed cervical cancer diagnosis: a systematic review. Eur Rev Med Pharmacol Sci. 2022;26:8467–8480. doi: 10.26355/eurrev_202211_30382. [DOI] [PubMed] [Google Scholar]
- Behnamfar F., Azadehrah M. Factors associated with delayed diagnosis of cervical cancer in Iran–a survey in Isfahan City. Asian Pac J Cancer Prev. 2015;16:635–639. doi: 10.7314/apjcp.2015.16.2.635. [DOI] [PubMed] [Google Scholar]
- Bhatla N., Berek J.S., Cuello Fredes M., et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- Bruni L., Saura-Lázaro A., Montoliu A., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med (baltim) 2021 doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- Canfell K. Towards the global elimination of cervical cancer. Papillomavirus Research. 2019 doi: 10.1016/j.pvr.2019.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfell K., Kim J.J., Brisson M., et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Clinical Research and Biostatistics. Unmatched Case-Control. Available at https://www2.ccrb.cuhk.edu.hk/stat/epistudies/cc2.htm. Accessed 6 Aug 2023.
- Denny L., de Sanjose S., Mutebi M., Anderson B.O., Kim J., Jeronimo J., Herrero R., Yeates K., Ginsburg O., Sankaranarayanan R. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet. 2017;389:861–870. doi: 10.1016/S0140-6736(16)31795-0. [DOI] [PubMed] [Google Scholar]
- Dereje N., Addissie A., Worku A., Assefa M., Abraha A., Tigeneh W., Kantelhardt E.J., Jemal A. Extent and predictors of delays in diagnosis of cervical cancer in Addis Ababa, Ethiopia: a population-based prospective study. JCO Glob Oncol. 2020;6:277–284. doi: 10.1200/JGO.19.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunyo P., Effah K., Udofia E.A. Factors associated with late presentation of cervical cancer cases at a district hospital: a retrospective study. BMC Public Health. 2018;18:1–10. doi: 10.1186/s12889-018-6065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel-Klingner T.M., Luckett R., Bazzett-Matabele L., et al. Clinical and sociodemographic factors associated with late-stage cervical cancer diagnosis in Botswana. BMC Womens Health. 2021;21:1–9. doi: 10.1186/s12905-021-01402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasmeyer L., Mcharo R.D., Torres L., et al. Long-term follow-up on HIV infected and non-infected women with cervical cancer from Tanzania: staging, access to cancer-directed therapies and associated survival in a real-life remote setting. BMC Cancer. 2022 doi: 10.1186/S12885-022-09966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Cancer Today. https://gco.iarc.fr/today/home. Accessed 2 May 2023.
- Kangethe J.M., Monroe-Wis A., Muiruri P.N., Komu J.G., Mutai K.K., Nzivo M.M., Pintye J. Utilisation of cervical cancer screening among women living with HIV at Kenya’s national referral hospital. South Afr J HIV Med. 2022 doi: 10.4102/SAJHIVMED.V23I1.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa R., Irene Y., Woldetsadik E., Kidane E., Higgins M., Dejene T., Wells J. Survival of women with cervical cancer in East Africa: a systematic review and meta-analysis. J Obstet Gynaecol. 2023;43 doi: 10.1080/01443615.2023.2253308. [DOI] [PubMed] [Google Scholar]
- Kemper K.E., McGrath C.J., Eckert L.O., Kinuthia J., Singa B., Langat A., Drake A.L. Correlates of cervical cancer screening among women living with HIV in Kenya: a cross-sectional study. Int. J. Gynecol. Obstet. 2022;156:151–158. doi: 10.1002/ijgo.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp J.M., De Neve J.W., Bussmann H., et al. Lifetime prevalence of cervical cancer screening in 55 low-and middle-income countries. JAMA - Journal of the American Medical Association. 2020;324:1532–1542. doi: 10.1001/jama.2020.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measuring Equity with Nationally Representative Wealth Quintiles. Available at https://www.researchgate.net/publication/267327645_Measuring_Equity_with_Nationally_Representative_Wealth_Quintiles. Accessed 30 Oct 2023.
- Mlange R., Matovelo D., Rambau P., Kidenya B. Patient and disease characteristics associated with late tumour stage at presentation of cervical cancer in northwestern Tanzania. BMC Womens Health. 2016 doi: 10.1186/S12905-016-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaka A.D., Garimoi C.O., Were E.M., Roland M., Wabinga H., Lyratzopoulos G. Social, demographic, and healthcare factors associated with stage at diagnosis of cervical cancer: cross-sectional study in a tertiary hospital in northern Uganda. BMJ Open. 2016;6:e007690. doi: 10.1136/bmjopen-2015-007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenda V., Mburu W., Bor J.P., Nyangasi M., Arbyn M., Weyers S., Tummers P., Temmerman M. Cervical cancer programme, Kenya, 2011–2020: lessons to guide elimination as a public health problem. Ecancermedicalscience. 2022 doi: 10.3332/ECANCER.2022.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng’Ang’A A., Nyangasi M., Nkonge NG., Gathitu E., Kibachio J., Gichangi P., Wamai RG., Kyobutungi C. Predictors of cervical cancer screening among kenyan women: results of a nested case-control study in a nationally representative survey. BMC Public Health. 2018;18:1–10. doi: 10.1186/s12889-018-6054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambe A., Lubeya M.K. Cervical cancer and HIV in zambian women. Lancet Glob Health. 2021;9:e734–e735. doi: 10.1016/S2214-109X(21)00230-8. [DOI] [PubMed] [Google Scholar]
- Olsen J, Christensen K, Murray J, Ekbom A (2010) The Cross-Sectional Study. 79–79.
- Osok D., Karanja S., Kombe Y., Njuguna E., Todd J. Assessing factors associated with survival among Cervical cancer patients in Kenya: a retrospective follow-up study. East Afr Health Res J. 2018;2:118–127. doi: 10.24248/EAHRJ-D-18-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.R., Kim S.Y., Shin D.W., Yang H.K., Park J.H. Influence of socioeconomic status, comorbidity, and disability on late-stage cancer diagnosis. Osong Public Health Res Perspect. 2017;8:264–270. doi: 10.24171/j.phrp.2017.8.4.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira D, Garey SL (2020) Cancer, Cervical. In: Encyclopedia of Behavioral Medicine. StatPearls Publishing, pp 350–351.
- Perez-Guzman P.N., Chung M.H., De Vuyst H., Dalal S., Mutai K.K., Muthoni K., Kigen B., Kilonzo N., Hallett T.B., Smit M. The impact of scaling up cervical cancer screening and treatment services among women living with HIV in Kenya: a modelling study. BMJ Glob Health. 2020;5:1886. doi: 10.1136/bmjgh-2019-001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisy MK., Boni SP., Coffie PA., Tanon A., Innocent A., Horo A., Dabis F., Bekelynck A., Jaquet A. Barriers to early diagnosis of cervical cancer: a mixed-method study in Côte d’Ivoire, West Africa. BMC Womens Health. 2023 doi: 10.1186/S12905-023-02264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifu B., Fikru C., Yilma D., Tessema F. Predictors of time to death among cervical cancer patients at tikur anbesa specialized hospital from 2014 to 2019: a survival analysis. PLoS One. 2022 doi: 10.1371/JOURNAL.PONE.0264369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengayi-Muchengeti M., Joko-Fru W.Y., Miranda-Filho A., et al. Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study. Int J Cancer. 2020;147:3037–3048. doi: 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- Setia M. Methodology series module 2: case-control studies. Indian J Dermatol. 2016;61:146. doi: 10.4103/0019-5154.177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzle D., Tanaka L.F., Lee K.K., et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9:e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Susser E., Schwartz S., Morabia A., Bromet E.J. Applications of the case-control study. psychiatric. Epidemiology. 2006:192–202. [Google Scholar]
- Tapera O., Nyakabau A.M., Simango N., Guzha B.T., Jombo-Nyakuwa S., Takawira E., Mapanga A., Makosa D., Madzima B. Gaps and opportunities for cervical cancer prevention, diagnosis, treatment, and care: evidence from midterm review of the Zimbabwe cervical cancer prevention and control strategy (2016–2020) BMC Public Health. 2021 doi: 10.1186/s12889-021-11532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekalign T., Teshome M. Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0267571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenny S., Kerndt C.C., Hoffman M.R. Case control studies. Encyclopedia of Pharmacy Practice and Clinical Pharmacy. 2023:356–366. [Google Scholar]
- Voruganti T., Moineddin R., Jembere N., Elit L., Grunfeld E., Lofters A.K. Comparing stage of diagnosis of cervical cancer at presentation in immigrant women and long-term residents of Ontario: a retrospective cohort study. CMAJ Open. 2016;4:E424–E430. doi: 10.9778/cmajo.20160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanyenze R.K., Bwanika J.B., Beyeza-Kashesya J., Mugerwa S., Arinaitwe J., Matovu J.K.B., Gwokyalya V., Kasozi D., Bukenya J., Makumbi F. Uptake and correlates of cervical cancer screening among HIV-infected women attending HIV care in Uganda. Glob Health Action. 2017 doi: 10.1080/16549716.2017.1380361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeramanthri T.S., Dawkins H.J.S., Baynam G., Bellgard M., Gudes O., Semmens J.B. Editorial: Precision public health. Front Public Health. 2018;6 doi: 10.3389/fpubh.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. A Global Strategy for elimination of cervical cancer. Available at https://www.who.int/activities/a-global-strategy-for-elimination-of-cervical-cancer. Accessed 1 Mar 2020.
- Wilailak S., Kengsakul M., Kehoe S. Worldwide initiatives to eliminate cervical cancer. Int. J. Gynecol. Obstet. 2021;155:102–106. doi: 10.1002/ijgo.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Cervical cancer. Available at https://www.who.int/news-room/fact-sheets/detail/cervical-cancer. Accessed 6 Aug 2023.
- Zeleke S., Anley M., Kefale D., Wassihun B. Factors associated with delayed diagnosis of cervical cancer in tikur anbesa specialized hospital, Ethiopia, 2019: cross-sectional study. Cancer Manag Res. 2021;13:579–585. doi: 10.2147/CMAR.S285621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zeng Q., Cai W., Ruan W. Trends of cervical cancer at global, regional, and national level: data from the global burden of disease study 2019. BMC Public Health. 2021;21:1–10. doi: 10.1186/s12889-021-10907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]