Abstract
Background
Anti-Program-Death-1 (PD-1) is a standard adjuvant therapy for patients with resected melanoma. We hypothesized that there are discrepancies in survival, recurrence pattern and toxicity to adjuvant PD-1 between different ethnicities and melanoma subtypes.
Objective
We performed a multicenter cohort study incorporating 6 independent institutions in Australia, China, Japan, and the United States. The primary outcomes were recurrence free survival (RFS) and overall survival (OS). Secondary outcomes were disease recurrence patterns and toxicities.
Results
In total 534 patients were included. East-Asian/Hispanic/African reported significantly poorer RFS/OS. Nonacral cutaneous or melanoma of unknown primary reported the best RFS/OS, followed by acral, and mucosal was the poorest. Within the nonacral cutaneous or melanoma of unknown primary subtypes, East-Asian/Hispanic/African reported significantly poorer RFS/OS than Caucasian. In the multivariate analysis incorporating ethnicity/melanoma-subtype/age/sex/stage/lactate dehydrogenase/BRAF (v-Raf murine sarcoma viral oncogene homolog B)-mutation/adjuvant radiotherapy, East-Asian/Hispanic/African had independently significantly poorer outcomes (RFS: HR, 1.71; 95% CI, 1.19-2.44 and OS: HR, 2.34; 95% CI, 1.39-3.95), as was mucosal subtype (RFS: HR, 3.25; 95% CI, 2.04-5.17 and OS: HR, 3.20; 95% CI, 1.68-6.08). Mucosal melanoma was an independent risk factor for distant metastasis, especially liver metastasis. East-Asian/Hispanic/African had significantly lower incidence of gastrointestinal/musculoskeletal/respiratory/other-rare-type-toxicities; but higher incidences of liver toxicities.
Limitations
A retrospective study.
Conclusions
Ethnicity and melanoma subtype are associated with survival and recurrence pattern in melanoma patients treated with adjuvant anti-PD-1. Toxicity profile differs by ethnicity and may require a precision toxicity surveillance strategy.
Key words: adjuvant PD-1, efficacy, ethnicity, melanoma subtype, toxicity
Capsule Summary.
-
•
Anti-Program-Death-1 monotherapy is the standard postdefinitive adjuvant therapy in melanoma but its efficacy and toxicity for acral or mucosal subtypes and Asian or Hispanic or African ethnicities are poorly profiled.
-
•
We provide first-hand data for survival, recurrence pattern, and immune-related-adverse-event profiles in previously underrepresented populations thus guiding daily clinical practice for these patients.
Introduction
Anti-Program-Death 1 (PD-1) antibody monotherapy is the standard of care in patients with melanoma after definitive surgery based on positive results of several large randomized control trials.1, 2, 3 These trials recruited largely Caucasian patients predominantly from Australia, Europe, and North America, mostly with nonacral cutaneous (NAC) or melanoma of unknown primary (UP). Based on the unproven presumption that these clinical trial data are generalizable worldwide, anti-PD-1 is also widely used in Asia, Africa, and South America. However, the efficacy, recurrence pattern, and toxicity data in patients with darker skin color and acral or mucosal melanomas remain poorly defined.
We previously reported distinct efficacy and toxicity profiles with immunotherapy in East-Asian/Hispanic/African populations with metastatic melanoma and in acral or mucosal or uveal melanoma subtypes.4 We therefore hypothesize that there are also discrepancies in survival, recurrence pattern, and toxicity to PD-1 monotherapy in the adjuvant setting. To address this clinically relevant issue, we assembled a cohort of patients with melanoma (n = 534) from 6 independent melanoma centers in Australia, China, Japan, and the United States, who received adjuvant anti-PD-1 monotherapy after definitive surgery. The purpose of this study is to provide evidence to facilitate clinical decision-making in the adjuvant setting in patients with different ethnic backgrounds and melanoma subtypes.
Materials and methods
Patients
The inclusion and exclusion criteria are as follows: patients with melanoma who underwent definitive surgery and received anti-PD-1 monotherapy afterward (without previous anti-PD-1 exposure, therapy initiated between July 2015 and April 2022), both within and outside clinical trial settings (no neoadjuvant therapy allowed), were included at Massachusetts General Hospital, Melanoma Institute Australia, National Cancer Center Hospital Japan, Peking University Cancer Hospital, Saitama Medical University International Medical Center, and Vanderbilt University Medical Center.
The last follow-up was in September 2022. This study has been approved by local institutional review board and conducted in accordance with Declaration of Helsinki.
Data collection and recurrence/immune-related adverse event/survival assessment
The following clinical data were collected: baseline demographics (age, sex, and self-identified ethnicity), melanoma pertinent information (subtype, anatomic location of the primary lesion, BRAF (v-Raf murine sarcoma viral oncogene homolog B) V600 mutational status, stage [by AJCC 8th edition for cutaneous melanoma],5 baseline, and at-recurrence lactate dehydrogenase [LDH]), definitive surgery, adjuvant radiotherapy, adjuvant PD-1 monotherapy, immune-related adverse events (irAEs), recurrence pattern and treatment after recurrence, recurrence free survival (RFS) and overall survival (OS) from the initiation of adjuvant anti-PD-1 monotherapy. Recurrence was determined by local radiologists or treating physicians. The irAEs were graded based on medical notes, laboratory test results, clinical trial data, or by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. The irAEs were categorized by severity (grading), the use of glucocorticoids, and also based on organ or system involvement, including endocrine, gastrointestinal (GI), hepatic, musculoskeletal, skin, respiratory, and other rare types. Medical records of each patient were reviewed and data were independently quality controlled.
Outcomes
Thus, the primary outcomes included RFS and OS by ethnicity (Caucasians vs East-Asian/Hispanics/Africans) and by melanoma subtype (NAC/UP vs acral versus mucosal subtypes). Secondary outcomes included recurrence pattern and irAEs.
Statistical analysis
For the primary outcomes, we used Bonferroni correction to control the overall type I error at an α level of 0.05 (2-sided). Secondary outcomes and exploratory outcomes were not adjusted for multiple comparisons and thus all considered as exploratory. Detailed statistical analysis description can be found in the Supplementary methods section.
Results
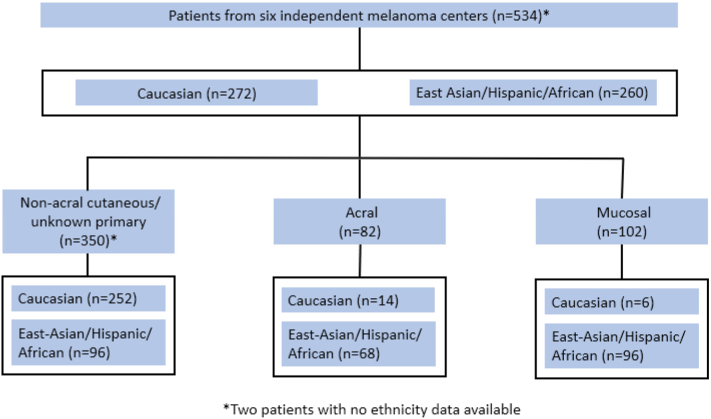
In total, 534 patients with melanoma from 6 independent melanoma centers from Australia, China, Japan, and the United States, who underwent definitive surgery and received anti-PD-1 monotherapy as adjuvant therapy were included in this study (Fig 1). The median follow-up time was 35 months (IQR, 25-44). Of the entire cohort, 96 (18%) patients were with stage I or II, 395 (74%) stage III, and 33 (6%) stage IV melanomas. In total 433 patients with BRAF mutation status data available, among whom 95 (22%) were BRAF V600 mutant. Similar to previous reports,6,7 most Caucasian patients had melanomas of the NAC/UP subtypes, whereas the East-Asian/Hispanic/African decedents had a substantial higher proportion of acral and mucosal melanomas (Fig 1). Detailed baseline characteristics are in Supplementary Tables I and II, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Fig 1.
Flow chart.
Survival outcomes
In total, 328 survival events were observed, including 211 for RFS and 117 for OS. (Table I and Supplementary Figs 1, A and B, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Table I.
Survival outcomes in different subgroups
| Survival | Ethnicity∗ |
Melanoma subtype |
Ethnicity in NAC/UP subtypes |
|||||
|---|---|---|---|---|---|---|---|---|
| Entire cohort |
Caucasian |
East-Asian Hispanic/African |
NAC/UP |
Acral |
Mucosal |
Caucasian |
East-Asian/ Hispanic/ African |
|
| (n = 534) | (n = 272) | (n = 260) | (n = 350) | (n = 82) | (n = 102) | (n = 252) | (n = 96) | |
| RFS | ||||||||
| Median (mo, 95% CI) | 58.5 (45.9-NR) | NR (58.5-NR) | 26.7 (19.9-NR) | NR (58.5-NR) | 40.6 (29.1-NR) | 17.8 (12.3-26.7) | NR (NR) | 33.9 (18.3-NR) |
| 1-y rate (%, 95% CI) | 75.8 (72.3-79.6) | 83.6 (79.3-88.1) | 67.5 (62.0-73.5) | 80.1 (75.9-84.4) | 78.3 (69.7-88.0) | 59.3 (50.4-69.8) | 84.7 (80.3-89.3) | 67.6 (58.9-77.7) |
| 2-y rate (%, 95% CI) | 63.7 (59.6-68.1) | 73.3 (68.0-78.9) | 52.9 (46.7-59.8) | 69.2 (64.4-74.4) | 65.1 (54.7-77.6) | 43.1 (34.1-54.4) | 74.8 (69.5-80.6) | 53.5 (43.9-65.2) |
| Nominal P | NA | 7∗10-8 | 3∗10-8 | < .001 | ||||
| Adjusted P | NA | 3∗10-7 | 1∗10-7 | NA | ||||
| OS | ||||||||
| 2-y rate (%, 95% CI) | 86.0 (83.0-89.2) | 93.0 (89.9-96.2) | 77.9 (72.6-83.6) | 90.7 (87.6-94.0) | 84.1 (75.9-93.3) | 71.1 (62.4-81.0) | 93.2 (90.1-96.5) | 83.4 (75.7-91.8) |
| 4-y rate (%, 95% CI) | 72.2 (67.5-77.2) | 83.9 (78.9-89.3) | 54.9 (45.8-65.7) | 82.3 (77.7-87.3) | 66.2 (53.5-82.0) | 46.1 (35.7-59.4) | 85.5 (80.5-90.7) | 73.9 (64.1-85.3) |
| Nominal P | NA | 9∗10-10 | 5∗10-10 | .002 | ||||
| Adjusted P | NA | 4∗10-9 | 2∗10-9 | NA | ||||
NA, Not applicable; NR, not reached; NAC, non-acral cutaneous; OS, overall survival; RFS, recurrence free survival; UP, unknown primary.
Two patients with no ethnicity data available thus excluded from ethnicity-based analysis.
RFS and OS in different ethnicities and melanoma subtypes
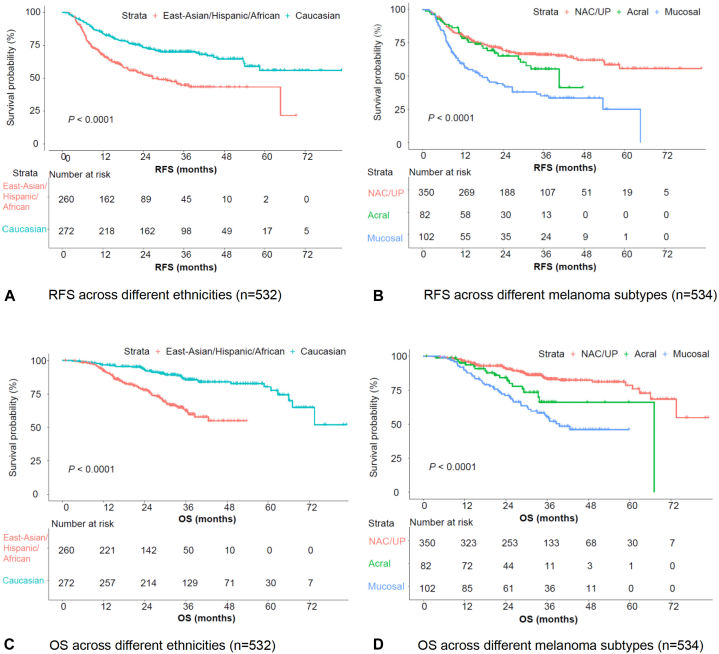
Caucasians had significantly longer RFS compared to East-Asian/Hispanic/African (P < .001), the median RFS was not reached (NR) versus 26.7 months, the 1-year RFS rate was 83.6% versus 67.5%, and the 2-year RFS rate was 73.3% versus 52.9%, in Caucasian and East-Asian/Hispanic/African, respectively (Table I, Figs 2, A and C). Patients with NAC/UP subtype had the longest RFS, followed by acral; patients with mucosal melanoma had the poorest survival outcomes (P < .001) (Table I, Figs 2, B and D). The median RFS was NR versus 40.6 versus 17.8 months, the 1-year RFS rate was 80.1% versus 78.3% versus 59.3%, the 2-year RFS rate was 69.2% versus 65.1% versus 43.1%, in NAC/UP, acral, and mucosal subtypes, respectively (Table I, Fig 2B). Similar findings were observed for OS (Table I, Figs 2, C and D).
Fig 2.
RFS and OS across different ethnicities and melanoma subtypes. A, RFS across different ethnicities (n = 532). B, RFS across different melanoma subtypes (n = 534). C, OS across different ethnicities (n = 532). D, RFS across different melanoma subtypes (n = 534).
Similar to previous reports,8 patients with BRAF V600 mutant melanoma had significantly poorer RFS than wild type, but this did not translate into an OS inferiority with mitogen-activated protein kinase inhibitors as a choice in the palliative setting. No other demographic/clinical characteristics were found to be significantly correlated with either RFS or OS (Supplementary Table III, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Multivariate analysis of survival outcomes
In the multivariate analysis incorporating ethnicity, melanoma subtype, age, sex, stage, LDH, BRAF mutation status, and adjuvant radiotherapy, East-Asian/Hispanic/African ethnicity (RFS: HR, 1.70; P = .004; OS: HR, 2.34; P = .001) and mucosal subtype (RFS: HR, 3.25; P < .001; OS: HR, 3.20; P < .001) were both independently associated with significant poorer survivals (Table II).
Table II.
Multivariate analysis of RFS and OS (n = 532)
| RFS |
OS |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Ethnicity | ||||
| Caucasian | 1 | 1 | ||
| East-Asian/ Hispanic/African | 1.70 (1.19-2.44) | .004 | 2.34 (1.39-3.95) | .001 |
| Melanoma subtype | ||||
| NAC/UP | 1 | 1 | ||
| Acral | 1.01 (0.64-1.59) | > .95 | 1.46 (0.790-2.679) | .23 |
| Mucosal | 3.25 (2.04-5.17) | < .001 | 3.20 (1.68-6.08) | < .001 |
Other covariates incorporated included sex (male vs female), age (continuous variable), baseline stage (I or II, III, vs IV), baseline LDH (normal vs elevated), BRAF (V600 mutant vs wild type), adjuvant radiotherapy (yes vs no).
Subgroup analysis within the NAC/UP subtype
RFS and OS across different ethnicities and across different anatomic locations of the primary lesions
Due to the different levels of eumelanin expression in patients with different ethnic background and different ultraviolet exposure levels across different anatomic sites of the human body, we analyzed the survival correlations of both ethnicity (in patients with ethnicity data available, n = 348) and anatomic sites of primary lesions (in patients with anatomic site data available, n = 349) within the NAC/UP subtype (n = 350).
East-Asian/Hispanic/African had significantly poorer survival outcomes than Caucasian (Table I), and patients with melanomas originated from head or neck region had numerically poorer RFS, but P-values varied because of varying sample sizes (Supplementary Table IV, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Multivariate analysis
In the multivariate analysis (for the NAC/UP subtypes only) incorporating ethnicity, age, sex, stage, LDH, BRAF mutation status, adjuvant radiotherapy, and anatomic location of the primary lesion, East-Asian/Hispanic/African ethnicity was independently associated with significantly poorer survivals (RFS: HR, 2.27; P < .001; OS: HR, 3.49; P < .001); melanoma originated from upper limb (HR, 0.22; P < .001) and UP (HR, 0.46; P = .03) both were independently correlated with better RFS, but not OS (Supplementary Table V, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Recurrence pattern and subsequent treatment
By last follow-up, 39.5% (211 per 534) patients have developed disease recurrence, among whom 33.2% (70 per 211) developed local recurrence only, 51.7% (109 per 211) developed distant metastasis only, 15.2% (32 per 211) had both local recurrence and distant metastasis. There were 173 patients with LDH data available at the time point of disease recurrence, and 26.6% (46 per 173) had elevated LDH levels. Time of disease recurrence varied widely (median 9.5 months, range 0.9-64.7), 60.2% (127 per 211) developed disease recurrence within 1 year after surgery (Supplementary Fig 2, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
We further explored the correlates of the development of distant metastasis (Supplementary Table VI, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6). Multivariate analyses incorporating all covariates with statistical significance in the univariate analyses demonstrated that mucosal subtype was independently correlated with distant metastasis at disease recurrence (OR, 4.046; P = .03), and East-Asian/Hispanic/African patients had a trend toward the development of distant metastasis (OR 1.93, P =.07) (Supplementary Table VII, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6). At the organ/system level, the most commonly involved were lung (29.6%, 63/211), lymph node/soft tissue (25.6%, 54/211), and liver (18.5%, 39/211) (Supplementary Table VIII, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6). East-Asian/Hispanic/African patients (OR, 4.63; P < .001) and patients with acral melanoma (OR, 2.61; P = .04) had independently higher incidence of lymph node or soft tissue involvement, and patients with mucosal melanoma had independently higher incidence of liver metastasis (OR, 2.64; P = .04) (Supplementary Table IX, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
With regard to the treatment after adjuvant anti-PD-1 monotherapy failure, 42.7% (88 per 206 with data available) received local or regional and 83.3% (169 per 203 with data available) received systemic treatment after disease recurrence. Among those who received systemic treatment due to unresectable disease recurrence, TVEC, BRAFi/MEKi combo, PD-1/antiangiogenic agent combination therapies provided the longest progression free survival, whereas those who received conventional chemotherapy had shorter progression free survival (Supplementary Table X, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Immune-related adverse events
There were 531 (99.4%) patients within the entire cohort with irAE data available. In total, 80 patients (15.1%) discontinued anti-PD-1 monotherapy due to irAE(s). We previously showed that irAE discrepancies remained constant across melanoma subtypes,4 thus we performed ethnicity-specific toxicity analyses.
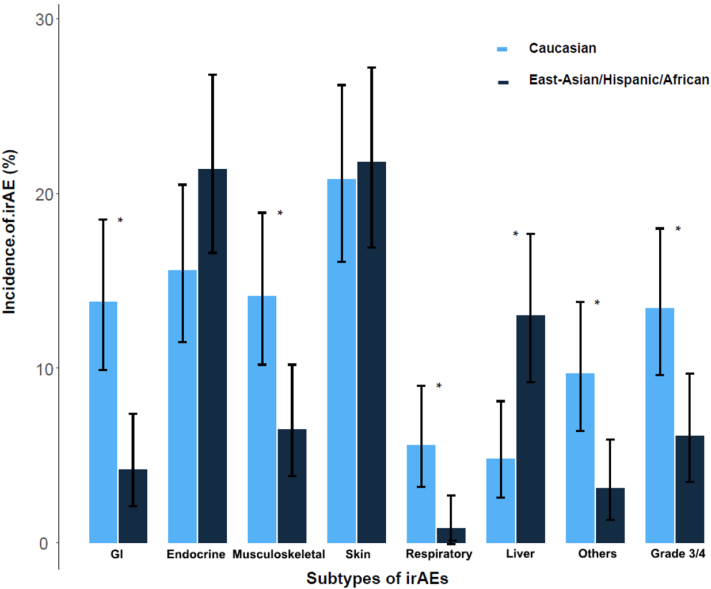
The overall incidence of irAEs was higher with marginal significance in East-Asian/Hispanic/African (62.6% vs 54.3%; P = .05). Compared with Caucasian, East-Asian/Hispanic/African had significantly lower incidences of GI, musculoskeletal, respiratory, and other rare types of irAEs, but higher incidences of liver and endocrine irAEs (marginal significance) (Fig 3, Supplementary Table XI, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6). East-Asian/Hispanic/African had lower incidence of grade 3 per 4 severe irAEs (6.1% vs 13.4%; P = .005), were less likely to receive systemic glucocorticoids (Supplementary Table XII, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6), develop multiple irAEs (8.4% vs 23.4%; P < .001), or discontinue anti-PD-1 adjuvant therapy due to irAEs (6.5% vs 23.4%; P < .001).
Fig 3.
IrAE incidence by ethnicity (n =531, ∗with statistical significance).
Of the entire cohort, 31.6% (168 per 531) of patients developed long-lasting irAEs that did not resolve by the last follow-up. The most common types of long-lasting irAEs included endocrine (16.0%), cutaneous (11.7%), and musculoskeletal (3.0%). Ethnic discrepancies were also noticed, namely East-Asian/Hispanic/African had significantly higher incidence of long-lasting cutaneous but lower musculoskeletal and other-rate-type of irAEs compared with their Caucasian counterparts (Supplementary Table XIII, available via Mendeley at https://www.mendeley.com/reference-manager/reader-v2/a1075b43-7834-364c-abb9-a532c63ac9ff/18aa4ded-4948-42eb-4681-ffee0979fed6).
Discussion
This large multicenter international study demonstrates the first effort of investigation of the effect of ethnicity and melanoma subtype on the survival outcome, recurrence pattern, and irAEs to adjuvant anti-PD-1 monotherapy. We are first to show the efficacy and irAE profile of adjuvant anti-PD-1 in previously underrepresented melanoma populations. With clearly defined primary endpoints and a multivariate analysis, we demonstrate that both ethnicity and melanoma subtype are associated with survival outcomes and recurrence patterns, and East-Asian/Hispanic/African patients have distinct toxicity profiles.
This study focuses on populations that were underrepresented in previous phase III randomized control trials and demonstrates that those trial data are not well generalizable across the globe. We show for the first time that both ethnicity and melanoma subtypes matter in the postdefinitive-surgery adjuvant setting. Specifically, East-Asian/Hispanic/African and patients with mucosal melanoma have poorer survival than their Caucasian counterparts and patients with NAC/UP. Of note, East-Asian/Hispanic/African had a higher prevalence of acral and mucosal melanomas and did serve as a proxy for these subtypes. Therefore, we performed a multivariate analysis incorporating both ethnicity and melanoma subtype (Table II) and found that ethnicity was independently associated with poorer survival outcomes. In another word, the survival discrepancies observed in Fig 2, A and C were associated with both higher proportion of mucosal or acral subtypes and the decreased survival of East-Asian/Hispanic/African in NAC/UP. Even within the NAC/UP subtype, after adjustment for primary site and other known confounders, East-Asian/Hispanic/African have poorer survival than Caucasians. Our observation suggests that NAC/UP melanomas in patients with darker skin color, based on its distinct response to immunotherapy (presumably because of different eumelanin levels and lower tumor mutational burden,9,10 human leukocyte antigen heterogeneity11 and microbiome discrepancies, etc.), might be a special entity and is still largely understudied. We acknowledge that that all but 2 patients were of Asian ethnicity and, therefore, the observed differences in outcome were between Asian and Caucasian patients; and this study did not aim to reveal the mechanism. Further clinical trials and translational research focusing on NAC/UP melanoma subtypes should thus put specific emphasis on these subgroups of patients. Besides, our study corroborates earlier smaller cohort studies of acral (shorter follow-up, thus OS data not available) and mucosal melanomas.12,13 We acknowledge that the limited Caucasian patient sample size in acral or mucosal melanoma subgroups prevents us from performing between-ethnicity comparison in these subtypes. Thus, it remains unclear whether there are ethnic differences in these subtypes of melanoma which are intrinsically less associated with ultraviolet exposure. Of note, sentinel lymph node biopsy is not routinely performed in mucosal melanoma, therefore, there may be a subgroup of stage I or II patients that were indeed with occult lymph node metastasis and therefore should have been categorized as stage III.
It remains debatable what the best management strategy should be after the failure of adjuvant anti-PD-1 monotherapy. In the routine clinical practice, the clinicians tend to take the tumor evolving kinetics as an essential part of information during therapeutic decision-making. To date, there is a lack of data addressing the detailed recurrence pattern and the efficacy of subsequent treatments in such divergent melanoma populations. Our report represents the first international collaborative efforts to reveal the disease kinetics (at the organ or system involvement level), treatments, and survival outcomes. We report that mucosal melanoma has a higher likelihood of developing distant metastasis, especially liver metastasis; and East-Asian/Hispanic/African decedents and acral melanoma have higher incidence of lymph node or soft tissue metastasis. Although we speculate that this phenomenon can be caused by the homological or lymphatic circulating route differences of the primary melanomas originated from different anatomic sites, the exact mechanism underlying remains unclear. Further studies are warranted. The real-world efficacy data of different therapeutic agents after adjuvant anti-PD-1 monotherapy failure is instructive. Although no direct comparison can be made based on the limited sample size and varying recurrence patterns, in concert with previous reports after anti-PD-1 failure in the metastatic setting,14,15 we notice that PD-1- and CTLA-4-based combo, mitogen-activated protein kinase inhibitors combo, T-VEC, and participation in clinical trials are all conveying reasonable benefits. By contrast, traditional chemotherapy, even with previous immunotherapy exposure, remains associated with dismal outcomes, as previously observed after anti-PD-1 monotherapy failure in the metastatic setting.16,17
We note that patients with different ethnic backgrounds demonstrate variating irAEs profiles. Specifically, East-Asian/Hispanic/African, although with a higher likelihood of developing irAEs overall, their irAEs tend to be less severe. At the organ or system level, they have a higher incidence of liver involvement, but experience fewer GI, musculoskeletal, respiratory, and other rare types of irAEs. Although the direct comparison between the irAE profiles of patients receiving anti-PD-1 monotherapy in different settings (ie, adjuvant vs metastatic) is not completely comparable, we note similar between-ethnicity irAE differences in both settings, suggesting that tumor factors may play a less important role in toxicity than host factors. Given that East-Asian/Hispanic/African patients have a higher tendency to develop long-lasting irAEs and gained limited survival benefit, it is worth careful and thorough discussions over the benefits and risks before the use of adjuvant anti-PD-1 monotherapy in these patients.
We acknowledge that this is a retrospective cohort study, and therefore prone to multiple biases and confounders. Although we put emphasis on objective laboratory measures of irAEs, eg, liver and thyroid function tests; we acknowledge that some biases, eg, patient selection bias, underreporting of irAEs in routine medical notes, and different irAE surveillance and management strategies across different centers, etc., cannot be entirely eliminated. We acknowledge that the East-Asian/Hispanic/African group is predominated by East-Asian populations, thus may not fully capture features of Hispanics/African populations. Also, due to the geographic locations of participant centers, our results may not fully represent situations in Africa/Europe/South America either. Thus, further cross-continent international efforts are warranted.
Conclusion
We show that both ethnicity and melanoma subtype independently impact survival outcomes and recurrence patterns in patients with melanoma who received anti-PD-1 monotherapy in the adjuvant setting after definitive surgery, and that there are ethnicity-specific toxicity profiles. Specifically, East-Asian/Hispanic/African decedents and those with mucosal melanoma have poorer survival outcomes and higher likelihood of distant metastasis at disease recurrence. This novel survival, recurrence pattern, and toxicity data for these ethnicities and melanoma subtypes should factor into discussions of the risks and benefits of adjuvant PD-1 blockade. Our data also suggest that toxicity surveillance strategies could be personalized according to ethnicity. Translational research aimed at understanding the underpinnings of ethnicity-specific efficacy and toxicity to adjuvant anti-PD-1 monotherapy is critical for the oncology field.
Conflict of interests
Dr Yamazaki has received honoraria from Ono pharmaceutical, Bristol-Myers Squibb, MSD, Novartis, and Maruho; has received research grant from Ono pharmaceutical, Bristol-Myers Squibb, Novartis, and Amgen. Dr Ogata has received honoraria from MSD, Novartis, Ono pharmaceutical, and Bristol-Myers Squibb. Dr Nakamura serves on a scientific advisory board and educational steering committee for Novartis, has received honoraria from BMS, Kyowa-Kirin, Maruho, MSD, Novartis, Ono Pharma, and Tanabe-Mitsubishi Pharma, and received institutional grants from Kaken Pharma, Ono, Pola Pharma, and Torii. Dr Namikawa has received honoraria from Ono pharmaceutical, Novartis, Bristol-Myers Squibb, and MSD; serves as an advisory role for Novartis and MSD. Dr Guo serves as consultant or is on advisory boards for MSD, Roche, Pfizer, Bayer, Novartis, Simcere Pharmaceutical Group, Shanghai Junshi Biosciences, and Oriengene. Dr Flaherty serves on the Board of Directors of Clovis Oncology, Strata Oncology, Vivid Biosciences, and Scorpion Therapeutics;Scientific Advisory Boards of PIC Therapeutics, Apricity, Tvardi, ALX Oncology, xCures, Monopteros, Vibliome, and Soley Therapeutics; consultant to Takeda, Novartis and Transcode Therapeutics. Dr Long serves as a consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, BMS, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics, MSD, Novartis, OncoSec, PHMR Ltd, Pierre-Fabre, Provectus, Qbiotics, Regeneron. Dr Menzies serves as a consultant for BMS, MSD, Novartis, Roche, Pierre-Fabre and QBiotics. Dr D.B. Johnson has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte. Dr Sullivan serves as consultant for Amgen, Asana Biosciences, BMS, Merck, Novartis, Array BioPharma, Compugen, and Replimune; he receives research support from Amgen and Merck. Dr Boland has a sponsored research agreements with Takeda Oncology, Palleon Pharmaceuticals, InterVenn Biosciences, and Olink Proteomics; serves as a consultant for Merck, InterVenn Biosciences, and Ankyra Therapeutics; served as a speaker for Novartis; and served on a scientific advisory board and steering committee for Nektar Therapeutics. Dr Si has received speakers’ honoraria from MSD, Roche, Novartis, Shanghai Junshi Biosciences and Oriengene. Drs Bai, Gerstberger, Park, Umeda and Authors Lawless, Czapla, Jung, R. Johnson, Li have no conflicts of interest to declare.
Footnotes
Drs Guo, Flaherty, Nakamura, Namikawa, Long, Menzies, Johnson, Sullivan, Boland, and Si are joint senior authors.
Funding sources: Dr Xue Bai was supported by Beijing Municipal Natural Science Foundation (7214217) and Beijing Hospitals Authority (QMS20211101). Dr. Lu Si was supported by National Natural Science Foundation of China (81972566). Dr. Jun Guo was supported by National Natural Science Foundation of China (81972562).
This study has been partially presented in a poster session in ESMO annual meeting, Paris, France, September 9-13, 2022.
IRB approval status: This study has been approved by the IRB of Beijing Cancer Hospital (Peking University Cancer Hospital), No. 2021YJZ16.
References
- 1.Eggermont A.M.M., Blank C.U., Mandala M., et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann K.F., Othus M., Patel S.P., et al. Adjuvant Pembrolizumab versus IFNalpha2b or ipilimumab in Resected High-Risk Melanoma. Cancer Discov. 2022;12(3):644–653. doi: 10.1158/2159-8290.CD-21-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luke J.J., Rutkowski P., Queirolo P., et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 4.Bai X., Shoushtari A.N., Betof Warner A., et al. Benefit and toxicity of programmed death-1 blockade vary by ethnicity in patients with advanced melanoma: an international multicentre observational study. Br J Dermatol. 2022;187(3):401–410. doi: 10.1111/bjd.21241. [DOI] [PubMed] [Google Scholar]
- 5.Amin M.B., Greene F.L., Edge S.B., et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 8th ed. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 6.Chi Z., Li S., Sheng X., et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. 2011;11:85. doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A.E., Karnell L.H., Menck H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Eckardt J., Schroeder C., Martus P., et al. TMB and BRAF mutation status are independent predictive factors in high-risk melanoma patients with adjuvant anti-PD-1 therapy. J Cancer Res Clin Oncol. 2023;149(2):833–840. doi: 10.1007/s00432-022-03939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang B., Chi Z., Chen Y., et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter Phase II trial. Clin Cancer Res. 2020;26(16):4250–4259. doi: 10.1158/1078-0432.CCR-19-3922. [DOI] [PubMed] [Google Scholar]
- 10.Shoushtari A.N., Chatila W.K., Arora A., et al. Therapeutic implications of detecting MAPK-activating alterations in cutaneous and unknown primary melanomas. Clin Cancer Res. 2021;27(8):2226–2235. doi: 10.1158/1078-0432.CCR-20-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowell D., Krishna C., Pierini F., et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med. 2019;25(11):1715–1720. doi: 10.1038/s41591-019-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muto Y., Kambayashi Y., Kato H., et al. Adjuvant anti-PD-1 antibody therapy for advanced melanoma: A multicentre study of 78 Japanese cases. Acta Derm Venereol. 2022;102 doi: 10.2340/actadv.v102.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques S.K., McKeown J., Grover P., et al. 809P Outcomes of patients with resected stage III/IV acral or mucosal melanoma treated with adjuvant anti-PD-1 therapy. Ann Oncol. 2022;33:S915–S916. doi: 10.1016/j.annonc.2022.07.935. [DOI] [PubMed] [Google Scholar]
- 14.Olson D.J., Eroglu Z., Brockstein B., et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol. 2021;39(24):2647–2655. doi: 10.1200/JCO.21.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pires da Silva I., Ahmed T., Reijers I.L.M., et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22(6):836–847. doi: 10.1016/S1470-2045(21)00097-8. [DOI] [PubMed] [Google Scholar]
- 16.Bai X., Kim M., Kasumova G., et al. Radiological dynamics and SITC-defined resistance types of advanced melanoma during anti-PD-1 monotherapy: an independent single-blind observational study on an international cohort. J Immunother Cancer. 2021;9(2) doi: 10.1136/jitc-2020-002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldinger S.M., Buder-Bakhaya K., Lo S.N., et al. Chemotherapy after immune checkpoint inhibitor failure in metastatic melanoma: a retrospective multicentre analysis. Eur J Cancer. 2022;162:22–33. doi: 10.1016/j.ejca.2021.11.022. [DOI] [PubMed] [Google Scholar]