Graphical abstract
Keywords: Clear cell ovarian cancer, Paraneoplastic cerebellar degeneration
Highlights:
-
•
Paraneoplastic cerebellar degeneration (PCD) is rare condition associated with gynecologic malignancy.
-
•
PCD presents with progressive cerebellar dysfunction in the setting of malignancy and confers a poor neurologic prognosis.
-
•
PCD associated with ovarian clear cell cancer may have more favorable neurologic outcomes versus other histologies.
-
•
The mainstay of PCD treatment is treatment of malignancy; symptom management may improve quality of life.
1. Introduction
Gynecologic clear cell carcinoma is an uncommon malignancy that has the potential to arise from multiple primary sites, including fallopian tubes, peritoneum, ovaries (5–10 % of all ovarian carcinomas), endometrium (1–5.5 % of all endometrial carcinomas), and vagina (5–10 % of vaginal carcinomas). (Gadducci et al., 2010) The prognosis of clear cell carcinoma is generally poor compared to serous ovarian carcinoma. (Gadducci et al., 2010) Treatment generally includes total hysterectomy, bilateral salpingo-oophorectomy with staging or debulking. Though many of these tumors are inherently resistant to platinum-based chemotherapies, platinum-based chemotherapy remains standard of care (Offman and Longacre, 2012, Armstrong, xxxx).
Unlike other gynecologic malignancies, ovarian clear cell carcinoma is associated with paraneoplastic syndromes, including hypercalcemia, retinopathy and paraneoplastic cerebellar degeneration (PCD). (Offman and Longacre, 2012, Cybulska et al., 2011, Aly and Emmady, 2023) PCD is rare and has been associated with neuroendocrine tumors, lymphoma, breast, ovarian, and endometrial cancers. (Aly and Emmady, 2023) In PCD, antibodies directed at tumor cells cross-react with similar proteins found on Purkinje cells in the cerebellum resulting in their degeneration. (Aly and Emmady, 2023) Patients present with cerebellar symptoms including gait instability, limb dysmetria, severe nausea and vomiting, as well as speech and vision difficulties; these symptoms can be the first sign of an underlying malignancy. (Aly and Emmady, 2023) In these cases, anti-Yo antibodies are often identified as a marker of the disease process and may indicate an unfavorable outcome. (Aly and Emmady, 2023) The clinical course of PCD can vary; however, most patients develop symptoms progressively over weeks to months. (Aly and Emmady, 2023) Permanent disability by six months with little neurologic improvement is the typical outcome. (Aly and Emmady, 2023) Due to the rare nature of the disease, clinical suspicion is uncommon, and diagnosis is frequently delayed.
Table 1 highlights the previously reported primary gynecologic malignancies associated with PCD, along with patient symptoms, imaging findings, and outcomes (Cao et al., 1999, Liontos et al., 2021, Marchand et al., 2007, Campero and Selman, 2017, Tanaka et al., 2005, Johns et al., 1999, Erez et al., 2007, Juárez-Vignon Whaley et al., 2021, Elomrani et al., 2014, Negishi et al., 2014, Panegyres and Graves, 2012, Russo et al., 2013, Butt et al., 2019). Here, we present a patient diagnosed with PCD in the setting of gynecologic clear cell carcinoma and highlight how symptomatology and neurologic outcomes in this disease process may be associated and provide clinicians with management considerations.
Table 1.
Summary of all published cases of gynecologic cancer related paraneoplastic cerebellar degeneration.
| Author, year | Pt age (years) | Pt primary disease site and histology | Disease markers | PCD presenting symptoms | Imaging |
Treatments |
Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Cancer Treatment | Immune-modulating treatment | Supportive Care Treatment | |||||||
| Cao, 1999 | 65 | Ovary Serous carcinoma | Anti-Yo antibodies | -Slurred speech -Ataxia -Dysarthria -Diplopia -Horizontal nystagmus-Absent gag reflex-Brisk reflexes with exception of diminished ankle reflexes |
Brain MRI with enhancement of the folia of the cerebellum and diffuse mild cerebellar atrophy | Surgical staging Not a candidate for adjuvant chemotherapy a |
IVIG | Physical therapy | -Immobile and cared for in nursing home-Reliant on gastrostomy tube feeds |
| Michalis Liontos, 2021 | 70 | Endometrium Serous carcinoma | Anti-Yo antibodies | -Symmetrical lower extremity numbness -Blurry vision -Nystagmus-Ataxia-Dysarthria |
Normal brain MRI | Chemotherapy only | IVIGCorticosteroidsPlasmapheresis | – | Death 11 months after cancer diagnosis |
| Marchand, 2007 | 60 | Ovary Serous carcinoma | Anti-Yo antibodiesIncreased IgG index with monoclonal kappa band | -Dysarthria -Dysgraphia -Ataxia -Nystagmus-Diminished reflexes-Babinski sign |
Normal brain MRI except for a meningioma | Interval surgical debulking with chemotherapy | – | – | No change in neurologic function at 6 months |
| Campero, 2017 | 65 | Fallopian tube Serous carcinoma | Anti-Yo antibodies | -Dysarthria -Dizziness -Horizontal nystagmus -Weight loss -Absent ankle reflex-Dysmetria-Ataxia |
Normal brain MRI and EEG | Surgical stagingPatient declined adjuvant chemotherapy | – | – | No change in neurologic function at 6 months |
| Tanaka, 2005 | 63 | Fallopian tube Serous carcinoma | Anti-Yo antibodies | -Dizziness -Dysarthria -Ataxia -Weight loss-Vertical nystagmus-Dysdiadochokinesis |
– | Surgical debulkingPatient declined adjuvant chemotherapy | – | – | Mild improvement in dysarthria |
| Johns, 1999 | 74 | Endometrium Serous carcinoma | Anti-Yo antibodies | -Ataxia -Dysarthria -Vertigo -Emesis -Left lateral gaze nystagmus -Dysphagia-Dysmetria-Diffuse decreased strength |
Normal brain CT and MRI | Surgical staging | -- | Physical rehabilitation | No improvement in neurologic function at 6 months |
| Erez, 2007 | 76 | Endometrium Serous carcinoma | Anti-Yo antibodies | -Dizziness -Ataxia -Weight loss -Dysmetria -Dysdiadochokinesis-Titubation-Hypophonia |
– | Surgical staging | Plasmapheresis | – | Slight improvement in neurologic function, especially speech |
| Juárez-Vignon Whaley, 2021 | 62 | Ovary Unspecified carcinoma | Anti-CV2 antibodies | -Dysarthria -Dizziness -Right lateropulsion -Tinnitus -Gait instability |
Normal brain MRI | Interval surgical debulking with chemotherapy | CorticosteroidsPlasmapheresis | Physical rehabilitation | Not recorded |
| Elomrani, 2014 | 80 | Unspecified gynecologic organ Serous carcinoma | Anti-Yo antibodies | -Vertigo-Emesis-Nystagmus | Normal brain MRI | Chemotherapy only | Corticosteroids | Antiemetics | Initial disappearance of neurologic symptoms followed by return of symptoms and death within a month |
| Negishi, 2014 | 62 | Ovary Clear cell carcinoma | Anti-Yo antibodies | -Gait instability -Dysarthria-Vertical nystagmus-Vertigo |
Normal brain CT and MRI | Surgical stagingAdjuvant paclitaxel only | IVIGCorticosteroidsTacrolimus | – | Regained ability to feed self and walk with walker |
| Panegyres, 2012 | 75 | Endometrium Clear cell carcinoma | Anti-Yo antibodiesAnti-GAD antibodies | -Vertigo -Emesis -Nystagmus -Past-pointing -Dysdiadochokinesis-Ataxia-Dysarthria |
Normal brain MRI | Surgical stagingNot a candidate for adjuvant chemotherapy | IVIG | Nursing home support | -Improved dysarthria -Bed bound and dependent on nursing home staff for ADLs |
| Russo, 2013 | 64 | Ovary Serous carcinoma | Anti-Yo antibodies | -Dysmetria -Ataxia -Dysgraphia -Nystagmus-Diplopia-Dysphagia |
Normal brain MRI | Interval surgical debulking with chemotherapy | Corticosteroids Cyclophosphamide bIVIG |
-- | Palliative care |
| Butt, 2019 | 69 | Ovary Serous carcinomaBreast DCIS |
Negative | -Ataxia -Dysarthria -Recurrent falls-Dysdiadochokinesis-Nystagmus |
– | Surgical stagingAdjuvant carboplatin only | – | – | No improvement of neurologic symptoms |
a Chemotherapy refers to platinum-taxane doublet unless otherwise specified.
b Cyclophosphamide was used as an immunosuppressant rather than as an anti-cancer agent.
Abbreviations: MRI: magnetic resonance imaging; IVIG: intravenous immunoglobulin; EEG: electroencephalogram; CT: computerized tomography; DCIS: ductal carcinoma in situ; ADLs: activities of daily living.
2. Case presentation
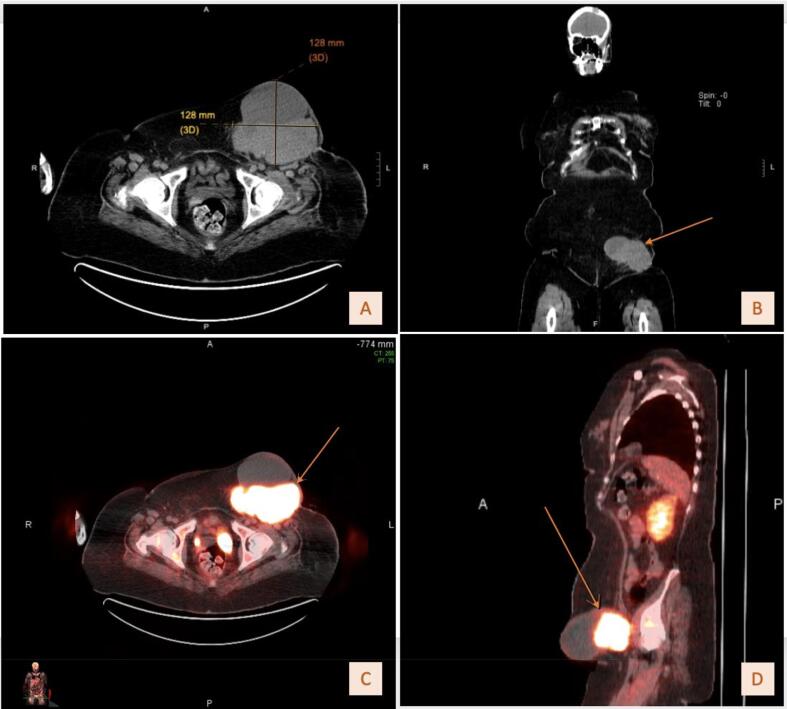
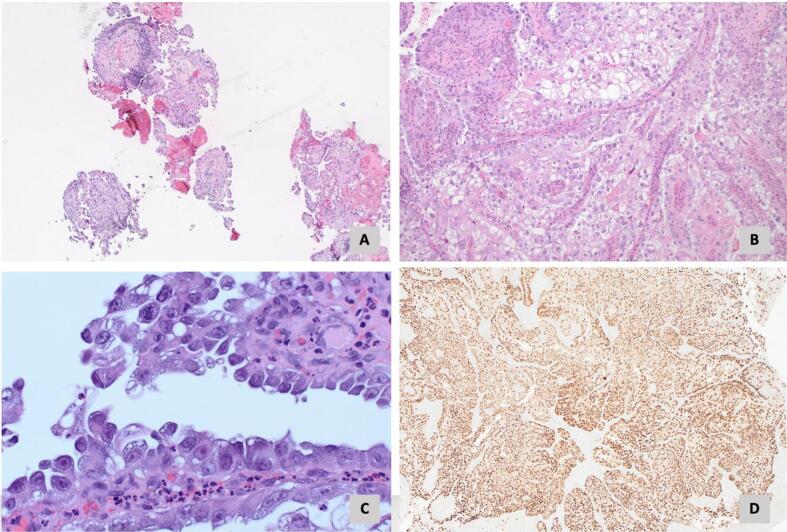
The patient is a 58-year-old G0P0 female who presented with drainage from a chronic non-healing wound in her left groin, unintentional weight loss, and a gradually worsening tremor. Her family history was significant for ovarian cancer in her mother. Her physical exam demonstrated an easily palpable left inguinal mass with overlying draining ulceration. During her pre-treatment workup, the patient noted gradually worsening neurologic symptoms, including an upper extremity tremor that impaired her ability to complete activities of daily living (ADLs). An initial magnetic resonance imaging (MRI) of the head was performed and displayed chronic age-related white matter changes. Computerized tomography (CT) of the abdomen and pelvis identified a complex groin mass (Fig. 1A-1B) with inguinal lymphadenopathy. A transvaginal pelvic ultrasound showed no abnormalities of the gynecologic organs. Positron Emission Tomography (PET) revealed hypermetabolism of this area (Fig. 1C-1D). Biopsy demonstrated a poorly differentiated adenocarcinoma of gynecologic or renal origin (Fig. 2A-2D). Molecular signature testing indicated a 90 % probability of an ovarian clear cell origin. Table 2 details serum tumor marker levels, next generation sequencing results, and the tumor immunohistochemical profile. Due to active venous thromboembolic disease, she underwent four cycles of neoadjuvant carboplatin and paclitaxel with excellent treatment response followed by optimal interval tumor debulking, which included radical resection of the groin mass, inguinal and pelvic lymphadenectomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and vertical rectus abdomino-myocutaneous flap with groin reconstruction. Final pathology revealed focal residual disease within the groin mass and benign gynecologic organs, pertinent only for adenomyosis. The patient then completed two additional cycles of adjuvant carboplatin and paclitaxel chemotherapy and was without evidence of disease.
Fig. 1.
A. Initial axial CT of left inguinal mass measuring 12.8 cm x 12.8 cm. B. Initial coronal CT of left inguinal mass. C. Axial FDG-PET/CT fusion. D. Sagittal FDG-PET/CT fusion.
Fig. 2.
A. Biopsy of left inguinal mass demonstrating papillary architecture (H&E stain, 40X). B. Cells demonstrating abundant clear cytoplasm and pleomorphic nuclei (H&E stain, 100X). C. Hobnail cells typical of clear cell carcinoma (H&E stain, 500X). D. PAX-8 immunostain (40X).
Table 2.
Tumor and serum testing data.
| Test / Marker | Result / Variant |
|---|---|
| Immunohistochemical Staining | |
| CK7 | Strongly positive |
| CK20 | Negative |
| ER | Negative |
| Gata3 | Negative |
| P53 | Patchy nuclear staining |
| PAX8 | Strongly positive |
| SOX10 | Negative |
| Next Generation Sequencing | |
| Gene | |
| PIK3CA | T1025S, R88Q |
| PTEN | D301f*3 |
| BCOR | E1017* |
| BRAF | G596R |
| NFE2L2 | E82D |
| U2AF1 | S34F |
| ARID1A | R750* |
| Biomarker | |
| Microsatellite stability | Microsatellite-stable |
| Tumor mutational burden | 4 mutations/Megabase (low) |
| Serum tumor markers | |
| CA-19–9 | 164.8 Units/mL (elevated) |
| CA-125 | 37 Units/mL (slightly elevated) |
During her cancer treatment, persistent neurologic symptoms prompted a repeat MRI of the head, which revealed mild cerebellar atrophy. A neurologic examination identified profound ataxic speech, ataxic gait, and action tremor of the bilateral upper extremities with notable end point ataxia on finger-to-nose testing. Following completion of her cancer treatment, her tremor and speech difficulties stabilized. Given the timing, constellation of symptoms, and response to treatment of malignancy, the patient was diagnosed with PCD.
Supportive care interventions included Botulinum toxin injections of the bilateral sternocleidomastoid and bilateral trapezius muscles for control of essential tremor and cervical dystonia. Oral primidone, which activates gamma-aminobutyric acid receptors, tizanidine, which inhibits motor neurons, and propranolol, a beta blocker, were used as adjuncts in treating her tremors and spasticity. (Pal, 2011) During her ovarian cancer treatment and thereafter, she has continued with regular physical and occupational therapy to improve ability to complete ADLs. She is now able to ambulate with the use of a walker and continues with regular physical and occupational therapy. Though she remains free of evidence of malignancy, she continues to suffer from tremors, slurred speech, and ataxia; importantly, these symptoms have remained stable in the setting of her disease status.
3. Discussion
Clear cell ovarian cancer associated PCD is uncommon. There are thirteen reports of gynecologic cancer associated PCD (Table 1) and to our knowledge, the present case is the second report of a clear cell ovarian cancer associated PCD. (Negishi et al., 2014) Though this patient’s disease distribution was not classic, the histopathology and genomic profile were consistent with clear cell carcinoma of ovarian origin (Table 2). (Offman and Longacre, 2012) We suspect that her disease may have originated from a focus of endometriosis given the known association between these diseases (Offman and Longacre, 2012) and concurrent histologic findings of adenomyosis on hysterectomy specimen. (Gonzales et al., 2012) This patient experienced a similar constellation of symptoms to other reports of PCD associated with gynecologic cancers. A stark contrast to prior reports describing rapid onset neurological disability, this patient’s milder symptom severity, more gradual symptom onset, and cancer remission may have clinical significance regarding the PCD prognosis.
PCD can progress at a variable pace and survival may depend on the type and origin of the underlying malignancy. For example, patients with PCD associated with breast cancer lived longer compared with those with underlying gynecologic malignancies (median survival 100 vs 22 months, respectively). (Rojas et al., 2000) The majority of ovarian cancer associated PCD reports describe tumors with serous histology and rapid onset, progressively worsening severe neurological dysfunction and permanent physical disability or death. (Cao et al., 1999, Marchand et al., 2007, Russo et al., 2013, Butt et al., 2019) In contrast, the present patient and a previously described patient with clear cell ovarian cancer both demonstrated more favorable PCD outcomes despite their poorer prognosis histologies. (Offman and Longacre, 2012, Negishi et al., 2014) Negishi et al., (2014) describes a patient with ovarian clear cell carcinoma and PCD, whose vertigo and dysarthria improved with stabilization during treatment of the malignancy. (Negishi et al., 2014) Similar to the patient presented, she continued to have minimal persistent cerebellar symptoms and regained the ability to independently perform ADLs. (Negishi et al., 2014) Additionally, patients from both cases achieved remission from disease, which may be associated with improved PCD outcomes; however, the patient Negishi and colleagues (2014) described achieved improvement in PCD prior to disease remission. (Negishi et al., 2014) These cases provide insight that the clear cell histology may be favorable as it relates to PCD recovery and are consistent with the experiences of this patient.
Brain imaging (e.g., CT or MRI) is used to exclude vascular or malignant etiology in suspected PCD cases, and anti-Yo antibodies in the cerebrospinal fluid can aid in diagnosis. Despite the severe symptomatology, imaging is frequently non-specific and non-diagnostic. Although PCD is an autoimmune mediated disease, cerebellar inflammation is a rare imaging finding. (de Andrés et al., 2006) Because diagnosis is frequently delayed, cerebellar inflammation has often resolved and cerebellar atrophy is the predominant finding on MRI. (de Andrés et al., 2006) De Andres and colleagues (2006) suggest the disease process is advanced if imaging abnormalities are detected. The present case is consistent with previously published reports in that initial brain MRI was unremarkable, and it was only late in the disease course that mild cerebellar atrophy was identified.
Treatment of PCD includes treating the underlying malignancy and immune modulating therapies, (Aly and Emmady, 2023) including intravenous immunoglobulins (IVIG), systemic steroids, or the use of other immunosuppressive medications. Reported efficacy of these therapies are variable. (Aly and Emmady, 2023) Negishi et al., (2014) described a patient with ovarian clear cell carcinoma who regained the ability to perform ADLs following treatment with IVIG, methylprednisolone and tacrolimus. (Negishi et al., 2014) Once her functional status improved, she received chemotherapy and achieved remission from malignancy. In contrast, the current patient presented did not receive immune modulating therapies and achieved a relatively good outcome. As both treatment for PCD and outcomes are variable, it is difficult to correlate symptom resolution with specific treatment regimens.
Interventions aimed at quality-of-life improvement for patients with PCD include pharmacotherapy and early rehabilitation during cancer treatment to improve physical function, especially with walking and completion of ADLs. (Kato et al., 2017) Drug intervention focused on symptom control may assuage the lasting effects of PCD in this patient, specifically anti-convulsant barbiturates and beta blockers for residual tremor and muscle relaxants for limb spasticity. Early initiation of physical and occupational therapy for this patient may have contributed to her favorable recovery.
4. Conclusion
PCD is a rare and challenging diagnosis for clinicians to establish, with significant life-long implications for patients and their families. Analysis of various histologies, primary sites and clinical features of malignancies with PCD demonstrate the variability in presentation, onset, and prognosis. This case highlights a rare favorable neurologic outcome and hypothesizes potential contributing factors. Clear cell carcinoma histology, resolution of the underlying malignancy and a therapeutic approach aimed at symptom management may have contributed to this patient’s improved neurologic state. A comprehensive understanding of PCD neurologic findings in concordance with histological analysis can support clinicians in making this diagnosis as well as provide guidance for management of patients with long term and debilitating neurologic effects.
CRediT authorship contribution statement
Madeline Tierney: Writing – review & editing, Writing – original draft, Visualization, Methodology, Conceptualization. Emma Landenwich: Writing – review & editing, Writing – original draft, Visualization, Methodology. Dava Piecoro: Visualization, Resources, Data curation. James Liau: Resources, Data curation. Erin Burke: Resources, Data curation. Charles S Dietrich III: Writing – review & editing, Supervision, Resources, Data curation. Megan L Hutchcraft: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Conceptualization.
References
- Aly R., Emmady P.D. StatPearls; 2023. Paraneoplastic cerebellar degeneration. Updated July 2023, Available at https://www.ncbi.nlm.nih.gov/books/NBK560638/ [PubMed] [Google Scholar]
- Armstrong DK, Alvarez RD, Backes FJ, Barroilhet L, Behbakht K, Berchuck A, et al. NCCN Clinical Practice Guidelines in Oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer; Version 2.2023. Available at https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- Butt E., Tadross J.A., Chadda K.R., Latimer J. Rare case of paraneoplastic cerebellar degeneration secondary to high-grade serous carcinoma of tubo-ovarian origin. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-229777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M., Selman A.E. Paraneoplastic cerebellar degeneration in a patient with a primary fallopian tube adenocarcinoma: a case report and brief review. Gynecol Oncol Rep. 2017;20:90–92. doi: 10.1016/j.gore.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Abbas J., Wu X., Dooley J., van Amburg A.L. Anti-yo positive paraneoplastic cerebellar degeneration associated with ovarian carcinoma: case report and review of the literature. Gynecol Oncol. 1999;75:178–183. doi: 10.1006/gyno.1999.5553. [DOI] [PubMed] [Google Scholar]
- Cybulska P., Navajas E.V., Altomare F., Bernardini M.Q. Clear cell carcinoma of the endometrium causing paraneoplastic retinopathy: case report and review of the literature. Case Rep Obstet Gynecol. 2011;2011 doi: 10.1155/2011/631929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrés C., Esquivel A., de Villoria J.G., Graus F., Sánchez-Ramón S. Unusual magnetic resonance imaging and cerebrospinal fluid findings in paraneoplastic cerebellar degeneration: a sequential study. J Neurol Neurosurg Psychiatry. 2006;77:562–563. doi: 10.1136/jnnp.2005.073379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomrani F., Ouziane I., Boutayeb S., Bensouda Y., Mrabti H., Errihani H. Ovarian cancer revealed by paraneoplastic cerebellar degeneration: a case report. Pan Afr Med J. 2014;18:2. doi: 10.11604/pamj.2014.18.2.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez Y., Rojansky N., Shveiky D., Ben-Meir A., Benshushan A. Endometrial carcinoma first presenting as paraneoplastic cerebellar degeneration. Gynecol Oncol. 2007;105:826–827. doi: 10.1016/j.ygyno.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Gadducci A., Cosio S., Spirito N., Cionini L. Clear cell carcinoma of the endometrium: a biological and clinical enigma. Anticancer Res. 2010;30:1327–1334. [PubMed] [Google Scholar]
- Gonzales M., de Matos L.A., da Costa Gonçalves M.O., Blasbalg R., Dias J.A., Jr, Podgaec S., et al. Patients with adenomyosis are more likely to have deep endometriosis. Gynecol Surg. 2012;9:259–264. [Google Scholar]
- Johns J.B., Odunsi K.O., Fleischman S., Azodi M., Schwartz P.E. Serous adenocarcinoma of the uterus presenting as paraneoplastic cerebellar degeneration. Gynecol Oncol. 1999;73:326–330. doi: 10.1006/gyno.1998.5324. [DOI] [PubMed] [Google Scholar]
- Juárez-Vignon Whaley J.J., Carrera-Muiños A., Hernandez-Gutierrez K.G., Rodriguez-Cid J.R., Otero-Cerdeira M.E., Garcia-Montes V. Paraneoplastic cerebellar degeneration with anti-CV2/CRMP5 antibodies in ovarian cancer: case report and review of the literature. Case Rep Oncol. 2021;14:1799–1805. doi: 10.1159/000519969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hashida G., Konaka K. Rehabilitation for a patient with anti-yo antibody-positive paraneoplastic cerebellar degeneration caused by breast cancer: a case report and literature review. Medicine (Baltimore) 2017;96:e8468. doi: 10.1097/MD.0000000000008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liontos M., Fiste O., Drakopoulou D., Thomakos N., Goula K., Zagouri F., et al. Paraneoplastic cerebellar degeneration in platinum-responsive endometrial cancer: a case report and review of literature. Gynecol Oncol Rep. 2021;37 doi: 10.1016/j.gore.2021.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand V., Graveleau J., Lanctin-Garcia C., Bourbouloux E., Bridji B., Resche I., et al. A rare gynecological case of paraneoplastic cerebellar degeneration discovered by FDG-PET. Gynecol Oncol. 2007;105:545–547. doi: 10.1016/j.ygyno.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Negishi Y., Sakai K., Noguchi Y., Iwasaki N., Kawai N. Paraneoplastic cerebellar degeneration caused by ovarian clear-cell carcinoma. J Obstet Gynaecol Res. 2014;40:614–617. doi: 10.1111/jog.12212. [DOI] [PubMed] [Google Scholar]
- Offman S.L., Longacre T.A. Clear cell carcinoma of the female genital tract (not everything is as clear as it seems) Adv Anat Pathol. 2012;19:296–312. doi: 10.1097/PAP.0b013e31826663b1. [DOI] [PubMed] [Google Scholar]
- Pal P.K. Guidelines for management of essential tremor. Ann Indian Acad Neurol. 2011;14(Suppl 1):S25–S28. doi: 10.4103/0972-2327.83097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panegyres P.K., Graves A. Anti-yo and anti-glutamic acid decarboxylase antibodies presenting in carcinoma of the uterus with paraneoplastic cerebellar degeneration: a case report. J Med Case Rep. 2012;6:155. doi: 10.1186/1752-1947-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas I., Graus F., Keime-Guibert F., Rene R., Delattre J.Y., Ramon J.M., et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-yo antibodies. Neurology. 2000;55:713–715. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- Russo A.E., Scalone S., Leonardi G.C., Scalisi A., Giorda G., Sorio R. Paraneoplastic cerebellar degeneration associated with ovarian cancer. Oncol Lett. 2013;5:681–683. doi: 10.3892/ol.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Suzuki N., Takao M., Ichikawa A., Susumu N., Aoki D. Paraneoplastic cerebellar degeneration with fallopian tube adenocarcinoma. Gynecol Oncol. 2005;99:500–503. doi: 10.1016/j.ygyno.2005.06.064. [DOI] [PubMed] [Google Scholar]