ABSTRACT
In Japan, systemic chemotherapy is the standard treatment for unresectable, advanced, or recurrent gastric cancer. However, numerous patients with gastric cancer do not receive late-line treatment because of the rapid progression of gastric cancer. Additionally, late-line treatments, such as nivolumab, trifluridine tipiracil (FTD/TPI), or irinotecan, have limited effects on improving clinical symptoms and delaying the onset of symptoms associated with cancer progression. Recently, a combination of FTD/TPI and ramucirumab was reported to have a high response rate in late-line treatment; however, owing to patient selection bias and a high rate of hematologic toxicity in that previous study, this regimen may not be feasible in real-world clinical applications. Our objective is to conduct a single-arm phase II study to assess the safety and efficacy of FTD/TPI plus ramucirumab combination therapy for gastric cancer after third-line treatment under real-world clinical conditions. This study will recruit 32 patients according to eligibility criteria and administer FTD/TPI (35 mg/m2) and intravenous ramucirumab (8 mg/kg). The primary endpoint will be the time to treatment failure. The secondary endpoints will include the overall survival time, progression-free survival time, overall response rate, disease control rate, relative dose intensity, and incidence of adverse events. The results will add new insights for improving the late-line treatment of advanced gastric cancer.
Key Words: gastric cancer, trifluridine tipiracil, ramucirumab, clinical trial
INTRODUCTION
Gastric cancer is the fifth most common cancer and the second leading cause of cancer-related deaths worldwide, despite its declining incidence in developed countries.1 In 2018, gastric cancer affected approximately 1,030,000 people, resulting in approximately 780,000 deaths worldwide.1 Systemic chemotherapy is the standard treatment for patients with unresectable, advanced, or recurrent gastric cancer to prolong survival times.2 Despite recent advances in chemotherapy for gastric cancer, such as pembrolizumab and nivolumab, the median overall survival for patients with advanced gastric cancer remains low at approximately 6–17 months with a 5-year survival rate of 8–10%.3-5
Based on the results of clinical trials in Japan, the 5th English edition of the Japanese gastric cancer treatment guidelines recommend combination therapy of fluoropyrimidine with platinum-containing regimens, such as S-1 plus cisplatin and S-1 plus oxaliplatin, as first-line chemotherapy.2 Second-line treatment comprises combination therapy of taxanes with ramucirumab followed by nivolumab monotherapy, trifluridine tipiracil (FTD/TPI), or irinotecan as later-line treatments.2 Trastuzumab or trastuzumab deruxtecan (T-DXd) has been approved for the treatment of human epidermal growth factor receptor 2 (HER2) positive gastric cancer.3,6
Trifluridine tipiracil is an oral anticancer drug consisting of FTD and TPI combined at a molar ratio of 1:0.5.7 A recent phase III trial (TAGS trial) demonstrated that FTD/TPI significantly prolonged overall survival, and the primary endpoint of median OS was 5.7 and 3.6 months in the FTD/TPI and placebo groups, respectively.8 Recently, FTD/TPI in combination with anti-angiogenic drugs (ie, ramucirumab and bevacizumab) has been suggested as a promising late-line treatment regimen for gastric and colorectal cancers.9-11 A phase II (open-label, single-arm, two cohorts) study on the combination of FTD/TPI with ramucirumab in gastric cancer revealed a disease control rate of 77%.11 In addition, a phase III study evaluating the effect of sustained use of ramucirumab beyond disease progression in gastric cancer is ongoing.10 However, owing to patient selection bias and a high rate of hematologic toxicity in the aforementioned study,11 combination therapy of FTD/TPI with ramucirumab may not be feasible in real-world clinical conditions. For example, the aforementioned study excluded patients with an Eastern Cooperative Oncology Group performance status ≥ 2 and those without measurable lesions. Therefore, this study aims to evaluate the safety and efficacy of combination therapy of FTD/TPI with ramucirumab against gastric cancer after third-line treatment under real-world clinical conditions, with modified doses of FTD/TPI to reduce hematologic toxicity.
METHODS
Objective
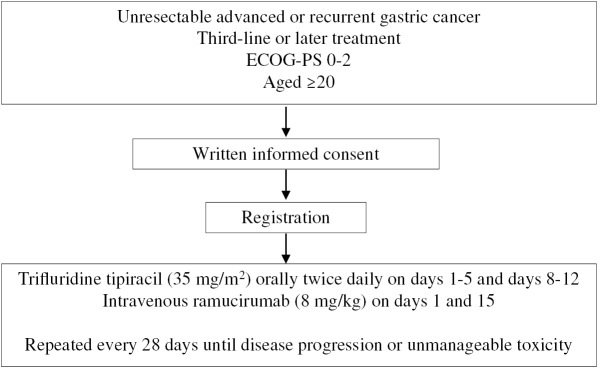
To explore the safety and efficacy of FTD/TPI and ramucirumab as third-line or later treatments in patients with advanced or recurrent gastric cancer (Fig. 1).
Fig. 1.
Treatment protocol
ECOG-PS: Eastern Cooperative Oncology Group performance score
Endpoints
The primary endpoint will be the time to treatment failure (TTF). The secondary endpoints will include the overall survival time, progression-free survival time, overall response rate (ORR), disease control rate (DCR), relative dose intensity, and incidence of adverse events. The ORR and DCR will be evaluated according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (ver1.1). The TTF will be measured from the date of treatment initiation to the date of the first documented disease progression, discontinuation of the study, or the date of death due to any causes, whichever occurs earlier.
Eligibility criteria
Inclusion criteria.
(1) Patients diagnosed with histological confirmation of gastric adenocarcinoma (either one of the general histological types or gastric carcinoma with lymphoid stroma).
(2) Patients diagnosed with advanced or recurrent gastric cancer, including esophago-gastric junctional cancer, that cannot be curatively resected.
(3) Patients who have received 2 or more treatment regimens of the standard of care systemic therapy (eg, fluoropyrimidines, platinum, taxane, ramucirumab, and anti-HER2 therapy or immune checkpoint inhibitors as needed).
(4) Patients aged 20 years or older on the day of providing informed consent.
(5) Adequate oral intake.
(6) Eastern Cooperative Oncology Group performance score (ECOG-PS) of 0–2.
(7) Patients presenting adequate organ function (meet the following criteria on laboratory test within 14 days prior to enrollment):
• Hemoglobin (Hb) ≥ 8.0 g/dL
• Neutrophil count ≥ 1,500/mm3
• Platelet count (PLT) ≥ 75,000/mm3
• Total bilirubin ≤ 1.5 mg/dL
• Serum aspartate transaminase (AST), alanine transaminase (ALT) ≤ 100 IU/L (≤ 200 IU/L in the setting of liver metastases)
• Serum creatinine (Cr) ≤ 1.5 mg/dL
• Urinary protein is ≤ 1+ on dipstick or routine urinalysis (If it is ≥ 2+, the patient is eligible if a 24-hour urine collection for protein demonstrates < 2000 mg or urine protein/urine creatinine ratio (UPC) is < 2.0).
(8) Patients who provide written informed consent.
Exclusion criteria.
(1) Patients who have previously received FTD/TPI.
(2) Patients presenting with concurrent active malignancies, excluding malignancies that are disease-free, for no less than 2 years without treatment.
(3) Patients with an active infectious disease or fever requiring systemic treatment (body temperature ≥ 38 °C).
(4) Females who are pregnant, breastfeeding, or are planning to conceive; and males who plan to have children during the study.
(5) Patients with grade 3 or higher peripheral neuropathy.
(6) Patients with diarrhea of grade 2 or higher.
(7) Patients with serious non-healing wounds, ulcers, bone fractures, or any surgery within 4 weeks prior to enrollment (excluding implanted port systems).
(8) Patients with the following complications:
• Severe liver diseases (eg, Child-Pugh class B or C, hepatic encephalopathy, marked ascites, hepatorenal syndrome). Even if HBsAg is positive, the patient can be enrolled if it is controlled using nucleic acid analog and HBV-DNA negative is then confirmed.
• History of thromboembolism (eg, myocardial infarction, cerebral infarction, deep venous thromboembolism, pulmonary thromboembolism) or receiving anticoagulation therapy for thrombosis.
• History, within 1 year prior to enrollment, or risk of gastrointestinal perforation or inflammatory bowel disease.
• Bleeding disorder or coagulopathy.
• Severe pulmonary disease (interstitial pneumonia, pulmonary fibrosis, or severe pulmonary emphysema).
• History of myocardial infarction or unstable angina within 6 months prior to enrollment.
• Uncontrolled hypertension.
(9) Patients with psychiatric diseases that may increase the risk associated with study participation or study drug administration.
(10) Patients with a history of allergy to ramucirumab or severe hypersensitivity to the components of the study drug.
(11) Patients who are judged ineligible for the study at the physician’s assessment.
Treatment procedure
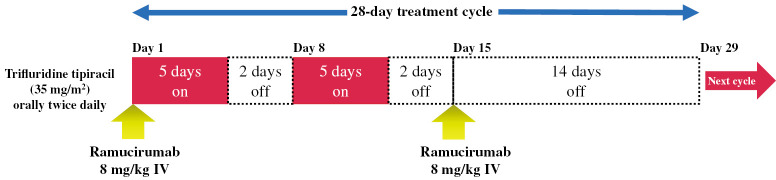
Patients will receive FTD/TPI (35 mg/m2) orally twice daily on days 1–5 and 8–12 of each 28-day treatment cycle, and intravenous ramucirumab (8 mg/kg) on days 1 and 15, until disease progression, occurrence of unacceptable toxicity, or patient’s refusal (Fig. 2). From cycle 2 onward, the investigator will confirm if the study assessment data obtained on the day of administration or the day before the initiation of the subsequent cycle meet the following criteria:
Fig. 2.
Dosing schedule
FTD/TPI.
• ECOG-PS of 0–2
• Hb ≥ 8.0 g/dL
• Neutrophil count ≥ 1,500/mm3
• PLT ≥ 75,000/mm3
• Total bilirubin ≤ 1.5 mg/dL
• AST, ALT ≤ 100 IU/L (≤ 200 IU/L in the setting of liver metastases)
• Serum Cr ≤ 1.5 mg/dL
• No active infectious disease with fever (body temperature ≥ 38 °C)
• Other non-hematological adverse events related to FTD/TPI, except in the case where the event is not serious and safe use of the drug can continue, grade ≤ 1 or baseline
Ramucirumab.
• Urinary protein ≤ 1+ on dipstick or routine urinalysis, or 24-hour urine collection for protein shows < 2000 mg, or UPC < 2.0
• Hypertension (blood pressure can be controlled with antihypertensive drugs)
• Other non-hematological adverse events related to Ramucirumab, except in the case where the event is not serious and safe use of the drug can continue, grade ≤ 1 or baseline
Dose suspension, resumption, and reduction criteria. The criteria for the suspension, resumption, and reduction of treatment procedures are listed in Table 1. The dose may be suspended or reduced at the discretion of the investigator to ensure patient safety in the case of adverse events, irrespective of its association with the study drug.
Table 1.
Criteria for modification of chemotherapy
| Adverse events | Suspension criteria | Resumption criteria | Dose reduction criteria | ||
| FTD/TPI | Ramucirumab | FTD/TPI | Ramucirumab | ||
| Neutrophil count | < 1,000/mm3 |
≥ 1,500/mm3 |
< 500/mm3 and delayed for more than 7 days |
||
| Platelet count | < 50,000/mm3 |
≥ 75,000/mm3 |
< 50,000/mm3 and delayed for more than 7 days |
||
| Febrile neutropenia | Grade ≥ 3 | resolved | Grade ≥ 3 | ||
| Other adverse eventsa |
Grade ≥ 3 | Grade ≤ 1 or baseline | Grade ≥ 3 | ||
| Urinary protein |
≥ 2 g and < 3 g / day, or UPC ≥ 2.0 and < 3.0 |
≤ 1+ on dipstick or < 2 g/day, or UPC < 2.0 |
≥ 2 g and < 3 g / day, or UPC ≥ 2.0 and < 3.0 |
||
| Hypertension | Symptomatic grade 2 or grade ≥ 3 | Can be controlled with antihypertensive drugs |
Symptomatic grade 2 or grade ≥ 3 | ||
| Other adverse eventsb |
Grade ≥ 3 | Grade ≤ 1 or baseline | Grade ≥ 3 | Grade ≥ 3 (second episode) |
|
FTD/TPI: trifluridine tipiracil
UPC: urine protein/urine creatinine ratio
a : FTD/TPI related, except in the case where the event is not serious and where safe use of the drug can continue.
b : Ramucirumab related, except in the case where the event is not serious and safe use of the drug can continue.
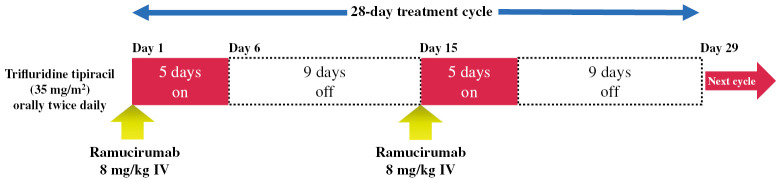
Dose modifications. A phase Ib/II study showed the efficacy and safety of biweekly FTD/TPI monotherapy in reducing hematologic toxicity caused by FTD/TPI (Days 1–5, every 2 weeks) in unresectable or recurrent colorectal cancer.12 Based on this study, we will allow FTD/TPI biweekly administration (orally twice daily on days 1–5 and 15–19 of each 28-day treatment cycle) as an option if the neutrophil count is < 500/mm3 during the previous course and FTD/TPI must be withdrawn or skipped. However, dose reduction will be considered first (Fig. 3).
Fig. 3.
Modified dosing schedule (Trifluridine tipiracil biweekly administration)
Data collection
The data of all participants will be stored in a web-based electronic data capture (EDC) system with limited access and a unique identification code. All data entered by the physician will be recorded in the EDC. If there is any doubt or discrepancy, the physician in charge will be questioned by data managers via the system.
Assessments
Table 2 shows descriptions of the required study assessments. Patient characteristics, including vital signs, ECOG-PS, and clinical backgrounds, will be assessed and registered within 14 days before treatment initiation after obtaining written informed consent from the patients. Tumor assessments using CT scans of the chest, abdomen, and pelvis, as well as serum tumor markers and serum tumor markers (CEA and CA19-9), will be performed within 28 days before enrollment in the study and repeated every 6 weeks (± 2 weeks allowed) from treatment initiation to discontinuation of protocol treatment. Disease progression will be confirmed using either radiological imagery for those who exhibit measurable lesions according to RECIST version 1.1, or clinical findings that are strongly suggestive of disease progression, such as an apparent increase in ascites, serial and exponential increase in serum tumor markers, or symptoms indicative of progressive disease in those who do not have a measurable lesion. Safety assessments will be repeated after each administration of the chemotherapeutic agents. The severity of adverse events will be graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Table 2.
Schedule of study assessments
| Assessments | Screening period | Treatment period (1 cycle = 28 days) |
End of treatment |
Follow-up period |
|||
| –28 days | –14 days | Day 1 | Day 8 | Day 15 | |||
| Demographics/ Medical history |
● | ||||||
| Vital signs, ECOG- PS, body weight |
● | ● | ◎ | ● | ● | ○ | |
| Adverse event assessment |
● | ● | ◎ | ● | ● | ○ | |
| Hematology/ Serum chemistry |
● | ● | ◎ | ● | ● | ○ | |
| Urinalysis | ● | ● | ◎ | ● | ● | ○ | |
| Tumor marker | ● | Every 6 weeks (± 2 weeks)*1 |
○*2 |
○*3 |
|||
| Imaging test | ● | Every 6 weeks (± 2 weeks)*1 |
○*2 |
○*3 |
|||
●: Mandatory, ◎: Mandatory in first cycle, ○: As required
ECOG-PS: Eastern Cooperative Oncology Group performance score
*1 : After the first 3 months, the following modified schedule is allowed; every 8 weeks (± 2 weeks) from 3 to 12 month and every 12 weeks (± 2 weeks) thereafter.
*2 : If the study is discontinued for reasons other than disease progression, it is advisable to perform the assessment.
*3 : If the study is discontinued for reasons other than disease progression, measurements should be performed every 6 weeks (±2 weeks) until disease progression or the start of new anticancer therapy.
Sample size plan
The TAGS trial demonstrated that the median duration of protocol treatment was 1.5 months,8 and the recent phase II study of the combination of FTD/TPI with ramucirumab reported that the TTF was 4.2 and 4.4 months in cohorts A and B, respectively.11 Therefore, we set a threshold and expected TTF of 1.7 and 4.2 months, respectively. Assuming accrual and follow-up periods of 24 and 12 months, respectively, and using a one-sided log-rank test with α-error = 0.05 and β-error = 0.1, we estimated a required sample size of 29 patients to detect statistically significant differences according to a calculation using the One Sample Binomial method of the Southwest Oncology Group. With the estimation of a loss of approximately 10% of the final subject, the target sample size is set at 32 patients for the study. The planned registration period is two years.
Ethics
The study protocol was approved by the Nagoya University Certified Review Board (number 2021-0343) and is registered in the Japan Registry of Clinical Trials (jRCT) as jRCTs041210105 (https://jrct.niph.go.jp/).
Participating institutions
Nagoya University Hospital, Ichinomiya Municipal Hospital, Okazaki City Hospital, Kainan Hospital, Kanagawa Cancer Center, Gifu Prefectural Tajimi Hospital, Konan Kosei Hospital, Tosei General Hospital, Komaki City Hospital, Yokkaichi Municipal Hospital, Toyohashi Medical Center, and Nishio Municipal Hospital.
Trial registration
The Japan Registry of Clinical Trials (jRCT): jRCTs041210105, registered on November 22, 2021 (https://jrct.niph.go.jp/).
DISCUSSION
Gastric cancer progresses more rapidly than other cancers, especially in patients with peritoneal dissemination, which greatly impairs the patients’ quality of life and makes it impossible to continue chemotherapy. Several patients with gastric cancer do not receive third-line or later treatments due to rapid disease progression.13,14 Therefore, late-line treatments that increase tumor response with tolerable toxicity under real-world clinical conditions are required. The results of this study will add new insights for improving the outcomes of advanced gastric cancer.
ACKNOWLEDGMENTS
This work was supported by the Nagoya University Hospital Funding for Clinical Development.
We thank Editage (https://www.editage.jp) for editing a draft of this manuscript.
CONFLICT OF INTEREST
The authors declare the following financial interests/personal relationships that may be considered potential competing interests:
Dr Nakanishi reports personal fees from Taiho Pharmaceutical Co, Ltd, Ono Pharmaceutical Co, Ltd, and Daiichi Sankyo Company, Ltd, outside the scope of the submitted work.
Dr Kodera reports grants and personal fees from Taiho Pharmaceutical Co, Ltd, Chugai Pharma, MSD, Nihon Kayaku, Yakult, Lilly Japan, Ono Pharmaceutical Co, Ltd, Covidien, Daiichi Sankyo Company, Ltd, Tsumura, Johnson & Johnson, and Abbvie; grants from Takeda, Kaken Pharma, EA Pharma, Otsuka, Sanofi, Abbot, Bayer, and Pfizer; and personal fees from Miyarisan, Amgen, and Olympus, outside the scope of the submitted work.
Dr Ando reports grants and personal fees from Chugai Pharmaceutical Co, Ltd, Kyowa Kirin Co, Ltd, Nippon Kayaku Co, Ltd, Yakult Honsha Co, Ltd, Ono Pharmaceutical Co, Ltd, Taiho Pharmaceutical Co, Ltd, Novartis Pharma KK, Daiichi Sankyo Company, Ltd, and Eisai Co, Ltd; personal fees from Eli Lilly Japan KK, Bayer Holding Ltd, Sawai Pharmaceutical Co, Ltd, MSD KK, Astellas Pharma Inc., Otsuka Holdings Co, Ltd, Sanwa Kagaku Kenkyusho Co, Ltd, Hisamitsu Pharmaceutical Co, Inc., SymBio Pharmaceuticals, Aptitude Health, and Alfresa Pharma Corporation; and grants from BeiGene, Ltd, outside the scope of the submitted work.
Dr Murotani reports personal fees from Taiho Pharmaceutical Co, Ltd, outside the scope of the submitted work.
The other authors declare that no financial or material support was received for this study.
Abbreviations
- FTD/TPI
trifluridine tipiracil
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed]
- 4.Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed]
- 5.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed]
- 6.Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed]
- 7.Emura T, Nakagawa F, Fujioka A, et al. An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med. 2004;13(2):249–255. doi: 10.3892/ijmm.13.2.249. [DOI] [PubMed]
- 8.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed]
- 9.Tabernero J, Taieb J, Prager GW, et al. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol. 2021;17(16):1977–1985. doi: 10.2217/fon-2020-1238. [DOI] [PubMed]
- 10.Sakai D, Boku N, Kodera Y, et al. An intergroup phase III trial of ramucirumab plus irinotecan in third or more line beyond progression after ramucirumab for advanced gastric cancer (RINDBeRG trial). J Clin Oncol. 2018;36(15_Suppl):TPS4138. doi: 10.1200/JCO.2018.36.15_suppl.TPS4138. [DOI]
- 11.Kawazoe A, Ando T, Hosaka H, et al. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(3):209–217. doi: 10.1016/S2468-1253(20)30396-4. [DOI] [PubMed]
- 12.Satake H, Kato T, Oba K, et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS Study). Oncologist. 2020;25(12):e1855-e1863. doi: 10.1634/theoncologist.2020-0643. [DOI] [PMC free article] [PubMed]
- 13.Ueno M, Doi A, Sunami T, Takayama H, Mouri H, Mizuno M. Delivery rate of patients with advanced gastric cancer to third-line chemotherapy and those patients’ characteristics: an analysis in real-world setting. J Gastrointest Oncol. 2019;10(5):957–964. doi: 10.21037/jgo.2019.05.07. [DOI] [PMC free article] [PubMed]
- 14.Iizumi S, Takashima A, Sakamaki K, Morita S, Boku N. Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol. 2018;81(6):981–989. doi: 10.1007/s00280-018-3569-9. [DOI] [PubMed]