Abstract
Background
Abnormal cystic fibrosis transmembrane conductance regulator (CFTR) function in cystic fibrosis (CF) has been linked to airway smooth muscle abnormalities including bronchial hyperresponsiveness. However, a role for CFTR in other types of smooth muscle, including myometrium, remains largely unexplored. As CF life expectancy and the number of pregnancies increases, there is a need for an understanding of the potential role of CFTR in myometrial function.
Methods
We investigated the role of CFTR in human and mouse myometrium. We used immunofluorescence to identify CFTR expression, and carried out contractility studies on spontaneously contracting term pregnant and non-pregnant mouse myometrium and term pregnant human myometrial biopsies from caesarean sections.
Results
CFTR was found to be expressed in term pregnant mouse myometrium. Inhibition of CFTR, with the selective inhibitor CFTRinh-172, significantly reduced contractility in pregnant mouse and human myometrium in a concentration-dependent manner (44.89 ± 11.02 term pregnant mouse, 9.23 ± 4.75 term-pregnant human; maximal effect at 60 μM expressed as a percentage of the pre-treatment control period). However, there was no effect of CFTRinh-172 in non-pregnant myometrium.
Conclusion
These results demonstrate decreased myometrial function when CFTR is inhibited, which may have implications on pregnancy and labour outcome and therapeutic decisions for labour in CF patients.
Keywords: Cystic fibrosis, CFTR, Pregnancy, Myometrium, Contractility, Human, Mouse
Highlights
-
•
CFTR is expressed in term mouse myometrium.
-
•
Decreased contractility occurs in vitro human myometrium when CFTR is inhibited.
-
•
CFTR inhibition decreases contractility in term pregnant but not non-pregnant mouse.
-
•
Altered CFTR function in cystic fibrosis may have implications on labour outcome.
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutation of the cystic fibrosis transmembrane conductance regulator (CFTR), which is primarily expressed in epithelial cells (Riordan et al., 1989). CFTR functions as a chloride channel, regulating electrolyte and fluid transport and mucociliary clearance in the airways. Resulting CF pathophysiology includes dehydration and airway obstruction, the ultimate cause of mortality resulting from repeated airway infections, bronchiectasis and irreversible lung damage (Elborn, 2016). Increasing evidence is emerging of a role for the cystic fibrosis transmembrane conductance regulator (CFTR) in smooth muscle physiology. Both human and animal studies have shown CFTR to be expressed in muscle cells, including airway smooth muscle (ASM) (Vandebrouk et al., 2006; Michoud et al., 2009), vascular smooth muscle (VSM) (Robert et al., 2004, 2007) and cardiomyocytes (Warth et al., 1996; Lader et al., 2000). In ASM, CFTR is localised to the apical membrane (Vandebrouk et al., 2006) and sarcoplasmic reticulum (Cook et al., 2016) and has been reported to regulate smooth muscle tone (Robert et al., 2004; Vandebrouck et al., 2006; Robert et al., 2007; Michoud et al., 2009; Wallace et al., 2013). A study on newborn CF pigs has shown a decrease in calcium re-uptake into the sarcoplasmic reticulum, and an increase in myosin light chain activation in ASM cells (Cook et al., 2016). Another study in human ASM cells reported that calcium release in response to histamine was significantly decreased (Michoud et al., 2009). Furthermore, we have previously observed a loss in the contractile capacity of the CF mouse trachea due to downregulation of the pathway specific to acetylcholine (ACh) activation (Wallace et al., 2013). Other reports demonstrate that CFTR activation promotes smooth muscle relaxation in pre-contracted rat tracheal and vascular smooth muscle (Robert et al., 2004; Vandebrouck et al., 2006; Robert et al., 2007). It is therefore becoming increasingly clear that lack of CFTR results in functional abnormalities in smooth muscle cells. Clinical presentation in people with CF linked to airway smooth muscle dysfunction include bronchial hyperresponsiveness, a condition sometimes referred to as ‘CF asthma’ involving augmented bronchoconstriction (Balfour-Lynn and Elborn, 2002).
The role of CFTR in other types of smooth muscle remains unexplored. For example, the presence of CFTR in myometrium and its potential functional role in contractility is yet to be determined. Over the past few decades, prognosis for CF patients has greatly improved with a current average life expectancy of 47.3 years (UK CF registry, 2018). As a result of improved survival, more CF women are choosing to have children. The increase in CF pregnancies emphasises the need for an understanding of the role CFTR plays in normal myometrial function. Studies have shown pregnancy to be feasible and well tolerated in the majority of CF patients (Renton et al., 2015). Limited data exist however on mode of delivery and labour outcomes in CF women due to small numbers; studies suggest an increase in the incidence of pre-term births and higher rates of primary caesarean delivery (Patel et al., 2015; Jelin et al., 2017). A link between complications in labour and abnormalities in myometrial contractility however has not been explored. A fundamental unanswered question is whether the myometrium expresses CFTR, and if CFTR can affect contractility.
To address the lack of data and understanding of the role of CFTR in myometrium, we investigated the role of CFTR in human and mouse myometrial function. We used immunofluorescence to identify the presence of CFTR protein in term pregnant mouse myometrium sections; We used the CFTR inhibitor CFTR (inh)-172, to investigate the effect of CFTR inhibition on spontaneous myometrial contractility in term-pregnant and non-pregnant mice and term-pregnant human myometrial tissue.
2. Methods
2.1. Tissue preparation
2.1.1. Mouse
Term pregnant mice and non-pregnant mice (C57BL/6 J, Charles River, United Kingdom) were humanly killed at 19 days of gestation or 8–12 weeks old, using CO2 anaesthesia and cervical dislocation in accordance with Schedule 1 of the UK Animal (Scientific Procedures) Act of 1986. The uterus was removed, cleaned and longitudinal strips (1 × 4 mm) were dissected (with or without endometrium). Individual strips were mounted between a fixed support and a force transducer using aluminium clips in a 1 ml organ bath and were continuously superfused with physiological saline solution (PSS) composition (mM): NaCl 154, KCl 5.6, MgSO4 1.2, HEPES 10.9, Glucose 8, CaCl2 2 (adjusted to pH 7.4)) at a rate of 1 ml min−1 and maintained at 36 °C as described previously (Osaghae et al., 2020). Tissues were placed under 2 mN resting tension and allowed to equilibrate for 45–60min, by which time contraction rate and force had stabilised. Non-pregnant mice were also studied (8-12-week-old C57BL/6 J, Charles River, UK) and similar 1 × 4 mm strips of intact myometrium (full thickness) were cut and mounted as described above.
2.1.2. Human
Non-labouring human myometrial biopsies were collected from women with uncomplicated singleton pregnancies undergoing elective caesarian section at term (37–39 weeks gestation), after written informed consent, at Liverpool Women's Hospital, UK. A total of 15 human samples were used (indications for caesarian section were previous caesarian section (10), previous traumatic vaginal delivery (2), breech presentation (2) and tocophobia (1)). The average BMI of the women was 31.06 (range 20.4–48) and average weight of the babies was 3598 g (range 2450–4560 g). Strips of myometrium were prepared, as described above and previously (Arrowsmith et al., 2016). A 2-3-h equilibration period was required for regular spontaneous contractions to become established and experimental protocols were commenced once contractions had stabilised.
2.2. Contractility
2.2.1. Effect of CFTRinh-172 on myometrial contraction
CFTRinh-172 (Sigma), a voltage-independent selective CFTR inhibitor, was used to modulate CFTR function. This drug was made up in DMSO (Sigma) before being diluted in PSS to the appropriate concentration. Each tissue was exposed to just one concentration of inhibitor. The appropriate vehicle control (0.005%–0.5% DMSO, depending on the antagonist concentration used) was added for 30min (mouse) or 40min (human) before superfusing with 1, 3, 10, 30, 60 or 100 μM CFTRinh-172 for 20min (mouse) or 1, 10, 30, 60 μM CFTRinh-172 for 30min (human). The tissues were returned to the vehicle control solution thereafter to determine whether the effect of CFTRinh-172 was reversible.
2.2.2. Role of endometrial CFTR
In order to determine if effects observed with CFTRinh-172 were due to CFTR present in the myometrium rather than the endometrium, we compared the inhibitory effect of 30 μM CFTRinh-172 in the absence and presence of endometrium. This concentration was chosen because it reliably produced approximately 50% inhibition of contraction in pregnant mouse myometrium in initial experiments.
2.2.3. Effect of oxytocin on CFTRinh-172 response
In term pregnant mouse myometrium, the effect of CFTRinh-172 was examined in the presence of oxytocin (OT; Sigma) since this hormone is instrumental in labour. Tissues were exposed to 0.1 nM OT for 60min prior to addition of 10 μM CFTRinh-172.
2.2.4. Non-specific effects of CFTRinh-172
In order to identify potential non-specific activity of CFTRinh-172 at L-type Ca2+ channels, which are crucial for myometrial contractions, we examined the effect of CFTRinh-172 on the response to high K+ solution (composition (mM): NaCl 120, KCl 40, MgSO4 1.2, HEPES 10.9, Glucose 8, CaCl2 2 (adjusted to pH 7.4)). This solution causes depolarisation and maximal opening of L-type Ca2+ channels, and a tonic contraction in the tissue, and CFTRinh-172 was examined in two protocols.
Protocol 1: paired protocol with repeated 8-min applications of high K+ solution, to examine the peak amplitude of the high K+ response in the absence and presence of 10 μM CFTRinh-172. In control experiments, to demonstrate the repeatability of the high K+ response, tissues were exposed to high K+ solution for 8 min, returned to normal PSS for 30 min, before another 8 min high K+ application. In test experiments, 10 μM CFTRinh-172 was applied for 10 min before the first or second high K+ application.
Protocol 2: paired protocol with 40-min repeat applications of high K+ solution, to examine the effect of 10 μM CFTRinh-172 on the amplitude of the steady state plateau phase of the high K+ response. Again, control experiments were carried out to demonstrate the repeatability of the high K+ response; tissues were exposed to high K+ solution for 40 min, returned to normal PSS for 60 min, before another 40 min high K+ application. In test experiments, 10 μM CFTRinh-172 was applied for 20 min midway through the high K+ application (20min high K+, followed by 20min high K+ containing 10 μM CFTRinh-172).
2.3. Immunofluorescence
Term pregnant mouse myometrial strips were fixed in 4% paraformaldehyde, embedded in 5% gelatine and snap frozen as described previously (Wallace et al., 2008). Alternate longitudinal 7 μm cryosections were incubated for 2 h with either anti-actin, α-smooth muscle-Cy3 monoclonal antibody 1a4 (Sigma) or CFTR monoclonal antibody CF3 (Abcam) 1:500 dilution with 5% goat serum and 1% saponin in PBS (Sigma). Strips were then incubated for 1 h with 1:100 goat anti-mouse Alexa Fluor 488 (Abcam) secondary antibody. In the control, the same protocol was followed but the primary antibody was omitted. Ten sections from each mouse strip were observed and photographed under a fluorescent microscope. Sections labelled with anti-actin were used to indicate the presence and location of smooth muscle which were superimposed on images taken of sections labelled with CFTR antibody.
2.4. Data analysis
2.4.1. Contractility
Experimental contractility data were recorded using LabScribe Data Aquistion software (iWorx, CB Sciences Inc., Dover, NH, USA) and imported into Origin, version 8.5 (MicroCal, Inc., Northampton, MA, USA) for analysis. In mouse tissue, the mean force amplitude, duration (measured at 50% of maximal amplitude), frequency and total integral of force (area under the curve, AUC) was measured over a 5-min period and compared to a control 5-min period. All values are presented as a percentage of the control period (100%). The effect of CFTRinh-172 was assessed during the last 5 min of antagonist application initially. However, as the maximal inhibitory effect of CFTRinh-172 was frequently not yet reached at this point, we also measured the responses once the maximal effect was attained. In human tissue, due to the lower frequency of spontaneous contraction, measurements were taken over a 30-min period.
In Protocol 1 high K+ experiments (8min applications), peak amplitude of the high K+ response and AUC for the 8 min period were measured. In the longer Protocol 2 experiments (40min application), the amplitude of the high K+ plateau was measured at 20min and 40min and expressed as % decrease over that time period.
Statistical differences were tested using the Student's t-test, one sample t-test or one-way ANOVA (or Mann Whitney/Kruskal-Wallis tests where data was not normally distributed) using PRISM (version 5.0; Graph Pad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant. Concentration–response curves were fitted to the logistic equation using non-linear regression, constraining the top of the curve to 100% and the bottom to zero (PRISM, version 5.0; Graph Pad Software Inc., San Diego, CA, USA). Where multiple measurements were taken from the same mouse/subject, the data were meaned and the contribution to n was 1.
3. Results
3.1. Control experiments
Intact strips of mouse myometrium (with endometrium) and human myometrium contracted spontaneously and regularly for more than 3 h (see Fig. 1, Fig. 3A), providing enough time for all protocols to be completed.
Fig. 1.
The effect of different concentrations of CFTRinh-172 (1–100 μM) on contractility of term pregnant and non-pregnant mouse myometrium in vitro.
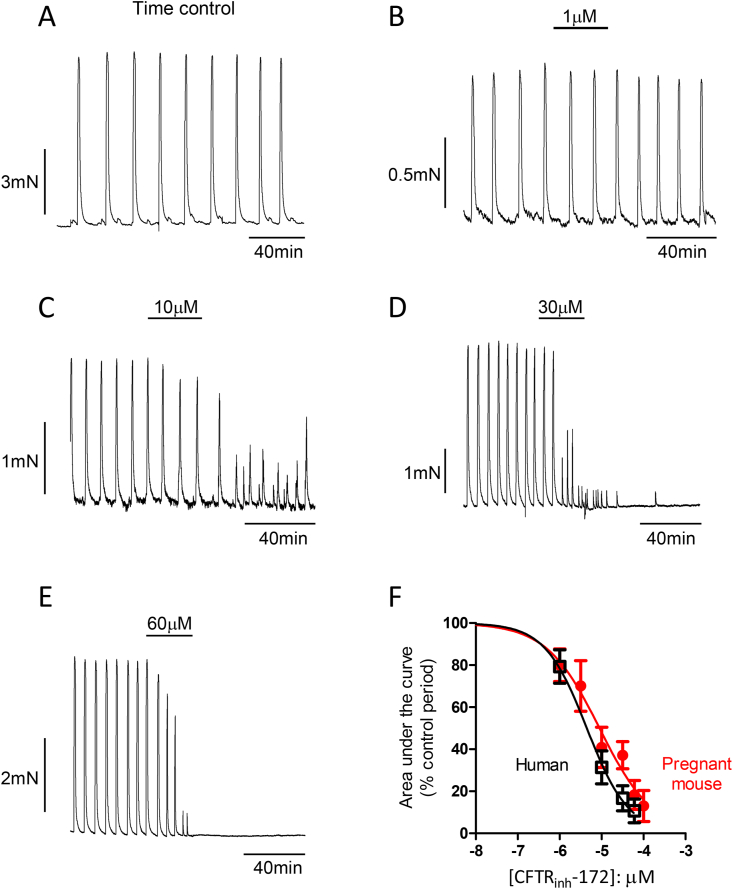
Fig. 3.
The effect of increasing concentrations of CFTRinh-172 (1–60 μM) on contractility of human myometrium in vitro. (A), shows control period of contraction, followed by increased concentrations of CFTRinh-172 (B–E). (F), shows the concentration-response curve of the AUC, with an pEC50 value of 5.35 ± 0.10 compared to pregnant mouse pEC50 value of 5.12 ± 0.14.
The antagonist CFTRinh-172 is made up in DMSO before being diluted in aqueous solution to the appropriate concentrations. The concentration of DMSO used as a vehicle was between 0.005% and 0.5% in mouse and between 0.005% and 0.3% in human. In non-pregnant mouse, term pregnant mouse and pregnant human myometrium, the amplitude, frequency and AUC of the spontaneous contractions were unaltered by the presence of DMSO (ANOVA: amplitude p = 0.12, AUC p = 0.29, frequency p = 0.36, see Supplementary Fig. 1). Duration of contractions was also unchanged in non-pregnant mouse myometrium and human myometrium, although duration was reduced in term pregnant mouse myometrium at 0.5% (ANOVA: p = 0.02). However, no change in duration was apparent at 0.005%–0.3% DMSO in the mouse myometrium (Supplementary Fig. 1E). Every strip of myometrial tissue was exposed to the relevant vehicle control (0.005%–0.5% DMSO) before addition of the antagonist and therefore in the analysis, the effect of the antagonist is compared to the contractility observed in the presence of the vehicle alone (the control period), thereby taking into account any effect of the vehicle.
3.2. Non-pregnant mouse myometrium
In non-pregnant mouse myometrium, CFTRinh-172 (1–100 μM) was found to have no significant effect on the amplitude, duration, frequency or AUC of spontaneous contractions when the response was measured in the last 5 min of antagonist application (Table 1(A)). When the maximal effect was measured, again 1–100 μM CFTRinh-172 had no significant effect on amplitude, frequency or duration. However, there was a small but significant decrease in AUC at 30 and 60 μM CFTRinh-172 (Table 1(A)).
Table 1.
Effect of 1–100 μM CFTRinh-172 on 4 parameters of contraction (amplitude, duration, frequency, AUC), expressed as a % of the pre-treatment control period, in (A) non-pregnant mouse myometrial strips, (B) term pregnant mouse myometrial strips and (C) human pregnant myometrial strips, measured during last 5min of antagonist application and at maximal effect. *p < 0.05, Kruskal-Wallis test;#p < 0.05, one-way ANOVA, compared to control period (100%).
| Amplitude % |
Duration % |
Frequency % |
AUC % |
N (mice/women) | |
|---|---|---|---|---|---|
| (A) | Non- pregnant mouse | ||||
| Last 5 min CFTRinh172 application | |||||
| 1 μM | 96.10 ± 2.61 | 96.68 ± 6.32 | 97.11 ± 10.78 | 84.97 ± 9.62 | 6 |
| 10 μM | 93.48 ± 1.43 | 100.80 ± 12.42 | 91.04 ± 3.79 | 83.15 ± 3.53 | 5 |
| 30 μM | 93.59 ± 1.92 | 104.20 ± 6.57 | 90.92 ± 3.07 | 78.67 ± 1.88 | 6 |
| 60 μM | 84.53 ± 8.51 | 124.40 ± 17.90 | 95.73 ± 4.69 | 79.88 ± 4.44 | 5 |
| 100 μM | 88.22 ± 3.24 | 110.70 ± 13.51 | 96.14 ± 5.00 | 82.09 ± 3.07 | 5 |
| Maximal effect | |||||
| 1 μM | 96.30 ± 3.16 | 98.34 ± 6.07 | 93.46 ± 11.18 | 82.53 ± 9.76 | 6 |
| 10 μM | 93.07 ± 1.46 | 98.81 ± 13.33 | 91.04 ± 3.79 | 82.93 ± 3.45 | 5 |
| 30 μM | 85.43 ± 8.32 | 104.90 ± 11.58 | 89.01 ± 8.51 | 70.46 ± 6.00# | 6 |
| 60 μM | 79.64 ± 10.32 | 134.20 ± 25.64 | 92.22 ± 8.16 | 71.48 ± 6.64# | 5 |
| 100 μM | 81.21 ± 5.75 | 94.62 ± 12.94 | 107.60 ± 12.40 | 77.61 ± 1.82 | 5 |
| (B) | Term pregnant mouse | ||||
| Last 5 min CFTRinh172 application | |||||
| 1 μM | 93.21 ± 4.56 | 98.89 ± 5.70 | 99.29 ± 4.39 | 84.99 ± 7.22 | 4 |
| 3 μM | 81.46 ± 10.46 | 84.96 ± 12.79 | 85.11 ± 13.22 | 77.01 ± 12.14 | 9 |
| 10 μM | 69.31 ± 11.14* | 66.91 ± 10.95* | 73.92 ± 11.81* | 49.97 ± 8.51* | 8 |
| 30 μM | 66.45 ± 7.73* | 79.71 ± 8.19 | 63.48 ± 9.41* | 42.61 ± 7.86* | 10 |
| 60 μM | 68.27 ± 6.54* | 80.16 ± 8.00 | 62.48 ± 7.48* | 38.05 ± 8.76* | 7 |
| 100 μM | 40.70 ± 15.05* | 54.48 ± 15.05* | 40.89 ± 13.24* | 26.72 ± 10.37* | 7 |
| Maximal effect | |||||
| 1 μM | 87.24 ± 3.72 | 97.19 ± 5.81 | 93.56 ± 8.51 | 79.98 ± 7.74 | 4 |
| 3 μM | 78.89 ± 10.12 | 90.76 ± 14.52 | 71.43 ± 11.60 | 70.09 ± 12.05 | 9 |
| 10 μM | 59.47 ± 13.44* | 63.42 ± 13.18 | 62.40 ± 12.18 | 40.87 ± 9.57* | 8 |
| 30 μM | 59.70 ± 8.41* | 77.64 ± 8.21 | 63.29 ± 7.94 | 37.16 ± 6.44* | 10 |
| 60 μM | 44.89 ± 11.02* | 69.08 ± 20.04 | 34.56 ± 8.73# | 18.25 ± 6.85* | 7 |
| 100 μM | 25.49 ± 12.87* | 43.63 ± 16.06# | 24.18 ± 11.47# | 13.03 ± 7.38* | 7 |
| (C) | Pregnant human | ||||
| Last 5 min CFTRinh172 application | |||||
| 1 μM | 90.83 ± 3.25 | 98.39 ± 4.86 | 97.95 ± 5.43 | 83.51 ± 5.51 | 9 |
| 10 μM | 82.03 ± 6.64* | 99.10 ± 6.13 | 84.11 ± 18.06 | 66.76 ± 6.30* | 9 |
| 30 μM | 68.23 ± 8.27* | 78.91 ± 5.25* | 131.30 ± 16.45 | 63.56 ± 6.46* | 8 |
| 60 μM | 69.70 ± 5.05* | 95.68 ± 13.09 | 93.26 ± 9.14 | 63.52 ± 4.05* | 8 |
| Maximal effect | |||||
| 1 μM | 79.88 ± 6.40 | 87.99 ± 7.39 | 121.40 ± 18.30 | 79.33 ± 7.96 | 9 |
| 10 μM | 20.80 ± 6.35* | 35.81 ± 9.42* | 118.10 ± 33.30 | 31.37 ± 7.87* | 9 |
| 30 μM | 22.48 ± 9.08* | 29.80 ± 11.87* | 100.50 ± 36.07 | 16.67 ± 5.93* | 8 |
| 60 μM | 9.23 ± 4.75* | 25.45 ± 13.25* | 118.1 ± 77.48 | 10.69 ± 5.63* | 8 |
3.3. Term pregnant mouse myometrium
In term pregnant mouse myometrium, CFTRinh-172 (1–100 μM) was found to decrease contractility in a concentration-dependent manner. Table 1(B) shows that the degree of inhibition measured in the last 5 min of antagonist application was smaller than that measured at the maximal effect point. In all further experiments, all measurements were made once the maximal inhibitory effect had been attained, as measurements taken in the last 5 min of CFTRinh-172 application underestimated the ability of the antagonist to inhibit myometrial contractility.
When measured at the point of maximal inhibitory effect, there was a significant decrease in amplitude and AUC at 10–100 μM CFTRinh-172 (Fig. 1, Table 1(B)), a significant decrease in frequency at 60–100 μM CFTRinh-172, but no significant change in duration of contractions except at 100 μM CFTRinh-172. When the concentration-response curve of the AUC data was plotted, it had a pEC50 value of 5.12 ± 0.14 (n = 4–10 mice; Fig. 3F). As data are expressed as a percentage of the control parameters, the raw data has been provided as Supplementary material.
The effect of CFTRinh-172 was reversible in most cases (54/74 strips either partially or fully recovered, see Fig. 1D), however it was clear that myometrium exposed to the lower concentrations of CFTRinh-172 recovered more frequently than those exposed to the highest concentration of 100 μM (5/8 at 1 μM; 10/13 at 3 μM; 11/14 at 10 μM; 8/11 at 30 μM; 8/11 at 60 μM; 3/10 at 100 μM).
In some myometrial samples, CFTRinh-172 (3–100 μM) caused spontaneous contractions to cease entirely (0/8 at 1 μM; 2/13 at 3 μM; 3/14 at 10 μM: 2/11 at 30 μM: 5/11 at 60 μM: 5/10 at 100 μM). The majority of these myometrial strips began contracting spontaneously again once the antagonist was washed out, although at the highest concentration (100 μM), only 1/5 strips recovered. In some experiments where contractions failed to recover, tissues did respond to 40 mM KCl indicating that the tissue was still responsive to depolarisation (see Supplementary Fig. 2).
3.3.1. Role of endometrial CFTR
CFTR is known to be expressed in the endometrium (Zheng et al., 2004). In order to ensure that the inhibitory effect of CFTRinh-172 on contractility demonstrated above, is due to CFTR present in the myometrium rather than the endometrium, we compared the effect of 30 μM CFTRinh-172 in the absence and presence of endometrium. The initial amplitude of spontaneous contraction was not significantly different in intact and myometrium only strips (Intact: 2.64 ± 0.40 mN (n = 10) versus myometrium only: 3.02 ± 0.45 mN (n = 11); Student's t-test, p = 0.53). We found no significant difference in the inhibition produced by 30 μM CFTRinh-172, whether the endometrium was present or not (Responses compared to control period (100%): Intact AUC 50.68 ± 5.66 % (n = 8) versus Myometrium only AUC 47.26 ± 8.07% (n = 8)). As no difference was apparent, all other experiments in mouse myometrium were carried out using intact tissue strips.
3.3.2. Effect of oxytocin on CFTRinh-172 response
Oxytocin (OT) is an important hormone in labour that augments myometrial contraction. We were therefore interested to determine whether the effect of CFTRinh-172 was altered in the presence of OT (0.1 nM). Preliminary experiments demonstrated that OT caused a long-lasting increase in frequency of contraction and AUC, that did not fade during the time course of the experiment (% increase compared to control period (n = 9, one sample t-test): Frequency, 128.28 ± 8.3% (p = 0.0091): AUC, 158.36 ± 20.4% (p = 0.021)).
We examined the inhibitory effect of 10 μM CFTRinh-172 in the absence and presence of 0.1 nM OT. As described above (Table 1(B)), at a concentration of 10 μM, CFTRinh-172 produces a significant inhibition of contraction amplitude and AUC (Response compared to control period (100%): Amplitude 59.47 ± 13.44%; AUC 40.87 ± 9.57%). However, in the presence of OT, only contraction amplitude was significantly decreased and to a lesser degree (Response compared to control period (100%): amplitude 83.27 ± 5.32%, n = 8, p = 0.004, Wilcoxon signed rank test), suggesting that the presence of OT attenuates the inhibitory effect of CFTRinh-172. When the inhibitory effects of CFTRinh-172 alone and CFTRinh-172 in the presence of OT were compared, we found a significant difference in AUC (but not amplitude, duration or frequency) (Fig. 2), demonstrating that CFTRinh-172 alone produces a significantly greater inhibition of AUC compared to in the presence of OT (40.87 ± 9.57% versus 73.71 ± 10.09%, p = 0.011, n = 8–9, Mann Whitney test).
Fig. 2.
Effect of 10 μM CFTRinh172 in the absence and presence of 0.1 nM oxytocin. Scatter plot demonstrating the significant inhibitory effect of 10 μM CFTRinh-172 on AUC, in the absence and presence of 0.1 nM oxytocin (Mann Whitney Test; p < 0.05).
3.4. Human myometrium
In pregnant human myometrium, CFTRinh-172 (1–60 μM) decreased contractility in a concentration-dependent manner (Fig. 3, Table1C, raw data in supplementary material). When measured at its maximum effect point, there was a significant decrease in amplitude, duration and AUC at concentrations of 10 μM CFTRinh-172 and above, but no significant change in frequency of contractions (Fig. 3, Table 1(C)). CFTRinh-172 exhibited a larger inhibitory effect in human myometrium compared to the mouse tissue, with amplitude of contraction being decreased to a significantly greater degree in human tissue at concentrations of 10 μM and above (10 μM 20.80 ± 6.35% versus 59.47 ± 13.44%, p = 0.0164; 30 μM 22.48 ± 9.08% versus 59.70 ± 8.41%, p = 0.0038, 60 μM 9.23 ± 4.75% versus 44.89 ± 11.02%, p = 0.0082). AUC was also decreased to a greater degree in human tissue at concentrations of 10 μM and above, although only reached significance at 30 μM (16.67 ± 5.93% versus 37.16 ± 6.44%, p = 0.036). Duration of contraction was significantly decreased in human tissue but unaltered in mouse. When the concentration-response curve of the AUC data was plotted, it had a pEC50 value of 5.35 ± 0.10 (n = 8–9 biopsies, Fig. 3F). This pEC50 was not significantly different to the value obtained in the pregnant mouse tissue (p = 0.20). While Fig. 3F shows that the concentration-response curve for CFTRinh-172 is very similar in both term-pregnant mouse and human myometrium, the response in human tissue was largely irreversible (only 4/39 strips either partially or fully recovered). In many cases, contractions ceased entirely after exposure to CFTRinh-172 and this phenomenon increased as the concentration of CFTRinh-172 increased (0/10 at 1 μM, 3/11 at 10 μM, 4/9 at 30 μM and 7/9 at 60 μM). When unresponding tissues were challenged with high K+ stimulation, they did however still respond (see Supplementary Fig. 2).
3.5. Non-specific effects of CFTRinh-172
By examining the effect of CFTRinh-172 on the response to high K+ solution, we determined whether this CFTR inhibitor had any non-specific activity at L-type Ca2+ channels. Fig. 4A shows the 8 min repeat high K+ protocol (Protocol 1). In control experiments, the initial peak amplitude of the high K+ response was comparable in first and second applications (4.76 ± 0.51 mN versus 4.70 ± 0.57 mN, n = 6, demonstrated by dotted line) and AUC also remained unchanged (18.66 ± 2.41 mN versus 20.44 ± 2.42 mN). In the presence of 10 μM CFTRinh-172, the peak amplitude of the high K+ response was significantly decreased (2.68 ± 0.56 mN versus 2.37 ± 0.51 mN, n = 6, paired Student's t-test p = 0.02, Fig. 4B and C), although AUC was unchanged (Fig. 4D, p = 0.11).
Fig. 4.
The effect of CFTRinh-172 on the response to high K+ solution in term pregnant mouse. Control high K+ responses are shown in (A) (protocol 1) and (E) (protocol 2); High K+ responses in the presence of CFTRinh-172 are shown in (B) (protocol 1) and (F) (protocol 2). Dotted lines in A and B indicate maximum High K+ amplitude. Significant decrease in peak amplitude in the presence of CFTRinh-172 (difference before and in presence of CFTR inhibitor; paired Student's t-test) and area under the curve (AUC) are shown as scatterplots in (C) and (D). Significant decrease of the steady-state plateau amplitude in the presence of CFTRinh-172 compared to control (Mann Whitney test) is shown in (G).
The longer 40min high K+ protocol was designed to observe the effect of the inhibitor on the steady state plateau phase of the high K+ response (Protocol 2). The amplitude of the high K+ steady state plateau was stable between the 20- and 40-min time points in control experiments (Fig. 4E). When 10 μM CFTRinh-172 was added at 20 min, it caused a significant decrease of the steady-state plateau, leading to an 18.75 ± 3.7% decrease in amplitude from 20 to 40 min (Control: 4.52 ± 3.8% decrease, CFTRinh-172: 23.77 ± 5.8% decrease, n = 7, Mann Whitney test, p = 0.018: Fig. 4F and G.
3.6. Expression of CFTR
A total of 30 myometrial strips from three pregnant mice were incubated with anti-actin and CFTR monoclonal antibody, and when observed under a fluorescent microscope. Immunofluorescence with anti-actin and CFTR were clearly visible in all strips demonstrating the presence of CFTR on myometrial smooth muscle cells. Representative strips from all three mice are shown in Fig. 5 and Supplementary Fig. 3. Compared to the actin staining, there appeared to be less fluorescence visible in the cytosol indicating more membrane expression of CFTR. However, some intracellular expression is visible, indicating expression around intracellular organelles. No fluorescence was detected when the primary antibody was omitted.
Fig. 5.
Immunofluorescence showing expression of CFTR in (A), actin in (B), CFTR and actin images (A and B) merged to show relative expression patterns shown in (C). DAPI (4,6-diamidino-2-phenylindole), staining of myometrial nuclei is shown in (D) and control no primary antibody shown in (E).
4. Discussion
These data are the first to identify expression of the CFTR protein in myometrial smooth muscle and to establish a functional role for CFTR in contractility of both mouse and human myometrial smooth muscle, adding to the growing list of smooth muscles where a role for CFTR has been demonstrated (Warth et al., 1996; Lader et al., 2000; Robert et al., 2004, 2007; Vandebrouck et al., 2006; Michoud et al., 2009).
Inhibition of CFTR with the selective inhibitor CFTRinh-172 significantly reduces contractility of spontaneously contracting pregnant mouse and human myometrium, which suggests that in term pregnant myometrial smooth muscle, CFTR is acting to augment myometrial contractility. These data also provide further insight into organ specific roles for CFTR in regulating contractility.
Expression of CFTR in term pregnant was shown in immunofluorescence experiments using the CF3 antibody. There have been questions surrounding the specificity of CF3 regarding proteins in the cytosol of tumour cell lines (Tomati et al., 2019), however the fluorescence pattern in the current study indicated predominant membrane expression of CFTR. Furthermore, specific CFTR expression using CF3 has been demonstrated on blood monocyte derived macrophages in CF but not in non-CF samples (Zhang et al., 2018). The Intracellular expression of CFTR we observed, suggests expression around organelles in the cytosol. This is supported by a study in new born pigs demonstrating localisation of CFTR to the sarcoplasmic reticulum in smooth muscle cells (Cook et al., 2016). Furthermore, CFTR expression has been demonstrated in several organelles in epithelial cells including the golgi network, endosomes, endoplasmic reticulum and mitochondria (Lukasiak and Zajac, 2021).
We have demonstrated that CFTR plays a role in contractility of both mouse and human term pregnant myometrium, but not in non-pregnant mouse myometrium, suggesting gestational differences in expression or function. Studies have shown that estrogen and progesterone regulate levels of CFTR expression (Ismail et al., 2015; Jin et al., 2016). Upregulation of estrogen during pregnancy may therefore provide a possible mechanism for the expression of CFTR in term pregnant myometrium shown in our study. Many protein expression changes are known to occur at term and govern the transition from a quiescent myometrium to the active, contractile state required to maintain labour (connexins, OT receptors, K+ channels, Ca2+-activated Cl− channels (CaCC) (Wray and Arrowsmith, 2021). Upregulation of CFTR could aid the requirement for increased myometrial contractility at term. In order to more closely mimic labouring myometrium, OT was applied to spontaneously contracting term pregnant tissues. Under these near-physiological conditions, CFTRinh-172 was still able to inhibit contractility, although to a lesser degree.
The effects of CFTRinh-172 on contractility were generally reversible. On occasion, CFTRinh-172 caused contractions to cease entirely and this was also reversible in many cases, although contractions were less likely to be restored with higher antagonist concentrations. The inhibitory effect of CFTRinh-172 was greater in human tissue than in the mouse tissue and less reversible. This difference could be explained by the fact that mouse studies were carried out on term pregnant but non-labouring myometrium. It is conceivable that further increases to CFTR expression may occur once labour starts and its role in enhancing myometrial contractility may increase.
The inhibitor CFTRinh-172 had a small but significant non-specific effect on L-type Ca2+ channel activity as observed in high K+ experiments. However, since the spontaneous contractility of the non-pregnant myometrium was essentially unaltered by CFTRinh-172, it would appear that these non-CFTR-specific effects are small, since even 100 μM CFTRinh-172 doesn't alter contractility in the non-pregnant tissue. These data are also consistent with high K+ responses in tracheal smooth muscle of cftr−/− mice, which do not significantly differ to non-CF mouse responses (Wallace et al., 2013). A previous study investigating the specificity of CFTRinh-172 in cell lines, has shown that concentrations higher than 5 μM can inhibit other channels namely the volume-sensitive outwardly rectifying Cl− conductance (VSORC) (Melis et al., 2014). However, a study using tissue preparations and concentrations similar to the current study, have shown specific inhibition of CFTR activator relaxation responses (Vandebrouck et al., 2006). Thus, we conclude on balance, our data show specific effects on the myometrium of CFTR inhibition.
CFTR channels predominantly conduct Cl− ion efflux from the cell. Myometrial smooth muscle cells also express other Cl− channels, CaCC, and it has been demonstrated that Cl− ion efflux from the cell via CaCC leads to spontaneous transient inward currents (STICs), which cause increased Ca2+ entry into the cell because of membrane depolarisation (Jones et al., 2004; Bernstein et al., 2014; Wray and Arrowsmith, 2021). These receptors are upregulated at term (Song et al., 2009), suggesting a role in parturition. We suggest that a CFTR channel-mediated Cl− ion efflux could lead to depolarisation of the plasma membrane and result in increased contractility due to increased opening of voltage-dependent L-type Ca2+ channels in the membrane and thus increased Ca2+ influx. The decreased contractility we observed with CFTRinh-172 is consistent with prevention of this depolarisation and lowering of intracellular Ca2+ levels.
Myometrial smooth muscle contractility is sensitive to changes in pH. In pregnant human myometrium, alkalinisation enhances contractility and acidification decreases contractility (Phoenix and Wray, 1993; Pierce et al., 2003). Similar changes are also observed in rat myometrium (Heaton et al., 1993; Hanley et al., 2015), although some species differences are apparent, with mouse myometrium displaying the opposite behaviour of increased contractility to acidification and decreased contractility with alkalinisation (Hong et al., 2013; Kyeong et al., 2016; Almohanna et al., 2016). In addition to conducting Cl− ions, CFTR channels have also been shown to regulate extracellular pH via control of bicarbonate (HCO3−) efflux, therefore activation of wildtype CFTR channels can generate extracellular alkalinisation (Massey et al., 2021). In mouse myometrial muscle strips, CFTR activity acts to enhance smooth muscle contractility (since inhibition of CFTR resulted in inhibition of spontaneous contractions). This is consistent with an outward Cl− ion current causing membrane depolarisation, increasing Ca entry and increasing contractility. It is not however consistent with CFTR mediating bicarbonate transport and causing extracellular alkalinisation, since that would be expected to cause decreased contractility in the mouse model and therefore inhibition with CFTRinh-172 would not also lead to decreased contractility. In human myometrial muscle strips however, extracellular alkalinisation enhances contractility. Therefore, it is possible that the inhibition seen with CFTRinh-172 in human myometrium may be due to dual action of CFTR, regulating Cl− ions and bicarbonate movement and perhaps explains the larger and less reversible inhibition in human myometrium compared to mouse. Further studies are required to confirm these hypotheses.
Future work may include the use of other CFTR inhibitors to further confirm the specificity of CFTR inhibition. A direct link between expression and function could also be investigated further by altering CFTR expression in myometrial strips or isolated smooth muscle cells to determine the effect on contractility. Data indicate upregulation of CFTR in myometrial tissues in pregnancy. Further work would examine CFTR expression in human tissue and non-pregnant mice, to investigate the potential gestational differences as indicated by the functional data.
There is evidence that dysfunctional labour requiring caesarean section is associated with an increasingly acidic myometrial environment (Quenby et al., 2004) and decreasing the acidic uterine environment in labour, by giving bicarbonate has been shown to improve labour outcome in a small randomized controlled trial (Wiberg-Itzel et al., 2018). It is interesting to speculate whether in CF women in labour, the lack of a functional CFTR channel might amplify the acidic environment during contractions and lead to an increased risk of operative delivery. Certainly, the caesarean section rate in CF women can be high (up to 50% (Thorpe-Beeston et al., 2013):), particularly in those with poor lung function and pre-term deliveries (Ashcroft et al., 2020). But worries about declining maternal health will be the reason for many of these operative deliveries and no link has yet been identified between complications in labour and abnormal myometrial contractility in CF women.
Interestingly, term labour with successful outcomes in CF women has been reported (Ashcroft et al., 2020). Such outcomes appear conflicting to results obtained in this study where we observed complete inhibition of contraction in the presence of inhibitor in human myometrial strips. This may be explained by the physiological effect of oxytocin; as demonstrated in this study, the addition of inhibitor in the presence of oxytocin resulted in a reduced effect on contractility. It is also possible that compensatory mechanisms may occur in vivo that would not occur in an in vitro preparation. Furthermore, it was not reported whether term births were oxytocin assisted. There may have been evidence of dysfunctional labour that was not reported. It must also be noted that residual chloride function can occur in certain CF genotypes (Veeze et al., 1994; Wallace et al., 2003). Reported genotypes varied in Ashcroft's study, and detail was not provided on the genotypes of women with term labour.
As life expectancy for CF patients continues to increase (UK CF registry, 2018), more women with CF will choose to start families. According to recent studies (Ashcroft et al., 2020; Esan et al., 2022), 30–40 women with CF undertake pregnancy in the UK each year. There is also emerging evidence that modulator therapies increase the rate of pregnancies in women with CF Esan et al. (2022)]. As yet, there is only limited information on mode of delivery and labour outcomes in CF women or the effect of common CF therapeutics during pregnancy; this is the first study to address whether lack of a functional CFTR alters the ability of the myometrium to contract. The need for expedited preterm delivery due to declining maternal health, may increase the risk of caesarean delivery.
Further investigations are required in order to provide the best possible information and advice to women with CF as to the likely success of pregnancy and labour in their current circumstances.
Funding
This study used internal funds from the University of Liverpool and did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Clodagh Prendergast: Conceptualization, Methodology, Validation, Formal analysis, writing, Visualization, Data curation, Project administration. Susan Wray: Conceptualization, Methodology, Resources, Supervision, Funding acquisition. Daniella Dungate: Investigation, Formal analysis. Christine Martin: Investigation, Formal analysis. Andra Vaida: Investigation, Formal analysis. Elizabeth Brook: Investigation, Formal analysis. Cecilia Ani Chioma: Investigation, Formal analysis. Helen Wallace: Conceptualization, Methodology, Validation, Investigation, writing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphys.2024.100122.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Almohanna A., Noble K., Wray S. An investigation into the effects of extracellular acidification on mouse uterine contractions. Proc. Phys. Soc. 2016;37 PCA354 Poster Communications. [Google Scholar]
- Arrowsmith S., Neilson J., Bricker L., Wray S. Differing in vitro potencies of tocolytics and progesterone in myometrium from singleton and twin pregnancies. Reprod. Sci. 2016;23(1):98–111. doi: 10.1177/1933719115597788. [DOI] [PubMed] [Google Scholar]
- Ashcroft A., Chapman S.J., Mackillop L. The outcome of pregnancy in women with cystic fibrosis: a UK population-based descriptive study. BJOG. 2020;127(13):1696–1703. doi: 10.1111/1471-0528.16423. [DOI] [PubMed] [Google Scholar]
- Balfour-Lynn I.M., Elborn J.S. "CF asthma": what is it and what do we do about it? Thorax. 2002;57(8):742–748. doi: 10.1136/thorax.57.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K., Vink J., Fu X., Wakita H., Danielsson J., Wapner R., Gallos G. Calcium-activated chloride channels anoctamin 1 and 2 promote murine uterine smooth muscle contractility. Am. J. Obstet. Gynecol. 2014;211(688) doi: 10.1016/j.ajog.2014.06.018. e1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D.P., Rector M.V., Bouzek D.C., Michalski A.S., Gansemer N.D., Reznikov L.R., Li X., Stroik M.R., Ostedgaard L.S., Alaiwa M.H.A., Thompson M.A., Prakash Y.S., Krishnan R., Meyerholz D.K., Seow C.Y., Stoltz D.A. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am. J. Respir. Crit. Care Med. 2016;193(4):417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn J.S. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- Esan O.B., Schlüter D.K., Phillips R., Cosgriff R., Paranjothy S., Williams D., Norman R., Carr S.B., Duckers J., Taylor-Robinson D. Pregnancy rates and outcomes in women with cystic fibrosis in the UK: comparisons with the general population before and after the introduction of disease-modifying treatment, 2003–17. BJOG. 2022;129(5):743–751. doi: 10.1111/1471-0528.16957. [DOI] [PubMed] [Google Scholar]
- Hanley J.A., Weeks A., Wray S. Physiological increases in lactate inhibit intracellular calcium transients, acidify myocytes and decrease force in term pregnant rat myometrium. J. Physiol. 2015;15;593(20):4603–4614. doi: 10.1113/JP270631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.C., Wray S., Eisner D.A. Effects of metabolic inhibition and changes of intracellular pH on potassium permeability and contraction of rat uterus. J. Physiol. 1993;465:43–56. doi: 10.1113/jphysiol.1993.sp019665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.H., Sung R., Young C.K., Suzuki H., Choi W., Park Y.J., Ji I.W., Kim C.H., Myung S.C., Lee M.Y., Kang T.M., You R.Y., Lee K.J., Lim S.W., Yun H.Y., Son Y.J., Xu W.X., Kim H.S., Lee S.J. Mechanism of relaxation via TASK-2 channels in uterine circular muscle of mouse. KOREAN J. PHYSIOL. PHARMACOL. 2013;17:359–365. doi: 10.4196/kjpp.2013.17.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N., Giribabu N., Muniandy S., Salleh N. Estrogen and progesterone differentially regulate the levels of cystic fibrosis transmembrane regulator (CFTR), adenylate cyclase (AC), and cyclic adenosine mono-phosphate (cAMP) in the rat cervix. Mol. Reprod. Dev. 2015;82:463–474. doi: 10.1002/mrd.22496. [DOI] [PubMed] [Google Scholar]
- Jelin A.C., Sharshiner R., Aaron B., Caughey A.B. Maternal co-morbidities and neonatal outcomes associated with cystic fibrosis. J. Matern. Fetal Neonatal Med. 2017;30(1):4–7. doi: 10.3109/14767058.2016.1161747. [DOI] [PubMed] [Google Scholar]
- Jin H., Wen G., Deng S., Wan S., Xu J., Xuemei L., Xie R., Dong H., Tuo B. Oestrogen upregulates the expression levels and functional activities of duodenal mucosal CFTR and SLC26A. Exp. Physiol. 2016;101(11):1371–1382. doi: 10.1113/EP085803. [DOI] [PubMed] [Google Scholar]
- Jones K., Shmygol A., Kupittayanant S., Wray S. Electrophysiological characterization and functional importance of calcium-activated chloride channel in rat uterine myocytes. Pflügers Archiv. 2004;448:36–43. doi: 10.1007/s00424-003-1224-7. [DOI] [PubMed] [Google Scholar]
- Kyeong K.S., Hong S.H., Young C.K., Cho W., Myung S.C., Lee M.Y., You R.Y., Kim C.H., Kwon S.Y., Suzuki H., Park Y.J., Jeong E.H., Kim H.S., Kim H., Lim S.W., Xu W.X., Lee S.J., Ji I.W. Myometrial relaxation of mice via expression of two pore domain acid sensitive K+ (TASK-2) channels. KOREAN J. PHYSIOL. PHARMACOL. 2016;20(5):547–556. doi: 10.4196/kjpp.2016.20.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader A.S., Wang Y., Jackson G.R., Jr., Borkan S.C., Cantiello H.F. cAMP-activated anion conductance is associated with expression of CFTR in neonatal mouse cardiac myocytes. Am. J. Physiol. Cell Physiol. 2000;278(2):C436–C450. doi: 10.1152/ajpcell.2000.278.2.C436. [DOI] [PubMed] [Google Scholar]
- Lukasiak A., Zajac M. The distribution and role of the CFTR protein in the intracellular compartments. Membranes. 2021;11(11):804. doi: 10.3390/membranes11110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey M.K., Reiterman M.J., Mourad J., Luckie D.B. Is CFTR an exchanger?: regulation of HCO3−Transport and extracellular pH by CFTR. Biochem. Biophys. Rep. 2021;25 doi: 10.1016/j.bbrep.2020.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis N., Tauc M., Cougnon M., Bendahhou S., Giuliano S., Rubera I., Duranton C. Revisiting CFTR inhibition: a comparative study of CFTRinh-172 and GlyH-101 inhibitors. Br. J. Pharmacol. 2014;171:3716–3727. doi: 10.1111/bph.12726. www.brjpharmacol.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michoud M.C., Robert R., Hassan M., Moynihan B., Haston C., Govindaraju V., Ferraro P., Hanrahan J.W., Martin J.G. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2009;40(2) doi: 10.1165/rcmb.2006-0444OC. 217-22. [DOI] [PubMed] [Google Scholar]
- Osaghae B.E., Arrowsmith S., Wray S. Gestational and hormonal effects on magnesium sulfate's ability to inhibit mouse uterine contractility. Reprod. Sci. 2020;27(8):1570–1579. doi: 10.1007/s43032-020-00185-8. [DOI] [PubMed] [Google Scholar]
- Patel E.M., Swamy G.K., Heine R.P., Kuller J.A., James A.H., Grotegut C.A. Medical and obstetric complications among pregnant women with cystic fibrosis. Am. J. Obstet. Gynecol. 2015;212:98. doi: 10.1016/j.ajog.2014.07.018. e1–9. [DOI] [PubMed] [Google Scholar]
- Phoenix J., Wray S. Changes in frequency and force production of human myometrium with alteration of pH and metabolism. J. Reprod. Fertil. 1993;97:507–512. doi: 10.1530/jrf.0.0970507. [DOI] [PubMed] [Google Scholar]
- Pierce S.J., Kupittayanant S., Shmygol T., Wray S. The effects of pH change on Ca(++) signaling and force in pregnant human myometrium. Am. J. Obstet. Gynecol. 2003;188:1031–1038. doi: 10.1067/mob.2003.229. [DOI] [PubMed] [Google Scholar]
- Quenby S., Pierce S.J., Brigham S., Wray S. Dysfunctional labour and myometrial lactic acidosis. Obstet. Gynecol. 2004;103(4):718–723. doi: 10.1097/01.AOG.0000118306.82556.43. [DOI] [PubMed] [Google Scholar]
- Renton M., Priestly L., Bennett L., Mackillop L., Chapman S.J. Pregnancy outcomes in cystic fibrosis: a 10-year experience from a UK centre. Obstet. Med. 2015;8(2):99–101. doi: 10.1177/1753495X15575628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J.R., Rommens J.M., Kerem B.S., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.L., Drumm M.L., Iannuzzi M.C., Collins F.S., Tsui L.C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robert R., Thoreau V., Norez C., Cantereau A., Kitzis A., Mettey Y., Rogier C., Becq F. Regulation of the cystic fibrosis transmembrane conductance regulator channel by beta-adrenergic agonists and vasoactive intestinal peptide in rat smooth muscle cells and its role in vasorelaxation. J. Biol. Chem. 2004;279(20):21160–21168. doi: 10.1074/jbc.M312199200. 14. [DOI] [PubMed] [Google Scholar]
- Robert R., Savineau J.P., Norez C., Becq F., Guibert C. Expression and function of cystic fibrosis transmembrane conductance regulator in rat intrapulmonary arteries. Eur. Respir. J. 2007;30(5):857–864. doi: 10.1183/09031936.00060007. [DOI] [PubMed] [Google Scholar]
- Song J., Zhang X., Qi Z., Sun G., Chi S., Zhu Z., Ren J., Qiu Z., Liu K., Myatt L., Ma R.Z. Cloning and characterization of a calcium-activated chloride channel in rat uterus. Biol. Reprod. 2009;80:788–794. doi: 10.1095/biolreprod.108.071258. [DOI] [PubMed] [Google Scholar]
- Thorpe-Beeston J.G., Madge S., Gyi K., Hodson M., Bilton D. The outcome of pregnancies in women with cystic fibrosis--single centre experience 1998-2011. J. Obstet. Gynecol. 2013;120(3):354–361. doi: 10.1111/1471-0528.12040. [DOI] [PubMed] [Google Scholar]
- Tomati V., Caci E., Ferrera L., Pesce E., Sondo E., Cholon D.M., Quinney N.L., Boyles S.E., Armirotti A., Ravazzolo R., Galietta L.J.V., Gentzsch M., Pedemonte N. Thymosin α-1 does not correct F508del-CFTR in cystic fibrosis airway epithelia. JCI Insight. 2019;4(7) doi: 10.1172/jci.insight.98699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK CF registry 2018. https://www.cysticfibrosis.org.uk/sites/default/files/2020-12/2018%20Registry%20Annual%20Data%20Report.pdf Available from:
- Vandebrouck C., Melin P., Norez C., Robert R., Guibert C., Mettey Y., Becq F. Evidence that CFTR is expressed in rat tracheal smooth muscle cells and contributes to bronchodilation. Respir. Res. 2006;7(1):113. doi: 10.1186/1465-9921-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeze H.J., Halley D.J., Bijman J., de Jongste J.C., de Jonge H.R., Sinaasappel M. Determinants of mild clinical symptoms in cystic fibrosis patients: residual chloride secretion measured in rectal biopsies in relation to the genotype. J. Clin. Invest. 1994;93:461–466. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H.L., Connell M.G., Losty P.D., Jesudason E.C., Southern K.W. Embryonic lung growth is normal in a cftr-knockout mouse model. Exp. Lung Res. 2008;34(10):717–727. doi: 10.1080/01902140802389719. [DOI] [PubMed] [Google Scholar]
- Wallace H.L., Southern K.W., Connell M.G., Wray S., Burdyga T. Abnormal tracheal smooth muscle function in the CF mouse. Phys. Rep. 2013;1(6) doi: 10.1002/phy2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth J.D., Collier M.L., Hart P., Geary Y., Gelband C.H., Chapman T., Horowitz B., Hume J.R. CFTR chloride channels in human and simian heart. Cardiovasc. Res. 1996;31(4):615–624. [PubMed] [Google Scholar]
- Wiberg-Itzel E., Wray S., Akerud H. A randomized controlled trial of a new treatment for labor dystocia. J. Matern. Fetal Neonatal Med. 2018;31(17):2237–2244. doi: 10.1080/14767058.2017.1339268. [DOI] [PubMed] [Google Scholar]
- Wray S., Arrowsmith S. Uterine excitability and ion channels and their changes with gestation and hormonal environment. Annu. Rev. Physiol. 2021;83:331–357. doi: 10.1146/annurev-physiol-032420-035509. [DOI] [PubMed] [Google Scholar]
- Wray Prendergast, Arrowsmith Calcium-activated chloride channels in myometrial and vascular smooth muscle. S Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.751008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H.L., Barker P.M., Southern K.W. Nasal airway ion transport and lung function in young people with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:594–600. doi: 10.1164/rccm.200211-1302OC. [DOI] [PubMed] [Google Scholar]
- Zhang S., Shrestha C., Kopp T. Cystic fbrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-35151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.Y., Chen G.A., Wang H.Y. Expression of cystic fibrosis transmembrane conductance regulator in human endometrium. Hum. Reprod. 2004;19(12) doi: 10.1093/humrep/deh507. 2933-41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.