ABSTRACT
We previously reported that spinal cord injury following thoracic endovascular aortic repair for a thoracic aortic aneurysm is a micro embolism caused by a vulnerable mural thrombus. Conversely, patients who underwent thoracic endovascular aortic repair for aortic dissection develop spinal cord injury less frequently due to fewer mural thrombi. Paying attention to preserving blood flow toward the spinal cord, namely collateral circulation and steal phenomenon, prevents spinal cord injury following thoracic endovascular aortic repair for aortic dissection.
Key Words: spinal cord injury, thoracic endovascular aortic repair
INTRODUCTION
Spinal cord injury (SCI) is a devastating complication following thoracic aortic surgery. Scali et al reported a clear association between SCI development and survival following thoracic endovascular aortic repair (TEVAR). They presented a 2-year postoperative survival rate of 84% ± 1% in the no SCI group, compared with 77% ± 7% in the transient SCI group and 53% ± 7% in the permanent SCI group.1 TEVAR shows the same risk of SCI as open surgical repair (OSR), but the incidence of SCI is generally low, although preserving or reconstructing the intercostal arteries that supply blood flow to the spinal cord is impossible in TEVAR. A meta-analysis revealed a lower incidence of SCI after TEVAR for descending thoracic aortic disease, with an odds ratio of 0.42 (95% confidence interval [CI]: 0.28–0.63) in the comparison with OSR.2 This indicates the different mechanisms of SCI in these two procedures. Common risk factors for SCI after TEVAR include: (1) extensive aortic coverage (eg, >20 cm), (2) distal descending thoracic aortic coverage (eg, Th9–Th12), (3) collateral vessel occlusion (eg, left subclavian artery, hypogastric artery), and (4) perioperative hypotension. However, the VQI data analysis described above revealed a significantly higher incidence of transient ischemic attack/stroke, new hemodialysis, lower extremity arterial embolism, upper extremity arterial embolism, arterial occlusion, and intestinal ischemia in patients with SCI than those without SCI.1 These were all embolic complications, indicating that embolism may be the primary mechanism of SCI in TEVAR.
SHAGGY AORTA
The shaggy aorta is always a risk factor for embolism in aortic treatment but with no uniform definition, as described in the revised 2020 Japanese Guidelines for Aortic Aneurysm and Dissection, wherein the atheroma protruding into an irregular lumen with a thickness of ≥4–5 mm is described as extensive.3 Hosaka et al proposed the shagginess score as a risk factor for postTEVAR cerebral infarction in arch aortic lesions.4 The shagginess score was the ratio of the contour length of the irregular lumen to the expected diameter of the circle. The quantification of the morphological irregularity of the lumen defined the shaggy aorta. Similarly, Maeda et al proposed the shaggy score based on the aortic lumen morphology. Ulcer-like thrombus, thrombus thicker than 5 mm, and thrombus thicker than 2/3rd of the circumference were counted as one point each; their sum defined the shaggy score. The shaggy score was a significant predictor of postTEVAR embolic complications, but a high shaggy score was associated with SCI, although with no significance.5 This study indicates that embolism is a possible mechanism for SCI development following TEVAR. We evaluated the characteristics of thoracic aortic wall thrombi based on computed tomography (CT) values.6 The study results indicate embolism as a possible mechanism of SCI following TEVAR. We reported a vulnerable thrombus/plaque volume with a low CT value as a risk factor for SCI development following TEVAR. This study hypothesized microembolism as the mechanism of SCI following TEVAR.
INCIDENCE OF SCI AFTER TEVAR FOR AORTIC DISSECTION
Aortic dissection (AD) and aortic aneurysm dissection frequently have no or few mural thrombi in the true lumen to be treated with TEVAR. A meta-analysis comparing the results of TEVAR and OSR for chronic type B dissection7 in 39 reports revealed that 2.2% (11/1193) of patients developed SCI following TEVAR, with an odds ratio of 3.3 (95% CI: 0.97–11.25), with no significant difference, but with a trend toward fewer patients with TEVAR. Similarly, a meta-analysis of 48 studies of TEVAR for chronic type B dissociation revealed a very low incidence of SCI, 0.9% (95% CI: 0.3–1.6).8 This data confirmed that the SCI pathogenesis in TEVAR is microembolism. However, TEVAR for AD mainly aimed to cover the entry and false lumen blood flow from the (former) re-entry frequently remains after treatment. This partial intercostal arterial blood flow preservation may contribute to the low incidence of SCI following TEVAR for AD.
SCI FOLLOWING TEVAR IN OUR DEPARTMENT (AD VS THORACIC AORTIC ANEURYSM CASES)
Methods
We retrospectively reviewed patients who underwent TEVAR from October 2008 to June 2022 at our department.
Results
We found 95 patients with AD and 387 patients with thoracic aortic aneurysms. Table 1 shows the patient background. Patients with AD were significantly younger, having less dyslipidemia, coronary artery disease, and chronic obstructive pulmonary disease. Additionally, they had a significantly lower American Society of Anesthesiologists (ASA) class (Table 1). The zone classification of the proximal landing was significantly different and should be considered (cases were lacking to match the backgrounds in this study) (Table 2). Table 2 shows that the early postoperative results, the incidence of SCI, embolism, and inhospital mortality tended to be lower in the AD group, but with no significant differences. We present two cases without SCI, wherein most of the blood flow to the spinal cord was interrupted; however, in one case, TEVAR for AD resulted in a SCI.
Table 1.
Patient characteristics
| AD (n = 95) | TAA (n = 387) | P value | ||
|---|---|---|---|---|
| Male, n (%) | 73 (76.8) | 315 (81.4) | N.S. | |
| Age, years | 68 [57, 75] | 75 [71, 80] | <0.0001 | |
| HT, n (%) | 87 (91.6) | 336 (86.8) | N.S. | |
| DL, n (%) | 26 (23.4) | 164 (42.4) | 0.007 | |
| Diabetes, n (%) | 10 (10.5) | 67 (17.4) | N.S. | |
| CAD, n (%) | 12 (12.6) | 115 (29.7) | 0.0007 | |
| CVD, n (%) | 11 (11.6) | 60 (15.5) | N.S. | |
| CKD ≥ 3b, n (%) | 32 (33.7) | 114 (29.5) | N.S. | |
| Dialysis, n (%) | 6 (6.4) | 14 (3.7) | N.S. | |
| COPD, n (%) | 18 (19.0) | 151 (39.0) | 0.0002 | |
| ASA class, n (%) | 1: 0 2: 60 (63.2) 3: 24 (25.3) 4: 11 (11.6) |
1: 9 (2.3) 2: 200 (51.7) 3: 167 (43.2) 4: 11 (2.8) |
<0.0001 |
AD: aortic dissection
TAA: thoracic aortic aneurysm
HT: hypertension
DL: dyslipidemia
CAD: coronary artery disease
CVD: cerebrovascular disease
CKD: chronic kidney disease
COPD: chronic obstructive pulmonary disease
ASA: American Society of Anesthesiology
N.S.: not significant
Table 2.
Surgical data and early results
| AD (n = 95) | TAA (n = 387) | P value | ||
|---|---|---|---|---|
| Proximal landing zone, n (%) | 0: 5 (5.3) 1: 0 2: 26 (27.4) 3: 23 (24.2) 4: 22 (23.2) ET: 19 (20.0) |
0: 49 (12.7) 1: 10 (2.6) 2: 48 (12.4) 3: 53 (13.7) 4: 159 (41.1) ET: 68 (17.6) |
< 0.0001 | |
| Stroke, n (%) | 4 (4.2) | 19 (4.9) | 1.00 | |
| SCI, n (%) | 1 (1.1) | 23 (5.9) | 0.062 | |
| Embolism, n (%) | 1 (1.1) | 21 (5.4) | 0.10 | |
| In-hospital death, n (%) | 0 | 11 (2.9) | 0.13 |
AD: aortic dissection
TAA: thoracic aortic aneurysm
SCI: spinal cord injury
ET: elephant trunk
Case 1 (Figure 1)
Fig. 1.
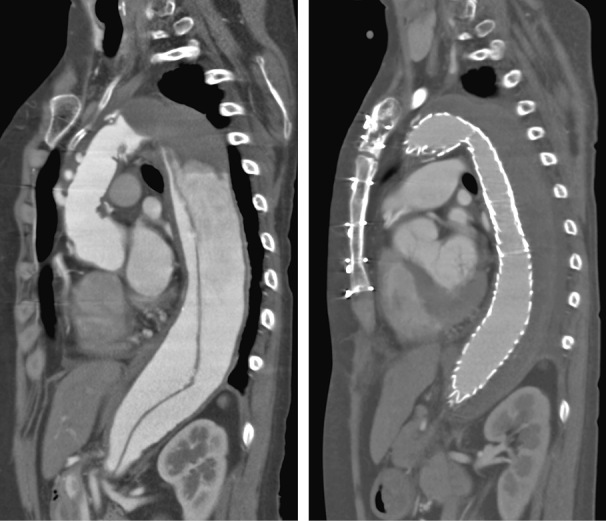
Case 1
The patient developed no SCI although all intercostal arteries were occluded during TEVAR.
SCI: spinal cord injury
TEVAR: thoracic endovascular aortic repair
In general, a long treatment length (aortic coverage) (> 15 cm or > 20 cm) is a risk factor for SCI in TEVAR. This case underwent a stent graft implantation into the entire length of the descending thoracic aorta after total arch replacement and elephant trunk placement, causing thrombus occlusion of the entire thoracic false lumen. TEVAR occluded all intercostal arteries but with no SCI.
Case 2 (Figure 2)
Fig. 2.
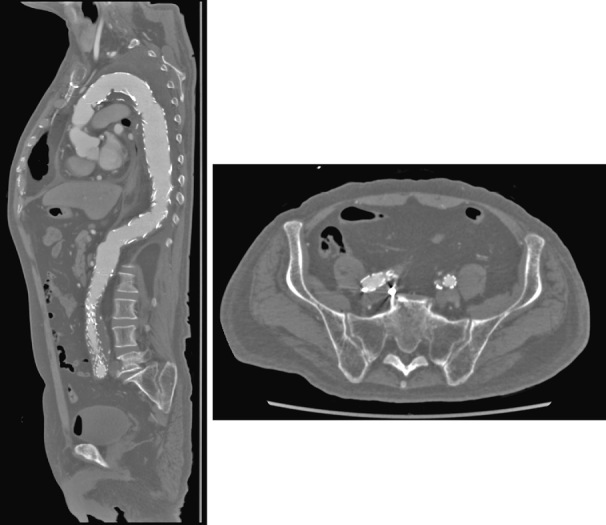
Case 2
The patient underwent endovascular aortic repair (EVAR) with a previous unilateral hypogastric embolization. The contralateral hypogastric artery was embolized due to type Ib endoleak a few years later. Afterward, the patient underwent total arch repair without the left subclavian reconstruction. The stent graft was implanted in the whole descending thoracic aorta. Results revealed no hypogastric artery, no left subclavian artery, and no intercostal artery after TEVAR, and the patient developed no SCI.
TEVAR: thoracic endovascular aortic repair
SCI: spinal cord injury
The occlusion of vessels, which can supply blood flow to the thoracic spinal cord (lumbar artery, left subclavian artery and internal iliac artery, to mention a few), in TEVAR is generally considered a risk factor for SCI. This is another case, wherein a stent graft was placed in the entire length of the descending thoracic aorta following total arch replacement with an elephant trunk. The left subclavian artery was not reconstructed during total arch replacement. The patient had an abdominal aortic aneurysm history treated with an endovascular repair (EVAR). The first surgery included hypogastric artery embolization on one side, followed by the remaining hypogastric artery due to an endoleak at the contralateral distal fixation site, causing bilateral hypogastric artery occlusion. Additionally, TEVAR occluded all intercostal arteries, and blood flow to the thoracic spinal cord seemed to be extremely poor but with no SCI. The closure of each source of blood flow may be staggered, thereby allowing the development of collateral blood supply to the spinal cord.
Case 3 (Figure 3)
Fig. 3.
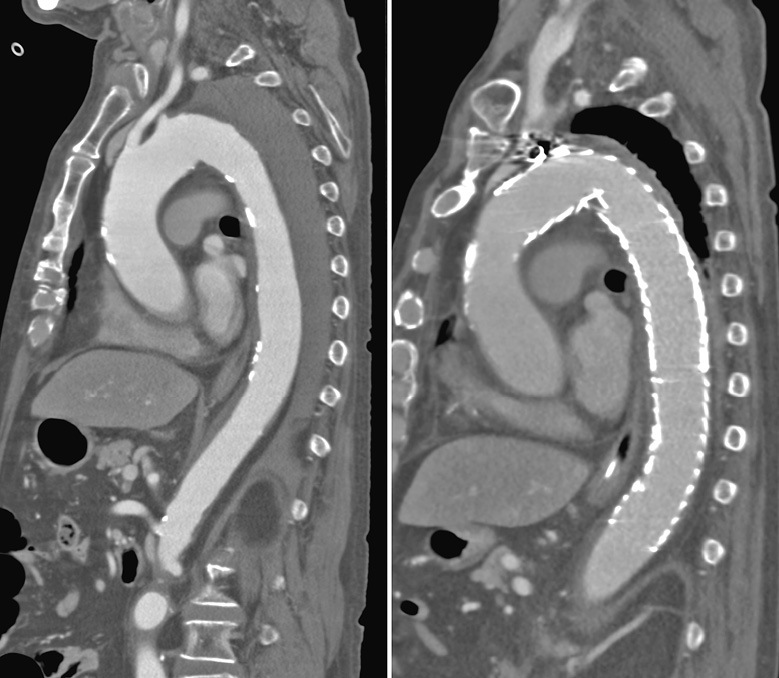
Case 3
Ruptured case. All intercostal arteries were in the thrombosed false lumen. The patient showed no neurological deficits preoperatively. Emergent zone 2 TEVAR without left subclavian reconstruction was performed. This is the only case that developed SCI after TEVAR for AD.
TEVAR: thoracic endovascular aortic repair
SCI: spinal cord injury
AD: aortic dissection
This is the only case of SCI in TEVAR for AD. The patient had a rupture due to acute AD and underwent emergency TEVAR. Thrombo-occlusive false lumen preoperatively occluded all the intercostal arteries but with no preoperative SCI symptoms. The patient underwent emergency zone 2 TEVAR with simple left subclavian artery closure. Upon hospital arrival, the patient was hemodynamically stable and had no hypotension, including intraoperative hypotension.However, the patient developed SCI postoperatively. Preserving the left subclavian artery as a potential source of blood flow could prevent SCI.
PREVENTION OF SCI AFTER TEVAR FOR AD
We believe that SCI prevention in TEVAR for AD will require: (1) preserving the blood supply to the spinal cord as much as possible and (2) preventing blood steal from side branch arteries (such as intercostal arteries) in the false lumen (Figure 4).
Fig. 4.
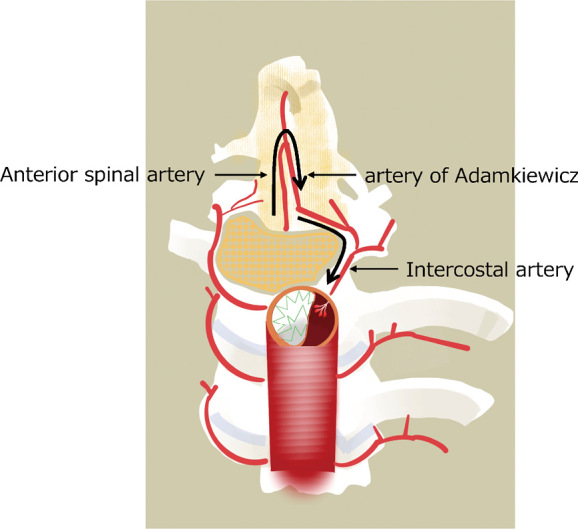
Illustration of steal phenomenon
Steal phenomenon after entry tear coverage by the endograft causing retrograde flow in an intercostal artery due to a decreased pressure within the false lumen.
Case 4
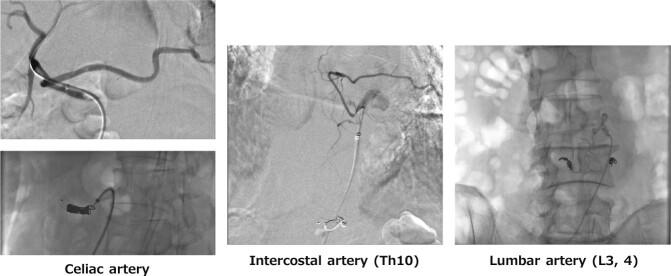
Dissecting distal arch and abdominal aortic aneurysm. The first step included the embolization of the celiac artery, Th10 intercostal artery, as weel as L3 and L4 lumbar arteries originating from the false lumen to prevent blood steal from the false lumen side branches (Figure 5). The second step included left common carotid-subclavian artery bypass, zone 2 TEVAR, and intimal tear embolization near the celiac artery. Finally, right hypogastric artery embolization and EVAR were performed. The patient underwent extensive aortic stent grafting but did not develop SCI.
Fig. 5.
Case 4 (Embolization of false lumen branches)
Branch arteries of the false lumen were embolized before zone 2 TEVAR and EVAR to prevent a steal phenomenon in the false lumen.
TEVAR: thoracic endovascular aortic repair
EVAR: endovascular aortic repair
PREOPERATIVE INTERCOSTAL ARTERY EMBOLIZATION OF THORACOABDOMINAL AORTIC ANEURYSM FOR SCI PROPHYLAXIS
A German group reported two cases of preoperative segmental artery embolization to prevent SCI during thoracoabdominal aortic aneurysm treatment.9 One patient underwent OSR and the other underwent endovascular repair without SCI and embolization-related complications. They preconditioned the spinal cord by preembolizing the intercostal arteries to develop the collateral vascular network to the spinal cord. Subsequently, they performed intercostal artery embolization before endovascular treatment of 50 patients with thoracoabdominal aortic aneurysms and evaluated the safety of this procedure.10 They evaluated the safety of intercostal artery embolization. It was technically challenging, with 22, 24, and 1 cases successfully embolized in one , two , and five sessions, respectively. Finally, 77.7% of intercostal arteries were embolized, with no cases of SCI. An international multicenter randomized controlled trial is being planned with these results, but the study was postponed due to delayed patient enrollment caused by the coronavirus disease-2019 pandemic.11 The study results are awaited.
SUMMARY
The pathogenesis of SCI during TEVAR for true aneurysms seemed to be mainly embolism-related. Therefore, the incidence of SCI in TEVAR for AD and aortic aneurysm dissection, wherein there is little thrombus in the true lumen wall, is much lower. However, the incidence of SCI is not zero and remains a devastating complication for patients. Preserving collateral vessels as much as possible and preventing blood steal in the false lumen are important to prevent SCI. Intercostal artery embolization before aortic surgery may be effective in preventing embolism or blood steal and in promoting collateral vessel development.
DISCLOSURE OF CONFLICTS OF INTEREST
Hiroshi Banno received scholarship donations from Japan Gore, Medtronic Japan, and Terumo Corporation. Other co-authors declare no conflicts of interest.
Abbreviations
- AD
aortic dissection
- SCI
spinal cord injury
- OSR
open surgical repair
- TEVAR
thoracic endovascular aortic repair
REFERENCES
- 1.Scali ST, Giles KA, Wang GJ, et al. National incidence, mortality outcomes, and predictors of spinal cord ischemia after thoracic endovascular aortic repair. J Vasc Surg. 2020;72(1):92–104. doi: 10.1016/j.jvs.2019.09.049. [DOI] [PubMed]
- 2.Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease. J Am Coll Cardiol. 2010;55(10):986–1001. doi: 10.1016/j.jacc.2009.11.047. [DOI] [PubMed]
- 3.Ogino H, Iida O, Akutsu K, et al. JCS/JSCVS/JATS/JSVS 2020 Guideline on Diagnosis and Treatment of Aortic Aneurysm and Aortic Dissection. Circ J. 2023;87(10):1410–1621. doi: 10.1253/circj.CJ-22-0794. [DOI] [PubMed]
- 4.Hosaka A, Motoki M, Kato M, Sugai H, Okubo N. Quantification of aortic shagginess as a predictive factor of perioperative stroke and long-term prognosis after endovascular treatment of aortic arch disease. J Vasc Surg. 2019;69(1):15–23. doi: 10.1016/j.jvs.2018.03.425. [DOI] [PubMed]
- 5.Maeda K, Ohki T, Kanaoka Y, Shukuzawa K, Baba T, Momose M. A novel shaggy aorta scoring system to predict embolic complications following thoracic endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2020;60(1):57–66. doi: 10.1016/j.ejvs.2019.11.031. [DOI] [PubMed]
- 6.Banno H, Kawai Y, Sato T, et al. Low-density vulnerable thrombus/plaque volume on preoperative computed tomography predicts for spinal cord ischemia. J Vasc Surg. 2021;73(5):1557–1565.e1. doi: 10.1016/j.jvs.2020.09.026. [DOI] [PubMed]
- 7.Boufi M, Patterson BO, Loundou AD, et al. Endovascular versus open repair for chronic type B dissection treatment: a meta-analysis. Ann Thorac Surg. 2019;107(5):1559–1570. doi: 10.1016/j.athoracsur.2018.10.045. [DOI] [PubMed]
- 8.Williams ML, de Boer M, Hwang B, et al. Thoracic endovascular repair of chronic type B aortic dissection: a systematic review. Ann Cardiothorac Surg. 2022;11(1):1–15. doi: 10.21037/ACS-2021-TAES-25. [DOI] [PMC free article] [PubMed]
- 9.Etz CD, Debus ES, Mohr FW, Kölbel T. First-in-man endovascular preconditioning of the paraspinal collateral network by segmental artery coil embolization to prevent ischemic spinal cord injury. J Thorac Cardiovasc Surg. 2015;149(4):1074–1079. doi: 10.1016/j.jtcvs.2014.12.025. [DOI] [PubMed]
- 10.Branzan D, Etz CD, Moche M, et al. Ischaemic preconditioning of the spinal cord to prevent spinal cord ischaemia during endovascular repair of thoracoabdominal aortic aneurysm: first clinical experience. EuroIntervention. 2018;14(7):828–835. doi: 10.4244/EIJ-D-18-00200. [DOI] [PubMed]
- 11.Petroff D, Czerny M, Kölbel T, et al. Paraplegia prevention in aortic aneurysm repair by thoracoabdominal staging with “minimally invasive staged segmental artery coil embolisation” (MIS2ACE): trial protocol for a randomised controlled multicentre trial. BMJ Open. 2019;9(3):e025488. doi: 10.1136/bmjopen-2018-025488. [DOI] [PMC free article] [PubMed]