Summary
Background
Benefits of Intermittent fasting (IF) on health-related outcomes have been found in a range of randomised controlled trials (RCTs). Our umbrella review aimed to systematically analyze and synthesize the available causal evidence on IF and its impact on specific health-related outcomes while evaluating its evidence quality.
Methods
We comprehensively searched the PubMed, Embase, Web of Science, and Cochrane databases (from inception up to 8 January 2024) to identify related systematic reviews and meta-analyses of RCTs investigating the association between IF and human health outcomes. We recalculated the effect sizes for each meta-analysis as mean difference (MD) or standardized mean difference (SMD) with corresponding 95% confidence intervals (CIs). Subgroup analyses were performed for populations based on three specific status: diabetes, overweight or obesity, and metabolic syndrome. The quality of systematic reviews was evaluated using A Measurement Tool to Assess Systematic Reviews (AMSTAR), and the certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system. This study is registered with PROSPERO (CRD42023382004).
Findings
A total of 351 associations from 23 meta-analyses with 34 health outcomes were included in the study. A wide range of outcomes were investigated, including anthropometric measures (n = 155), lipid profiles (n = 83), glycemic profiles (n = 57), circulatory system index (n = 41), appetite (n = 9), and others (n = 6). Twenty-one (91%) meta-analyses with 346 associations were rated as high confidence according to the AMSTAR criteria. The summary effects estimates were significant at p < 0.05 in 103 associations, of which 10 (10%) were supported by high certainty of evidence according to GRADE. Specifically, compared with non-intervention diet in adults with overweight or obesity, IF reduced waist circumference (WC) (MD = −1.02 cm; 95% CI: −1.99 to −0.06; p = 0.038), fat mass (MD = −0.72 kg; 95% CI: −1.32 to −0.12; p = 0.019), fasting insulin (SMD = −0.21; 95% CI: −0.40 to −0.02; p = 0.030), low-density lipoprotein cholesterol (LDL-C) (SMD = −0.20; 95% CI: −0.38 to −0.02; p = 0.027), total cholesterol (TC) (SMD = −0.29; 95% CI: −0.48 to −0.10; p = 0.003), and triacylglycerols (TG) (SMD = −0.23; 95% CI: −0.39 to −0.06; p = 0.007), but increased fat free mass (FFM) (MD = 0.98 kg; 95% CI: 0.18–1.78; p = 0.016). Of note, compared with the non-intervention diet, modified alternate-day fasting (MADF) reduced fat mass (MD = −0.70 kg; 95% CI: −1.38 to −0.02; p = 0.044). In people with overweight or obesity, and type 2 diabetes, IF increases high-density lipoprotein cholesterol (HDL-C) levels compared to continuous energy restriction (CER) (MD = 0.03 mmol/L; 95% CI: 0.01–0.05; p = 0.010). However, IF was less effective at reducing systolic blood pressure (SBP) than a CER diet in adults with overweight or obesity (SMD = 0.21; 95% CI: 0.05–0.36; p = 0.008).
Interpretation
Our findings suggest that IF may have beneficial effects on a range of health outcomes for adults with overweight or obesity, compared to CER or non-intervention diet. Specifically, IF may decreased WC, fat mass, LDL-C, TG, TC, fasting insulin, and SBP, while increasing HDL-C and FFM. Notably, it is worth noting that the SBP lowering effect of IF appears to be weaker than that of CER.
Funding
This work was supported by the National Key Research and Development Program of China (Q-JW), the Natural Science Foundation of China (Q-JW and T-TG), Outstanding Scientific Fund of Shengjing Hospital of China Medical University (Q-JW), and 345 Talent Project of Shengjing Hospital of China Medical University (T-TG).
Keywords: GRADE, Health, Intermittent fasting, Randomised controlled trial, Umbrella review
Research in context.
Evidence before this study
We searched PubMed, Embase, Web of Science, and the Cochrane Database of Systematic Reviews from inception to January 8, 2024, for meta-analyses of intermittent fasting (IF) on health outcome. Our study summarized existing studies exploring the role of IF interventions focused on six categories of health outcomes: anthropometric measures, lipid profile outcomes, glycemic profile outcomes, circulatory system index, appetite, and others. The results of some studies have been inconsistent, leading to doubts over the validity of the claimed efficacy of IF on human health, and the potential influence of biases such as publication bias in the literature. Inconsistencies in the results of various studies have raised concerns regarding the validity of the purported effectiveness of IF on human health. Additionally, the potential influence of biases, such as publication bias, within the existing literature further complicates the assessment the true impact of IF. Furthermore, extensive meta-analyses have produced conflicting evidence concerning the health outcomes associated with IF. Previous umbrella review (UR) examining the effects of IF on obesity-related health outcomes have failed to consider other specific conditions such as diabetes and metabolic syndrome. To address these shortcomings, our UR adopts a comprehensive approach, encompassing a thorough analysis of randomised controlled trials and focused on diverse health-related outcomes. Additionally, we incorporate subgroup analyses to discern potential variations in the effects of IF across different populations.
Added value of this study
To address the limitations of previous studies, we conducted a comprehensive updated UR. We performed the Grading of Recommendations, Assessment, Development, and Evaluations to assess the certainty of existing evidence. We found and analyzed 351 unique associations of the effect of IF on anthropometric measures, lipid profile outcomes, glycemic profile outcomes, circulatory system index, appetite, and others. Among identified outcomes, there was high certainty of evidence that decreased of waist circumference, fat mass, low-density lipoprotein cholesterol, triacylglycerols, total cholesterol, fasting insulin, and systolic blood pressure, while increased of high-density lipoprotein cholesterol and fat free mass by IF compare with non-intervention or continuous energy restriction (CER). Based on a sensitivity analysis, reduced of body mass index, fat mass, homeostatic model assessment for insulin resistance after IF intervention was graded as highly quality evidence.
Implications of all the available evidence
Our findings, supported by high certainty of evidence, propose promising insights for clinicians and scientists in helping them provide high-evidence-level recommendations when receiving patient counseling about IF, or using IF interventions to improve patient health. It is worth noting that IF appears to exhibit a less pronounced systolic blood pressure lowering effect compared to CER. However, additional research is necessary to thoroughly assess the impact of IF on various health outcomes and elucidate the underlying mechanisms involved.
Introduction
Intermittent fasting (IF), an eating pattern characterized by alternating periods of eating and fasting, has attracted significant attention in recent years due to its potential health benefits and lifespan extension.1 IF encompasses various categories. The first category is zero-calorie alternate-day fasting (ADF) or modified alternate-day fasting (MADF). ADF involves alternating days of complete fasting with days of unrestricted eating. MADF, where participants alternate between days of unrestricted eating and days of fasting with caloric intake ranging from 0% to 40% or 0–600 kcal per day for 3–5 days per week. Another category is the twice-per-week fasting diet (TWF), where individuals fast for 2 days per week (either consecutively or nonconsecutively) with caloric intake ranging from 0% to 40% or 0–600 kcal per day, and have 5 days of unrestricted eating. The third category time-restricted eating (TRE) involves fasting for 12–24 h per day.2, 3, 4, 5 Additionally, there is a category known as periodic fasting, which involves less frequent but longer periods of fasting. For instance, a 2–5 day pure water fast or a 4–7 day fasting simulated diet, designed to mimic the metabolic effects of fasting, fall into this category.6,7
Over the past years, numerous clinical trials have highlighted the potential health benefits of IF, particularly for conditions like obesity, diabetes, cancer, and cardiovascular diseases, through weight reduction and improvements in cardiometabolic parameters.8, 9, 10, 11 However, previous literature has presented conflicting results regarding the change in health outcomes following IF intervention compared to a controlled group. In 2021, an umbrella review (UR) that included 11 meta-analyses comprising 130 randomised controlled trials (RCTs), it was found that MADF for 1–2 months was associated with a reduction in body mass index (BMI) in healthy adults and those with overweight, obesity, or nonalcoholic fatty liver disease compared to a regular diet.4 However, several issues are still warranted to be solved. Firstly, in their screening process, only obesity-related outcomes were considered, neglecting relevant outcomes such as heart rate,12 total calorie intake,13 percentage change in body weight12 and body fat.14 Secondly, Patikorn et al. performed an UR and identified 11 systematic reviews, but only 10 references were cited, indicating potential oversight in citation accuracy. Additionally, there have been several high-quality meta-analyses published after the retrieval deadline (January 12, 2021) that could provide updated evidence.12,14, 15, 16, 17, 18, 19, 20 For instance, Gu et al. (2022) conducted a comprehensive review of 43 RCTs and found no significant results on fasting glucose after IF intervention compared to non-intervention diets,16 which contradicts the findings of the UR.4 Furthermore, of the 11 studies included, 6 (55%) did not provide a mean or SD,21, 22, 23, 24, 25, 26 which could lead to bias in effect size or hinder interpretation and applicability of findings. Interestingly, a recent meta-analysis conducted by Zhang et al. in 2022 yielded inconsistent results, showing notable alterations in body weight observed after IF in comparison to CER, the difference in BMI between the two intervention failed to reach statistical significance.27 It is worth noting that several high-quality studies with more health-related outcomes investigating this topic have been published in recent years.14,16,17,20
In recent years, UR have gained recognition as a valuable tool in evidence synthesis due to their ability to address methodological limitations and biases associated with individual meta-analyses. By systematically consolidating and analyzing multiple meta-analyses within a transparent and reproducible framework, UR provide a comprehensive evaluation of the credibility of evidence derived from a wide range of published studies.28,29 Recent UR have highlighted the impact of IF on health outcomes, particularly in specific populations such as healthy, individuals with obesity, people with diabetes, or those with metabolic syndrome.4,30 These findings suggest that IF holds promise as a potential intervention for improving health in these groups.
As far as our current knowledge goes, numerous meta-analyses have been conducted since the retrieval of research data by Patikorn et al. (January 12, 2021).4 It is essential for us to summarize and provide an updated overview of the evidence, considering the volume of these meta-analyses.31, 32, 33, 34, 35, 36, 37, 38 When a limited number of studies are included or when there is considerable heterogeneity among the results, it becomes challenging to elucidate the source of heterogeneity through subgroup analysis. Consequently, such limitations can reduce the level of evidence obtained.30,39 Moreover, previous URs have often focused on specific areas, such as solely investigating anthropometry or parameters related to metabolic diseases.4, 30, 40 Recognizing these limitations, we have conducted an updated UR that encompasses all health-related outcomes, synthesizing evidence from published systematic reviews and meta-analyses. Our aim is to evaluate the strength and validity of the evidence based on factors such as sample size, effect size, and an assessment of biases.41, 42, 43 The goal of this UR is to support evidence-based clinical decision-making regarding IF interventions.
Methods
We performed an UR, which was a rigorous process of gathering and evaluating multiple systematic reviews and meta-analyses that investigate the relationship between IF and various health outcomes. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines were followed to ensure transparent and comprehensive reporting of our UR findings (Supplementary Table S1).44 Additionally, the study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42023382004).
Search strategy
We conducted searches in PubMed, Embase, Web of Science, and the Cochrane Database of Systematic Reviews from inception up until November 14, 2022. Furthermore, one additional search was conducted on 8 January 2024 to ensure completeness. Our search strategy employed a combination of keywords related to fasting, health outcomes, and meta-analysis. Detailed information regarding the search strategy can be found in Supplementary Table S2.
Eligibility criteria
Two reviewers (M-LS and W-Y) independently screened titles and abstracts for relevance and assessed the full texts of potentially eligible articles. Any discrepancies were resolved by discussion with a third reviewer (Q-JW). Studies were included based on the following Population, Intervention, Comparator, Outcome, Study design (PICOS) criteria:
-
(1)
Population: Adults of any ethnicity in any country or setting;
-
(2)
Intervention: Any type of IF, including ADF, MADF, TRE, intermittent energy restriction (IER), modified periodic fasting, combination of caloric restriction (CR) and IF (Time-restricted feeding [TRF] or ADF), combination of resistance training (RT) and TRF, and TWF, at any duration. IER is defined as a general term for IF that includes ADF and TWF, which is synonymous with IF in this study.45 Modified periodic fasting refers to a diet that severely restricts energy intake, such as 800 kcal, or less than 25% of the estimated energy requirement, on fasting days.35 CR includes continuous energy restriction (CER), a Mediterranean diet, and Dietary Approaches to Stop Hypertension.16 TRF and TRE are two terms often used interchangeably.46 The study of Gu et al. included the RCT study with the combination of CR and ADF as the intervention group and a simple CR regimen as the control group. RT program was performed three days per week and consisted of alternating upper and lower body workouts. The study of Liang et al. included the RCT study with the combination of RT and TRF as the intervention group and the combination of RT and normal diet as the control group33,47;
-
(3)
Comparison: The control group in the study was assigned to one of nine diets: CER, ad libitum diet, unrestricted diet, normal diet, usual diet, continuous dieting, habitual diet, regular diet, or no-intervention diet. CER is characterized by consistently reducing calorie intake over a specific time frame.48,49 Continuous dieting is defined as continual consistent CR over time.50 Ad libitum diet means individuals have unrestricted access to food and can consume it according to their own preferences and appetite.15,51 Unrestricted diet refers to a dietary approach that does not impose any specific restrictions or limitations on food intake.37 Normal diet is used to describe a dietary pattern that is considered typical or standard within a given population or cultural context. Usual diet refers to the dietary habits and food choices that an individual typically consumes on a regular basis, reflecting their ongoing eating patterns.52,53 Habitual diet describes the long-standing eating habits that individuals consistently follow over time.12 Routine diet is often used in healthcare settings to indicate the normal dietary pattern for an individual without any prescribed restrictions or modifications13,54;
-
(4)
Outcomes: any health outcome or indicator, such as anthropometric measures (e.g., BMI, body fat, body weight, fat free mass [FFM], fat mass, hip circumference, lean mass, steps per day, total calorie intake, visceral fat mass, waist circumference [WC], and weekly energy intake), lipid profile outcomes (e.g., high density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], total cholesterol [TC], and triacylglycerols [TG]), glycemic profile outcomes (e.g., cortisol, fasting glucose, fasting insulin, hemoglobin A1c [HbA1c], and homeostatic model assessment of insulin resistance [HOMA-IR]), circulatory system index (e.g., systolic blood pressure [SBP], diastolic blood pressure, and heart rate), appetite (e.g., desire to eat, fullness, and hunger), and others (e.g., liver stiffness, prospective food consumption, serum alanine aminotransferase, serum aspartate aminotransferase, and tetosterone);
-
(5)
Study design: Systematic reviews and meta-analyses of RCTs.
We excluded (1) Studies focusing on observational studies, laboratory studies, or animal studies; (2) Systematic reviews or meta-analyses without a relevant exposure; or (3) Systematic reviews or meta-analyses that did not provide specific data (e.g., mean and SD) for quantitative synthesis.55
Data extraction and quality assessment
Data extraction and quality assessment were independently performed by 2 investigators (M-LS and W-Y) and verified by other 2 investigators (Q-JW and T-TG). Any discrepancies were resolved through consensus. The quality of meta-analyses was evaluated using the A Measurement Tool to Assess Systematic Reviews (AMSTAR).56
Data synthesis
Effect sizes were categorized based on the population, intervention, comparator, and outcomes to generate a list of unique associations with IF. For each association, we recalculated the effect sizes as mean difference (MD) or standardized mean difference (SMD) with corresponding 95% confidence intervals (CIs) using the DerSimonian and Laird random-effects model separately for RCTs.57 Statistical significance was defined as p < 0.05 in two-sided tests. Heterogeneity was assessed using the I2 statistic.
We conducted a sensitivity analysis for significant associations with moderate-to high-quality evidence level, by excluding a high risk of bias or small sample size (25th percentile) from the identified associations.4,58,59 The sensitivity analysis followed the approach used for the random-effects model. Statistical analyses were performed using Stata version 16.0 (StataCorp, College Station, TX).
Considering the heterogeneity among study participants, population-based subgroup analyses were conducted to explore the effects of IF on various health outcomes. These subgroup analyses were based on three specific status: diabetes, overweight or obesity, and metabolic syndrome. To compare the effect size of IF on these health outcomes, study level data were utilized, including outcomes of anthropometric indicators, lipid profile, glycemic profile, and circulatory system index. The quality of evidence for each subgroup was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria.
To assess the quality of evidence provided in the meta-analyses of RCTs, we used the GRADE criteria across five domains: (1) risk of bias in the individual studies, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias. We graded the strength of evidence (high, moderate, low, and very low) using GRADE criteria.60
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. M-LS, WY, SG, X-YW, Y-HZ, D-YZ, Q-JW, and T-TG had full access to all data in the study. Y-HZ, D-YZ, Q-JW, and T-TG had final responsibility for the decision to submit for publication.
Results
Study selection
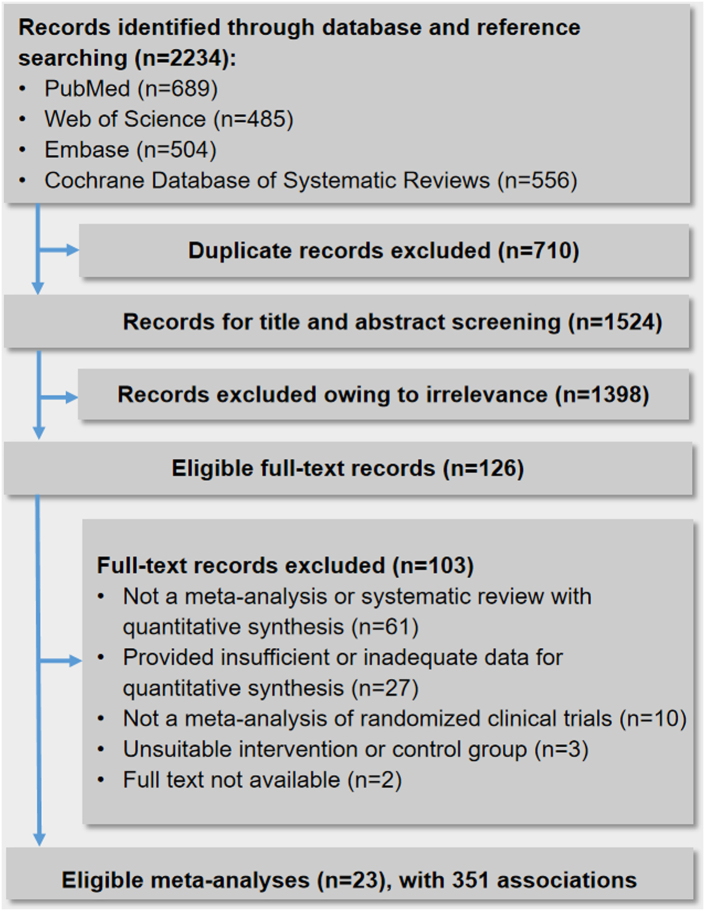
A flow chart depicting the search process and study selection is shown in Fig. 1. A total of 2234 records were identified by searching the four electronic databases. After filtering based on titles and abstracts, 126 records remained. One hundred and three records were excluded after reading the full text (Supplementary Table S3). The final selection yielded 23 meta-analyses to be included for the main analysis.12, 13, 14, 15, 16, 17, 18, 19, 20,31, 32, 33, 34, 35, 36, 37, 38,50,52,53,61, 62, 63
Fig. 1.
Flow diagram of the study selection process.
The basic information of included meta-analyses
Three hundred and fifty-one associations were involved in the 23 meta-analyses which were published between 2017 and 2023. The present UR population comprises healthy adults, premenopausal women, family history of breast cancer, and individuals with comorbidities such as overweight, obesity, prediabetes, diabetes, non-alcoholic steatohepatitis, non-alcoholic fatty liver disease, autosomal dominant polycystic kidney disease, or metabolic syndrome (Table 1). The median number of original RCTs in each association was 5 (ranged from 2 to 23). The identified associations mainly comprised three types of IF, including ADF/MADF, TWF, and TRE.
Table 1.
Characteristics of meta-analyses of randomized clinical trials studying intermittent fasting with health outcomes.
| Author, year, ref | Population | No. of studies | Mean age range (years) | Total participants | Type of IF | Duration of fasting (weeks) | Comparator | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Harris et al., 201761 | Adults with overweight or obesity | 5 | 21–69 | 317 | IER | 14–48 | CER | Body weight |
| Harris et al., 201852 | Adults with overweight or obesity | 4 | 40–50 | 287 | TWF, MADF | 12–18 | CER | Body weight |
| Roman et al., 201850 | Adults with overweight, obesity, or diabetes | 6 | 39.6–61.5 | 553 | Regular intermittent | NA | Continuous dieting | Lean mass, Body weight, WC, Hip circumference, Fat mass |
| 3 | 176 | Intensified intermittent | NA | Continuous dieting | Weight loss | |||
| Schwinghackl et al., 202053 | Patients with obesity or overweight, T2DM and at least 1 risk marker for metabolic syndrome | 17 | 31.7–67.6 | 1328 | TWF, MADF | 12–42 | Usual diet, CER | Body weight, Fat mass, WC, TG, SBP, LDL-C, FBG, HbA1c (%) |
| Cui et al., 202013 | Adults with overweight or obesity | 7 | 18–70 | 269 | ADF | 4–48 | RD | Body weight, BMI, Total calorie intake, TC, TG, LDL-C, HDL-C, FBG, HOMA-IR, Fat mass, Lean mass, SBP, DBP |
| He et al., 202162 | Adults with overweight or obesity, T2DM, metabolic syndrome | 11 | 28–71 | 850 | MADF, TWF | 12–48 | CER | Body weight, Fat mass, Fat free mass, WC, FBG, Fasting insulin, HOMA-IR, HbA1c |
| Chen et al., 202112 | Adults with overweight or obesity | 6 | 18–65 | 348 | TRE | 6–48 | Habitual diet | Body weight (%), BMI, Lean mass, Visceral fat mass, TC, TG, Fat mass, LDL-C, HDL-C, FBG, Fasting insulin, DBP, SBP, Heart rate |
| Wang et al., 202119 | Patients with T2DM or metabolic syndrome | 4 | 35.5–70.2 | 355 | MADF, TWF | 8–48 | CER | HbA1c, FBG, Body weight, BMI, TC, TG, LDL-C, HDL-C |
| Allaf et al., 202131 | Adults with overweight or obesity, T2DM, premenopausal women | 18 | 18–75 | 1125 | ADF, IF MADF, TRF, TWF | 4–24 | Ad libitum, CER | Body weight, Fat mass, WC, TC, TG, SBP, DBP, LDL-C, HDL-C, FBG, HbA1c, BMI |
| Wang et al., 202218 | Adults with overweight or obesity, T2DM patients | 11 | 18–71 | 750 | MADF, TWF | 4–96 | CER | Body weight, BMI, WC, Fat mass, Fat free mass |
| Zaki et al., 202220 | Patients with T2DM | 5 | 25–75 | 326 | TWF, MADF | NA | NA | Body weight, BMI, HbA1c |
| Gu et al., 202216 | Adults with overweight or obesity | 43 | 18–70 | 2483 | ADF, TRE, TWF, ADF+CR | 4–12 | Non-intervention diet | BMI, WC, Fat mass, Fat free mass, FBG, Fasting insulin, HOMA-IR, TG, TC |
| Kim et al., 202214 | Adults with overweight or obesity, T2DM, metabolic syndrome, or premenopausal women | 16 | 18–75 | 1438 | MADF, TRE, TWF | 8–52 | CER | Body weight, WC, Body fat (%), FBG, HbA1c (%), SBP, DPB, TG, HDL-C, LDL-C, BMI, Fat mass, Fat free mass, Fasting insulin, TC |
| Pascual et al., 202215 | Adults with overweight or obesity | 16 | 22–70.7 | 791 | ADF, MADF, TRE, TWF | 3–26 | CER | Body weight |
| 8 | 476 | ADF, MADF, TRE, TWF | 4–12 | Ad libitum | Body weight | |||
| Li et al., 202217 | Patients with metabolic syndrome | 4 | 34.5–71.7 | 268 | TWF, MADF | 1–25.7 | CER, RD | Body weight, BMI, WC, SBP, DBP, TC, TG, LDL-C, HDL-C, Fasting insulin, FBG, HOMA-IR |
| Zeng et al., 202238 | Patients with metabolic syndrome | 6 | 18–72 | 351 | TWF, MADF, TRE, IF | 8–16 | Non-intervention diet | Body weight, BMI, Fat mass, Fat free mass, WC, SBP, DBP, TC, TG, LDL-C, HDL-C, HOMA-IR, FBG, Fasting insulin |
| Lange et al., 202263 | Adults with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis | 12 | 42.5 | 908 | IF | NA | Non-intervention diet | Liver stiffness, Serum AST, Serum ALT |
| Zaman et al., 202337 | Adults with overweight or obesity, prediabetes, or T2DM | 15 | 27–74 | 927 | TRE | 3–48 | Unrestricted diet | Body weight, WC, Fat mass, Lean mass, HbA1c, HOMA-IR Fasting insulin, TC, TG, LDL-C, HDL-C, C-reactive protein, SBP, DBP, Heart rate, FBG |
| Liu et al., 202334 | Adults with normal weight or mildly obesity | 6 | 19–44 | 124 | TRE | 4–10 | Normal diet | Fat mass, Body weight, Fat free mass, Testosterone, Cortisol |
| Xu et al., 202336 | Adults with overweight or obesity, and T2DM | 16 | 33.5–71.1 | 1511 | TWF, ADF, MADF, IER | 4–48 | CER | WC, TG, HDL-C, FBG, SBP, DBP |
| Silverii et al., 202335 | Adults with obesity | 9 | 31.5–67.5 | 540 | MADF, ADF, TRE, TWF, MPF | 8–56 | CER, Ad libitum | Body weight, BMI |
| Elsworth et al., 202332 | Adults with overweight or obesity, T2DM, non-alcoholic fatty liver disease, autosomal dominant polycystic kidney disease, family history of breast cancer | 17 | 18.2–70.7 | 1111 | MADF, ADF, TRE, TWF | 2–39 | CER | Hunger, Fullness, Desire to eat Prospective food consumption, Body weight, Weekly energy intake, Steps per day |
| Liang et al., 202333 | Adults with overweight or obesity, T2DM, metabolic syndrome | 33 | 18–70 | 1725 | TRE, TRE + RT, TRE + Diet | 0.57–48 | Non-intervention diet | Body weight, SBP, BMI, Fat mass, Lean mass, LDL-C, HDL-C, TG, TC, FBG, Fasting insulin, HOMA-IR, DBP |
ADF, alternate-day fasting; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CER, continuous energy restriction; CR, continuous restriction; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; IER, intermittent energy restriction; IF, intermittent fasting; LDL-C, low-density lipoprotein cholesterol; MADF, modified alternate-day fasting; MPF, modified periodic fasting; NA, not available; RD, regular diet; Ref, Reference; RT, resistance training; SBP, systolic blood pressure; T2DM, diabetes mellitus type 2; TC, total cholesterol; TG, triacylglycerols; TRE, time restricted eating; TWF, twice-per-week fasting; WC, waist circumference.
A wide range of outcomes were investigated: anthropometric measures (n = 155, 44%), lipid profiles (n = 83, 23%), glycemic profiles (n = 57, 16%), circulatory system index (n = 41, 12%), appetite (n = 9, 3%), and others (n = 6, 2%) (Table 2 and Supplementary Table S4).
Table 2.
Summary of significant associations of intermittent fasting with health outcomes supported by moderate to high quality of evidence.
| Author, year, ref | Outcomes | Intermittent fasting/control | No. of studies | Metrics | Summary effects (95% CI) | p-value | I2, % | GRADE | AMSTAR |
|---|---|---|---|---|---|---|---|---|---|
| Anthropometric measures | |||||||||
| Schwinghackl et al., 202053 | Body weight | TWF, MADF/CER | 13 | MD | −0.55 (−1.01, −0.09) | 0.019 | 0.0 | Moderate | High |
| Schwinghackl et al., 202053 | Body weight | TWF/CER | 9 | MD | −1.37 (−2.24, −0.49) | 0.002 | 0.0 | Moderate | High |
| He et al., 202162 | Body weight | MADF, TWF/CER | 11 | MD | −0.95 (−1.63, −0.27) | 0.006 | 21.3 | Moderate | High |
| Li et al., 202217 | Body weight | TWF, MADF/RD | 4 | MD | −2.48 (−3.22, −1.74) | <0.001 | 0.0 | Moderate | High |
| Zaman et al., 202337 | Body weight | TRE/Unrestricted diet | 14 | MD | −2.25 (−3.09, −1.42) | <0.001 | 93.8 | Moderate | High |
| Zaman et al., 202337 | Body weight | TRE (7–9 h)/Unrestricted diet | 7 | MD | −2.30 (−4.37, −0.23) | <0.001 | 81.2 | Moderate | High |
| Liu et al., 202334 | Body weight | TRE/Normal diet | 4 | MD | −3.08 (−5.29, −0.86) | 0.006 | 0.0 | Moderate | High |
| Liang et al., 202333 | Body weight | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 23 | MD | −1.69 (−2.27, −1.11) | <0.001 | 97.5 | Moderate | Low |
| Liang et al., 202333 | Body weight | TRE/Non-intervention diet | 15 | MD | −1.77 (−2.52, −1.02) | <0.001 | 98.1 | Moderate | Low |
| Liang et al., 202333 | Body mass index | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 15 | MD | −0.46 (−0.67, −0.24) | <0.001 | 78.2 | Moderate | High |
| Liang et al., 202333 | Body mass index | TRE/Non-intervention diet | 8 | MD | −0.59 (−1.09, −0.09) | 0.021 | 78.4 | Moderate | High |
| Zaki et al., 202220 | Body mass index | TWF/NA | 3 | SMD | −0.40 (−0.68, −0.11) | 0.006 | 3.5 | Moderate | High |
| Li et al., 202217 | Body mass index | TWF, MADF/RD | 3 | MD | −0.90 (−1.00, −0.78) | <0.001 | 0.0 | Moderate | High |
| Allaf et al., 202131 | Body mass index | ADF, TWF/CER | 9 | MD | −0.43 (−0.76, −0.10) | 0.010 | 34.0 | Moderate | High |
| Cui et al., 2020 | Body mass index | ADF/RD | 4 | MD | −1.20 (−1.44, −0.96) | <0.001 | 0.0 | Moderate | High |
| Gu et al., 202216 | Waist circumference | ADF, TRE, ADF + CR/Non-intervention diet | 7 | MD | −1.02 (−1.99, −0.06) | 0.038 | 0.0 | High | High |
| Gu et al., 202216 | Waist circumference | ADF/Non-intervention diet | 3 | MD | −1.17 (−2.19, −0.15) | 0.024 | 0.0 | Moderate | High |
| Zaman et al., 202337 | Waist circumference | TRE/Unrestricted diet | 8 | MD | −2.21 (−4.36, −0.07) | 0.043 | 82.9 | Moderate | High |
| Liang et al., 202333 | Fat mass | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 14 | MD | −1.02 (−1.74, −0.31) | 0.005 | 97.1 | Moderate | High |
| Gu et al., 202216 | Fat mass | ADF, TRE, ADF + CR/Non-intervention diet | 12 | MD | −0.72 (−1.32, −0.12) | 0.019 | 0.0 | High | High |
| Gu et al., 202216 | Fat mass | ADF/Non-intervention diet | 4 | MD | −0.70 (−1.38, −0.02) | 0.044 | 0.0 | High | High |
| Zaman et al., 202337 | Fat mass | TRE/Unrestricted diet | 9 | SMD | −0.69 (−1.20, −0.17) | 0.009 | 84.7 | Moderate | High |
| Liu et al., 202334 | Fat mass | TRE/Normal diet | 6 | MD | −1.79 (−2.61, −0.97) | <0.001 | 0.0 | Moderate | High |
| He et al., 202162 | Fat mass | MADF (4:3)/CER | 3 | MD | −1.06 (−1.98, −0.13) | 0.025 | 0.0 | Moderate | High |
| Schwinghackl et al., 202053 | Fat mass | TWF/CER | 7 | MD | −0.82 (−1.35, −0.29) | 0.002 | 0.0 | Moderate | High |
| Schwinghackl et al., 202053 | Fat mass | TWF, MADF/CER | 10 | MD | −0.66 (−1.14, −0.19) | 0.010 | 0.0 | Moderate | High |
| Gu et al., 202216 | Fat free mass | ADF, TRE, TWF, ADF + CR/Non-intervention diet | 13 | MD | 0.98 (0.18, 1.78) | 0.016 | 0.0 | High | High |
| Zeng et al., 202238 | Fat free mass | MADF, TRE, TWF/Non-intervention diet | 3 | MD | −0.63 (−1.22, −0.04) | 0.036 | 0.0 | Moderate | High |
| Liang et al., 202333 | Lean mass | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 5 | MD | −0.67 (−1.12, −0.22) | 0.003 | 83.3 | Moderate | High |
| Zaman et al., 202337 | Lean mass | TRE/Unrestricted diet | 8 | MD | −0.69 (−1.26, −0.13) | 0.016 | 77.5 | Moderate | High |
| Lipid profile | |||||||||
| Xu et al., 202336 | HDL-cholesterol | TWF, MADF, ADF, IER/CER | 12 | MD | 0.03 (0.01, 0.05) | 0.010 | 0.0 | High | High |
| Kim et al., 202214 | LDL-cholesterol | TWF/CER | 6 | SMD | −0.20 (−0.38, −0.02) | 0.027 | 0.0 | High | High |
| Gu et al., 202216 | Total cholesterol | ADF/Non-intervention diet | 5 | SMD | −0.29 (−0.48, −0.10) | 0.003 | 0.0 | High | High |
| Gu et al., 202216 | Triacylglycerols | ADF, TRE, TWF, ADF + CR/Non-intervention diet | 15 | SMD | −0.23 (−0.39, −0.06) | 0.007 | 17.0 | High | High |
| Zaman et al., 202337 | Triacylglycerols | TRE/Unrestricted diet | 9 | MD | −18.19 (−32.07, −4.31) | 0.010 | 97.2 | Moderate | High |
| Zaman et al., 202337 | Triacylglycerols | TRE (7–9 h)/Unrestricted diet | 4 | MD | −30.65 (−52.39, −8.90) | 0.006 | 30.9 | Moderate | High |
| Zaman et al., 202337 | Triacylglycerols | TRE (10–12 h)/Unrestricted diet | 3 | MD | −27.51 (−40.12, −14.90) | <0.001 | 44.9 | Moderate | High |
| Glycemic profile | |||||||||
| Zaman et al., 202337 | Fasting glucose | TRE (7–9 h)/Unrestricted diet | 4 | MD | −2.82 (−4.51, −1.12) | 0.001 | 0.0 | Moderate | High |
| Liang et al., 202333 | Fasting glucose | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 15 | MD | −1.45 (−2.72, −0.18) | 0.025 | 67.7 | Moderate | High |
| Gu et al., 202216 | Fasting insulin | ADF, TRE, TWF/Non-intervention diet | 13 | SMD | −0.21 (−0.40, −0.02) | 0.030 | 0.0 | High | High |
| Gu et al., 202216 | Fasting insulin | TRE (16:8)/Non-intervention diet | 7 | SMD | −0.31 (−0.60, −0.02) | 0.035 | 0.0 | Moderate | High |
| Liang et al., 202333 | Fasting insulin | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 14 | MD | −0.81 (−1.59, −0.03) | 0.042 | 94.0 | Moderate | High |
| Gu et al., 202216 | HOMA-IR | ADF, TRE, TWF/Non-intervention diet | 8 | MD | −0.35 (−0.65, −0.04) | 0.030 | 0.0 | Moderate | High |
| Circulatory system index | |||||||||
| Kim et al., 202214 | Systolic blood pressure | TWF, MADF, TRE/CER | 9 | SMD | 0.21 (0.05, 0.36) | 0.008 | 0.0 | High | High |
| Liang et al., 202333 | Systolic blood pressure | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 11 | MD | −3.48 (−6.23, −0.73) | 0.013 | 90.6 | Moderate | High |
| Liang et al., 202333 | Diastolic blood pressure | TRE, TRE + RT, TRE + Diet/Non-intervention diet | 16 | MD | −1.46 (−2.67, −0.26) | 0.017 | 96.6 | Moderate | High |
| Liang et al., 202333 | Diastolic blood pressure | TRE/Non-intervention diet | 9 | MD | −1.90 (−3.73, −0.08) | 0.041 | 95.0 | Moderate | High |
| Chen et al., 202112 | Diastolic blood pressure | TRE/Habitual diet | 4 | MD | −5.10 (−6.27, −3.93) | <0.001 | 31.3 | Moderate | High |
| Zaman et al., 202337 | Diastolic blood pressure | TRE (4–6 h)/Unrestricted diet | 2 | MD | −5.41 (−6.25, −4.57) | <0.001 | 0.0 | Moderate | High |
| Other | |||||||||
| Lange et al., 202263 | Serum ALT | IF/Non-intervention diet | 4 | MD | −10.35 (−19.90, −0.80) | 0.034 | 0.0 | Moderate | Low |
| Liu et al., 202334 | Tetosterone | TRE/Normal diet | 4 | SMD | −0.51 (−0.93, −0.10) | 0.016 | 0.0 | Moderate | High |
AMSTAR, A Measurement Tool to Assess Systematic Reviews; ADF, alternate-day fasting; CER, continuous energy restriction; CR, continuous restriction; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HOMA-IR, homeostatic model assessment for insulin resistance; MADF, modified alternate-day fasting; MD, mean difference; NA, not available; RD, regular diet; Ref, reference; RT, resistance training; TRE, time-restricted eating; TWF, twice-per-week fasting; SMD, standardized mean difference.
Methodological quality of included meta-analyses
Supplementary Figure S1 summarizes the AMSTAR of included meta-analyses. Twenty-one (91%) meta-analyses were rated as having high confidence, one as having moderate confidence, and one as having low confidence. However, in our study, the AMSTAR assessment was downgraded mainly based on three specific reasons. Firstly, the research design employed at the outset of the study was not adequately described in the research methods section, which aligns with Item 1 of the AMSTAR checklist. Secondly, a comprehensive and detailed list of both included and excluded articles was not provided, as required by Item 5 of the checklist. Lastly, an assessment of the potential presence of publication bias was not conducted, as stipulated by Item 10 in the checklist.
Summary effect size
One hundred and three (29%) of the 351 associations were nominally statistically significant (p < 0.05) based on the random-effects model listed in Supplementary Table S4. As shown, there were five kinds of outcomes: anthropometric measures (n = 61, 59%), lipid profiles (n = 12, 12%), glycemic profiles (n = 15, 14%), circulatory system index (n = 13, 13%), and others (n = 2, 2%). A total of 202 associations (58%) had low heterogeneity (I2 ≤ 50%). Notably, fifty-three (15%) associations showed that IF had nominally statistically significant effects on diverse health outcomes with low heterogeneity, with a p < 0.05.
Anthropometric measures outcomes
In participants with overweight or obesity, fat mass and WC significantly decreased by 0.72 kg (95% CI: −1.32 to −0.12; p = 0.019) and 1.02 cm (95% CI: −1.99 to −0.06; p = 0.038) following 1–3 months of the ADF, TRE, and ADF plus CR diets, respectively, in comparison to non-intervention diet.16 IF (ADF, TRE, TWF, and ADF plus CR) were effective methods to increase FFM (MD = 0.98 kg; 95% CI: 0.18–1.78; p = 0.016) compared with a non-intervention diet.16 Six associations conducted on participants with overweight or obesity, type 2 diabetes (T2DM) patients, metabolic syndrome, and premenopausal women, compared IF (MADF, TRE, TWF, and ADF) with CER diets, reporting no significant effects with high quality evidence on weight and BMI14,16,18,45
Lipid profile outcomes
LDL-C significantly decreased by 0.20 (95% CI: −0.38 to −0.02; p = 0.027) following 8–52 weeks of TWF compared with CER.14 Pooled effect sizes across 15 studies revealed significant reductions in TG (SMD = −0.23; 95% CI: −0.39 to −0.06; p = 0.007) for IF (ADF, TRE, TWF, and ADF plus CR) intervention in comparison to non-intervention diet.16 IF (TWF, MADF, ADF, and IER) for 1–12 months was associated with increased HDL-C in adults with overweight or obesity, and T2DM compared with CER (MD = 0.03 mmol/L; 95% CI, 0.01–0.05; p = 0.010).36 Two non-significant effects with high quality evidence were observed for 4–12 weeks IF (ADF, TRE, TWF, and ADF plus CR) or 8–52 weeks IF (MADF, TRE, and TWF) than CER or no treatment for TC.14,16 However, limit the fasting mode to ADF, TC was decreased (SMD = −0.29; 95% CI: −0.48 to −0.10; p = 0.003) compared with a non-intervention diet.
Glycemic profile outcomes
Six effects (one significant and five non-significant) of our results provided high quality evidence on whether IF affected the level of glycemic profile outcomes than non-intervention diet or CER. The remaining results showed moderate, low or very low quality (n = 51, fourteen significant and thirty-seven non-significant) on whether IF affected the level of glycemic profile outcomes than habitual diet, non-intervention diet, RD, unrestricted diet, usual diet, ad libitum or CER. One high quality evidence association found IF (MADF, TRE, and TWF) for 1–3 months was associated with reduced fasting insulin in adults with overweight or obesity compared with non-intervention diet (SMD = −0.21; 95% CI, −0.40 to −0.02; p = 0.030).16 Five associations conducted on participants with overweight or obesity, T2DM patients, metabolic syndrome, and premenopausal women, compared IF (MADF, ADF, TRE, IER, TWF, and ADF plus CR) with CER diets or non-intervention diet, reporting no significant effects with high quality evidence on fasting glucose (n = 4) and fasting insulin (n = 1).14,16,36 Three significant associations conducted on participants with overweight or obesity, T2DM patients, and metabolic syndrome, compared IF (MADF, ADF, TRE, and TWF) with CER diets or non-intervention diet, reporting moderate (n = 1) and low (n = 2) evidence on HOMA-IR.16, 38, 62 Ten associations conducted on participants with obesity or overweight, T2DM and at least 1 risk marker for metabolic syndrome, premenopausal women, compared IF (TWF, MADF, and TRE) with usual diet, ad libitum, unrestricted, or CER diets, reporting no significant effects on HbA1c (three moderate, six low, and one very low).14, 19, 20, 31, 37, 53, 62
Circulatory system index
It is worth noting that one associations of SBP, which recruited 1438 participants with overweight or obesity (SMD = 0.21; 95% CI: 0.05–0.36; p = 0.008), found that IF was less effective at lowering SBP than CER.14 Non-significant effects with high quality evidence were observed for 8–52 weeks IF (MADF, TRE, and TWF) than CER for diastolic blood pressure.14 Three associations conducted on participants with overweight or obesity, prediabetes, or T2DM, compared TRE with habitual diet or unrestricted diet, reporting low evidence on heart rate.12,37
Appetite
None of our results provided over moderare quality evidence on whether IF affected the level of appetite outcomes than CER. Four associations (three low quality evidence and one very low quality evidence) show there is no difference were observed after 2–39 weeks IF intervention (MADF, TRE, or TWF) than CER for hunger.32 The remaining results showed low (n = 2) and very low (n = 3) quality on IF did not affect the level of appetite outcomes than CER.32 It is worth noting that three associations of hunger (n = 2) and desire to eat (n = 1), which recruited 1111 adults with overweight or obesity, and T2DM, non-alcoholic fatty liver disease, autosomal dominant polycystic kidney disease, and family history of breast cancer were without heterogeneity (I2 = 0%).32
Others
One non-significant association with high quality evidence was observed for 4 days-48 weeks IF than non-intervention diet for liver stiffness.63 Three associations (two significant and one non-significant) conducted on participants with normal weight, mildly obesity, overweight or obesity, T2DM, or metabolic syndrome, compared non-intervention diet or normal diet, reporting moderate evidence on Serum AST, Serum ALT, testosterone.34,63 Two non-significant associations with very low quality evidence were observed for 2–39 weeks IF than CER for prospective food consumption.32
Evidence quality
After applying the GRADE criteria, 43 (12%) and 144 (41%) associations were supported by high and moderate quality, respectively (Table 2 and Supplementary Table S5). Among these associations with high evidence quality, 10 associations showed statistical significance, including the following: IF (ADF, TRE, TWF, and ADF plus CR) for 4–12 weeks reduced WC,16 fat mass16 (n = 2), TG,16 TC,16 fasting insulin16 and increased FFM16 in adults with overweight or obesity compared with non-intervention diet; IF (MADF, TRE, and TWF) for 8–52 weeks reduced LDL-C and decreased SBP in adults with overweight or obesity or type 2 diabetics or metabolic syndrome patients or premenopausal women compared with a CER diet.14 IF (TWF, ADF, IER, and MADF) for 4–48 weeks increased HDL-C in individuals with overweight or obesity, and T2DM compared with CER diet.36
Sensitivity analyses
We performed several sensitivity analyses for evidence that significant effects were rated over moderate quality using the GRADE system. Firstly, after excluding RCTs with a high risk of bias, two associations were upgraded from moderate to high. IF (TRE, TRE plus RT, and TRE plus Diet) affected the reduction of BMI in adults with overweight or obesity, T2DM, metabolic syndrome compared with non-intervention diet.33 One to three months of IF (ADF, TRE, TWF, and ADF plus CR) affected the reduction of HOMA-IR in adults with overweight or obesity16 (Supplementary Table S6). Additionally, after excluding RCTs with a small sample size (25th percentile), one moderate quality association upgraded to high quality. IF (TRE, TRE plus RT, and TRE plus Diet) affected the reduction of fat mass in adults with overweight or obesity, T2DM, metabolic syndrome compared with non-intervention diet33 (Supplementary Table S7).
Subgroup analysis
The meta-analysis results classified based on population are reported in Supplementary Table S8. We emphasized that the effect of IF on health outcomes in comparing with non-intervention diet, unrestricted diet, continuous dieting, usual diet, or CER diet among patient with obesity, overweight, diabetic patients or metabolic syndrome. In the subgroup analysis according to participants with or without obesity, the subgroup with ten studies including participants with obesity showed significant reduction of fasting glucose (MD = −2.17 mmol/l; 95% CI: −2.78 to −1.55; p < 0.001) while the subgroup with five studies including participants without obesity did not show significant change of fasting glucose (MD = −0.96 mmol/l; 95% CI: −3.04 to 1.13; p = 0.369).33
Discussion
Our UR provides an evidence-based meta-analytic perspective on the effects of IF on various health outcomes. The findings indicate that IF is associated with favorable outcomes supported by high-quality evidence. These outcomes include reductions in WC, fat mass, LDL-C, TC, TG, fasting insulin, and SBP, while increase in HDL-C and FFM. The comprehensive insights provided by our UR hold potential value for clinicians in making informed medical decisions.
Our UR provides high-quality evidence supporting the effectiveness of IF in reducing fat mass when compared to CER or non-intervention diets. These findings are consistent with previous studies.64, 65, 66 A systematic review and meta-analysis consisting of 33 arms with 1610 participants showed that IF resulted in a significant reduction of fat mass (MD = −1.26 kg; 95% CI: −1.57 to −0.95; p < 0.05) when compared to control group.66 For example, an UR consisting of 11 meta-analyses and 130 RCTs showed that 1–2 months of fasting followed by MADF resulted in a reduction in healthy adults and individuals with overweight, obesity, or nonalcoholic fatty liver disease when compared to a regular diet.4 Nevertheless, a meta-analysis conducted by Zhang et al.27 in 2022 showed that there was no significant difference in fat mass after the IF intervention compared with CER based on 5 studies published between 2016 and 2021. The inconsistent results regarding the effect of IF on fat mass in different studies could be attributed to several factors, including variations in sample size and study duration. The average sample size of the meta-analyses varied, with Zhang et al.27 having a total sample size of 852 in their meta-analysis, Yang et al. having a sample size of 1,610, and our study having a sample size of 2483. These variations in sample size can influence the statistical power and precision of the results. The duration of the follow-up period in the studies also varied, with Zhang et al.27 ranging from 8 to 48 weeks, Yang et al. did not provide the duration of the study, and our study ranging from 4 to 12 weeks. The duration of follow-up can impact the observed effects on fat mass, as shorter follow-up durations may not be sufficient to capture significant changes in fat mass. On the other hand, longer follow-up durations allow for a more comprehensive assessment of the sustained effects of fasting on fat mass. Further research with larger sample sizes and longer follow-up durations is needed to provide more conclusive evidence on the effects of IF on BMI and body weight. The mechanisms underlying the role of IF in reducing the level of fat mass have been extensively studied. IF often leads to decreased overall caloric intake, improve insulin sensitivity and affect various hormones involved in appetite regulation and energy balance, which contribute to fat mass reduction.67, 68, 69
Regarding SBP, several studies have explored the changes in SBP after a period of IF compared to CER or non-intervention diets.12, 13, 14,53 A systematic review conducted by Cui et al. found that the ADF group showed statistically significant reductions in SBP compared to the regular diet group.13 Several studies showed no statistical difference between IF and CER on the effect to SBP,17,53 but a meta-study summarized the evidence from nine original studies showed that IF was less effective than CER in lowering blood pressure.14 However, the potential biological mechanisms underlying this effect are not well understood but early TRE may facilitate natriuresis, the excretion of salt in urine, by shifting salt intake to an earlier time of the day when sodium excretion is upregulated by the circadian system.70, 71, 72 IF can induce autophagy, which has been linked to improvements in cardiovascular health, including blood pressure regulation.73 IF increases the carriage of the bacterium Akkermansia muciniphila in gut, produces more propionate,74, 75, 76, 77 which has been shown to reduce blood pressure.78 On the contrary, another meta-analysis did not find beneficial effects of IF on SBP, possibly due to the small number of included RCTs.17 Further trials are needed to enhance the certainty of the evidence for this intervention.
Furthermore, our UR found high-certainty evidence supporting the positive effect of IF on fasting insulin levels. This finding is consistent with previous study that have demonstrated a slight reduction in fasting insulin concentrations caused by IF with IER regimens (MD = −0.89 μU/mL; 95% CI: −1.56 to −0.22; p = 0.009).21 The underlying mechanism behind the effect of IF on fasting insulin levels involves several metabolic pathways. IF helps regulate insulin levels by activating cellular repair processes, reducing overall calorie intake, improving insulin sensitivity, enhancing autophagic flux, promoting metabolic flexibility, and reducing in oxidative stress.79, 80, 81, 82, 83, 84
In our study, we found no perceptible alteration in fasting glucose levels.16 However, it is worth noting that there are studies with different findings. For instance, Chen et al.12 conducted a meta-analysis where participants underwent TRE for 6–48 weeks, and they observed a significant decrease in fasting blood glucose levels following the IF period compared to a habitual diet group. These contrasting results underscore the complexity of the relationship between IF and fasting blood glucose levels. Factors such as the duration of IF, the population, and individual metabolic differences may contribute to the observed discrepancies. Therefore, further research is necessary to gain a better understanding of the effects of IF on fasting blood glucose levels, taking into account various factors that may influence the outcomes.
In our study, we examined the effects of IF interventions (combined of MADF, TRE, and TWF, and respectively), on changes in hunger over time compared to a CER diet. Surprisingly, we found no significant fluctuate in hunger among participants who underwent the IF interventions (three low and one very low evidence). This finding may appear inconsistent with previous studies that have reported significant change in either group was a reduction in hunger at lunch at week 12 in early time restricted eating plus daily CR.85 The variation in study designs and methodologies could contribute to inconsistent results. Factors such as the duration of the interventions, research objects, and the specific protocols of each IF regimen, may differ across studies. These variations can lead to differences in hunger responses.
Subgroup analysis showed that for participants with obesity, the IF (TRE, TRE plus RT, TRE plus Diet) was able to lower fasting blood glucose compared to participants without obesity.33 There are several potential reasons for this differential response. Firstly, individuals with obesity often exhibit insulin resistance, which can lead to elevated blood glucose levels.86 TRE, by providing a structured eating window, may help improve insulin sensitivity and subsequently lower fasting blood glucose in this specific population.87 Additionally, obesity is associated with chronic inflammation, which can further contribute to impaired glucose metabolism.88,89 IF has been shown to have anti-inflammatory effects, which may help mitigate insulin resistance and improve glycemic control in individuals with obesity.90 It is important to note that these potential mechanisms are based on current scientific understanding and require further investigation to fully elucidate the underlying pathways.
The outcomes of our UR are not only related to obesity, diabetic, and metabolic syndrome patients but also to psychological disorders. However, the only two meta-analyses failed to provide sufficient data for recalculation and were excluded in the screening process.91,92 We present the results of these meta-analyses in the appendix materials (Supplementary Table S9). The evidence supporting the effectiveness of IF in alleviating depressive symptoms is still limited. However, the study conducted by Rodríguez et al. suggested a potential positive impact of IF on depressive symptoms.92 The underlying mechanisms that may explain this relationship include neuroplasticity and neurotrophic factors, autophagy and cellular repair, and hormonal regulation. It is important to note that while these mechanisms have been proposed, further research is necessary to fully understand the effects of IF on depression.
One of the strengths of our UR is its up-to-date comprehensive evaluation of published systematic reviews and meta-analyses on human health outcomes related to IF. In addition, it is important to note that our study was more methodologically rigorous than Patikorn et al.82 It did not incorporate a meta-analysis of observational studies on IF. In contrast, our UR did not impose any restrictions on the types of study designs encompassed initially. However, owing to the scarcity of available observational studies identified during the screening process,93 it was necessary to narrow down the range of study designs included in the review to solely randomised controlled studies. This adjustment was essential to ensure an adequate number of eligible studies for analysis. We conducted a thorough search in authoritative databases and implemented a rigorous screening, extraction, recalculating of effect sizes, methodological quality assessment, and evidence certainty evaluation in a systematic and independent manner by two authors. It's worth noting that all the included systematic reviews and meta-analyses achieved a moderate-to-high quality score according to AMSTAR. More than half of the associations examined in this UR received a quality assessment of over moderate quality according to the GRADE guidelines.
Nevertheless, there are several limitations to consider in our study. First, to account for the discrepancies in the populations, study designs, or other characteristics of the studies included in each meta-analysis, we used an I2 >50% as a criterion for downgrading by one or two levels for inconsistency,39,94 to assign the highest quality of evidence to robust associations without heterogeneity. Unfortunately, some associations (42%) showed high or very high heterogeneity. However, this phenomenon is common in several published UR.95, 96, 97 For example, in a previous UR on the influencing factors of statins on multiple non-cardiovascular outcomes, He et al. found that 52% of the identified associations had large heterogeneity.95 Second, about half (48%) of the meta-analyses in our study included less than 10 original studies, which may reduce the statistical power of Egger's and excess significance tests.98 Third, our work depended on prior meta-analyses, which might miss some individual studies. Fourth, we only included articles that provided data of mean with SD,55 whereas articles that provided insufficient or inadequate data for quantitative were excluded (Supplementary Table S3), which also explained why the meta-analyses included in the UR of Patikorn et al.4 were not included in our study. As a result, some systematic reviews and meta-analyses on the relationship between IF and health outcomes may have been overlooked. However, we made efforts to summarize findings from such meta-analyses to ensure that any relevant research was considered (Supplementary Table S8). In addition to the limitations mentioned earlier, one notable limitation is that our UR did not perform a quantitative analysis of the side effects of IF. However, some original studies have reported certain side effects associated with IF interventions. For instance, Cienfuegos et al. reported adverse events from 4- and 6-h TRE interventions, such as dizziness, nausea, headache, and diarrhea.51 Harvie et al. also reported side effects of IF interventions, including physical symptoms like feeling cold and constipation, as well as psychological symptoms like headache, lack of energy, irritability, and difficulty concentrating.99 These side effects should be considered when implementing IF interventions and further research is needed to thoroughly evaluate the incidence and severity of potential side effects. Lastly, due to the inherent limitations of UR, we did not investigate the specific type of IF that may be more effective for health outcomes. However, there are several relevant network meta-analyses registered on PROSPERO that may explore this aspect in future research.
In conclusion, this UR has systematically assimilated this vast amount of existing evidence where it has been published in a meta-analysis. All the evidence comes from RCTs and 53% of the associations were supported by over moderate quality using GRADE system. IF could beneficially affect a range of health outcomes (decreased WC, fat mass, LDL-C, TG, TC, fasting insulin, and SBP; increased HDL-C and FFM) for adults with overweight or obesity compared to CER or non-intervention diet. Further studies are warranted to evaluate the effects of IF on multiple health outcomes and investigate their underlying mechanisms.
Contributors
M-LS, WY, SG, Y-HZ, Q-JW, and T-TG contributed to the study design. M-LS, WY, MZ, and Z-YS collection of data. M-LS, WY, FC, and B-JZ analysis of data. M-LS, WY, X-YW, SG, KV, SF, M-HS, K-XL, QB, JX, QX, Y-HZ, LW, D-YZ, Q-JW, and T-TG wrote the first draft of the manuscript and edited the manuscript. All authors read and approved the final manuscript. M-LS, WY, X-YW, and SG contributed equally to this work.
Data sharing statement
The data supporting the conclusions of this article can be directed to the corresponding author.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
The UR study was supported by the National Key Research and Development Program of China (No. 2022YFC2704205 to Q-JW), the Natural Science Foundation of China (No. 82073647 and No. 82373674 to Q-JW and No. 82103914 to T-TG), Outstanding Scientific Fund of Shengjing Hospital of China Medical University (Q-JW), and 345 Talent Project of Shengjing Hospital of China Medical University (T-TG). We thank the research team for their daily efforts in study design, data collection, data analysis, data interpretation, and manuscript writing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102519.
Contributor Information
Yu-Hong Zhao, Email: zhaoyh@sj-hospital.org.
De-Yu Zhang, Email: zhangdy@sj-hospital.org.
Qi-Jun Wu, Email: wuqj@sj-hospital.org.
Ting-Ting Gong, Email: gongtt@sj-hospital.org.
Appendix A. Supplementary data
References
- 1.Persynaki A., Karras S., Pichard C. Unraveling the metabolic health benefits of fasting related to religious beliefs: a narrative review. Nutrition. 2017;35:14–20. doi: 10.1016/j.nut.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Hatting M., Rines A.K., Luo C., et al. Adipose tissue CLK2 promotes energy expenditure during high-fat diet intermittent fasting. Cell Metab. 2017;25(2):428–437. doi: 10.1016/j.cmet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton S.D., Moehl K., Donahoo W.T., et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring) 2018;26(2):254–268. doi: 10.1002/oby.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patikorn C., Roubal K., Veettil S.K., et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trepanowski J.F., Bloomer R.J. The impact of religious fasting on human health. Nutr J. 2010;9:57. doi: 10.1186/1475-2891-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandhorst S., Choi I.Y., Wei M., et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo V.D., Mattson M.P. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad R. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2020;382(18):1773. doi: 10.1056/NEJMc2001176. [DOI] [PubMed] [Google Scholar]
- 9.Lamos E.M., Malek R., Munir K.M. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2020;382(18):1771. doi: 10.1056/NEJMc2001176. [DOI] [PubMed] [Google Scholar]
- 10.de Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 11.Nencioni A., Caffa I., Cortellino S., Longo V.D. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11):707–719. doi: 10.1038/s41568-018-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J.H., Lu L.W., Ge Q., et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;63(15):2331–2347. doi: 10.1080/10408398.2021.1974335. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y., Cai T., Zhou Z., et al. Health effects of alternate-day fasting in adults: a systematic review and meta-analysis. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.586036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.K., Kang J.H., Kim E.M. Updated meta-analysis of studies from 2011 to 2021 comparing the effectiveness of intermittent energy restriction and continuous energy restriction. J Obes Metab Syndr. 2022;31(3):230–244. doi: 10.7570/jomes22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elortegui P.P., Rolands M.R., Eldridge A.L., et al. A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obesity (Silver Spring) 2023;31 Suppl 1(Suppl 1):9–21. doi: 10.1002/oby.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L., Fu R., Hong J., Ni H., Yu K., Lou H. Effects of intermittent fasting in human compared to a non-intervention diet and caloric restriction: a meta-analysis of randomized controlled trials. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.871682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Nian B., Li R., et al. Fasting and metabolic syndrome: a systematic review and meta-analyses. Crit Rev Food Sci Nutr. 2022;64:1–9. doi: 10.1080/10408398.2022.2119362. [DOI] [PubMed] [Google Scholar]
- 18.Wang J., Wang F., Chen H., et al. Comparison of the effects of intermittent energy restriction and continuous energy restriction among adults with overweight or obesity: an overview of systematic reviews and meta-analyses. Nutrients. 2022;14(11):2315. doi: 10.3390/nu14112315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Li Q., Liu Y., Jiang H., Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;179 doi: 10.1016/j.diabres.2021.109003. [DOI] [PubMed] [Google Scholar]
- 20.Zaki H.A., Iftikhar H., Abdalrubb A., et al. Clinical assessment of intermittent fasting with ketogenic diet in glycemic control and weight reduction in patients with type II diabetes mellitus: a systematic review and meta-analysis. Cureus. 2022;14(10) doi: 10.7759/cureus.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cioffi I., Evangelista A., Ponzo V., et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. doi: 10.1186/s12967-018-1748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng H., Zhu L., Kord-Varkaneh H., O Santos H., Tinsley G.M., Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020;77 doi: 10.1016/j.nut.2020.110801. [DOI] [PubMed] [Google Scholar]
- 23.Moon S., Kang J., Kim S.H., et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12(5):1267. doi: 10.3390/nu12051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J., Seo Y.G., Paek Y.J., Song H.J., Park K.H., Noh H.M. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154336. [DOI] [PubMed] [Google Scholar]
- 25.Pureza I., Macena M.L., Da S.J.A.E., Praxedes D.R.S., Vasconcelos L.G.L., Bueno N.B. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: a systematic review with meta-analysis. Clin Nutr. 2021;40(4):1788–1799. doi: 10.1016/j.clnu.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini M., Cioffi I., Evangelista A., et al. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):17–33. doi: 10.1007/s11154-019-09524-w. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Zhang C., Wang H., et al. Intermittent fasting versus continuous calorie restriction: which is better for weight loss? Nutrients. 2022;14(9):1781. doi: 10.3390/nu14091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusar-Poli P., Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. doi: 10.1136/ebmental-2018-300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51(20):1456–1458. doi: 10.1136/bjsports-2017-097621. [DOI] [PubMed] [Google Scholar]
- 30.Dinu M., Pagliai G., Angelino D., et al. Effects of popular diets on anthropometric and cardiometabolic parameters: an umbrella review of meta-analyses of randomized controlled trials. Adv Nutr. 2020;11(4):815–833. doi: 10.1093/advances/nmaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allaf M., Elghazaly H., Mohamed O.G., et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;1(1) doi: 10.1002/14651858.CD013496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsworth R.L., Monge A., Perry R., et al. The effect of intermittent fasting on appetite: a systematic review and meta-analysis. Nutrients. 2023;15(11):2604. doi: 10.3390/nu15112604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang X., Chen J., An X., et al. The optimal time restricted eating interventions for blood pressure, weight, fat mass, glucose, and lipids: a meta-analyses and systematic review. Trends Cardiovasc Med. 2023;S1050-1738(23):00087. doi: 10.1016/j.tcm.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Xu Y., Mu X., Shen J. The effects of time restricted feeding on weight loss and other changes of anthropometric parameters among physically active individuals. Sci Sports. 2023;39(1):87–95. [Google Scholar]
- 35.Silverii G.A., Cresci B., Benvenuti F., Santagiuliana F., Rotella F., Mannucci E. Effectiveness of intermittent fasting for weight loss in individuals with obesity: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2023;33(8):1481–1489. doi: 10.1016/j.numecd.2023.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Xu R., Cao Y., Wang P.Y., Chen X.L., Tao D. Intermittent energy restriction vs. continuous energy restriction on cardiometabolic risk factors in patients with metabolic syndrome: a meta-analysis and systematic review. Front Nutr. 2023;10 doi: 10.3389/fnut.2023.1090792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaman M.K., Teng N.I.M.F., Kasim S.S., Juliana N., Alshawsh M.A. Effects of time-restricted eating with different eating duration on anthropometrics and cardiometabolic health: a systematic review and meta-analysis. World J Cardiol. 2023;15(7):354–374. doi: 10.4330/wjc.v15.i7.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng L., Li H.-R., Liu M.-W., Rao W.M., He Q.-Q. Effects of intermittent fasting on cardiometabolic risk factors in patients with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2022;31(4):642–659. doi: 10.6133/apjcn.202212_31(4).0008. [DOI] [PubMed] [Google Scholar]
- 39.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Chew H.S.J., Ang W.H.D., Tan Z.Y.A., Ang W.W., Chan K.S., Lau Y. Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile. Nutr Rev. 2023;81(9):1180–1199. doi: 10.1093/nutrit/nuac103. [DOI] [PubMed] [Google Scholar]
- 41.Dragioti E., Solmi M., Favaro A., et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatr. 2019;76(12):1241–1255. doi: 10.1001/jamapsychiatry.2019.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannidis J.P. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181(8):488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 44.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Headland M., Clifton P.M., Carter S., Keogh J.B. Weight-Loss outcomes: a systematic review and meta-analysis of intermittent energy restriction trials lasting a minimum of 6 months. Nutrients. 2016;8(6):354. doi: 10.3390/nu8060354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoogian E.N.C., Chow L.S., Taub P.R., Laferrere B., Panda S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr Rev. 2022;43(2):405–436. doi: 10.1210/endrev/bnab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tinsley G.M., Forsse J.S., Butler N.K., et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 48.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br J Nutr. 2018;119(5):507–516. doi: 10.1017/S0007114517003890. [DOI] [PubMed] [Google Scholar]
- 49.Schroor M.M., Joris P.J., Plat J., Mensink R.P. Effects of intermittent energy restriction compared with those of continuous energy restriction on body composition and cardiometabolic risk markers - a systematic review and meta-analysis of randomized controlled trials in adults. Adv Nutr. 2024;15(1) doi: 10.1016/j.advnut.2023.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roman Y.M., Dominguez M.C., Easow T.M., Pasupuleti V., White C.M., Hernandez A.V. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: a systematic review and meta-analysis of randomized controlled trials. Int J Obes. 2019;43(10):2017–2027. doi: 10.1038/s41366-018-0204-0. [DOI] [PubMed] [Google Scholar]
- 51.Cienfuegos S., Gabel K., Kalam F., et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366–378.e3. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris L., Hamilton S., Azevedo L.B., et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16(2):507–547. doi: 10.11124/JBISRIR-2016-003248. [DOI] [PubMed] [Google Scholar]
- 53.Schwingshackl L., Zahringer J., Nitschke K., et al. Impact of intermittent energy restriction on anthropometric outcomes and intermediate disease markers in patients with overweight and obesity: systematic review and meta-analyses. Crit Rev Food Sci Nutr. 2021;61(8):1293–1304. doi: 10.1080/10408398.2020.1757616. [DOI] [PubMed] [Google Scholar]
- 54.Perichart-Perera O., Balas-Nakash M., Munoz-Manrique C., et al. Structured hypocaloric diet is more effective than behavioral therapy in reducing metabolic syndrome in Mexican postmenopausal women: a randomized controlled trial. Menopause. 2014;21(7):711–720. doi: 10.1097/GME.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 55.Rosson S., de Filippis R., Croatto G., et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: an umbrella review. Neurosci Biobehav Rev. 2022;139 doi: 10.1016/j.neubiorev.2022.104743. [DOI] [PubMed] [Google Scholar]
- 56.Shea B.J., Reeves B.C., Wells G., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 58.Dechartres A., Altman D.G., Trinquart L., Boutron I., Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014;312(6):623–630. doi: 10.1001/jama.2014.8166. [DOI] [PubMed] [Google Scholar]
- 59.Patikorn C., Saidoung P., Pham T., et al. Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials. BMC Med. 2023;21(1):196. doi: 10.1186/s12916-023-02874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mercuri M., Gafni A. The evolution of GRADE (part 3): a framework built on science or faith? J Eval Clin Pract. 2018;24(5):1223–1231. doi: 10.1111/jep.13016. [DOI] [PubMed] [Google Scholar]
- 61.Harris L., McGarty A., Hutchison L., Ells L., Hankey C. Short-term intermittent energy restriction interventions for weight management: a systematic review and meta-analysis. Obes Rev. 2017;19(1):1–13. doi: 10.1111/obr.12593. [DOI] [PubMed] [Google Scholar]
- 62.He S., Wang J., Zhang J., Xu J. Intermittent versus continuous energy restriction for weight loss and metabolic improvement: a meta-analysis and systematic review. Obesity (Silver Spring) 2021;29(1):108–115. doi: 10.1002/oby.23023. [DOI] [PubMed] [Google Scholar]
- 63.Lange M., Nadkarni D., Martin L., Newberry C., Kumar S., Kushner T. Impact of intermittent fasting on anthropometric and clinical outcomes in non-alcoholic fatty liver disease: systematic review and meta-analysis. J Hepatol. 2022;77:S168. doi: 10.1097/HC9.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Correia J.M., Santos I., Pezarat-Correia P., Minderico C., Mendonca G.V. Effects of intermittent fasting on specific exercise performance outcomes: a systematic review including meta-analysis. Nutrients. 2020;12(5):1390. doi: 10.3390/nu12051390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan S., Wang C., Zhao H., et al. Effects of fasting intervention regulating anthropometric and metabolic parameters in subjects with overweight or obesity: a systematic review and meta-analysis. Food Funct. 2020;11(5):3781–3799. doi: 10.1039/d0fo00287a. [DOI] [PubMed] [Google Scholar]
- 66.Yang F., Liu C., Liu X., et al. Effect of epidemic intermittent fasting on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.669325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fontana L., Partridge L., Longo V.D. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moro T., Tinsley G., Bianco A., et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patterson R.E., Sears D.D. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 70.Mattson M.P., Longo V.D., Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston J.G., Speed J.S., Jin C., Pollock D.M. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol. 2016;311(5):F991–F998. doi: 10.1152/ajprenal.00103.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varady K.A., Cienfuegos S., Ezpeleta M., Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18(5):309–321. doi: 10.1038/s41574-022-00638-x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang S., Liu X., Bawa-Khalfe T., et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 74.Bajic D., Niemann A., Hillmer A.K., et al. Gut microbiota-derived propionate regulates the expression of Reg3 mucosal lectins and ameliorates experimental colitis in mice. J Crohns Colitis. 2020;14(10):1462–1472. doi: 10.1093/ecco-jcc/jjaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su J., Braat H., Peppelenbosch M.P. Gut microbiota-derived propionate production may explain beneficial effects of intermittent fasting in experimental colitis. J Crohns Colitis. 2021;15(6):1081–1082. doi: 10.1093/ecco-jcc/jjaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gabel K., Marcell J., Cares K., et al. Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health. 2020;26(2):79–85. doi: 10.1177/0260106020910907. [DOI] [PubMed] [Google Scholar]
- 77.Vallianou N., Stratigou T., Christodoulatos G.S., Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: current evidence and perspectives. Curr Obes Rep. 2019;8(3):317–332. doi: 10.1007/s13679-019-00352-2. [DOI] [PubMed] [Google Scholar]
- 78.Hu T., Wu Q., Yao Q., Jiang K., Yu J., Tang Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev. 2022;81 doi: 10.1016/j.arr.2022.101706. [DOI] [PubMed] [Google Scholar]
- 79.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017;76(3):361–368. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- 81.Barnosky A.R., Hoddy K.K., Unterman T.G., Varady K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164(4):302–311. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 82.Patterson R.E., Laughlin G.A., LaCroix A.Z., et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet. 2015;115(8):1203–1212. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cienfuegos S., McStay M., Gabel K., Varady K.A. Time restricted eating for the prevention of type 2 diabetes. J Physiol. 2022;600(5):1253–1264. doi: 10.1113/JP281101. [DOI] [PubMed] [Google Scholar]
- 84.Forslund S.K. Fasting intervention and its clinical effects on the human host and microbiome. J Intern Med. 2023;293(2):166–183. doi: 10.1111/joim.13574. [DOI] [PubMed] [Google Scholar]
- 85.Thomas E.A., Zaman A., Sloggett K.J., et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity (Silver Spring) 2022;30(5):1027–1038. doi: 10.1002/oby.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tong Y., Xu S., Huang L., Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov Today. 2022;27(3):822–830. doi: 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 87.van den Burg E.L., van Peet P.G., Schoonakker M.P., van de Haar D.E., Numans M.E., Pijl H. Metabolic impact of intermittent energy restriction and periodic fasting in patients with type 2 diabetes: a systematic review. Nutr Rev. 2023;81(10):1329–1350. doi: 10.1093/nutrit/nuad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wondmkun Y.T. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611–3616. doi: 10.2147/DMSO.S275898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Artemniak-Wojtowicz D., Kucharska A.M., Pyrzak B. Obesity and chronic inflammation crosslinking. Cent Eur J Immunol. 2020;45(4):461–468. doi: 10.5114/ceji.2020.103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turner L., Charrouf R., Martinez-Vizcaino V., Hutchison A., Heilbronn L.K., Fernandez-Rodriguez R. The effects of time-restricted eating versus habitual diet on inflammatory cytokines and adipokines in the general adult population: a systematic review with meta-analysis. Am J Clin Nutr. 2024;119(1):206–220. doi: 10.1016/j.ajcnut.2023.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Berthelot E., Etchecopar-Etchart D., Thellier D., Lancon C., Boyer L., Fond G. Fasting interventions for stress, anxiety and depressive symptoms: a systematic review and meta-analysis. Nutrients. 2021;13(11):3947. doi: 10.3390/nu13113947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandez-Rodriguez R., Martinez-Vizcaino V., Mesas A.E., Notario-Pacheco B., Medrano M., Heilbronn L.K. Does intermittent fasting impact mental disorders? A systematic review with meta-analysis. Crit Rev Food Sci Nutr. 2022;63:1–16. doi: 10.1080/10408398.2022.2088687. [DOI] [PubMed] [Google Scholar]
- 93.Horne B.D., Muhlestein J.B., May H.T., et al. Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 2012;109(11):1558–1562. doi: 10.1016/j.amjcard.2012.01.379. [DOI] [PubMed] [Google Scholar]
- 94.Balshem H., Helfand M., Schunemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 95.He Y., Li X., Gasevic D., et al. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann Intern Med. 2018;169(8):543–553. doi: 10.7326/M18-0808. [DOI] [PubMed] [Google Scholar]
- 96.Huang Y., Chen Z., Chen B., et al. Dietary sugar consumption and health: umbrella review. BMJ. 2023;381 doi: 10.1136/bmj-2022-071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poole R., Kennedy O.J., Roderick P., Fallowfield J.A., Hayes P.C., Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bellou V., Belbasis L., Tzoulaki I., Evangelou E., Ioannidis J.P. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9. doi: 10.1016/j.parkreldis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Harvie M.N., Sims A.H., Pegington M., et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016;18(1):57. doi: 10.1186/s13058-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.