Abstract
Introduction and importance
The Intrauterine Contraceptive Device (IUD), a widely used contraceptive since 1965, has demonstrated efficacy but is associated with complications such as bleeding, pain, and rare occurrences of perforation. This case report details an IUD migration into the peritoneal cavity, leading to acute appendicitis.
Case presentation
A 33-year-old woman, with a history of IUD insertion 16 months prior, presented with pelvic pain. Gynecological examination and computed tomography, revealed the IUD intraperitoneal migration. The patient underwent laparoscopic extraction of the IUD which was embedded in the appendix and appendectomy, with an uneventful recovery.
Clinical discussion
This case emphasizes the complexity of IUD migration and its rare association with acute appendicitis, underscoring the importance of vigilant monitoring and prompt intervention.
We also explored factors contributing to IUD perforation risk, imaging modalities for detection, and emphasizes the necessity of surgical removal upon confirmation. We highlight the fact that despite the atypical presentation with minimal symptoms, we should always consider emergency situations. Surgical intervention, particularly laparoscopy, may be the standard approach for managing migrated IUDs.
Conclusion
We insist about the critical need for thorough assessment and vigilance in managing IUD-related complications, emphasizing timely intervention to ensure patient safety. This case contributes valuable insights into the complexities surrounding IUD migration, urging healthcare professionals to remain attentive to potential injuries in patients with a history of IUD insertion and abdominal pain.
Keywords: Intrauterine device, Migration, Perforation, Case report, Appendicitis
Highlights
-
•
The Intrauterine Contraceptive Device (IUD) is a widely used contraceptive method, but its popularity is accompanied by potential complications.
-
•
Uterine perforation is a rare but serious complication of IUD insertion leading to severe complications that may require intensive treatments, including surgery.
-
•
We highlight a novel occurrence of IUD migration into the peritoneal cavity, causing acute appendicitis. We performed a laparoscopic extraction.
-
•
This case also highlights the importance risk factor consideration and IUD continuous follow-up to avoid complications, such as acute appendicitis to ensure patient safety.
1. Introduction
The Intrauterine Contraceptive Device (IUD), introduced in 1965, has gained widespread use [1]. Despite its popularity IUD had their proper side effects including bleeding, pain, and ectopic pregnancies [2,3].
Perforation of the uterus by an IUD is a rare but serious complication, with an incidence ranging from 1 in 350 to 1 in 2500 insertions [4]. Uterine perforation can extend to adjacent organs, including the bladder and the intestinal tract, leading to severe complications that often necessitate intensive treatments, including surgery [5]. Perforation can occur during the insertion process or manifest later [1,6]. We present a novel case of intrauterine device migration into the peritoneal cavity. The diagnostic tools employed for this migration were gynecological examination and computed tomography. Laparoscopy was subsequently utilized to extract the IUD, which had become embedded in the appendix resulting in an acute appendicitis.
This case report has been reported in line with the SCARE Criteria [7].
2. Case report
A 33-year-old woman, who had a history of three vaginal deliveries and one abortion, underwent long-term contraception with an intrauterine device (IUD) in March 2019. The patient reported no immediate issues after the IUD insertion. After 16 months, she consulted her gynecologist for pelvic pain. Upon general examination, the patient was afebrile, no tachycardia, the gynecological examination revealed no abnormal bleeding or the retrieval string of the IUD. Pelvic ultrasound indicated an empty uterine cavity. Abdominal examination revealed tenderness in the hypogastrium.
Laboratory results showed a white blood cell count of 5700/mm3 and the rest of the tests being unremarkable. Given the clinical context, abdominal tenderness and the absence of the IUD on gynecological examination, an abdominal CT scan was performed, revealing a laterally displaced IUD to the right with surrounding fat infiltration, without pneumoperitoneum (Fig. 1, Fig. 2).
Fig. 1.

CT scan showing IUD localization (black arrow).
Fig. 2.
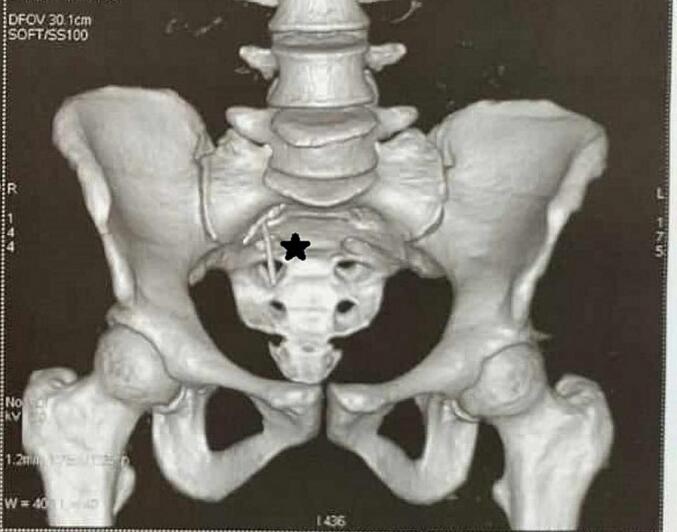
CT scan showing IUD localization (black star).
The patient was taken to the operating room for a laparoscopic approach, the intrauterine device was situated as indicated by the CT scan data, showing inflammation localized in the right iliac fossa in proximity to the appendix and cecum with adhesions to the mesoappendix. Careful dissection revealed strong adhesion of the intrauterine device to the mesoappendix, along with an inflamed appendix. The IUD was embedded in the appendix (Fig. 3). The IUD was successfully extracted, and an appendectomy was performed. The postoperative course was uneventful, and the patient was discharged on the first postoperative day.
Fig. 3.

Laparoscopic view T‑copper IUD (black star) causing acute appendicitis (red star). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At the follow-up after 2 months, the patient reported no further pain and expressed satisfaction with the outcome.
The pathologist report confirmed the presence of an acute suppurative appendicitis with periappendicitis.
3. Discussion
In the realm of contraceptive methods, intrauterine devices (IUDs) are widely used due to their efficacy and convenience. However, their insertion can carry potential risks [1], and one of the serious complications is myometrial perforation, leading to various surgical emergencies such as peritonitis, bladder/bowel injury [2,8], adhesions and even more rarely acute appendicitis. This case report highlights the complexities surrounding IUD migration and its involvement with acute appendicitis, emphasizing the need for vigilant monitoring and prompt intervention.
Therefore several factors may increase the risk of perforation, including lack of experience during insertion, low estrogen levels, uterine anatomical abnormalities, recent childbirth or women during breastfeeding period and multiple abortions [9,10]. Our patient had a history of only one abortion but shortly after that the device was implanted, we can only hypothesis that it is related due to the fragility of myometrial wall.
It is important to mention that when IUD migration is suspected, detecting a missing IUD string is crucial, as it may indicate a potential perforation. Imaging modalities such as ultrasound and abdominopelvic X-ray are key exam for evaluation [8,11]. If the IUD is confirmed to be in the abdominopelvic cavity, immediate surgical removal is imperative [12]. For this patient after ultrasound we requested an abdominal CT scan for it is more adequate that a plain X- ray to localize the exact position of the IUD and to evaluate the complications caused by its migration.
Perforation can manifest as a partial embedment in the myometrial wall or complete perforation [13], causing the device to migrate to the abdominopelvic cavity. The pouch of Douglas is identified as a common site for IUD migration in cases of complete perforation [2,13] following by the bladder and the intestinal tract. Thus, few reported cases have linked IUD migration to acute appendicitis [14,15], with patients presenting appendicitis-related symptoms such as nausea, vomiting, fever, and abdominal pain. These symptoms may be accompanied by clinical signs, including abdominal tenderness in the right lower quadrant and laboratory abnormalities such as elevated white blood cell count or C-reactive protein levels [4,16]. Unlike previously reported cases, this report highlights a unique scenario where the patient remains almost asymptomatic, emphasizing the importance of considering emergent situations even in the absence of overt typical clinical manifestations.
Surgical intervention is the standard approach for managing a migrated intrauterine contraceptive device [17], with options such as laparoscopy based on the specific complication observed and the experience of the surgeon [18] or laparotomy. Laparoscopy offers several advantages, including minimal tissue trauma, shorter procedural duration, quicker postoperative recovery, and a reduced risk of adhesions [19,20]. In the presented case, the migrated intrauterine device was successfully extracted, and an appendectomy was concurrently performed. The procedure and subsequent follow-up were free of complications.
4. Conclusion
The intrauterine device (IUD) is a highly effective contraceptive method, with its insertion being a medical procedure requiring minimal knowledge and experience. Perforation is among the rarest and most serious complications. Laparoscopy remains the most effective diagnostic and therapeutic method.
Consideration should be given to potential injury to structures adjacent to the uterus, when there is a history of IUD insertion and a patient presents with abdominal pain.
Acute appendicitis due to an intrauterine device is an exceedingly uncommon occurrence.
This review underscores the critical importance of thorough assessment and vigilance in managing IUD-related complications, emphasizing the need for timely intervention to mitigate risks and ensure patient safety.
Consent
Written informed consent was obtained from the patients for publication of this case series and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethical approval
Our institutions “la Rabta Hospital” and “School of Medicine of Tunis” require no ethical approval for case series. It is required for studies on human participants. This is just a case series with written patient approval.
Funding
No funding.
Author contribution
Amine Sebai: conceptualization, data curation, redaction, project manager.
Elaifia Rany: conceptualization, data curation, redaction, project manager.
Souhaib Atri: conceptualization, redaction.
Mahdi hammami: resources, visualization.
Haddad Anis: resources, visualization.
Montassar Kacem: supervision, validation, visualization.
Guarantor
Elaifia Rany.
Research registration number
Not applicable.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
References
- 1.Heinonen P.K., Merikari M., Paavonen J. Uterine perforation by copper intrauterine device. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984;17:257–261. doi: 10.1016/0028-2243(84)90068-6. [DOI] [PubMed] [Google Scholar]
- 2.Key T.C., Kreutner A.K. Gastrointestinal complications of modern intrauterine devices. Obstet. Gynecol. 1980;55:239–244. [PubMed] [Google Scholar]
- 3.Nelson A., Massoudi Mph N. New developments in intrauterine device use: focus on the US. OAJC. 2016;7:127–141. doi: 10.2147/OAJC.S85755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohana E., Sheiner E., Leron E., Mazor M. Appendix perforation by an intrauterine contraceptive device. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;88:129–131. doi: 10.1016/S0301-2115(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 5.Freijo B.A., Freijo B.A., Fernández J.A.L., Bayon T.M., Blanes E.V., Mialaret L.S., et al. Removal of migrated intrauterine devices to appendix, small intestine and omentum: case reports. SCR. 2021:1–3. doi: 10.31487/j.SCR.2021.02.20. [DOI] [Google Scholar]
- 6.Heartwell S.F., Schlesselman S. Risk of uterine perforation among users of intrauterine devices. Obstet. Gynecol. 1983;61:31–36. [PubMed] [Google Scholar]
- 7.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A., et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109:1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boortz H.E., Margolis D.J.A., Ragavendra N., Patel M.K., Kadell B.M. Migration of intrauterine devices: radiologic findings and implications for patient care. RadioGraphics. 2012;32:335–352. doi: 10.1148/rg.322115068. [DOI] [PubMed] [Google Scholar]
- 9.Andersson K., Ryde-Blomqvist E., Lindell K., Odlind V., Milsom I. Perforations with intrauterine devices. Contraception. 1998;57:251–255. doi: 10.1016/S0010-7824(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 10.Caliskan E., Oztürk N., Dilbaz B.O., Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003;8:150–155. [PubMed] [Google Scholar]
- 11.Zamani Bonab M., Anvari Aliabad R., Alimohammadi S. Migration of intrauterine device caused asymptomatic acute appendicitis: a case report. Clin. Case Reports. 2021;9 doi: 10.1002/ccr3.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledward R.S., Healey C., Eadie R. Removal of extrauterine Saf-T-coil through laparoscope. BMJ. 1972;1:508. doi: 10.1136/bmj.1.5798.508-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakin D., Stern W.Z., Rosenblatt R. Vol. 36. 1981. Complete and Partial Uterine Perforation and Embedding Following Insertion of Intrauterine Deives. I. Classification, Complications, Mechanism, Incidence, and Missing String: Obstetrical & Gynecological Survey; p. 335. [DOI] [PubMed] [Google Scholar]
- 14.Chang H.-M. Intrauterine contraceptive device appendicitis: a case report. WJG. 2005;11:5414. doi: 10.3748/wjg.v11.i34.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson S.A., Gatlin A., Mazur M. Appendiceal perforation by Copper-7 intrauterine contraceptive device. Am. J. Obstet. Gynecol. 1981;141:586–587. doi: 10.1016/S0002-9378(15)33284-1. [DOI] [PubMed] [Google Scholar]
- 16.Maree G., Mohammad S., Saleh R., Hoshma A., Makhluf H. Appendiceal perforation caused by an intrauterine contraceptive device: a case report. Case Rep. Women’s Health. 2022;36 doi: 10.1016/j.crwh.2022.e00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaislasuo J., Suhonen S., Gissler M., Lahteenmaki P., Heikinheimo O. Uterine perforation caused by intrauterine devices: clinical course and treatment. Hum. Reprod. 2013;28:1546–1551. doi: 10.1093/humrep/det074. [DOI] [PubMed] [Google Scholar]
- 18.Adiyeke M., Sanci M., Karaca I., Gökçü M., Töz E., Ocal E. Surgical management of intrauterine devices migrated towards intra-abdominal structures: 20-year experience of a tertiary center. Clin. Exp. Obstet. Gynecol. 2015;42:358–360. [PubMed] [Google Scholar]
- 19.Toumi O., Ammar H., Ghdira A., Chhaidar A., Trimech W., Gupta R., et al. Pelvic abscess complicating sigmoid colon perforation by migrating intrauterine device: a case report and review of the literature. Int. J. Surg. Case Rep. 2018;42:60–63. doi: 10.1016/j.ijscr.2017.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosley F.R., Shahi N., Kurer M.A. Elective surgical removal of migrated intrauterine contraceptive devices from within the peritoneal cavity: a comparison between open and laparoscopic removal. JSLS. 2012;16:236–241. doi: 10.4293/108680812X13427982377265. [DOI] [PMC free article] [PubMed] [Google Scholar]


