Abstract
Ulcerative colitis is a common type of inflammatory bowel disease that affects millions of individuals around the world. Traditional UC treatment has focused on suppressing immune responses rather than treating the underlying causes of UC, which include oxidative stress, inflammation, and microbiota dysbiosis. Diosmin (DIO), a naturally occurring flavonoid, possesses antioxidant and anti-inflammatory properties. This study aimed to assess the efficacy of DIO in treating dextran-sulfate sodium (DSS)-induced colitis, and to investigate some of its underlying mechanisms, with an emphasis on Akkermansia muciniphila abundance, inflammatory markers, and intestinal barrier function. C57BL/6 mice were given 4% (w/v) DSS to induce colitis. DSS-induced mice were administered DIO (100 and 200 mg/kg) or sulfasalazine orally for 7 days. Every day, the disease activity index (DAI) was determined by recording body weight, diarrhea, and bloody stool. Changes in fecal A. muciniphila abundance, colonic MUC1 and MUC2 expression, as well as oxidative stress and inflammatory markers were all assessed. Histopathological changes, colonic PIK3PR3 and ZO-1 levels, and immunohistochemical examinations of occludin and claudin-1, were investigated. DIO administration resulted in a dose-dependent decrease in DAI, as well as increase in A. muciniphila abundance and MUC2 expression while decreasing MUC1 expression. DIO also dramatically reduced colonic oxidative stress and inflammation by regulating the NF-κB and Nrf2 cascades, restored intestinal barrier integrity by inhibiting PIK3R3 and inducing ZO-1, and improved occludin/claudin-1 gene expression and immunostaining. This study provides the first evidence that DIO preserves intestinal barrier integrity and increases A. muciniphila abundance in DSS-induced colitis. However, more research is required to explore the impact of DIO on the overall composition and diversity of the gut microbiota. Likewise, it will be important to fully understand the molecular mechanisms by which A. muciniphila maintains intestinal barrier function and its potential use as an adjuvant in the treatment of UC.
Keywords: Ulcerative colitis, Diosmin, Akkermansia muciniphila, Inflammation, Oxidative stress, Intestinal barrier integrity
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that causes stomach pain, intestinal inflammation, and bloody diarrhea [1], all of which significantly reduce patients' quality of life [2]. UC is becoming more common year after year, enhancing the possibility of colorectal cancer [2]. UC pathogenesis is complex, with oxidative stress, intestinal mucosal damage, inflammation, and microbial dysregulation all playing critical roles [[3], [4], [5]]. When the gut's immune system is active, free radicals and pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) are produced resulting in inflamed intestinal mucosa [6]. Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a genetic factor that improve cell homeostasis in UC by combating reactive oxygen species (ROS) and inflammation [7]. While Heme oxygenase-1 (HO-1) is an antioxidant enzyme that elevates in response to oxidative stress [8]. Hence, pharmacological Nrf2/HO-1 pathway activation could be a promising UC treatment option.
Surprisingly, abnormalities in the intestinal mucosal barrier function, which is a defining hallmark of UC, result in a defective mucus layer or increased epithelial barrier permeability, allowing microbial invasion [9]. The intestinal mucosal barrier is formed by epithelial cell tight junctions (TJs), which are composed of transmembrane proteins (occludins and claudins) and accessory proteins (zonula occluden, ZO-1) that prevent spreading of pathogens and hazardous antigens across the epithelium [10]. Akkermansia muciniphila (A. muciniphila) is a Verrucomicrobia gram-negative anaerobic bacteria that lowers pro-inflammatory cytokines production and promotes gut flora normalization [11]. Furthermore, A. muciniphila secretes glycoside hydrolases, proteases, sulfate, and sialic acid, which breakdowns mucin 1 (MUC1), resulting in decreased colon inflammation [12]. Likewise, it stimulates mucin 2 (MUC2) expression in intestinal epithelial cells, assisting in the maintenance of appropriate intestinal barrier function [13].
Corticosteroids, mesalamine, and immunosuppressive medicines, which are currently utilized to treat UC, are linked with serious adverse effects and do not give long-term cures [14]. Natural products are promising alternatives to conventional therapy for the UC treatment [15]. Flavonoids are polyphenolic substances having anti-inflammatory and antioxidant properties, with good safety and few adverse effects [16]. Diosmin (DIO), a flavone glycoside, exhibits potent venoprotective [17], antioxidant and anti-inflammatory properties [18]. However, only few studies have looked at the effect of DIO on UC [19,20], and they found that it suppressed inflammation, apoptosis, and oxidative damage in colitis. However, no previous research has been undertaken to assess the modulatory effects of DIO on A. muciniphila abundance and intestinal barrier function alterations that occur during UC. Accordingly, the purpose of this study is to evaluate the efficacy of DIO in alleviating experimentally induced colitis, with a focus on A. muciniphila abundance, and intestinal barrier function. Our findings suggest that DIO therapy, by preserving the integrity of the intestinal barrier, and increasing the abundance of A. muciniphila, could reduce colon damage and prevent the progression of UC in DSS-induced colitis while maintaining gut health. DIO was also found to reduce oxidative stress and inflammation by modifying the Nrf2 and NF-κB pathways.
2. Materials and methods
2.1. Chemicals and reagents
DIO (Dioven®, AMRIYA PHARM. CO., Cairo, Egypt, batch number: 2120001), sulfasalazine (SSZ, Colosalazine-EC®, The Arab Company for gelatin and pharamaceutical products, Alexandria, Egypt, batch number: 622400588113) and dextran sulfate sodium (DSS, CHEM-LAB, Zedelgem, Belgium with a molecular weight of 36,000–50,000 Da, CAS no. 26.4361710) were purchased. All the analytical kits were provided from Biodiagnostic Company (Giza, Egypt).
2.2. Animals
Male C57BL/6 mice (20–25 g) were acquired from Theodor Bilharz Research Institute (TBRI)'s animal house in Giza, Egypt. Throughout the experiment, mice were accommodated at the animal house with free access to water and regular chow under controlled circumstances at 25 °C, with a 12 h/12 h light/dark cycle and 50–60% humidity. All efforts were made to treat the mice humanely, to adhere to ethical standards, and to utilize only the number of animals necessary to generate acceptable scientific results. In addition, we attempted to standardize experimental conditions in our laboratory as much as possible at the beginning of experiment design in order to minimize the effect of confounding factors and ensure that it had no effect on the research outcomes. This was accomplished through a variety of means, including randomization and the use of mice of the same age, gender, and inbred genetic strain. Mice are also kept in the same temperature and humidity-controlled conditions, in the same bedding cages, eating the same food, drinking the same water, and being handled in the same way. Water was autoclaved, and drinking bottles, bedding, and cages were sterilized on a regular basis to ensure that only normal opportunists and commensals were present. Lastly, randomly assigning mice to different groups distributes known and unknown confounding variables equally, resulting in more reliable and accurate results.
2.3. Colitis model and experimental protocol
Thirty mice were randomly assigned to five groups of six mice each. Normal mice in Group 1 were given the treatment vehicle (2% Cremophore-El; Sigma-Aldrich, St. Louis, MO, USA) for 7 days. Group 2 received 4% (w/v) DSS in drinking water for 7 days [21], and groups 3−5 included DSS-induced colitis mice that were orally treated with SSZ (200 mg/kg) [22] or DIO at dosages of 100 and 200 mg/kg, respectively [23] for 7 days. Body weight, bleeding, and stool character were recorded every day, and on day eight of the experiment, fecal samples were collected aseptically, mice were weighted, euthanized by quick decapitation under light anesthesia with thiopental (50 mg/kg, i.p.), and the colon was dissected. The distance from the junction of the ileocecal canal and the anal border was examined to assess the degree of morphometric inflammation. The distal colon was preserved in 4% formalin, submerged in paraffin, and cut into 5 μm-thick sections. Another section of the colon was taken and washed with ice-cold saline before being dried, weighed, and homogenized in ice-cold saline to provide a 10% homogenate for biochemical analysis, ELISA, and RNA extraction.
2.4. Disease activity index (DAI)
The DAI was computed as the sum of three parameters: body weight loss (0, ≤ 1%; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4 ≥ 15%), diarrhea (0, normal; 2, loose stools; 4, watery diarrhea), and blood in the stool (0, no bleeding; 2, slight bleeding; 4, extensive bleeding) [24].
2.5. Assessment of colonic oxidative stress markers
Colon tissue homogenates were utilized to assess reduced glutathione (GSH), catalase (CAT), malondialdehyde (MDA), and nitric oxide (NO) concentrations using commercial kits (Biodiagnostic, Giza, Egypt).
2.6. Assessment of colonic PIK3R3 and ZO-1 levels
Tissue contents for Phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3) and Zonula occludens-1 (ZO-1) were determined using ELISA kits (Sunlong Biotech Co., LTD, China; CAT no: SL0896Mo, CAT no: SL0895Mo, respectively) and the results were expressed as ng/mg protein.
2.7. Quantitative RT- PCR analysis
Total RNA was isolated from colon tissues in an RNase-free environment using the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions to examine the expression of NF-κB, TNF-α, IL-6, Nrf2, HO-1, MUC1, MUC2, occludin, and claudin-1. The RNA amount and purity were determined using a Thermo Fisher Scientific NanoDrop 2000 spectrophotometer (Wilmington, DE, USA). Thermo Scientific RevertAid First Strand cDNA Synthesis Kit was used to reverse-transcribe 1 μg of RNA. The PCR reactions were carried out using a StepOne™ Real-Time PCR System using 10 μl 2 × PowerUp™ SYBR™ Green/ROX PCR Master Mix (Applied Biosystems, ThermoFisher Scientific, USA). To determine the abundance of A. muciniphila in feces, bacterial DNA was isolated from feces using the QIAamp DNA Stool Mini Kit (Qiagen Hidden, Germany) according to the manufacturer's instructions. Five μl of isolated DNA was utilized, along with 10 μl of 2x SYBR Green PCR Master Mix (Power Up™ SYBR™ Green Master Mix, Thermo Scientific, USA) and 5 pmole of each primer. The primer sequences used are listed in Table 1. The comparative cycle threshold (Ct) (2−ΔΔCT) method was used to calculate the relative expression [25]. All NF-κB, TNF-α, IL-6, Nrf2, HO-1, MUC1, MUC2, occludin, and claudin-1 expression levels were normalized to beta-actin as an invariant endogenous control. Meanwhile, the content of Fecal A. muciniphila was calculated and normalized to the internal reference gene (universal bacterium).
Table 1.
Primer sequences for quantitative real-time PCR analysis.
| Target gene(s) | Amplicon length (bp) | Primer sequence |
|---|---|---|
| NF-κB | 78 | Forward primer: 5′-CTGGTGGACACATACAGGAAGAC-3′ |
| Reverse primer: 5′-ATAGGCACTGTCTTCTTTCACCTC-3′ | ||
| TNF-α | 246 | Forward primer: 5′-ACCCTCACACTCACAAACCA-3′ |
| Reverse primer: 5′-GGCAGAGAGGAGGTTGACTT-3′ | ||
| IL-6 | 542 | Forward primer: 5′-CTGGTGACAACCACGGCCTTCCCTA-3′ |
| Reverse primer: 5′-ATGCTTAGGCATAACGCACTAGGTT-3′ | ||
| HO-1 | 127 | Forward primer: 5′-TTCAGAAGGGCCAGGTGACC-3′ |
| Reverse primer: 5′-AAGTAGACAGGGGCGAAGACTGG-3′ | ||
| Nrf2 | 225 | Forward primer: 5′-ATGATGGACTTGGAGCTGCC -3′ |
| Reverse primer: 5′-TTGTAACTGAGCGAAAAAGGCTTT-3′ | ||
| Mucin 1 | 133 | Forward primer: 5′-ACCTACCATCCTATGAGCGAG-3′ |
| Reverse primer: 5′-GGTTTGTGTAAGAGAGGCTGC-3′ | ||
| Mucin 2 | 70 | Forward primer: 5′-TGAAGACCTGCGGCTGTGT-3′ |
| Reverse primer: 5′-CAGTCGAACTCGAAGTGCTCC-3′ | ||
| Occludin | 138 | Forward primer: 5′-TCACTTTTCCTGCGGTGACT-3′ |
| Reverse primer: 5′-GGGAACGTGGCCGATATAATG-3′ | ||
| Claudin-1 | 210 | Forward primer: 5′-ATGCAAAGATGTTTTGCCAC-3′ |
| Reverse primer: 5′-TACAAATTCCCATTGCAGCCC-3′ | ||
| Beta actin | 118 | Forward primer: 5′GGGAATGGGTCAGAAGGACT-3′ |
| Reverse primer: 5′-CTTCTCCATGTCGTCCCAGT-3′ | ||
| A. muciniphila | 329 | Forward primer: 5′-CAGCACGTGAAGGTGGGGAC-3′ |
| Reverse primer: 5′-CCTTGCGGTTGGCTTCAGAT-3′ | ||
| Universal bacteria | 118 | Forward primer: 5′GGGAATGGGTCAGAAGGACT-3′ |
| Reverse primer: 5′-CTTCTCCATGTCGTCCCAGT-3′ |
2.8. Histopathological examination of colon tissue
Tissues from mice's distal colon were fixed in 4% buffered formalin and embedded in paraffin before being cut into 5 μm slices and stained with hematoxylin and eosin (H&E) according to conventional techniques. The sections were analyzed with a computerized image analysis system (Axiovision version 4.8, Zeiss Germany) and captured at a final magnification of x200. The colon was examined under a microscope for damage based on the degree of inflammation and the presence of edema and/or ulceration; 0, normal; 1, minor inflammation; 2, minor inflammation with edema; 3, moderate inflammation with ulceration; and 4, severe inflammation with ulceration.
2.9. Immunohistochemical examinations (IHC) for TNF-α, IL-6, occludin, and claudin-1
Deparaffinized paraffin slices were rehydrated and washed in Tris-buffered saline after being deparaffinized in xylene. Endogenous peroxidase activity was inhibited by incubating for 5 min in 3% H2O2 in methanol. Non-specific binding sites were blocked for 20 min with non-serum Protein Block (DAKO, Carpinteria, CA). Antigen retrieval for monoclonal anti-TNF-α, IL-6, occludin, and claudin-1 staining (Santa Cruz Biotechnology, USA) was accomplished by microwaving sections in citrate buffer (pH = 6) for 15 min. Following a PBS wash, a secondary antibody (Agilent Dako, CA, USA) was administered for 60 min. 3,3′-diaminobenzidine chromagen (Agilent Dako, CA, USA) was used to visualize the reaction. After that, the slides were counterstained with hematoxylin, mounted, and examined. The percentages of cytoplasmic TNF-α, IL-6, occludin, and claudin-1 positively stained cells in 10 successive fields for each mouse (x400) were measured.
2.10. Statistical analysis
The data are presented as Mean ± SEM. All data were analyzed using the SPSS software (Version 16.0, Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to perform comparison between multiple groups. When differences were identified, the Tukey's post hoc test was used to describe specific differences between each pair of means with appropriate adjustment for multiple testing, where it keeps experiment-wise alpha at the specified level (conventionally 0.05). In all analyses, the confidence interval was set at 95%, and P values less than 0.05 were considered statistically significant.
3. Results
3.1. Effect of DIO on body weight and DAI in DSS-induced colitis
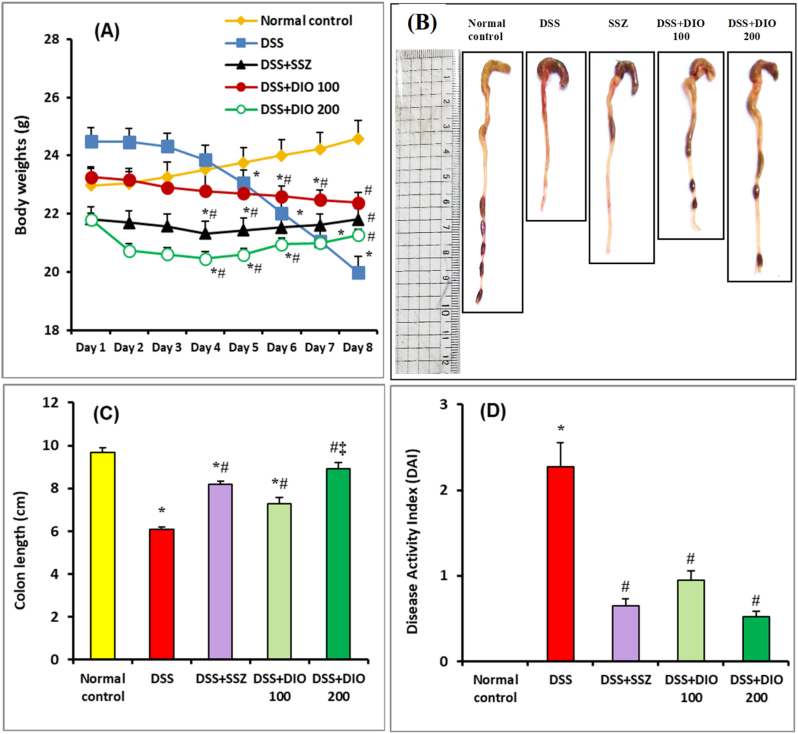
The current investigation successfully established DSS-induced acute colitis in mice, as evidenced with characteristic colitis manifestations that include bloody diarrhea, ulceration, weight loss, and colon shortening. During the experiment, the normal mice showed a steady increase in body weight. DSS, on the other hand, significantly (p < 0.05) reduced body weights when compared to normal control mice (Fig. 1A). When compared to DSS-induced mice, SSZ (200 mg/kg), DIO (100 mg/kg) or DIO (200 mg/kg)-treated groups showed a considerable (p < 0.05) recovery of body weights (Fig. 1A). Furthermore, when compared to the normal control group, DSS administration resulted in noticeable colon shortness as an indication of colon inflammation (Fig. 1B and C). When compared to DSS-induced group, SSZ (200 mg/kg) or DIO (100 mg/kg) treatment dramatically improved colon shortening. In particular, better recovery of colon shortening was observed after administration of DIO (200 mg/kg) (Fig. 1B and C). When mice were administered DSS alone, they experienced a substantial increase in DAI scores (p < 0.05), an indicator of colitis severity, when compared to normal control mice (Fig. 1D). Treatment with either SSZ (200 mg/kg) or DIO (100 mg/kg) significantly (p < 0.05) reduced DAI scores when compared to DSS-induced group (Fig. 1D). However, DAI score improvement was superior in the group treated with DIO (200 mg/kg).
Fig. 1.
Effect of DIO on clinical manifestations in mice with DSS-induced colitis. Daily body weight variations (A), representative images of colon length (B), quantitative analysis of colon length (C), and DAI recorded on the 8th day of the experiment (D). The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, ‡ Significantly different from normal control, DSS-induced colitis and DSS + DIO 100 mg/kg groups at p < 0.05, respectively. DSS: dextran sulfate sodium; DAI: disease activity index; SSZ: sulfasalazine; DIO: diosmin.
3.2. Effect of DIO on colonic oxidative stress status in DSS-induced colitis
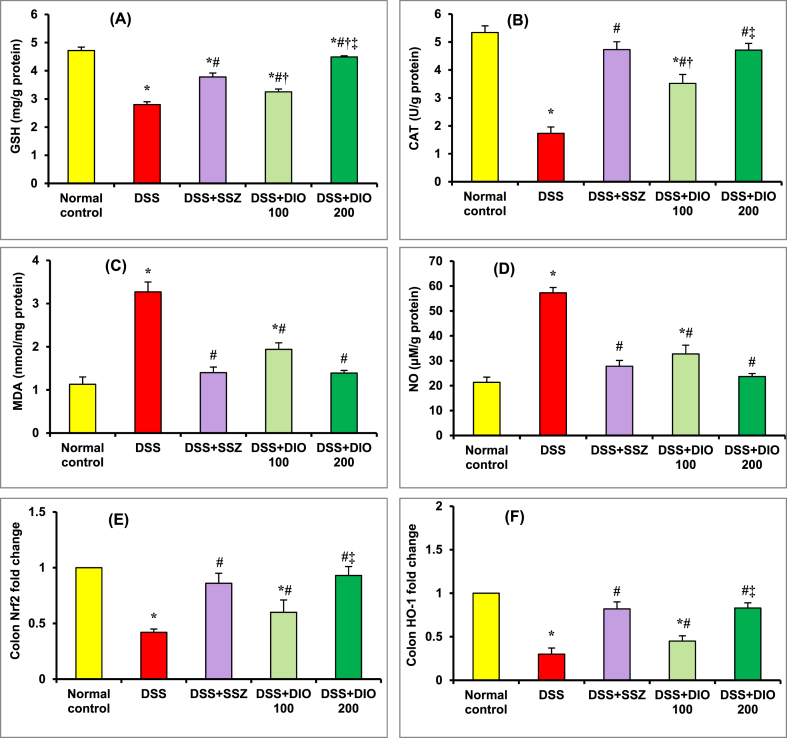
Fig. 2 (A-F) depicts the effect of DIO on the oxidative stress indicators, alongside the expression of Nrf2 and HO-1 in mice colon tissue. Compared to the normal control group, DSS-induced colitis mice demonstrated a significant (p < 0.05) decline in colonic GSH (Fig. 2A) and CAT (Fig. 2B) contents, as well as Nrf2 (Fig. 2E), and HO-1 (Fig. 2F) expression, along with a substantial (p < 0.05) increase in MDA (Fig. 2C) and nitric oxide (NO) (Fig. 2D) contents, revealing a decrease in free radical scavenging and an increase in oxidative stress. Mice treated with either SSZ (200 mg/kg) or DIO (100 mg/kg) showed considerable replenishment in colonic GSH and CAT contents, as well as a significant improvement in Nrf2 and HO-1 expression, and a meaningful (p < 0.05) loss of colonic MDA and NO contents compared to the DSS-induced colitis group, suggesting recovery of the ability to eliminate free radicals, particularly with the higher dose of DIO (200 mg/kg).
Fig. 2.
Effect of DIO on colonic GSH (A), CAT (B), MDA (C) and NO (D) contents, as well as Nrf2 (E) and HO-1 (F) expression in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal control, DSS-induced colitis, DSS + SSZ and DSS + DIO 100 mg/kg groups at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin; GSH: reduced glutathione; CAT: catalase; MDA: malondialdehyde; NO: nitric oxide; Nrf2: Nuclear factor erythroid 2-related factor 2; HO-1: Heme oxygenase-1.
3.3. Effect of DIO on fecal A. muciniphila abundance and intestinal barrier integrity in DSS-induced colitis
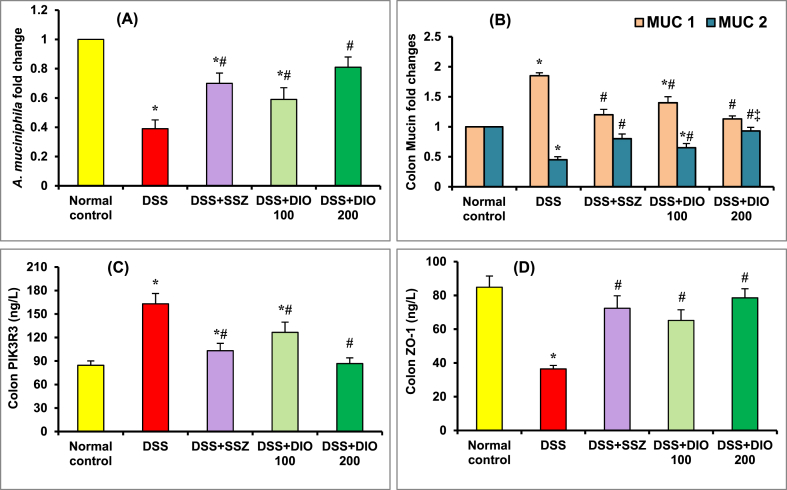
The abundance of fecal A. muciniphila was considerably (p < 0.05) decreased in the DSS-induced colitis group than in the normal control group, suggesting microbiota dysbiosis (Fig. 3A), which coincides with an impressive (p < 0.05) increment in colonic MUC1 expression along with a significant (p < 0.05) depletion in MUC2 expression (Fig. 3B). Furthermore, the colon PIK3R3 content was significantly (p < 0.05) increased (Fig. 3C), whereas the colon ZO-1 (Fig. 3D) content was significantly decreased, indicating intestinal TJ disruption. In comparison to the DSS-induced colitis group, administering SSZ (200 mg/kg) or DIO (100 mg/kg) remarkably (p < 0.05) enhanced fecal A. muciniphila abundance, colon MUC2, and ZO-1 content, while significantly reducing MUC2 expression and PIK3R3 content, with DIO at 200 mg/kg showing superb improvement (Fig. 3A–D).
Fig. 3.
Effect of DIO on colonic A. muciniphila abundance (A) and MUC1, MUC2 (B) expressions as well as PIK3R3 (C) and ZO-1 (D) contents in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, ‡ Significantly different from normal, DSS-induced colitis and DSS + DIO 100 mg/kg groups at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin; A. muciniphila: Akkermansia muciniphila; MUC1: mucin 1; MUC2: mucin 2; PIK3R3: Phosphoinositide-3-kinase regulatory subunit 3; ZO-1: zonula occlude.
3.4. Effect of DIO on histopathological alterations in DSS-induced colitis
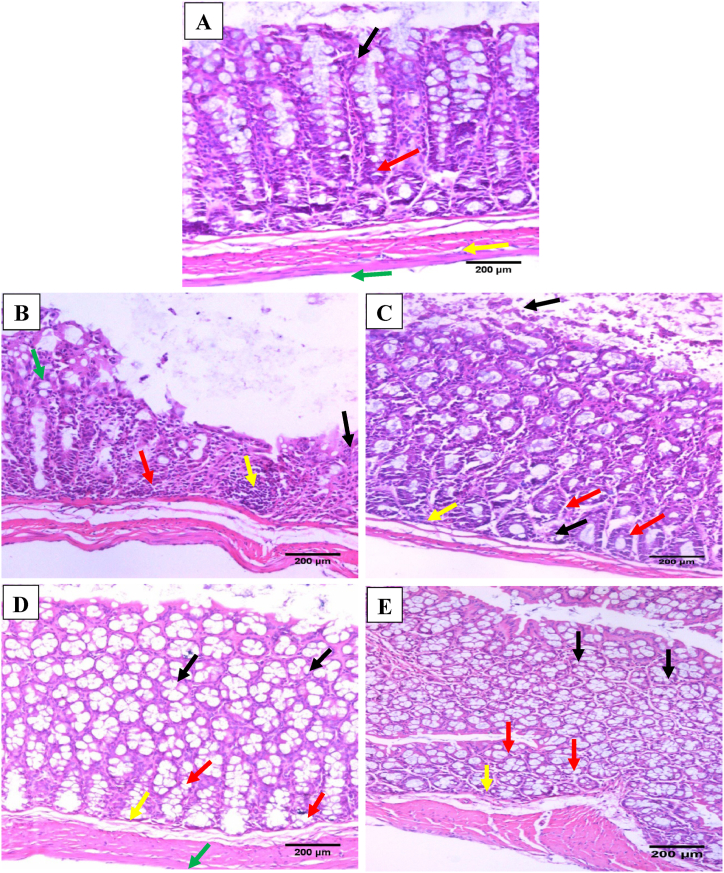
The histological examination is presented in Fig. 4, where colon sections from normal mice exhibited intact colonic architecture with regular glandular arrangement and no obvious histological abnormalities (Fig. 4A). Nonetheless, the colonic mucosa in the DSS group showed epithelial atrophy as a lack of glands and mucosal flattening, with ulcers (epithelial necrosis involving the entire thickness of the mucosa), sudden complete loss of glands substituted by granulation tissue, acute and chronic inflammatory infiltrate glands in lamina propria, glandular distortion, and erosion of epithelial mucosa (Fig. 4B). Treatment with SSZ (200 mg/kg) and DIO (100 mg/kg) drastically lowered the extent and severity of colonic cell damage and restored the architect of colonic mucosa with almost normal regular glandular arrangement, while their mucinous compartment was preserved with linear crypts and regular glandular arrangement with mild inflammatory cell infiltrate (Fig. 4C and D). DIO treatment at 200 mg/kg revealed almost normal mucosa, preserved colon architecture, and no inflammatory cell infiltrate (Fig. 4E).
Fig. 4.
Effect of DIO on colon histological alterations (H & E; x200) in normal control (A), 4% (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with DIO at doses of 100 mg/kg (D), and 200 mg/kg (E). Black arrows indicated mucosal epithelial lining cells, red arrows indicated linear crypts, yellow arrows indicated lamina propria, and green arrows indicated muscle layer. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In terms of histological grading of colon damage, DSS generated serious edematous inflammation in the colon, with a significantly higher microscopic score (p < 0.05) of colonic injury than normal control mice. Table 2 shows that the mucosa was ulcerated, edematous, and hemorrhagic. While, DIO (100 and 200 mg/kg/day) or SSZ (200 mg/kg/day) therapy greatly decreased (p < 0.05) the severity of the gross lesions score when compared to the DSS-induced colitis group. In addition, the DIO-treated group (200 mg/kg/day) showed the best protective effect.
Table 2.
Effect of diosmin on colonic histopathological scoring of inflammation, edema, and ulceration.
| Animal groups | Histopathological scoring |
||
|---|---|---|---|
| Inflammation | Edema | Ulceration | |
| Normal control | 2.00 ± 0.68 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DSS | 51.67 ± 3.07* | 43.33 ± 1.67* | 52.50 ± 2.14* |
| DSS + SSZ | 22.50 ± 1.12*# | 20.00 ± 2.24*# | 23.17 ± 1.01*# |
| DSS + DIO 100 | 30.00 ± 3.65*# | 25.83 ± 2.71*# | 26.67 ± 1.67*# |
| DSS + DIO 200 | 14.33 ± 1.48#‡ | 10.00 ± 1.29#†‡ | 7.67 ± 0.92#†‡ |
Values are presented as means of 6 mice ± SEM. Statistical analysis was carried out using one-way ANOVA followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal control, DSS-induced colitis, DSS + SSZ and DSS + DIO 100 mg/kg groups at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin.
3.5. Effect of DIO on inflammatory markers in DSS-induced colitis
The DSS group had significantly higher mRNA levels of NF-κB, TNF-α, and IL-6 (p < 0.05) compared to normal control mice, indicating increased colon inflammation. Treatment with SSZ or DIO (100 mg/kg) significantly reduced NF-κB, TNF-α, and IL-6 gene expressions (p < 0.05) compared to the DSS-induced colitis group. While, treatment with DIO at 200 mg/kg showed a substantial decline in their expressions (p < 0.05) when compared to either SSZ or DIO (100 mg/kg) treated groups (Table 3), with normalization of colonic NF-κB, TNF-α, and IL-6 gene expressions.
Table 3.
Effect of diosmin on colonic inflammatory markers (NF-κB, TNF-α and IL-6) and epithelial TJ proteins (occludin and claudin-1) gene expression.
| Animal groups | Inflammatory markers |
Epithelial TJ proteins |
|||
|---|---|---|---|---|---|
| NF-κB | TNF-α | IL-6 | Occludin | Claudin-1 | |
| Normal control | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| DSS | 2.27 ± 0.15* | 4.49 ± 0.29* | 3.90 ± 0.39* | 0.26 ± 0.03* | 0.41 ± 0.02* |
| DSS + SSZ | 1.51 ± 0.13*# | 2.70 ± 0.31*# | 2.19 ± 0.04*# | 0.53 ± 0.04*# | 0.71 ± 0.03*# |
| DSS + DIO 100 | 1.60 ± 0.06*# | 2.51 ± 0.15*# | 2.09 ± 0.14*# | 0.57 ± 0.03*# | 0.76 ± 0.04*# |
| DSS + DIO 200 | 1.13 ± 0.12#‡ | 1.92 ± 0.22# | 1.35 ± 0.12#† | 0.72 ± 0.04#†‡ | 0.87 ± 0.02#† |
Values are presented as means of 6 mice ± SEM. NF-κB, TNF-α and IL-6 gene expressions were detected using qRT-PCR. Statistical analysis was carried out using one-way ANOVA followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal control, DSS-induced colitis, DSS + SSZ and DSS + DIO 100 mg/kg groups at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin, NF-κB: nuclear factor kappa B; IL-6: interleukine 6; TNF-α: tumor necrosis factor-alpha.
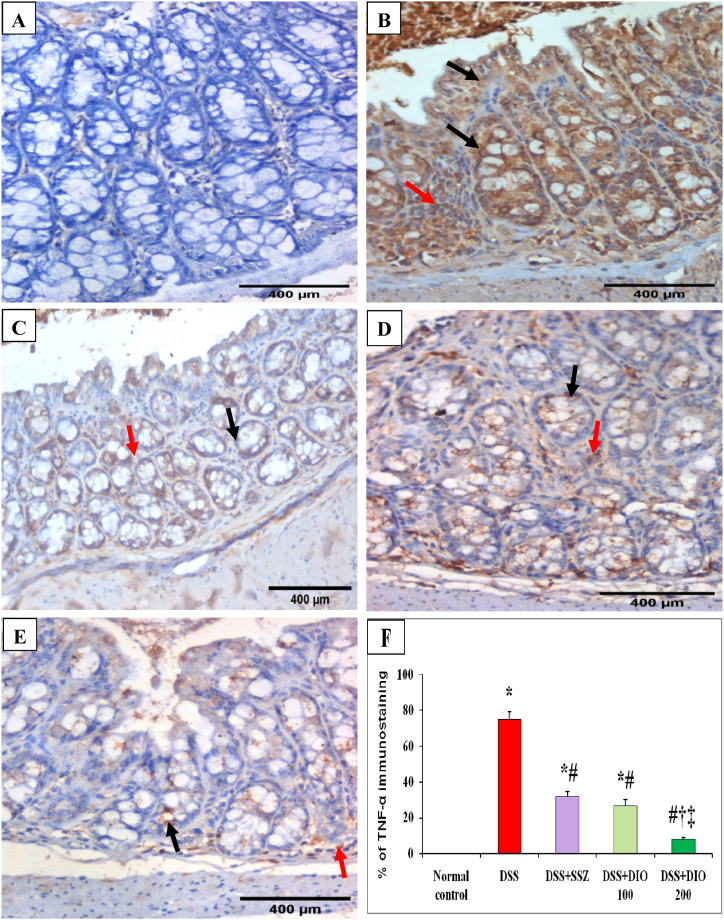
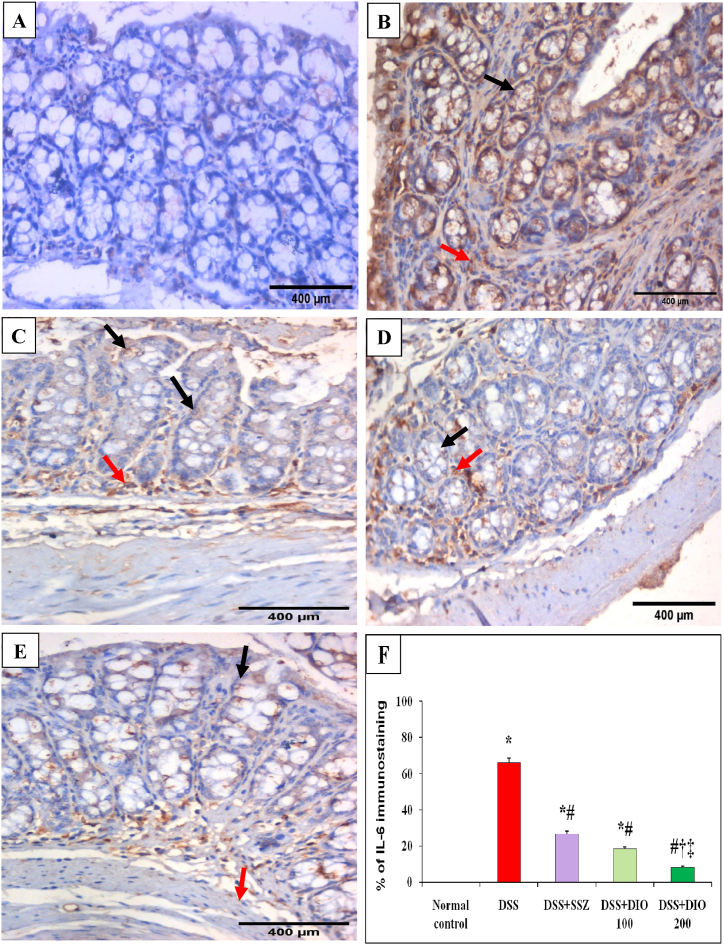
Likewise, colon sections from normal control mice exhibited negative IHC expression of TNF-α and IL-6 in intestinal epithelial cells or lamina propria (Fig. 5, Fig. 6A). Meanwhile, colonic mucosa from DSS-induced colitis group displayed marked cytoplasmic brownish IHC expression of TNF-α and IL-6 (75% and 65.83%, respectively) in the atrophic mucosal epithelial lining cells with a moderate expression in lamina propria mononuclear cells (Fig. 5, Fig. 6B, F). Treatment with either SSZ or DIO (100 mg/kg) showed moderate IHC expressions of TNF-α (31.67% and 26.67%, respectively) (Fig. 5C, D, F) and IL-6 (26.67% and 18.33%, respectively) (Fig. 6C, D, F) in intestinal epithelial cells and lamina propria mononuclear cells. Meanwhile, treatment with DIO at a dose of 200 mg/kg resulted in excellent recovery of intestinal TNF-α and IL-6, with lower IHC expressions of TNF-α (8%) (Fig. 5 E, F) and IL-6 (7.83%) (Fig. 6 E, F) in intestinal epithelial cells.
Fig. 5.
Effect of DIO on colonic TNF-α immunostaining changes (DAB, IHC, TNF-α, x400) in normal control (A), 4% (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with DIO at doses of 100 mg/kg (D), and 200 mg/kg (E), and (F) % of TNF-α immunostaining. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin; TNF-α: Tumor necrosis factor-alpha. Black arrows indicated mucosal epithelial lining cells and red arrows indicated number of lamina propria mononuclear cell. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal, DSS-induced colitis, DSS + SSZ or DSS + DIO 100 mg/kg groups at p < 0.05, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Effect of DIO on colonic IL-6 immunostaining changes (DAB, IHC, IL-6, x400) in normal control (A), 4% (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with DIO at doses of 100 mg/kg (D), and 200 mg/kg (E), and (F) % of IL-6 immunostaining. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin; IL-6: interleukin 6. Black arrows indicated mucosal epithelial lining cells and red arrows indicated number of lamina propria mononuclear cell. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal, DSS-induced colitis, DSS + SSZ or DSS + DIO-100 mg/kg groups at p < 0.05, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Effect of DIO on epithelial TJ proteins in DSS-induced colitis
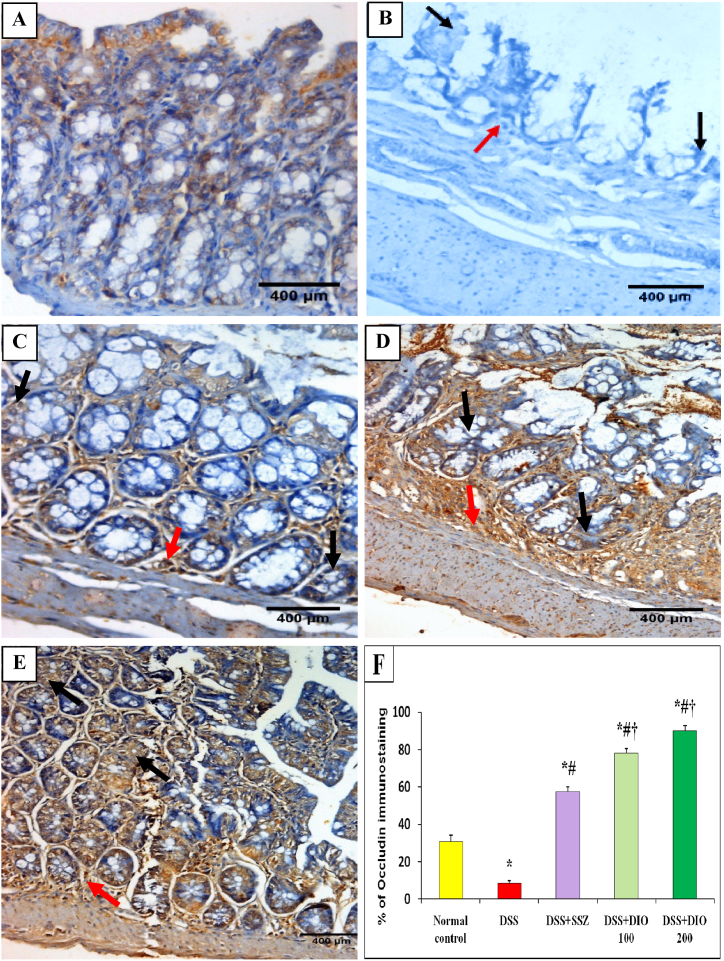
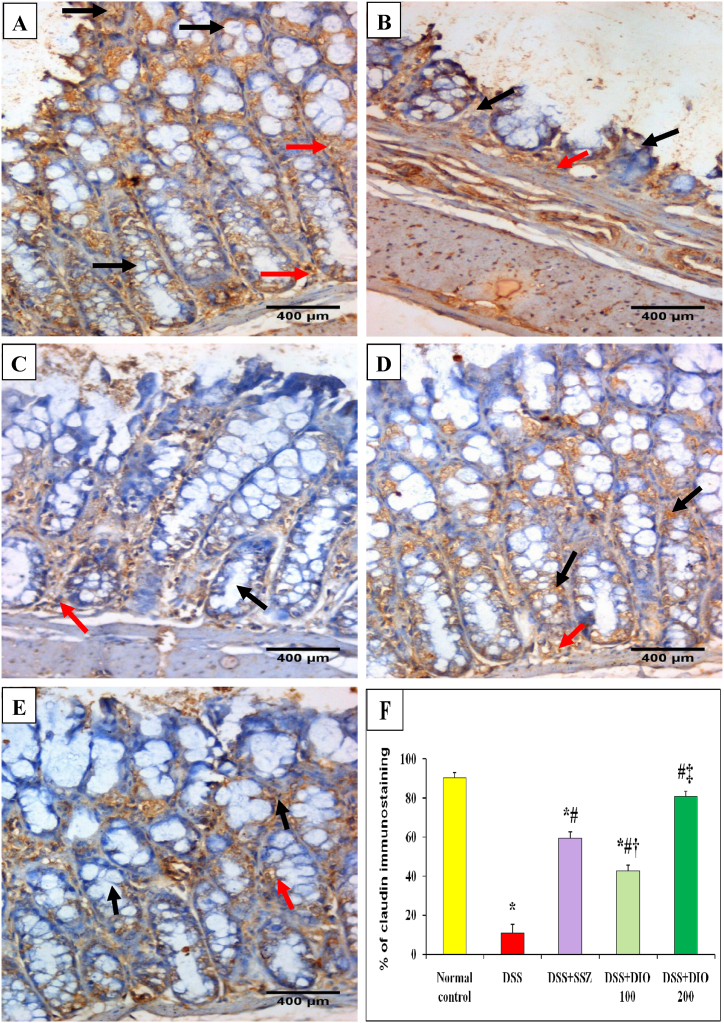
The effects of DIO on TJ proteins occludin and claudin-1 were studied in colon tissues. DSS-induced UC showed decreased mRNA expression (p < 0.05) of occludin and claudin-1 in colon tissues. Meanwhile, treatment with SSZ or DIO (100 mg/kg) increased their expressions significantly as compared to the DSS-induced colitis group. Treatment with DIO (200 mg/kg) restored their gene expression (Table 3). In terms of occludin IHC expression, colon sections from normal mucosa showed mild and scattered cytoplasmic brownish expression (30.83%) in intestinal epithelial cells and mononuclear cells in the lamina propria (Fig. 7A and F). Colonic mucosa from DSS-induced colitis group showed mild cytoplasmic brownish expression of occludin (8.33%) in atrophic mucosal epithelial lining cells, with a low number in lamina propria mononuclear cells (Fig. 7B and F). Administration of either SZZ or DIO (100 mg/kg) produced moderate occludin expression (57.50% and 78.33%, respectively) in intestinal epithelial cells and lamina propria mononuclear cells (Fig. 7C and D). However, treatment with DIO (200 mg/kg) revealed a marked cytoplasmic brownish expression of occludin (90%) in intestinal epithelial cells and lamina propria mononuclear cells, indicating better restoration of intestinal TJs (Fig. 7E and F). Concerning IHC expression of claudin-1, colon sections from normal mice revealed marked cytoplasmic brownish expression (90%) in intestinal epithelial cells and lamina propria mononuclear cells (Fig. 8A and F). Colonic mucosa from the DSS-induced colitis group exhibited mild cytoplasmic brownish expression of claudin-1 (10.84%) in the atrophic mucosal epithelial lining cells with a moderate expression in lamina propria mononuclear cells (Fig. 8B and F). Treatment with SSZ or DIO (100 mg/kg) resulted in moderate claudin-1 expressions (59.17% and 42.50%, respectively) in intestinal epithelial cells and mononuclear cell lamina propria (Fig. 8C and D). However, DSS-induced mice showed improved restoration of intestinal claudin-1 after treatment with DIO at 200 mg/kg, with claudin-1 expression (80.83%) in intestinal epithelial cells and lamina propria mononuclear cells (Fig. 8E and F).
Fig. 7.
Effect of DIO on colonic occludin immunostaining changes (DAB, IHC, occludin, x400) in normal control (A), 4% (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with DIO at doses of 100 mg/kg (D), and 200 mg/kg (E), and (F) % of occludin immunostaing. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin. Black arrows indicated epithelium appearance and red arrows indicated edema and leucocytes infiltration. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, † Significantly different from normal control, DSS-induced colitis or DSS + SSZ groups at p < 0.05, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8.
Effect of DIO on colonic claudin-1 immunostaining changes (DAB, IHC, Claudin-1, x400) in normal control (A), 4% (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with DIO at doses of 100 mg/kg (D), and 200 mg/kg (E), and (F) % of claudin-1 immunostaining. DSS: dextran sulfate sodium; SSZ: sulfasalazine; DIO: diosmin. Black arrows indicated mucosal epithelial lining cells and red arrows indicated number of lamina propria mononuclear cell. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. *, #, †, ‡ Significantly different from normal control, DSS-induced colitis, DSS + SSZ or DSS + DIO 100 mg/kg groups at p < 0.05, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
UC is the most prevalent form of IBD, which affects millions of individuals worldwide. UC usually starts in adulthood and continues throughout the individual's life [26]. Previous medical approaches for treating UC symptoms have mostly focused on lowering immunological responses [27], nonetheless they generally do not treat the underlying reasons, which include a damaged mucus layer in the digestive tract and microbiota dysbiosis, resulting in a loss of intestinal barrier functions [28]. Therefore, it is critical to find a safe therapeutic avenue that can modulate gut microbiota dysbiosis and restore intestinal barrier integrity to manage UC.
In the present study, drinking 4% (w/v) DSS successfully established colitis in mice, which mimics the pathological changes seen in UC patients, such as severe diarrhea, loose stools, and apparent fecal blood, resulting in considerable weight loss, and inflammatory cell infiltration [29,30]. Numerous investigations have shown that oral administration of DSS to mice can result in colitis that precisely resembles the histological features and pathogenesis of UC in humans [21,31]. Herein, mice colon length decreased significantly after drinking DSS, and this decrease was associated with severely inflamed, edematous and ulcerated colons. Furthermore, histological examination of the whole colonic mucosa indicated degeneration, erosion, congestion, inflammatory cell infiltration, necrosis, and goblet cell hyperplasia. Conversely, oral DIO administration restored the high DAI score by reversing the clinical symptoms of DSS-induced colitis, as seen by reduced body weight loss, improved stool consistency, and decreased rectal bleeding. These findings are congruent with those of Shalkami et al. [20], who reported that administering two doses of DIO to mice resulted in a dose-dependent decrease in DAI and colon damage index scores. Furthermore, histological examinations revealed that DIO treatment restored colon tissue integrity, as seen by decreased colon inflammation, diminished epithelial erosion, and increased colon length. Notably, DIO's ability to improve ulcer healing and decrease bloody diarrhea may be related to increased venous tone, decreased capillary permeability, and inflammation reduction [32]. As a result, these findings suggest that DIO may have a protective role against experimentally induced colitis.
Oxidative stress and inflammation are important factors in the development of UC, as they cause intestinal tissue damage and microbiota dysbiosis [33]. Furthermore, they are thought to be important triggers in the development of UC to colon cancer [34,35]. Free radical overproduction combines with fatty acids in intestinal cell membranes, leading to lipid peroxidation and depletion of antioxidant enzymes such as GSH and CAT [36,37]. GSH is assumed to be a scavenger of free radicals or cellular oxidation blocker, and its deficiency is associated with oxidative stress [38]. Furthermore, GSH and CAT depletion triggered a reactive oxygen metabolite cascade, resulting in extensive lipid peroxidation and polyunsaturated lipid breakdown [39,40]. Increased oxidative stress was detected in the colitis model in the present study, which was supported by elevated MDA content as well as decreased GSH content and CAT activity in colon tissues, and these findings are compatible with those of Shahid et al. [41]. Furthermore, oxidative stress status was induced by a decrease in colonic Nrf2 and HO-1 expression, which was coupled with the rise in colonic NO content. Excess NO generation in UC enhances the generation of ROS and reactive nitrogenous species, resulting in colonic damage [42]. Nrf2 and NF-κB play critical roles in the body's reaction towards oxidative overload and inflammation [43]. Nrf2 deficiency enhances NF-κB expression, resulting in increased generation of inflammatory cytokines like TNF-α and IL-6, whereas NF-κB can influence downstream target gene expression by regulating Nrf2 transcription and activity [44]. Previous research has implicated the Nrf2/HO-1 and NF-κB signaling pathways in the pathogenesis of various diseases including UC, and that their modulation by treatment abrogates these diseases [41,[45], [46], [47], [48]]. TNF-α and IL-6 are important inflammatory mediators involved in intestinal tract inflammation, and TNF-α expression is linked to the development of UC, resulting in IL-6 production in response to immunological dysregulation [49,50].Therefore, their inhibition is an important target in the regulation of UC development [51]. The current study found that inflammatory markers such as NF-κB, TNF-α and IL-6 were considerably raised in the colon tissues of mice with DSS-induced colitis. These data suggest that the colons of the colitis group were inflamed, which is corroborated by histological and immunohistochemical findings. Herein, administration of DIO, particularly at the high dose, revealed potent antioxidant, free radical-scavenging, and anti-inflammatory activities, as evidenced by the prevention of GSH, CAT, Nrf2, and HO-1 depletion, with a substantial reduction in colonic MDA and NO contents, NF-κB expression as well as TNF-α and IL-6 immunostaining and gene expression. The restoration of antioxidant defense enzymes (GSH and CAT) and the reduction of oxidative stress (MDA and NO) with suppression of inflammatory cytokines are strong indicators of DIO's anti-UC effect. These results supports previous findings that DIO has powerful antioxidant and anti-inflammatory properties in the treatment of experimentally induced colitis models [19,20]. Furthermore, the Nrf2/HO-1 signaling pathway was dramatically inhibited in the DSS-induced colitis group and significantly upregulated in the treated groups, indicating that the Nrf2/HO-1 signaling pathway plays a role in the relief of colitis pathogenesis [52]. The Nrf2/HO-1 pathway protects against oxidative stress-induced damage by reactivating endogenous antioxidant enzymes such as GSH, CAT, and HO-1 [53]. Importantly, the activation of the Nrf2 pathway may have played a role in the reduction in inflammatory responses in the DIO-treated groups. NF-κB is typically found in the cytoplasm in inactive state by binding to the IκB inhibitory protein, and its activation is dependent on free radical-induced oxidative stress. When ROS levels rise, NF-κB enters the nucleus and interacts with DNA, causing the expression of several proinflammatory factors such as TNF-α and IL-6 [54]. According to Pan et al. [55], the loss of Nrf2 could lead to more aggressive inflammation by inducing pro-inflammatory cytokines and NF-κB. Thus, upregulating Nrf2 with DIO could inhibit NF-κB, activating antioxidant defenses and limiting proinflammatory cytokine transmission. These findings are consistent with previous studies [56,57]. Likewise, we suggest that DIO, particularly at high doses, reduced both inflammation and oxidative stress in murine colitis by activating the Nrf2 pathway. Aside from that, further investigations are essential to examine the complete mechanisms by which DIO alleviates UC. Recent studies suggested that Thymosin β4 (Tβ4), a multifunctional and widely distributed peptide, plays a pivotal role in several physiological and pathological processes in the body, including increasing angiogenesis and proliferation while inhibiting apoptosis and inflammation [58]. Tβ4 acts as an endogenous iron chelator, influencing anti-oxidative processes related to free iron ions and ROS production, its administration increases the expression of oxidative stress genes [58], and it could be used as a new and early biomarker of cellular stress [59]. Tβ4 is expressed in the human intestine and modulates the immune system [60], and it is believed to be useful in the treatment of gastrointestinal disorders [61]. Tβ4 modulates inflammatory cytokines and chemokines, and has therapeutic benefits on liver injury and Crohn's disease [62,63]. However, its role in treating UC has not been studied. Given its rich biological activity, antioxidative and anti-inflammatory abilities, future study should focus on the role of this essential peptide in the treatment of UC.
Pathogenic gut microbiota disruption causes an impaired intestinal barrier and promotes the onset of UC, where the gut microbiota composition, diversity, and richness are altered in UC patients [64], thus, addressing gut microbiota can potentially be used to treat UC. Aside from gut microbiota, the mucus layer not solely acts as a physical barrier but it additionally has potent antimicrobial properties. A. muciniphila is a key player in the colonic mucus-associated microbiota that resides in the intestine's mucus layer, a niche close to host cells, and is required for mucus production in the human gut to maintain a healthy mucus layer and intestinal wall thickness [65]. Our findings revealed that DSS reduced A. muciniphila abundance in the feces, which resulted in a decline in colonic MUC2 expression and an increase in colonic MUC1 expression. These findings are consistent with those of [66], who reported mucus layer thinning and inflammation aggravation in UC patients. Additionally, a prior study found that mice with DSS-induced colitis had thinner mucus, whereas MUC2 deficient animals were more prone to both spontaneous UC and DSS-induced colitis [67], Moreover, A. muciniphila abundance is reduced in UC patients, which is associated with higher inflammatory scores [[68], [69], [70]]. It is worth noting that oral DIO administration restored normal A. muciniphila abundance and decreased the DSS-induced elevation of MUC1, with a significant increase in colonic MUC2 expression, indicating increased intestinal mucosa thickness, modulating gut microbiota dysbiosis, and thus reducing inflammation.
Interestingly, A. muciniphila in the gut is positively associated with restoration of gut barrier function via mucus layer thickness modulation [71]. The presence of A. muciniphila in the mucus layer, close to the epithelial layer, most likely has a significant impact on the immune modulatory mechanisms developed by this bacterium. Because A. muciniphila levels are low in many inflammatory diseases, its absence during inflammation may prevent immune suppression at the mucosal epithelial border [71]. The initial pathological change that strengthens injury and inflammation is thought to be functional loss of epithelial cell barrier integrity, which results in the migration of luminal antigens into the submucosa, exposing lamina propria immune cells to these antigens and eliciting an inflammatory response in the intestine [72]. One of A. muciniphila's key mechanisms is based on its regulation of the host intestinal barrier via promotion of TJ protein expression (ZO-1 and Claudin-1 mRNA) [73], thereby inhibiting the entry of intestinal bacteria-borne endotoxin into the blood and relieving chronic inflammation in vivo [71]. Several studies have found that A. muciniphila could improve intestinal barrier integrity [74]. As a result, we could hypothesize that increasing A. muciniphila abundance by DIO has a significant impact on intestinal barrier function in DSS-induced colitis. To emphasize this point, the effect of DIO therapy on intestinal barrier integrity was examined. It is worth noting that TJ proteins attached to the intestinal epithelial membrane provide a robust protective system in the host [75]. TJs proteins, which consist of ZO-1, occludin, and claudin-1, regulate the permeability and the functioning of the intestinal epithelial barrier, as well as immunological responses [76]. Alterations in TJ proteins damage the intestinal epithelial barrier, allowing bacteria in the bowel lumen to stimulate aberrant immunological reactions, with excessive bacterial antigen leakage from the mucosa eventually destroying the TJs [77,78]. As previously reported by Tang et al. [79], DSS-induced colitis revealed a substantial rise in PIK3R3 as well as a noticeable reduction in ZO-1 content and immunostaining of occludin and claudin-1, resulting in intestinal barrier dysfunction. DIO, on the other hand, decreased colonic PIK3R3 content while increasing colonic ZO-1 content and immunostaining for occludin and claudin-1, implying that the intestinal epithelial barrier function was maintained. According to Ibrahim et al. [75], damage to the ZO-1 dependent small intestinal barrier constitutes a starting point that leads to resistance breakdown and the development of chronic inflammatory diseases. TNF-α destroys the intestinal barrier through altering the form and functioning of the TJ [80]. Furthermore, PIK3R3 negatively regulated ZO-1 transcription via the NF-κB pathway [75]. Thus, we might speculate that DIO's inhibitory impact on the NF-κB pathway contributes to the integrity of the intestinal epithelial barrier, although the likely mechanism deserves further investigation. Notably, at a dosage of 100 mg/kg, DIO provided anti-colitis protection similar to conventional therapy, SSZ. Meanwhile, DIO at 200 mg/kg was shown to be more effective than SSZ at reducing DAI and histological alterations, oxidative stress, and inflammation while also preserving A. muciniphila abundance and intestinal epithelial integrity.
Polyphenolic compounds, including flavonoids, have been proposed to reduce the risk of chronic diseases caused by oxidative stress and inflammation. Flavonoids, a secondary metabolite of plants, are gaining popularity due to their great health advantages, safety, nontoxicity, and tolerability [81]. DIO is a flavonoid found naturally in citrus species and plays an important function in human nutrition and health [82]. The development of quick, accurate, and objective methods for estimating dietary intake markers, such as flavonoids, could lead to a better understanding of the complex relationship between disease and dietary exposures [83]. Metabolomics, a comprehensive approach to identifying all metabolites and low-molecular-weight molecules in a biological specimen, is a promising emerging technology that has the potential to inform precision medicine practice. There is a lot of advancement in this area, because a numerous of progress has been made in the analytical and spectroscopic methods of identifying compounds (natural as well as pollutants, and metabolites of all types) in a variety of tissues in recent years [84]. In medical and pharmacology studies, NMR has become the “gold standard” platform technology. This technique provided the next step in a more detailed investigation of the issue related to new potentially active or toxic compounds in various types of bio-material (such as a tissue) at the molecular level. We can look into the direction of metabolomics, targeted and untargeted analyses [85], and its extensive potential to supplement complementary plant/animal material studies. Furthermore, there are recent advances in the methodology used in NMR-based metabolomics studies, such as sensitivity and spectrum resolution [86]. Consideration of this new approach in the same topic of research in our future work will help improve understanding of the relationship between flavonoids/flavanols and health outcomes.
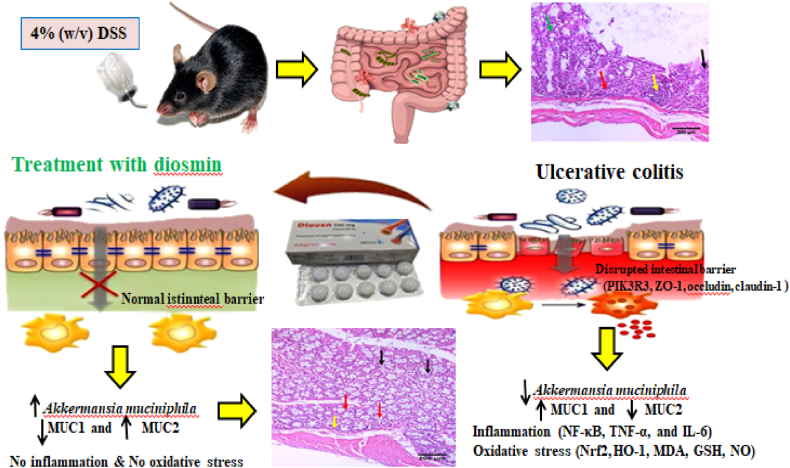
5. Conclusion
The findings of this study revealed that DIO provided significant protection against DSS-induced colitis in mice. This was supported by improved DAI, colon damage index scores, and histological findings. This appears to be due to the activation of the Nrf2/HO-1 antioxidant pathway and the downregulation of the NF-κB pathway. In addition, this is the first evidence suggesting that DIO maintains intestinal barrier integrity and enhances A. muciniphila abundance in DSS-induced colitis (Fig. 9). Notably, DIO at 200 mg/kg was more successful than SSZ at the same dosage. Overall, this study opens the door for future clinical trials using DIO as a safe adjuvant in the treatment and prevention of colitis. However, more research into the effect of DIO on the overall composition and diversity of the gut microbiota is needed before clinical therapy may be undertaken. In the future, it will be critical to thoroughly understand the molecular mechanisms by which A. muciniphila maintains intestinal barrier integrity and its potential use as an adjuvant in the treatment of UC.
Fig. 9.
A schematic illustration of DIO's mode of action in alleviating DSS-induced colitis.
Ethics statement
The experimental procedures complied to the ethical guidelines of the TBRI Ethics Committee for the Care and Use of Laboratory Animals (ethical approval number: PT 615).
Data availability statement
The data sets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Maha Badr Salem: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Naglaa Mohamed El-Lakkany: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Formal analysis, Conceptualization. Sayed Hassan Seif el-Din: Writing – review & editing, Visualization, Validation, Supervision, Formal analysis. Olfat Ali Hammam: Investigation, Formal analysis. Safia Samir: Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Walsh A.J., Bryant R.V., Travis S.P.L. Current best practice for disease activity assessment in IBD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:567–579. doi: 10.1038/NRGASTRO.2016.128. [DOI] [PubMed] [Google Scholar]
- 2.Duijvestein M., Battat R., Vande Casteele N., D'Haens G.R., Sandborn W.J., Khanna R., Jairath V., Feagan B.G. Novel therapies and treatment strategies for patients with inflammatory bowel disease. Curr. Treat. Options Gastroenterol. 2018;16:129–146. doi: 10.1007/S11938-018-0175-1. [DOI] [PubMed] [Google Scholar]
- 3.Mehandru S., Colombel J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021;18:83–84. doi: 10.1038/S41575-020-00399-W. [DOI] [PubMed] [Google Scholar]
- 4.Xie M., He C.S., Huang J.K., Lin Q.Z. Phase II study of pazopanib as second-line treatment after sunitinib in patients with metastatic renal cell carcinoma: a Southern China Urology Cancer Consortium Trial. Eur. J. Cancer. 2015;51:595–603. doi: 10.1016/J.EJCA.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Citi S. Intestinal barriers protect against disease. Science. 2018;359:1097–1098. doi: 10.1126/SCIENCE.AAT0835. [DOI] [PubMed] [Google Scholar]
- 6.Ansari M.N., Rehman N.U., Karim A., Soliman G.A., Ganaie M.A., Raish M., Hamad A.M. Role of oxidative stress and inflammatory cytokines (TNF-α and IL-6) in acetic acid-induced ulcerative colitis in rats: ameliorated by otostegia fruticosa. Life. 2021;11:1–17. doi: 10.3390/LIFE11030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian T., Wang Z., Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consoli V., Sorrenti V., Grosso S., Vanella L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules. 2021;11 doi: 10.3390/BIOM11040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoni L., Nuding S., Wehkamp J., Stange E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014;20:1165–1179. doi: 10.3748/WJG.V20.I5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisel M.B., Dhawan P., Baumert T.F. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547–561. doi: 10.1136/GUTJNL-2018-316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai R., Xue X., Zhang L., Yang X., Zhao L., Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front. Cell. Infect. Microbiol. 2019;9 doi: 10.3389/FCIMB.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paone P., Cani P.D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232–2243. doi: 10.1136/GUTJNL-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etienne-Mesmin L., Chassaing B., Desvaux M., De Paepe K., Gresse R., Sauvaitre T., Forano E., Van De Wiele T., Schüller S., Juge N., Blanquet-Diot S. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol. Rev. 2019;43:457–489. doi: 10.1093/FEMSRE/FUZ013. [DOI] [PubMed] [Google Scholar]
- 14.Cascorbi I. Inflammation: treatment progress and limitations. Clin. Pharmacol. Ther. 2017;102:564–567. doi: 10.1002/CPT.792. [DOI] [PubMed] [Google Scholar]
- 15.Gupta M., Mishra V., Gulati M., Kapoor B., Kaur A., Gupta R., Tambuwala M.M. Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology. 2022;30:397–434. doi: 10.1007/S10787-022-00931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullah A., Munir S., Badshah S.L., Khan N., Ghani L., Poulson B.G., Emwas A.H., Jaremko M. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25 doi: 10.3390/MOLECULES25225243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyseng-Williamson K.A., Perry C.M. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71–100. doi: 10.2165/00003495-200363010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Yao X., Gu X., Jin S., Shi K., Gao X., Wang Q., Zhao J., Zhang H., Lai X. Anticancer and anti-inflammatory effect of diosmin against dalton ascitic lymphoma induced leukemia. J. Oleo Sci. 2021;70:665–673. doi: 10.5650/JOS.ESS21022. [DOI] [PubMed] [Google Scholar]
- 19.Crespo M.E., Gálvez J., Cruz T., Ocete M.A., Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651–653. doi: 10.1055/S-2006-960838. [DOI] [PubMed] [Google Scholar]
- 20.Shalkami A.S., Hassan M.I.A., Bakr A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2018;37:78–86. doi: 10.1177/0960327117694075. [DOI] [PubMed] [Google Scholar]
- 21.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-Induced colitis in mice. Curr. Protoc. Im. 2014;104 doi: 10.1002/0471142735.IM1525S104. 15.25.1-15.25.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y.-H. 2016. Role of SENP1 in HBx-Induced Cell Migration and Stemness-Related Properties in Hepatocellular Carcinoma. [Google Scholar]
- 23.Abdel Ghany T.M., Ganash M., Alawlaqi M.M., Al-Rajhi A.M.H. Antioxidant, antitumor, antimicrobial activities evaluation of musa paradisiaca L. Pseudostem exudate cultivated in Saudi Arabia. Bionanoscience. 2019;9:172–178. doi: 10.1007/s12668-018-0580-x. [DOI] [Google Scholar]
- 24.Song M., Park H.J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharmacol. 2014;154:311–318. doi: 10.1016/J.JEP.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 25.Rao X., Lai D., Huang X. A new method for quantitative real-time polymerase chain reaction data analysis. J. Comput. Biol. 2013;20:703–711. doi: 10.1089/cmb.2012.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., Kaplan G.G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142 doi: 10.1053/J.GASTRO.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein C.N., Fried M., Krabshuis J.H., Cohen H., Eliakim R., Fedail S., Gearry R., Goh K.L., Hamid S., Khan A.G., LeMair A.W., Malfertheiner, Ouyang Q., Rey J.F., Sood A., Steinwurz F., Thomsen O.O., Thomson A., Watermeyer G. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm. Bowel Dis. 2010;16:112–124. doi: 10.1002/IBD.21048. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J. Teaching the basics of autophagy and mitophagy to redox biologists-Mechanisms and experimental approaches. Redox Biol. 2015;4:242–259. doi: 10.1016/J.REDOX.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichele D.D., Kharbanda K.K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017;23:6016–6029. doi: 10.3748/WJG.V23.I33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Guo W., Wu J., Luo Q., Tao F., Gu Y., Shen Y., Li J., Tan R., Xu Q., Sun Y. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013;85:1504–1512. doi: 10.1016/J.BCP.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Xu X., Lin S., Yang Y., Gong X., Tong J., Li K., Li Y. Histological and ultrastructural changes of the colon in dextran sodium sulfate-induced mouse colitis. Exp. Ther. Med. 2020;20 doi: 10.3892/ETM.2020.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struckmann J.R., Nicolaides A.N. Flavonoids. A review of the pharmacology and therapeutic efficacy of Daflon 500 mg in patients with chronic venous insufficiency and related disorders. Angiology. 1994;45:419–428. doi: 10.1177/000331979404500602. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Peng P., Ding N., Jia W., Huang C., Tang Y. Oxidative stress, inflammation, gut dysbiosis: what can polyphenols do in inflammatory bowel disease? Antioxidants. 2023;12 doi: 10.3390/ANTIOX12040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piechota-Polanczyk A., Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014;387:605–620. doi: 10.1007/S00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi E.J., Jung J.Y., Kim G.H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014;8:454–458. doi: 10.3892/ETM.2014.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyankumarraju M., Puppala E.R., Ahmed S., Jagadeesh Kumar G., Tene K., N P S., Sahu B.D., Barua C.C., Naidu V.G.M. Zanthoxylum alatum Roxb. seed extract ameliorates stress aggravated DSS-induced ulcerative colitis in mice: plausible role on NF-κB signaling axis. J. Ethnopharmacol. 2021;279 doi: 10.1016/J.JEP.2021.114385. [DOI] [PubMed] [Google Scholar]
- 37.Puppala E.R., Yalamarthi S.S., Aochenlar S.L., Prasad N., Syamprasad N.P., Singh M., Nanjappan S.K., Ravichandiran V., Tripathi D.M., Gangasani J.K., Naidu V.G.M. Mesua assamica (King&Prain) kosterm. Bark ethanolic extract attenuates chronic restraint stress aggravated DSS-induced ulcerative colitis in mice via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1 signaling pathways. J. Ethnopharmacol. 2023;301 doi: 10.1016/J.JEP.2022.115765. [DOI] [PubMed] [Google Scholar]
- 38.Franco R., Cidlowski J.A. Glutathione efflux and cell death. Antioxidants Redox Signal. 2012;17:1694–1713. doi: 10.1089/ARS.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 40.Rana S.V., Sharma S., Prasad K.K., Sinha S.K., Singh K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J. Med. Res. 2014;139 568./pmc/articles/PMC4078495/ [PMC free article] [PubMed] [Google Scholar]
- 41.Shahid M., Raish M., Ahmad A., Bin Jardan Y.A., Ansari M.A., Ahad A., Alkharfy K.M., Alaofi A.L., Al-Jenoobi F.I. Sinapic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apoptosis. Molecules. 2022;27 doi: 10.3390/MOLECULES27134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J., Ma X., He Y., Wang Y., Zhong T., Zhang Y. Anti-inflammatory and anti-oxidant properties of Melianodiol on DSS-induced ulcerative colitis in mice. PeerJ. 2022;10 doi: 10.7717/PEERJ.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W., Khor T.O., Xu C., Shen G., Jeong W.S., Yu S., Kong A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008;76:1485–1489. doi: 10.1016/J.BCP.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao W., Guo L., Yang Y., Wang Y., Xia S., Gong H., Zhang B.K., Yan M. Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell Dev. Biol. 2022;9 doi: 10.3389/FCELL.2021.809952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raish M., Shahid M., Bin Jardan Y.A., Ansari M.A., Alkharfy K.M., Ahad A., Abdelrahman I.A., Ahmad A., Al-Jenoobi F.I. Gastroprotective effect of sinapic acid on ethanol-induced gastric ulcers in rats: involvement of Nrf2/HO-1 and NF-κB signaling and antiapoptotic role. Front. Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.622815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad A., Alkharfy K.M., Bin Jardan Y.A., Shahid M., Ansari M.A., Alqahtani S., Jan B.L., Al-Jenoobi F.I., Raish M. Sinapic acid mitigates methotrexate-induced hepatic injuries in rats through modulation of Nrf-2/HO-1 signaling. Environ. Toxicol. 2021;36:1261–1268. doi: 10.1002/TOX.23123. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad A., Alkharfy K.M., Jan B.L., Ahad A., Ansari M.A., Al-Jenoobi F.I., Raish M. Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp. Lung Res. 2020;46:53–63. doi: 10.1080/01902148.2020.1726529. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad Ansari M., Shahid M., Ahmad S.F., Ahmad A., Alanazi A., Malik A., Bin Jardan Y.A., Attia S.M., Bakheet S.A., Raish M. Sinapic acid alleviates 5-fluorouracil-induced nephrotoxicity in rats via Nrf2/HO-1 signalling. Saudi Pharmaceut. J. 2023;31:1351–1359. doi: 10.1016/J.JSPS.2023.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ngo B., Farrell C.P., Barr M., Wolov K., Bailey R., Mullin J.M., Thornton J.J. Tumor necrosis factor blockade for treatment of inflammatory bowel disease: efficacy and safety. Curr. Mol. Pharmacol. 2010;3:145–152. doi: 10.2174/1874467211003030145. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Duan X., Liu X., Liu Y., Fan H., Xu M., Chen Q., Tang Q. Rho kinase blockade ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of the NF-κB and IL-6/STAT3 pathways. Inflammation. 2020;43:857–867. doi: 10.1007/S10753-019-01171-2. [DOI] [PubMed] [Google Scholar]
- 51.Park S.C., Jeen Y.T. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9:18–27. doi: 10.5009/GNL14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/J.JCMGH.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansari M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017;93:646–653. doi: 10.1016/J.BIOPHA.2017.06.085. [DOI] [PubMed] [Google Scholar]
- 54.Akanda M.R., Kim I.S., Ahn D., Tae H.J., Nam H.H., Choo B.K., Kim K., Park B.Y. Anti-inflammatory and gastroprotective roles of rabdosia inflexa through downregulation of pro-inflammatory cytokines and MAPK/NF-κB signaling pathways. Int. J. Mol. Sci. 2018;19 doi: 10.3390/IJMS19020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan H., Wang H., Wang X., Zhu L., Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012;2012 doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saber S., Khalil R.M., Abdo W.S., Nassif D., El-Ahwany E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019;364:120–132. doi: 10.1016/J.TAAP.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Lu M.C., Ji J.A., Jiang Y.L., Chen Z.Y., Yuan Z.W., You Q.D., Jiang Z.Y. An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci. Rep. 2016;6 doi: 10.1038/SREP26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachowicz J.I., Pichiri G., Piludu M., Fais S., Orrù G., Congiu T., Piras M., Faa G., Fanni D., Torre G.D., Lopez X., Chandra K., Szczepski K., Jaremko L., Ghosh M., Emwas A.H., Castagnola M., Jaremko M., Hannappel E., Coni P. Thymosin β4 is an endogenous iron chelator and molecular switcher of ferroptosis. Int. J. Mol. Sci. 2022;23 doi: 10.3390/IJMS23010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coni P., Piras M., Mateddu A., Piludu M., Orru G., Scano A., Cabras T., Piras V., Lachowicz J.I., Jaremko M., Faa G., Castagnola M., Pichiri G. Thymosin β4 cytoplasmic/nuclear translocation as a new marker of cellular stress. A Caco2 case study. RSC Adv. 2020;10:12680–12688. doi: 10.1039/C9RA10365A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemolato S., Cabras T., Cau F., Fanari M.U., Fanni D., Manconi B., Messana I., Castagnola M., Faa G. Different thymosin Beta 4 immunoreactivity in foetal and adult gastrointestinal tract. PLoS One. 2010;5 doi: 10.1371/JOURNAL.PONE.0009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinwar P.D. Overwhelming post splenectomy infection syndrome - review study. Int. J. Surg. 2014;12:1314–1316. doi: 10.1016/J.IJSU.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Zheng X.Y., Lv Y.F., Li S., Li Q., Zhang Q.N., Zhang X.T., Hao Z.M. Recombinant adeno-associated virus carrying thymosin β4 suppresses experimental colitis in mice. World J. Gastroenterol. 2017;23:242–255. doi: 10.3748/WJG.V23.I2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah R., Reyes-Ordillo K., Cheng Y., Varatharajalu R., Ibrahim J., Lakshman M.R. Thymosin β 4 prevents oxidative stress, inflammation, and fibrosis in ethanol- and LPS-induced liver injury in mice. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9630175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knox N.C., Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiome as a target for IBD treatment: are we there yet? Curr. Treat. Options Gastroenterol. 2019;17:115–126. doi: 10.1007/S11938-019-00221-W. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Siles M., Enrich-Capó N., Aldeguer X., Sabat-Mir M., Duncan S.H., Garcia-Gil L.J., Martinez-Medina M. Alterations in the abundance and Co-occurrence of Akkermansia muciniphila and faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front. Cell. Infect. Microbiol. 2018;8 doi: 10.3389/FCIMB.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kühn F., Klar E. Surgical principles in the treatment of ulcerative colitis. Viszeralmedizin. 2015;31:246–250. doi: 10.1159/000438894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersson J., Schreiber O., Hansson G.C., Gendler S.J., Velcich A., Lundberg J.O., Roos S., Holm L., Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300 doi: 10.1152/AJPGI.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lo Presti A., Del Chierico F., Altomare A., Zorzi F., Cella E., Putignani L., Luca Guarino M.P., Monteleone G., Cicala M., Angeletti S., Ciccozzi M. Exploring the genetic diversity of the 16S rRNA gene of Akkermansia muciniphila in IBD and IBS. Future Microbiol. 2019;14:1497–1509. doi: 10.2217/FMB-2019-0175. [DOI] [PubMed] [Google Scholar]
- 69.Lee Y., Sugihara K., Gillilland M.G., Jon S., Kamada N., Moon J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020;19:118–126. doi: 10.1038/S41563-019-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Earley H., Lennon G., Balfe Á., Coffey J.C., Winter D.C., O'Connell P.R. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019;9 doi: 10.1038/S41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ottman N., Reunanen J., Meijerink M., Pietila T.E., Kainulainen V., Klievink J., Huuskonen L., Aalvink S., Skurnik M., Boeren S., Satokari R., Mercenier A., Palva A., Smidt H., De Vos W.M., Belzer C. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arijs I., De Hertogh G., Lemaire K., Quintens R., Van Lommel L., Van Steen K., Leemans P., Cleynen I., Van Assche G., Vermeire S., Geboes K., Schuit F., Rutgeerts P. Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS One. 2009;4 doi: 10.1371/JOURNAL.PONE.0007984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J., Fu L., Yin F., Yin L., Song X., Guo H., Liu J. Diosmetin maintains barrier integrity by reducing the expression of ABCG2 in colonic epithelial cells. J. Agric. Food Chem. 2023;71:8931–8940. doi: 10.1021/ACS.JAFC.3C00912. [DOI] [PubMed] [Google Scholar]
- 74.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., Myridakis A., Delzenne N.M., Klievink J., Bhattacharjee A., Van Der Ark K.C.H., Aalvink S., Martinez L.O., Dumas M.E., Maiter D., Loumaye A., Hermans M.P., Thissen J.P., Belzer C., De Vos W.M., Cani P.D. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/NM.4236. [DOI] [PubMed] [Google Scholar]
- 75.Ibrahim S., Zhu X., Luo X., Feng Y., Wang J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int. Immunopharm. 2020;85 doi: 10.1016/J.INTIMP.2020.106610. [DOI] [PubMed] [Google Scholar]
- 76.Lechuga S., Naydenov N.G., Feygin A., Cruise M., Ervasti J.M., Ivanov A.I. Loss of β-cytoplasmic actin in the intestinal epithelium increases gut barrier permeability in vivo and exaggerates the severity of experimental colitis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/FCELL.2020.588836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/S00018-012-1070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lechuga S., Ivanov A.I. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1183–1194. doi: 10.1016/J.BBAMCR.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang K., Kong D., Peng Y., Guo J., Zhong Y., Yu H., Mai Z., Chen Y., Chen Y., Cui T., Duan S., Li T., Liu N., Zhang D., Ding Y., Huang J. Ginsenoside Rc attenuates DSS-induced ulcerative colitis, intestinal inflammatory, and barrier function by activating the farnesoid X receptor. Front. Pharmacol. 2022;13 doi: 10.3389/FPHAR.2022.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruder B., Atreya R., Becker C. Tumour necrosis factor alpha in intestinal homeostasis and gut related diseases. Int. J. Mol. Sci. 2019;20 doi: 10.3390/IJMS20081887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gervasi T., Calderaro A., Barreca D., Tellone E., Trombetta D., Ficarra S., Smeriglio A., Mandalari G., Gattuso G. Biotechnological applications and health-promoting properties of flavonols: an updated view. Int. J. Mol. Sci. 2022;23 doi: 10.3390/IJMS23031710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barreca D., Mandalari G., Calderaro A., Smeriglio A., Trombetta D., Felice M.R., Gattuso G. Citrus flavones: an update on sources, biological functions, and health promoting properties. Plants. 2020;9 doi: 10.3390/PLANTS9030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Charles D., Gethings L.A., Potts J.F., Burney P.G.J., Garcia-Larsen V. Mass spectrometry-based metabolomics for the discovery of candidate markers of flavonoid and polyphenolic intake in adults. Sci. Rep. 2021;11 doi: 10.1038/S41598-021-85190-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emwas A.H., Szczepski K., Poulson B.G., Chandra K., McKay R.T., Dhahri M., Alahmari F., Jaremko L., Lachowicz J.I., Jaremko M. NMR as a “gold standard” method in drug design and discovery. Molecules. 2020;25 doi: 10.3390/MOLECULES25204597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emwas A.H., Roy R., McKay R.T., Tenori L., Saccenti E., Nagana Gowda G.A., Raftery D., Alahmari F., Jaremko L., Jaremko M., Wishart D.S. NMR spectroscopy for metabolomics research. Metabolites. 2019;9 doi: 10.3390/METABO9070123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandra K., Al-Harthi S., Sukumaran S., Almulhim F., Emwas A.H., Atreya H.S., Jaremko Ł., Jaremko M. NMR-based metabolomics with enhanced sensitivity. RSC Adv. 2021;11:8694–8700. doi: 10.1039/D1RA01103K. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author upon reasonable request.