Abstract
Herpes simplex virus type 1 glycoproteins gB, gD, and gHgL were expressed by transient transfection of Cos cells. Polykaryocyte formation above the background level seen in untransfected controls was observed only if all three components were expressed. Thus, gB, gD, and gHgL are necessary and sufficient to induce membrane fusion.
Membrane fusion is essential for the entry, cell-associated spread, and syncytial formation of enveloped viruses and is mediated by envelope glycoproteins (19, 23). Herpes simplex virus (HSV) encodes at least 10 glycoproteins (18), and many attempts have been made to identify those that are responsible for membrane fusion. Virions lacking glycoprotein B, D, H, or L fail to infect cells, but infectivity can be partially restored by the artificial fusogen polyethyleneglycol, implying that the glycoproteins are all required for membrane fusion (3, 7, 13, 17). Similarly, expression of these four glycoproteins is required for polykaryocyte formation by syncytial strains (3, 5, 13, 17), but mutants lacking dispensable glycoproteins gE, gI, or gM or the UL45 gene product are also deficient in polykaryocyte formation, implying a role for these proteins in plasma membrane fusion but not in virion entry (1, 5, 9, 14, 21). The studies of mutant viruses therefore implicate multiple HSV type 1 (HSV-1) membrane glycoproteins in the fusion process, but the assays used are indirect, and it is uncertain how many species are directly involved. Attempts to induce cell fusion by expressing HSV-1 glycoproteins individually or in combination have given conflicting results. There are reports that gB or gD will increase polykaryocyte formation in cells which constitutively express these proteins (2, 4), a finding that is difficult to reconcile with the results of studies of virus mutants described above. In contrast, the expression of many different combinations of HSV-1 glycoproteins with vaccinia virus or adenovirus vectors has failed to induce cell fusion (5, 15). The current view is that gB, gD, gH, and gL are required for postattachment virion entry and for virus-induced polykaryocyte formation, but it is unclear whether they are all involved directly in membrane fusion, and it is possible that other membrane proteins are also required.
To investigate whether HSV gB, gD, gH, and gL were sufficient to induce fusion, we expressed these glycoproteins under the adenovirus major late promoter of the Cos cell expression vector pSMH3. The construction of pSMH3gH (expressing gH) and pSMH3gL (expressing gL) has been described previously (24). Glycoproteins gH and gL are known to form a heterodimer (12) and will be considered as a single component in this study. Ligation of the gB coding region from pING14.2gB (nucleotides 52765 to 56099) (1) into the EcoRI site of pSMH3 generated pSMH3gB, while gD coding sequences (nucleotides 138345 to 139762) derived from pRVF6 (21) were excised as a HindIII-NruI fragment and ligated between the HindIII and EcoRV sites of pSMH3 to generate pSMH3gD. Immunofluorescence was used to confirm that all the constructs expressed the expected glycoproteins on the surfaces of transfected Cos7 cells (data not shown).
Cos cells seeded at 5 × 104 cells/10-cm2 dish were transfected with 0.5 μg of each plasmid, 2 μg of pSMH3gD alone, or no DNA, by a DEAE–dextran-mediated method (24). Two days after transfection, an overlay of 2 × 105 Vero cells was added to each dish. After a further 24 h, monolayers were examined for multinucleate cells by phase-contrast microscopy. On monolayers expressing gB, gD, and gHgL, polykaryocytes with 10 to 50 nuclei were routinely observed, but these were rarely seen on control wells transfected with the gD plasmid alone or with no DNA.
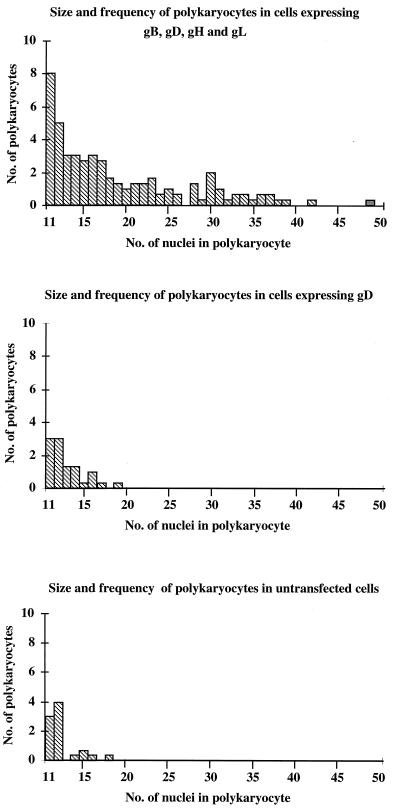
To quantify the extent of fusion induced, experimental and control wells were coded and counted blind by three independent observers. The number of polykaryocytes with more than 10 aggregated nuclei and the size of each polykaryocyte as judged by the number of nuclei it contained were scored. Figure 1 shows the means of the results obtained by the three observers comparing 8-cm2 areas expressing the four proteins gB, gD, and gHgL, gD alone, or no HSV proteins in a single experiment. While an average of 46.3 polykaryocytes was seen on the area expressing all four proteins, the largest polykaryocyte having 49 nuclei, the mean number of polykaryocytes seen on control wells was only 10.6 or 8.6, with the largest having 19 nuclei.
FIG. 1.
Polykaryocyte induction by HSV-1. Cos cells were transfected with the gB, gD, gH, and gL genes; the gD gene; or no DNA. Three independent counters scored the number and size of polykaryocytes with more than 10 nuclei that were observed on an 8-cm2 area, for each condition. Mean results are shown.
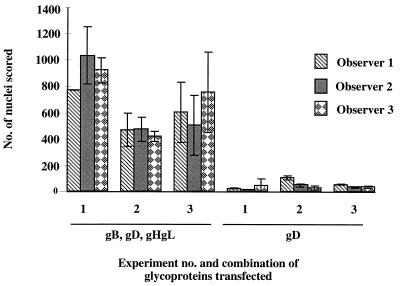
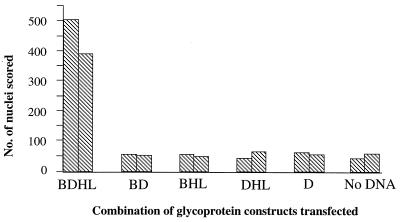
These data strongly indicated that gB, gD, and gHgL could induce a significant increase in fusion compared to controls. However, because the frequency of polykaryocytes observed was low, and the number scored was somewhat subjective, later results were expressed as the total number of nuclei involved in polykaryocytes with more than 10 nuclei. Although there was variation in the number of nuclei scored between observers and between experiments (Fig. 2), transfection of plasmids expressing gB, gD, and gHgL gave 10- to 20-fold more nuclei involved in fusion than in controls. Having demonstrated that gB, gD, and gHgL were sufficient to induce membrane fusion in this system, we determined whether they were all necessary by omitting each component individually. Figure 3 shows that expression of all four proteins was required to promote fusion above background levels, although the efficiency of polykaryocyte formation is relatively low. This result may reflect the difficulty of getting adequate levels of expression of all four glycoproteins simultaneously in every transfected cell. Indeed, immunofluorescence studies suggest that not every transfected COS cell expresses the full complement of HSV glycoproteins, and therefore not every transfected COS cell is likely to give rise to a syncytium.
FIG. 2.
Variation in fusion scored in three experiments. Shown is the total number of nuclei in polykaryocytes with more than 10 nuclei, scored by each observer, on 8-cm2 areas transfected with plasmids expressing HSV-1 gB, gD, and gHgL or only gD.
FIG. 3.
Effect of omitting individual glycoproteins on polykaryocyte formation. The total number of nuclei in polykaryocytes with more than 10 nuclei was scored on 4-cm2 areas transfected with HSV-1 genes encoding gB, gD, gH, and gL, various subsets of these genes, or no DNA. The mean scores of three independent observers are plotted.
HSV-1 glycoproteins B, D, and HL are thought to form a complex (10, 11), and studies with mutant viruses suggest that all these proteins are required for membrane fusion. Our studies confirm this view. The situation may be different for other members of the herpesvirus family, since there are reports that both the varicella-zoster virus gHgL complex and human cytomegalovirus gB are individually capable of mediating polykaryocyte formation (6, 20). That the requirements for fusion may differ in other herpesviruses is perhaps not surprising; it has been known for some time that in the alphaherpesvirus pseudorabies, gD is dispensable for cell-cell spread (16), a process that is assumed to involve plasma membrane fusion.
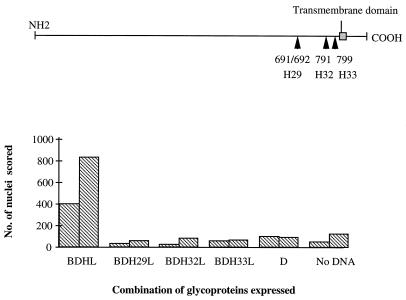
We consider the assay described here to be a measure of HSV-1 membrane fusion, and consistent with this view, we found that mutant gH proteins containing insertions at amino acid residues 691, 791, and 799 and known to be nonfunctional in HSV-1 entry and spread (8) were also nonfunctional in the fusion assay (Fig. 4). It is unclear, however, whether our system more closely resembles virus-cell or cell-cell fusion. The fusion scored is that between plasma membranes; thus, the degree of curvature and the lipid composition of the fusing membranes are similar to those of the membranes participating in cell-cell fusion. However, there is no absolute requirement for glycoproteins gE, gI, or gM, all of which have been implicated in cell-cell fusion. Furthermore, we found that the level of fusion was not increased when a syncytial mutation (Ala 855→Val) was introduced into the gB expression plasmid (data not shown), despite the fact that this mutation results in a dramatic increase in fusion in the context of virus infection (1). It would be interesting to see whether the pseudorabies homologs gB and gHgL could mediate fusion in the absence of gD in an analogous system.
FIG. 4.
Ability of gH mutants to mediate fusion. (Top panel) Diagram of mutant gH proteins containing insertions at amino acid residues 691, 791, and 799. (Bottom panel) The total number of nuclei in polykaryocytes with more than 10 nuclei was scored on 4-cm2 areas transfected with plasmids expressing gB, gD, and gL, together with either wild-type or mutant gH proteins. The mean scores of two independent observers are plotted.
We describe an assay which allows the measurement of HSV-1-induced membrane fusion in the absence of HSV-1 infection. Our results show that HSV-1 gB, gD, and gHgL are necessary and sufficient, and this assay should facilitate analysis of the fusion process.
Acknowledgments
This work was supported by the Wellcome Trust, United Kingdom.
REFERENCES
- 1.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoprotein gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 2.Butcher M, Raviprakash K, Ghosh H P. Acid pH-induced fusion of cells by herpes simplex virus glycoproteins. J Biol Chem. 1990;265:5862–5868. [PubMed] [Google Scholar]
- 3.Cai W, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62:2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Avitabile E, Fini S, Stirpe D, Arsenakis M, Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988;166:598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- 5.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duus K M, Grose C. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J Virol. 1996;70:8961–8971. doi: 10.1128/jvi.70.12.8961-8971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galdiero M, Whiteley A, Bruun B, Bell S, Minson T, Browne H. Site-directed and linker insertion mutagenesis of herpes simplex virus type 1 glycoprotein H. J Virol. 1997;71:2163–2170. doi: 10.1128/jvi.71.3.2163-2170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haanes E J, Nelson C M, Soule C L, Goodman J L. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J Virol. 1994;68:5825–5834. doi: 10.1128/jvi.68.9.5825-5834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handler C G, Cohen G H, Eisenberg R J. Cross-linking of glycoprotein oligomers during herpes simplex virus type 1 entry. J Virol. 1996;70:6076–6082. doi: 10.1128/jvi.70.9.6076-6082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6075. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson A C, Johnson D C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean C A, Robertson L M, Jamieson F E. Characterisation of the UL10 gene product of herpes simplex virus type 1 and investigation of its role in vivo. J Gen Virol. 1993;74:975–983. doi: 10.1099/0022-1317-74-6-975. [DOI] [PubMed] [Google Scholar]
- 15.Novotny M J, Parish M L, Spear P G. Variability of herpes-simplex virus-1 gL and anti-gL antibodies that inhibit cell-fusion but not viral infectivity. Virology. 1996;221:1–13. doi: 10.1006/viro.1996.0347. [DOI] [PubMed] [Google Scholar]
- 16.Rauh I, Mettenleiter T C. Pseudorabies virus proteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 19.Stegmann T, Doms R W, Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- 20.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralising antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 21.Visalli R J, Brandt C R. The HSV-1 UL45 gene product is not required for growth in Vero cells. Virology. 1991;185:419–423. doi: 10.1016/0042-6822(91)90790-i. [DOI] [PubMed] [Google Scholar]
- 22.Watson R J, Weis J H, Salstrom J S, Enquist L W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982;218:381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- 23.White J M. Membrane fusion. Science. 1992;258:971–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 24.Wilson D W, Davis-Poynter N, Minson A C. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J Virol. 1994;68:6985–6993. doi: 10.1128/jvi.68.11.6985-6993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]