Abstract
Introduction:
Remote ischemic conditioning upregulates endogenous protective pathways in response to ischemia-reperfusion injury. This study tested the hypothesis that limb remote ischemic per- conditioning (RIPerC) exerts cardioprotective effects via the renin-angiotensin system (RAS)/inducible nitric oxide synthase (iNOS)/apelin pathway.
Methods:
Renal ischemia-reperfusion injury (I/R) was induced by bilateral occlusion of the renal pedicles for 60 minutes, followed by 24 hours of reperfusion; sham-operated rats served as controls. RIPerC was induced by four cycles (5 minutes) of limb ischemia-reperfusion along with bilateral renal ischemia. The functional disturbance was evaluated by renal (BUN and creatinine) and cardiac (troponin I and lactate dehydrogenase) injury biomarkers.
Results:
Renal I/R injury increased renal and cardiac injury biomarkers that were reduced in the RIPerC group. Histopathological findings of the kidney and heart were also suggestive of amelioration injury-induced changes in the RIPerC group. Assessment of cardiac electrophysiology revealed that RIPerC ameliorated the decline in P wave duration without significantly affecting other cardiac electrophysiological changes. Further, renal I/R injury increased the plasma (322.40±34.01 IU/L), renal (8.27±1.10 mIU/mg of Protein), and cardiac (68.28±10.28 mIU/mg of protein) angiotensin-converting enzyme (ACE) activities in association with elevations in the plasma and urine nitrite (25.47±2.01 & 16.62±3.05 μmol/L) and nitrate (15.47±1.33 & 5.01±0.96 μmol/L) levels; these changes were reversed by RIPerC. Further, renal ischemia-reperfusion injury significantly (P=0.047) decreased the renal (but not cardiac) apelin mRNA expression, while renal and cardiac ACE2 (P<0.05) and iNOS (P=0.043) mRNA expressions were significantly increased compared to the sham group; these effects were largely reversed by RIPerC.
Conclusion:
Our results indicated that RIPerC protects the heart against renal ischemia- reperfusion injury, likely via interaction of the apelin with the RAS/iNOS pathway.
Keywords: Limb remote ischemic per-conditioning, Renal ischemia reperfusion, Myocardial injury, Apelin, Renin-angiotensin system, iNOS
Introduction
Renal ischemia-reperfusion (I/R) is a major cause of acute kidney injury (AKI) that is usually induced in some surgeries (kidney transplantation and coronary artery bypass grafting) and multiple clinical situations, including trauma, severe infection, sepsis, and hemorrhagic shock.1,2 Renal I/R is characterized by sudden restriction of blood supply to the kidney followed by the subsequent restoration of perfusion and re-oxygenation.3,4
The consequences of renal I/R injury are local as well as remote organ destruction such as liver,5 lung,6,7 and heart.8 The physiological and pathophysiological interaction between the kidney and the cardiovascular system provides essential indicators for maintaining homeostasis.9 Indeed, several studies have demonstrated cardiac dysfunction contributes to a high morbidity/mortality rate after renal I/R injury.10 It is often followed by a change in renin-angiotensin system (RAS) components and redox imbalance that induces renal and extra-renal organ disturbances.11,12
Oxidative stress, as one of the direct effects of the hypoxia process, is further developed during reperfusion.11 Activity of angiotensin-converting enzyme (ACE), which can increase the concentration of angiotensin II (Ang II) in the plasma by cleaving angiotensin I (Ang I), was shown to be significantly increased in renal and cardiac after renal I/R injury.13 The excess Ang II can intensify renal ischemia and promote renal and heart injury by increasing systemic inflammation, reactive oxygen species formation, altering the source of nitric oxide (NO) availability, and inducing nitrosative and oxidative stress.14,15 ACE2, the first known homolog of ACE, was indicated to be a negative regulator of RAS because of its ability to convert Ang II to Ang (1–7), a peptide with vasodilatory and anti-fibrotic effects.16
One of the newly-recognized endogenous peptides that has attracted researchers’ attention in the last decade is apelin. Apelin is an inotropic and cardioprotective ligand that shows positive effects by activating the G-protein-coupled receptor (APJ) in the pathology of cardiovascular diseases.17 Apelin receptor has about 50% homology with angiotensin II type 1 (AT1) receptor. However, a counter-regulatory role for apelin in relation to the renin-angiotensin system is suggested by antagonizing the effects of angiotensin II on vascular tone, blood pressure, and fluid homeostasis.18 Therefore, there are direct cellular and biological interactions between these two systems. On the other hand, apelin exhibits renal and cardioprotective effects through modulation of the nitric oxide synthesis (NOS)/NO-generation pathway.19
Hormesis or conditioning is a technique by which harmful stimuli below the threshold of injury are applied to an organ or system to develop cellular tolerance against more severe damage.20 Moreover, it was demonstrated that conditioning could be done in a distant (remote) non-vital organ, such as a limb, but still exert beneficial effects on vital organs. Remote ischemic per-conditioning (RIPerC) is an excellent example of episodes of occlusion and reperfusion of blood flow on a limb, making temporary limb ischemia and remotely promoting endogenous protective pathways in the heart and kidney or other organs.21,22
The exact endogenous mechanisms of RIPerC are not completely understood yet. However, signal transmission from theremote site to target organs is reported to be modulated by humoral factors, immune cells, and the autonomic nervous system.22 Therefore, this study tested the hypothesis that limb remote ischemic per-conditioning (RIPerC) exerts cardioprotective effects via the RAS/inducible nitric oxide synthase (iNOS)/apelin pathway.
Materials and Methods
Animals
This study was done on 30 male Sprague-Dawley rats weighing 250-270 g. They were purchased from the Animal Center of Shiraz University of Medical Sciences. Animal work took place in a specific animal room (in separate cages) at a controlled temperature of 22 to 25 °C with lighting (12 hours light/dark cycles) and was acclimatized to water and food without restriction.
Surgical procedure
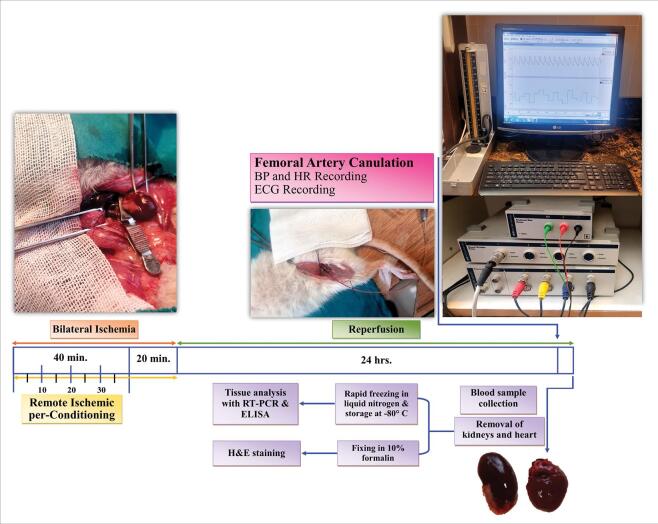
Rats were anesthetized with Ketamine (50 mg/kg; Alfasan, Woerden, the Netherlands) and xylazine (10 mg/kg; Bayer, Leverkusen, Germany) and were then put on a surgical heating table to retain the body temperature at 37 °C. The procedure of renal ischemia is described in our previous study.23 Briefly, a midline abdominal incision was made, and the renal pedicles were exposed. Rats were randomly allocated to three groups (n=10 per group): (1) Sham, in which renal pedicels did not clamp; (2) BIR (bilateral ischemic-reperfusion) group, in which both renal pedicles were occluded for 60 minutes using microaneurysm vascular clamp; (3) BIR+RIPerC (remote ischemic per-conditioning) groups, in which four cycles of 5 minutes ischemia of the left femoral artery followed by 5 minutes reperfusion were applied at the beginning of renal ischemia. After the termination of ischemia, the clamps were token up and confirmed for appropriate reperfusion of the blood flow to the ischemic kidney. The abdominal incision was then sutured by 2/0 stitches in 2 layers.
Experimental protocol
At the end of the surgery, the animals were kept in metabolic cages as previously described,24 and 24 hours urine volume was collected. After 24 hours reperfusion, all rats were weighed and immediately re-anesthetized. Animals were placed on a wooden plate to prevent additional electrical signals which could interfere with our ECG recording. Then, a filled cannula [normal saline (SAMEN, Mashhad, Iran) with 15 IU/mL heparin (Alborzdarouco, Tehran, Iran)] was inserted into the right femoral artery and connected to a pressure transducer. ECG electrodes were connected to the hands of animals (negative: right hand and positive: left hand) and the left limb. After 15 minutes of equilibration, arterial blood pressure (BP) and heart rate (HR) were continuously recorded for 10 minutes using a PowerLab/8SP data acquisition system (AD Instruments, BellaVista, NSW, Australia). Blood samples were taken from the abdominal vein to measure renal and cardiacfunction markers. Subsequently, the kidneys and heart were removed and weighed immediately. Two coronal sections were cut from the middle part of the kidney and heart.The left kidney and one piece of heart tissue were stored at -80 °C for measuring ACE activity, NO metabolites, and RT-PCR. The right kidney and another slice of the heart were kept in 10% formalin for hematoxylin-eosin (H & E) staining.
Measurement of renal and cardiac functional biomarkers
Plasma concentrations of creatinine (Cr), blood urea nitrogen (BUN), and lactate dehydrogenase (LDH) were measured using Technicon® RA-1000 auto-analyzer (Technicon Corporation, Tarry-town, USA) in Namazi Hospital Laboratory, Shiraz, Iran.Troponin I level is a specific index of myocardium injury. Cardiac troponin I (cTn-I) was measured using an enzyme-linked fluorescent assay (ELFA) kit (VIDAS®, bioMérieux, North Carolina, USA).
Echocardiographic (ECG) analysis
ECG long lead II was recorded while the rats were lying on a wooden plate to avoid additional electrical signals which could interfere with our recording. Intervals of R-R and J-T (distance between S wave and the end of T wave), durations and amplitudes of P, Q, R, S as well as T waves, and the heart rate were analyzed by ECG analyzing software of a Power Lab system (AD Instruments, Australia). QT interval was corrected using normalized Bazett's equation QTc= QT/(RR/f)1/2, where f is the normalization factor according to RR duration in the sham group.25
ACE activity assay
The ACE activity was measured, as previously described by Beneteau et al with minor modifications.26 In brief, ACE activity was determined with an artificial substrate [furanacryloyl-L-phenylalanylglycylglycine (FAPGG, Sigma-Aldrich, St. Louis, Missouri, United States)] in a reaction mixture containing 25 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, HEPES, Sigma-Aldrich], 0.5 mM FAPGG, 300 mM NaCl (Sodium chloride, Sigma-Aldrich), and the desired dilution of the serum or tissue homogenate at pH 8.2. The reaction rate at 340 nm can be determined using the FAPGG extinction coefficient (ε=0.989 μM-1). Measurements were performed in 96-well plates at 25 °C. Changes in the optical density (340 nm) were measured at 1 min intervals for 10 min with a microplate reader (Epoch 2, BioTek, Winooski, Vermont, United States). One unit of ACE activity is defined as the amount of enzyme that will cause the oxidation of 1.0 µmol of FAPGG to FAP per minute at 25 °C. The ACE activity in the serum and tissue samples was expressed as µmol of FAPGG oxidized/min/mL of the serum or mg of protein (Units/mL of serum or Units/mg of protein).
Measurement of NO metabolite
The amount of NO can be measured by determining the concentrations of nitrite and nitrate end products in the plasma and tissue samples by the Griess method.27 Initially, plasma samples were deproteinized using 99% ethanol and centrifuged. Quantitation of nitrite was based on its reaction with a mixture of 0.1% NEDD [N-(1-Naphthyl)ethylenediamine, Sigma-Aldrich] (25 µL) in 5% HCl (Hydrochloric acid, Sigma-Aldrich) (25 µL) and 2% sulphanilamide (Sigma-Aldrich) (25 µL), which were added to 50 µL of deproteinized samples in duplicate and kept in a water bath at 37 °C for 15 minutes. The optical densities were measured at 540 nm using a microplate reader (Epoch 2, BioTek). After that, nitrate was quantitated by reducing it to nitrite via the addition of vanadium (III) chloride (Sigma-Aldrich) (50 µL) to each sample, incubating for 15 min at 37 °C, and rereading absorbances at 540 nm. Total nitrite/nitrate (NO2-/NO3-) concentrations were calculated as µmol per liter of the plasma and urine using the standard curve generated from the serial concentrations of NaNO2 (0-100 µmol/L) (Sodium nitrite, Sigma-Aldrich).
Quantitative RT-PCR analysis
Total RNA extraction, cDNA synthesis, and SYBR Green based-real-time PCR methods were performed to evaluate the expression levels of ACE2, iNOS, and apelin genes in rat kidney and heart samples. For RNA extraction, 150 mg of each kidney and heart tissue sample in BIR, BIR+RIPreC, and sham groups was homogenized by adding 800 µL Trizol (YTzol, Yekta Tajhiz Azma, Tehran, Iran) in sterile falcon tubes on water and ice (4 °C). Homogenized solutions were centrifuged for 5 minutes at 4000 rpm, and their supernatants were transferred to an RNase-free 2 mL microtube. In the next step, 400 µL chloroform (Sigma-Aldrich) was added, and the solution was kept at 4 °C for 5 minutes and centrifuged for 20 minutes at 13500 rpm and 4 °C to form aqueous and organic phases. The aqueous phase was removed to a new RNase-free microtube, 800 µL ethanol 100% was added, and it was kept overnight at -20 °C. The next day, this solution was centrifuged for 20 minutes at 13500 rpm and 4 °C. The supernatant was removed, 800 µL ethanol 75% was added, and the mixture was centrifuged for 8 minutes at 8000 rpm and 4 °C. Then, the supernatants were removed, and microtubes were dried under the hood station for 20 minutes. Total RNA sediment was solved in 50 µL diethylpyrocarbonate (DEPC)-treated water (Sinaclon, Karaj, Iran) and put on a dry block at 55-60 °C for 10 minutes to open the secondary structure of RNA. The purity and concentration of RNA were measured using NanoDropTM (Thermo ScientificTM, Waltham, Massachusetts, United States) at 260/280 nm, and cDNA synthesis was done for the rat kidney and heart samples using AddScript cDNA Synthesis kit, based on the manufacturer’s instructions (AddScript cDNA Synthesis, AddBio Inc., Yuseong-gu, Daejeon, Korea).
ACE2, iNOS, and apelin expression levels in the kidney and heart tissue samples in all three groups were determined using an in-house SYBR green Real-time PCR protocol by Step One Real-Time Instrument (ABI, StepOnePlus, Foster City, United States). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was considered as an internal control to eliminate test errors.
The real-time PCR mix was composed of PCR master mix (Hot-start Taq DNA Polymerase, SYBR Green I dye, dNTPs mixture, protein stabilizers, and enhancers) (Add SYBR Master, AddBio Inc.), forward and reverse primers (10 pmol), and template cDNA. The real-time conditions of the studied transcripts were 95 °C/10 min, 40 cycles at 95 °C/15 s, 58°C/20 s, and 72 °C/30 s. Melting curves of the target and internal control genes were analyzed to confirm the specificity of PCR reactions. All real-time tests were repeated three times. The reverse and forward primer sequences for transcript amplification are shown in Table 1.
Table 1. Primer sequences for transcript amplification .
| Gene name | Gene ID | Sequence (5′→3′) | Product length (bp) |
| GAPDH | 24383 | F: AGTGCCAGCCTC GTCTCATA | 91 |
| R: GAGAAGGCAGCCCTGGTAAC | |||
| ACE2 | 302668 | F: GTGTCGTGATGGGAACGGTA | 124 |
| R: TTGCCAATGTCCATGGAGTCA | |||
| iNOS | 24599 | F: GGGAGAGTGAGCTGGTGTTT | 108 |
| R: TGCAGTCCCGAGCATCAAAT | |||
| Apelin | 58812 | F: CTCTGGCTCTCCTTGACTGC | 109 |
| R: TCGAAGTTCTGGGCTTCACC |
Kidney and heart histological evaluation
Renal and heart tissue samples were fixed in 10% formalin solution (Formaldehyde 37%, Merck Chemicals GmbH, Darmstadt, Germany). Slides of the samples were prepared at 5 µm thickness and stained with the routine hematoxylin and eosin (H&E) method.
In a blinded fashion, an expert pathologist examined each section in at least ten randomly selected non-overlapping fields under the light microscope. The renal histopathology was quantified for the degree of Bowman’s space enlargement, brush border loss in proximal tubules, cast formation, and tubular necrosis. The myocardium structural disturbance was assessed based on the score of interstitial edema, minimum congestion, and leucocyte infiltration. The level of each manifestation was graded according to the changes involved, scoring 0 with no changes, 1 with less than 20%, 2 with 20–40%, 3 with 40–60%, 4 with 60–80%, and 5 with greater than 80%. The sum of all numerical scores in each group was reported as the total histopathological score.7,28,29
Statistical analysis
All data are expressed as mean ± SEM. Multiple comparisons between groups were performed using one-way ANOVA, followed by Dunnett's and Tukey's post hoc tests. Differences were considered statistically significant at P<0.05. Statistical analyses were performed using GraphPad Prism software package version 9. (GraphPad Software Inc. La Jolla, California, USA).
Results
Renal and cardiac injury biomarkers
As shown in Table 2, renal BIR caused a significant increase in the plasma levels of Cr and BUN compared to values obtained from the plasma of sham-operated animals. Cardiac injury markers, including LDH and troponin I were significantly higher in the BIR group than the sham group. The renal and cardiac functions improved when cyclic limb remote ischemia started along with the renal ischemia. Therefore, the plasma levels of Cr and LDH significantly increased, and BUN and troponin I slightly declined in the RIPerC group compared with the BIR group.
Table 2. Effect of remote ischemic per-conditioning on renal and cardiac injury biomarkers .
| Variables | Experimental groups | |||
| Sham | BIR | BIR+RIPerC | ||
| Renal injury markers | Cr level (mg/dL) | 0.49 ± 0.02 | 3.91 ± 0.42**** | 2.19 ± 0.28***,### |
| BUN level (mg/dL) | 16.63 ± 1.54 | 120.70 ± 5.15**** | 109.30 ± 7.58*** | |
| Cardiac injury markers | LDH (U/L) | 404.80 ± 120.60 | 1816 ± 334.80** | 1187 ± 114.40# |
| Troponin I (ng/mL) | 0.18 ± 0.07 | 0.58 ± 0.21** | 0.23 ± 0.06 | |
Data are expressed as mean ± SEM, n=10.
** P<0.01, ***P<0.0001 and ****P<0.0001 vs. the sham group. #P<0.05 and ###P<0.001 vs. the BIR group.
Cr, creatinine; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; BIR, bilateral ischemic reperfusion; RIPerC, remote ischemic per-conditioning.
Blood pressure and electrocardiogram data
As determined in Table 3, mean blood pressure (MBP) significantly decreased in the BIR group compared with the sham group. The heart rate increased, but not significantly, in this group. ECG long lead II was recorded to evaluate whether the renal BIR induced functional disturbance in myocardia. ECG analysis data showed that P duration significantly decreased, whereas QRS, QTc, and J-T intervals significantly increased in the BIR group in comparison with the basic intervals in the sham group. Remote ischemic per-conditioning slightly modulated the transmissionof the cardiac impulse. P duration was significantly higher in the BIR+RIPerC compared with the BIR group. Moreover, QRS and QTc intervals slightly declined in the BIR+RIPerC group; however, there were significant differences with the basis durations in the sham group. The P-R interval did not change in the three studied groups (Table 3).
Table 3. Effect of remote ischemic per-conditioning on the mean blood pressure, heart rate, intervals, and voltages of electrocardiogram waves .
| Variables | Experimental groups | ||
| Sham | BIR | BIR+RIPerC | |
| MBP (mm Hg) | 104.50 ± 8.40 | 88.55 ± 3.70** | 102.90 ± 4.15# |
| HR (BPM) | 215.10 ± 7.75 | 221.30 ± 9.85 | 232.50 ± 9.01 |
| P-R Interval (ms) | 53.43 ± 1.71 | 52.75 ± 1.55 | 55.83 ± 1.06 |
| P Duration (ms) | 23.53 ± 1.08 | 14.80 ± 0.78*** | 19.42 ± 1.05*,# |
| QRS Interval (ms) | 15.26 ± 0.87 | 20.27 ± 1.02** | 19.10 ± 0.52* |
| QTc Interval (ms) | 41.18 ± 2.22 | 62.50 ± 2.44*** | 57.26 ± 3.65** |
| J-T Interval (ms) | 22.40 ± 2.40 | 40.31 ± 2.90** | 42.83 ± 2.70*** |
| P Amplitude (mV) | 1.62 ± 0.25 | 1.40 ± 0.25 | 1.70 ± 0.50 |
| Q Amplitude (mV) | -4.25 ± 0.72 | -3.38 ± 0.90 | -2.70 ± 0.30 |
| R Amplitude (mV) | 4.46 ± 0.50 | 4.67 ± 1.13 | 4.22 ± 0.51 |
| S Amplitude (mV) | -3.20 ± 0.90 | -3.40 ± 0.96 | -2.67 ± 0.22 |
| ST Height (mV) | -2.84 ± 0.80 | -3.37 ± 0.80 | -2.33 ± 0.40 |
| T Amplitude (mV) | 4.07 ± 1.27 | 2.24 ± 0.90 | 2.10 ± 0.58 |
Data are expressed as mean ± SEM, n=10.
*P<0.05, **P<0.01, and ***P<0.001 vs. the sham group. #P<0.05 vs. the BIR group.
MBP, mean blood pressure; HR, heart rate; BPM, beat per minute; BIR, bilateral ischemia-reperfusion; RIPerC, remote ischemic per-conditioning.
Bilateral renal ischemia (60 minutes) with 24 hours reperfusion led to a slight decline in P and T amplitude as well as ST depression in the BIR group compared to the sham group; however, the difference was not significant. RIPerC restored the P and T amplitude and ST depression to the basal voltage in the BIR+RIPerC group. No significant differences in Q, R, and S voltages were observed among the three experimental groups (Table 3).
ACE activity in the plasma and tissue
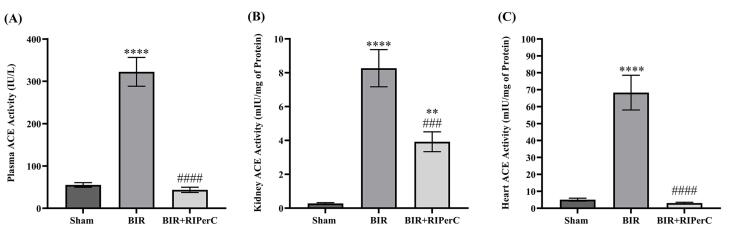
The activity of the ACE was measured in three types of samples (plasma, heart, and kidney) 24 hours after reperfusion. Our data showed that bilateral renal ischemia (60 minutes) stimulated the activity of ACE. Therefore, the activity of ACE in the kidney, as well as plasma and heart tissues, was significantly elevated in the BIR group compared to that in the sham group (in all three P<0.0001). Cyclic remote ischemia at the time of ischemia prevented the increase in the ACE activity seen after renal ischemia. For this reason, there was a significant difference in the ACE activity between the BIR+RIPerC and BIR groups in the plasma and heart (P<0.0001) as well as in the kidney tissue (P<0.001). However, the kidney ACE activity in the BIR+RIPerC group was higher than the sham group (P<0.01) (Fig. 1).
Fig. 1.
Effect of limb remote ischemic per-conditioning on the activity of the angiotensin-converting enzyme (ACE) in the plasma (A), kidney tissue (B), and heart tissue (C). Data are expressed as mean ± SEM, n=10. **P<0.01 and ****P<0.0001 vs. the sham group. ###P<0.001 and ####P<0.0001 vs. the BIR group.
NO metabolites (nitrite and nitrate) in the renal and cardiac tissues
We measured the concentration of NO metabolites (nitrite and nitrate) in the kidney and heart tissues. Our data confirmed that NO production was induced after renal ischemia in the kidney as well as heart tissue. Thus, tissue NO2– (nitrite) levels were elevated in the BIR group compared with the sham group (P<0.05). In addition, the kidney and heart NO3– (nitrate) levels slightly increased after renal ischemia in the BIR group. While nitrite and nitrate content decreased in the BIR+RIPerC group, there was no significant difference between the mean values of NO2– and NO3– levels in the BIR and BIR+RIPerC groups (Table 4).
Table 4. Effect of remote ischemic per-conditioning on the concentration of NO metabolites (nitrite and nitrate) in the kidney and heart tissues .
| Variables | Experimental groups | |||
| Sham | BIR | BIR+RIPerC | ||
| Kidney | NO2¯ level (µmol/L) | 53.01 ± 6.79 | 76.43 ± 7.52* | 72.96 ± 3.44 |
| NO3¯ level (µmol/L) | 2.04 ± 0.06 | 2.11 ± 0.11 | 1.91 ± 0.03 | |
| Heart | NO2¯ level (µmol/L) | 43.14 ± 7.14 | 65.60 ± 4.61* | 58.04 ± 3.99 |
| NO3¯ level (µmol/L) | 1.77 ± 0.08 | 1.90 ± 0.12 | 1.78 ± 0.02 | |
Data are expressed as mean ± SEM, n=10.
* P<0.05 vs. the sham group.
NO metabolites (nitrite and nitrate) in the biological fluids
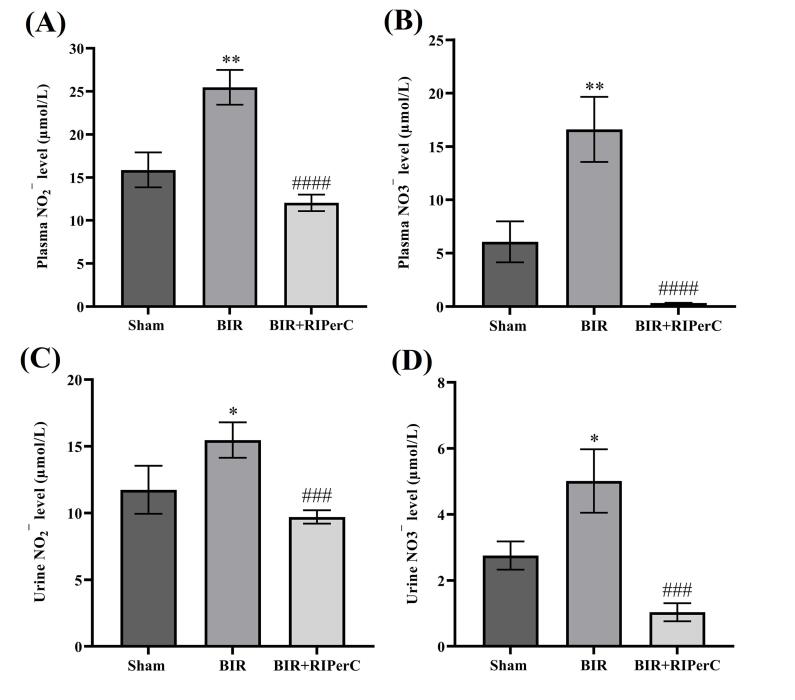
We also investigated the level of nitrite and nitrate in the biological fluids (plasma and urine) samples in the experimental groups as an index of NO synthesis. There was a significant increase in the plasma and urine nitrite and nitrate concentration in the BIR compared with the sham group (P<0.01 and P<0.05, respectively). Increased plasma and urine nitrite and nitrate levels induced by renal BIR were significantly decreased in BIR+RIPerC in comparison with the BIR group (P<0.0001 and P<0.001, respectively) (Fig. 2).
Fig. 2.
Effect of remote ischemic per-conditioning on NO metabolites (nitrite and nitrate) in the biological fluids (NO2¯ and NO3¯ levels in the plasma (A and B) and urine (C and D) samples, respectively). Data are expressed as mean ± SEM, n=10. *P<0.05 and **P<0.01 vs. the sham group. ###P<0.001 and ####P<0.0001 vs. the BIR group. BIR, bilateral ischemic reperfusion; RIPerC, remote ischemic per-conditioning.
The mRNA levels of apelin, ACE2, and iNOS in the renal and heart tissues
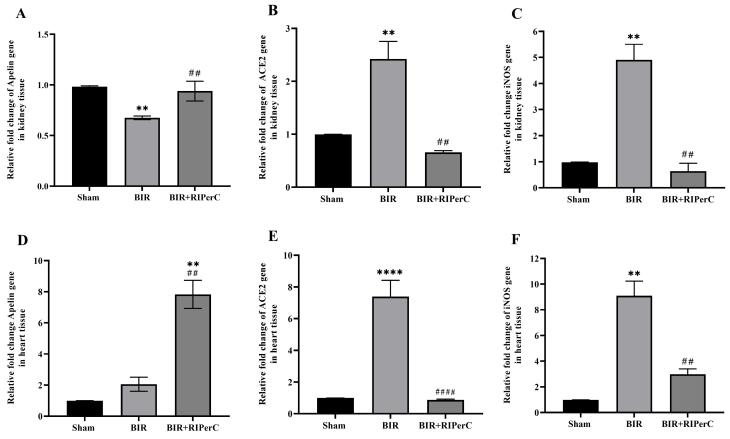
After 24 hours of reperfusion, the apelin mRNA expression was downregulated in the renal tissue (Fig. 3A), not the cardiac tissue (Fig. 3D) of the BIR group. However, the expression levels of ACE2 and iNOS in renal and myocardium tissues were significantly higher in the BIR groups than in the sham group (Fig. 3B-C and Fig. 3E-F). Remote ischemic per-conditioning modulated the expression of these genes. The apelin expression was upregulated, and iNOS and ACE2 expression were downregulated in BIR+RIPerC compared to the BIR group in both studied tissues (Fig. 3A-F).
Fig. 3.
Effect of remote ischemic per-conditioning on the apelin (A and D), ACE2 (B and E), and iNOS (C and F) mRNA expression in the renal and heart tissues. Data are expressed as mean ± SEM, n=10. **P<0.01 and ****P<0.0001 vs. the sham group. ##P<0.01 and ####P<0.0001 vs. the BIR group. BIR, bilateral ischemic reperfusion; RIPerC, remote ischemic per-conditioning.
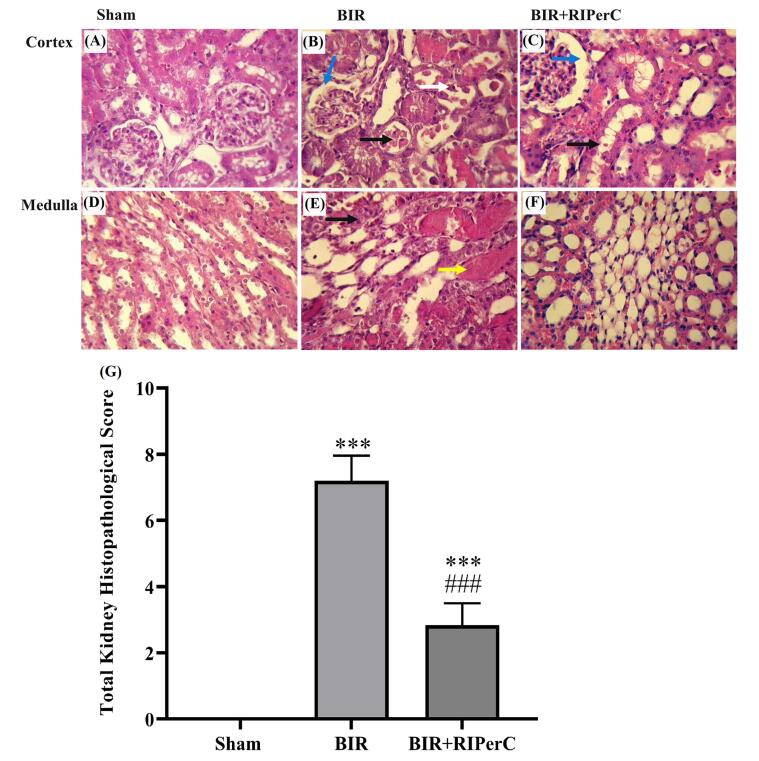
Renal and cardiac histopathological findings
Renal glomerular, tubular, and vascular damages in the experimental groups are shown in detail in Fig. 4. The normative appearance was found in the sham group (Fig. 4A and 4D) in the cortex and medulla area. Compared with those in the sham group, the rats in the BIR group showed significant renal histopathologic changes, including bowman space enlargement, epithelial tubular cells injury, acute tubular necrosis, intratubular cast, and vascular congestion (Fig. 4B and 4E). In the BIR+RIPerC group, minimal structural changes in the glomeruli and tubules were observed (Fig. 4C and 4F). Quantifiable results showed that the total histopathological score significantly increased in the BIR group and modulated in the BIR+RIPerC group (Fig. 4G).
Fig. 4.
Histopathological finding of the renal cortex and medulla at the end of 24 h reperfusion: sham group (A and D) showed normal appearance. Obvious tissue injuries were observed in the BIR group (B and E); a higher degree of Bowman space enlargement (blue arrow), loss of brush border in the proximal tubules (black arrow), cast formation (yellow arrow), and tubular necrosis (white arrow). Remote ischemic per-conditioning improved structural changes in the BIR+RIPerC group (C and F). The level of each manifestation was graded according to the changes involved, scoring 0 with no changes, 1 with less than 20%, 2 with 20–40%, 3 with 40–60%, 4 with 60–80%, and 5 with greater than 80%. The sum of all numerical scores in each group was stated as the total histopathological score (G). H & E staining (magnification ×400) and data are expressed as mean ± SEM, ***P<0.01 vs. the sham group and ###P<0.001 vs. the BIR group. BIR, bilateral ischemic reperfusion; RIPerC, remote ischemic per-conditioning
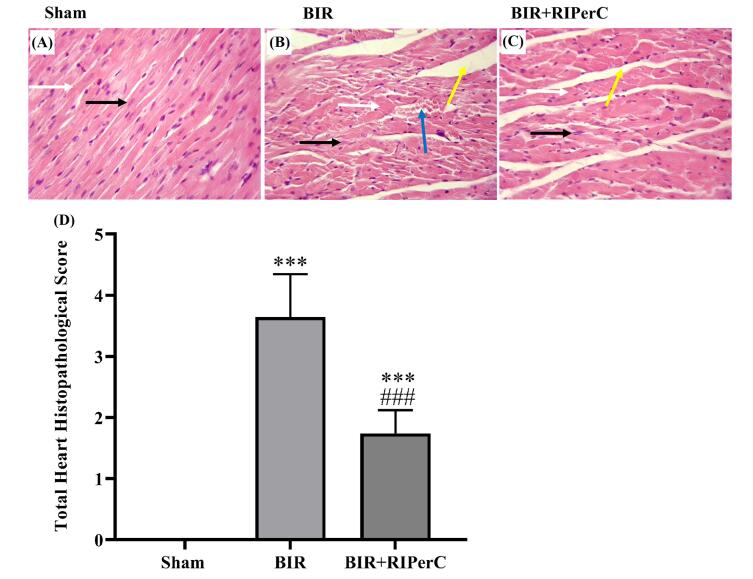
Bilateral renal ischemic reperfusion-induced slight structural damage in the heart tissue. The appearance of the sham group did not show alterations. Myocardial tissue destruction was characterized by interstitial edema, minimum congestion, and leucocyte infiltration in the BIR group. The combination of renal ischemia with cyclic remote ischemia (femoral artery ischemia) attenuated the severity of lesions in the BIR+RIPerC group. The total histopathological score decreased in the BIR+RIPerC group (Fig. 5A-D).
Fig. 5.
Histopathological finding of the heart tissue at the end of 24 h reperfusion: sham group (A) showed a normal appearance. Obvious tissue changes were detected in the BIR group (B) with a higher degree of interstitial edema and congestion. Remote ischemic per-conditioning improved the structural changes in the BIR+RIPerC group (C). Muscle fiber (white arrow), central nucleus (black arrow), congestion (blue arrow), and interstitial edema (yellow arrow). The level of each manifestation was graded according to the changes involved, scoring 0 with no changes, 1 with less than 20%, 2 with 20–40%, 3 with 40–60%, 4 with 60–80%, and 5 with greater than 80%. The sum of all numerical scores in each group was stated as the total histopathological score (D). H & E staining (magnification ×400) and data are expressed as mean ± SEM, ***P<0.01 vs. the sham group and ###P<0.001 vs. the BIR group. BIR, bilateral ischemic reperfusion; RIPerC, remote ischemic per-conditioning
Discussion
Renal ischemia/reperfusion-induced AKI is an outcome of various clinical situations, including transplantation, partial nephrectomy, sepsis, hydronephrosis, or elective urological surgery. Recent studies have demonstrated that cardiac failure is the most common complication after clinical and experimental AKI induced by renal ischemia/reperfusion.10,30
The obtained results in this study demonstrated that renal ischemia induced cardiac functional disturbance and structural damage. In addition, we detected significant elevation in ACE activity, No metabolites, and mRNA levels of iNOS and ACE2 in the post-renal I/R (ischemic/reperfusion) heart tissue.
Renal I/R injury caused histological and functional alterations in renal and heart tissues.30-32 We also found that tissue damages were associated with high plasma levels of renal and cardiac injury biomarkers, such as BUN, Cr, LDH, and troponin I.
Remote ischemic conditioning (RIC) is an adaptive phenomenon that causes tissue resistance to long-term ischemia or ischemia-reperfusion after one or more brief conditioning ischemia-reperfusion cycles.33,34 The phenomenon of RIC was first reported by Murry et al.35 Some researchers separately showed renal and cardioprotective effects of remote ischemic per-conditioning.36,37 To the best of our knowledge, no study has reported the mechanism involved in the protective effect of RIPerC on acute heart injury due to renal I/R injury. For the first time, we investigated the protective effects of this procedure on cardiac injury induced by renal I/R injury through interactive effects of apelin, RAS axis, and iNOS. In the first step of this research, we found that renal as well as heart dysfunctions improved when renal ischemia-reperfusion was done along with remote ischemic per-conditioning. The post-renal I/R myocardium injury improved with RIPerC. According to our previous study, histopathological findings demonstrated that remote ischemic per-conditioning significantly decreased epithelial tubular cell injury, tubular necrosis, intratubular cast, and vascular congestion.38
Moreover, other cardiac functional data indicated that RIPerC causes amelioration of ECG parameters, mean blood pressure, and heart rate. Prolonged QTc and QRS intervals in the BIR groups are similar to those of other studies on the cardiac effect of renal I/R injury.39,40 These results and the rise in troponin I and LDH activity confirmed myocardial injury after renal I/R injury.
However, the mechanisms involved in the cardiac injury induced by renal I/R are not completely understood. In the second part of the study, we set out to investigate the interactive effect of RAS, iNOS, and apelin in our experimental groups. Our result showed that apelin plays a critical role in post-renal I/R cardiac dysfunction.
Various studies have indicated that endogenous apelin/APJ receptor has potential therapeutic effects in kidney and cardiovascular diseases.17,41,42 Wang et al showed that apelin mRNA downregulated at 1-day post‐myocardial infarction (MI) in humans and murine. In addition, apelin-knockout mice were susceptible to MI after cardiac ischemia-reperfusion.43 Moreover, Gholampour et al showed that apelin expression increased after renal I/R injury.44 However, our result indicated that the expression level of the apelin gene decreased in the kidney, but not the heart, after renal I/R injury, whereas apelin mRNA upregulated in the heart and kidney of the BIR+RIPerC rats.
During renal ischemia and re-oxygenation of the tissue, the parenchymal cells, including tubular epithelial cells and vascular endothelial cells, generate free radicals, which are reactive nitrogen species (RNS) or reactive oxygen species (ROS). ROS/RNS stimulates inducible isoforms of NOS, which causes about 1000-fold higher NO formation than eNOS.11,45
Our results were in line with those of Meng et al study that indicated mRNA levels of iNOS increased after renal I/R in the kidney sample.46 In addition, nitrate and nitrite concentration as an index of NO production increased in biological fluids (blood and urine). Previous studies have confirmed systemic redox imbalance and systemic inflammation after renal I/R.10,47,48 Therefore, in the current study, upregulation of cardiac iNOS mRNA may be related to response to these systemic conditions. Overexpression of cardiac iNOS was accompanied by increased nitrate and nitrite levels in the post-renal I/R myocardium. In two separate studies, Güvenç et al and Feng et al demonstrated the deleterious effect of NO derived from iNOS.49,50 On the other hand, in accordance with other studies, some cardiovascular protective effects of the apelin peptide depend on NO derived from eNOS.19,51 An et al reported that apelin overexpression increased the eNOS levels through diminishing iNOS levels during ischemia-reperfusion injury in diabetic myocardium.52 In the current study, we found decreased apelin expression along with increased iNOS expression in the tissue samples of the BIR group.
Interestingly, our result demonstrated that iNOS expression following NO production decreased when renal ischemic reperfusion was performed along with remote ischemic per-conditioning. Accordingly, nitrate and nitrite concentration declined in the kidney and heart tissues and biological fluids.
In the third step, we measured different components of the cardiac and renal RAS in the experimental groups to evaluate the association between the RAS axis and apelin activity.
Renal I/R seems to change the balance of the RAS axis.3,53 This is supported by our data which showed that ACE activity markedly increased in the plasma as well as cardiac and renal tissues 24 h after reperfusion. Efrati et al demonstrated that captopril, as an ACE inhibitor, declined angiotensin-II production following the development of renal dysfunction at the early stage of reperfusion, differentially inhibited inflammation, significantly decreased intrarenal NO, and reduced histopathologic signs of renal damage after renal I/R.13 Interestingly, in our work, ACE2 mRNA was upregulated in both tissue samples in the BIR group. Whereas da Silveira Kátia et al detected that gene expression of ACE2 remained unchanged in the renal I/R2h group, it was significantly downregulated in the I/R4h group.54 The increased level of ACE2 mRNA in the heart and renal tissue may be a compensatory response to the local and systemic increase of the ACE activity in ischemic conditions, even though the elevation of the mRNA level of ACE2 could not suppress the deleterious effect of ACE activity in the ischemic condition.
Clear evidence exists of antagonism between the apelin system and the RAS. Previous studies have confirmed that apelin is an endogenous and cardioprotective peptide that exhibits beneficial effects through the modulation of RAS axis components in the pathology of cardiovascular diseases.55 Apelin depletion progressed the myocardium dysfunction and structural remodeling through angiotensin II pathways.56 Our data clearly indicated that remote ischemic per-conditioning suppressed the cardiac ACE activity and ACE2 expression.
Conclusion
Remote ischemic per-conditioning is a potential phenomenon to promote endogenous protective pathways against renal I/R injury. The findings of the current study revealed that renal I/R induced kidney and heart structural and functional disturbance by a significant increase in injury biomarkers. Histological damages in the renal and heart tissues were in line with the increased ACE activity and NO metabolites in the tissues and biological samples. On the other hand, the level of apelin mRNA was downregulated, but iNOS and ACE2 genes were upregulated in the renal and heart tissues. In total, our results indicated that RIPerC protects the heart against renal I/R injury, probably through the interaction of the apelin with the RAS/iNOS pathway.
Research Highlights
What is the current knowledge?
√ Remote ischemic conditioning upregulates endogenous protective pathways in response to ischemia-reperfusion injury.
√ The physiological and pathophysiological interaction between the kidney and the cardiovascular system provides essential indicators for maintaining homeostasis.
What is new here?
√ Limb remote ischemic per conditioning improves the myocardium dysfunction induced by renal ischemia-reperfusion injury through the interaction of the apelin with the RAS/iNOS pathway.
Acknowledgments
The authors would like to thank the Center for Development of Clinical Research of Nemazee Hospital, Shiraz, Iran, and Dr. Nasrin Shokrpour for editorial assistance.
Competing interests
The authors declare no conflict of interest.
Ethical Statement
The experimental protocols were approved by the local ethics committee of Shiraz University of Medical Sciences (Approval ID: IR.SUMS.REC.1399.894).
Data Availability Statement
Data reported in this manuscript are available upon reasonable request from the corresponding author.
Funding
This study was supported by the Vice-Chancellor for Research, Shiraz University of Medical Sciences (Academic Grant Number: 21395).
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–64. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Roozbeh J, Malekmakan L, Monavarian M, Daneshian A, Karimi Z. Survival of Kidney Retransplant Compared With First Kidney Transplant: A Report From Southern Iran. Exp Clin Transplant. 2018;16:386–90. doi: 10.6002/ect.2016.0130. [DOI] [PubMed] [Google Scholar]
- 3.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal InjPrev. 2015;4:20–7. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karimi Z, SoukhakLari R, Rahimi-Jaberi K, Esmaili Z, Moosavi M. Nanomicellar curcuminoids attenuates renal ischemia/reperfusion injury in rat through prevention of apoptosis and downregulation of MAPKs pathways. Mol Biol Rep. 2021;48:1735–43. doi: 10.1007/s11033-021-06214-2. [DOI] [PubMed] [Google Scholar]
- 5.Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in Hepatic TNF-α Levels, Antioxidant Status, and Oxidation Products after Renal Ischemia/Reperfusion Injury in Mice. J Surg Res. 2002;107:234–40. doi: 10.1006/jsre.2002.6513. [DOI] [PubMed] [Google Scholar]
- 6.Karimi Z, Ketabchi F, Alebrahimdehkordi N, Fatemikia H, Owji SM, Moosavi SM. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren Fail. 2016;38:1503–15. doi: 10.1080/0886022x.2016.1214149. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi SS, Janfeshan S, Karimi Z. Acute lung injury induced by acute uremia and renal ischemic-reperfusion injury: The role of toll-like receptors 2 and 4, and oxidative stress. Iran J Basic Med Sci. 2022;25:643–51. doi: 10.22038/ijbms.2022.64025.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sayed SS, Shahin RM, Fahmy A, Elshazly SM. Quercetin ameliorated remote myocardial injury induced by renal ischemia/reperfusion in rats: Role of Rho-kinase and hydrogen sulfide. Life Sci. 2021;287:120144. doi: 10.1016/j.lfs.2021.120144. [DOI] [PubMed] [Google Scholar]
- 9.Amann K, Wanner C, Ritz E. Cross-Talk between the Kidney and the Cardiovascular System. J Am Soc Nephrol. 2006;17:2112–9. doi: 10.1681/ASN.2006030204. [DOI] [PubMed] [Google Scholar]
- 10.Kelly KJ. Distant Effects of Experimental Renal Ischemia/Reperfusion Injury. J Am Soc Nephrol. 2003;14:1549–58. doi: 10.1097/01.ASN.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 11.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–78. doi: 10.1016/S0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 12.Fatemikia H, Ketabchi F, Karimi Z, Moosavi SM. Distant effects of unilateral renal ischemia/reperfusion on contralateral kidney but not lung in rats: the roles of ROS and iNOS. Can J PhysiolPharmacol. 2016;94:477–87. doi: 10.1139/cjpp-2015-0285. [DOI] [PubMed] [Google Scholar]
- 13.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I, et al. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant. 2012;27:136–45. doi: 10.1093/ndt/gfr256. [DOI] [PubMed] [Google Scholar]
- 14.Panico K, Abrahão MV, Trentin-Sonoda M, Muzi-Filho H, Vieyra A, Carneiro-Ramos MS. Cardiac Inflammation after Ischemia-Reperfusion of the Kidney: Role of the Sympathetic Nervous System and the Renin-Angiotensin System. Cell PhysiolBiochem. 2019;53:587–605. doi: 10.33594/000000159. [DOI] [PubMed] [Google Scholar]
- 15.Crassous P-A, Couloubaly S, Huang C, Zhou Z, Baskaran P, Kim DD, et al. Soluble guanylyl cyclase is a target of angiotensin II-induced nitrosative stress in a hypertensive rat model. Am J Physiol Heart Circ Physiol. 2012;303:H597–H604. doi: 10.1152/ajpheart.00138.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee P, Gheblawi M, Wang K, Vu J, Kondaiah P, Oudit GY. Interaction between the apelinergic system and ACE2 in the cardiovascular system: therapeutic implications. Clin Sci (Lond) 2020;134:2319–36. doi: 10.1042/cs20200479. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira AA, Vergara A, Wang X, Vederas JC, Oudit GY. Apelin pathway in cardiovascular, kidney, and metabolic diseases: Therapeutic role of apelin analogs and apelin receptor agonists. Peptides. 2022;147:170697. doi: 10.1016/j.peptides.2021.170697. [DOI] [PubMed] [Google Scholar]
- 18.Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol. 2006;41:778–81. doi: 10.1016/j.yjmcc.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, et al. Apelin activates l-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides. 2007;28:2023–9. doi: 10.1016/j.peptides.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, Preckel B. Remote Ischemic Conditioning to Protect against Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis. PloS One. 2012;7:e42179. doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholampour F, Roozbeh J, Janfeshan S, Karimi Z. Remote ischemic per-conditioning protects against renal ischemia-reperfusion injury via suppressing gene expression of TLR4 and TNF-α in rat model. Can J PhysiolPharmacol. 2019;97:112–9. doi: 10.1139/cjpp-2018-0543. [DOI] [PubMed] [Google Scholar]
- 23.Karimi Z, Soukhaklari R, Malekmakan L, Esmaili Z, Moosavi M. The effect of nanomicellar curcuminoids on renal ischemia/reperfusion injury and the expressions of COX-2 and Na+/K+-ATPase in rat’s kidney. J Physiology and Pharmacology. 2022;26:424–32. doi: 10.52547/phypha.27.1.3. [DOI] [Google Scholar]
- 24.Moosavi SM, Karimi Z. Cooperative mechanisms involved in chronic antidiuretic response to bendroflumethiazide in rats with lithium-induced nephrogenic diabetes insipidus. Acta Physiol Hung. 2014;101:88–102. doi: 10.1556/APhysiol.101.2014.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Europ J Pharmacol. 2010;641:187–92. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Beneteau B, Baudin B, Morgant G, Giboudeau J, Baumann FC. Automated kinetic assay of angiotensin-converting enzyme in serum. Clin Chem. 1986;32:884–6. [PubMed] [Google Scholar]
- 27.García-Robledo E, Corzo A, Papaspyrou S. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar Chem. 2014;162:30–6. doi: 10.1016/j.marchem.2014.03.002. [DOI] [Google Scholar]
- 28.Najafi H, Owji SM, Kamali-Sarvestani E, Moosavi SM. A1 -Adenosine receptor activation has biphasic roles in development of acute kidney injury at 4 and 24 h of reperfusion following ischaemia in rats. Exp Physiol. 2016;101:913–31. doi: 10.1113/ep085583. [DOI] [PubMed] [Google Scholar]
- 29.Masjedi F, Gol A, Dabiri S, Javadi A. Investigating the Preventive Effect of Garlic on Blood Glucose Levels and Histopathology of Pancreas in Streptozotocin-induced Diabetic Rats. Physiology and Pharmacology. 2009;13:179–90. [Google Scholar]
- 30.Junho CVC, González-Lafuente L, Navarro-García JA, Rodríguez-Sánchez E, Carneiro-Ramos MS, Ruiz-Hurtado G. Unilateral Acute Renal Ischemia-Reperfusion Injury Induces Cardiac Dysfunction through Intracellular Calcium Mishandling. Int J Mol Sci. 2022;23:2266. doi: 10.3390/ijms23042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golmohammadi MG, Shahbazi A, Asl MMC, Banaei SJBRAC. Calcitriol and erythropoietin protect against cardiac injury induced by renal ischemia-reperfusion. Biointerface Res Appl Chem. 2020;10:6718–27. doi: 10.33263/BRIAC106.67186727. [DOI] [Google Scholar]
- 32.Karimi Z, Janfeshan S, Kargar Abarghouei E, Hashemi SS. Therapeutic effects of bone marrow mesenchymal stem cells via modulation of TLR2 and TLR4 on renal ischemia-reperfusion injury in male Sprague-Dawley rats. Bioimpacts. 2021;11:219–26. doi: 10.34172/bi.2021.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal S, Randhawa PK, Singh N, Jaggi AS. Preconditioning at a distance: Involvement of endothelial vasoactive substances in cardioprotection against ischemia-reperfusion injury. Life Sci. 2016;151:250–8. doi: 10.1016/j.lfs.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 34.McDonough A, Weinstein JR. The role of microglia in ischemic preconditioning. Glia. 2020;68:455–71. doi: 10.1002/glia.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 36.Sawashita Y, Hirata N, Yoshikawa Y, Terada H, Tokinaga Y, Yamakage M. Remote ischemic preconditioning reduces myocardial ischemia–reperfusion injury through unacylated ghrelin-induced activation of the JAK/STAT pathway. Basic Res Cardiol. 2020;115:50. doi: 10.1007/s00395-020-0809-z. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Chen R, Xue S, Zhu H, Sun X, Sun X. Protective effects of three remote ischemic conditioning procedures against renal ischemic/reperfusion injury in rat kidneys: a comparative study. Irish J Med Sci. 2015;184:647–53. doi: 10.1007/s11845-014-1227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gholampour F, Roozbeh J, Janfeshan S, Karimi Z. Remote ischemic per-conditioning protects against renal ischemia–reperfusion injury via suppressing gene expression of TLR4 and TNF-α in rat model. Can J PhysiolPharmacol. 2019;97:112–9. doi: 10.1139/cjpp-2018-0543. [DOI] [PubMed] [Google Scholar]
- 39.Amini N, Sarkaki A, Dianat M, Mard SA, Ahangarpour A, Badavi M. Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacol Rep. 2019;71:1059–66. doi: 10.1016/j.pharep.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Trentin-Sonoda M, da Silva RC, Kmit FV, Abrahão MV, Monnerat Cahli G, Brasil GV, et al. Knockout of Toll-Like Receptors 2 and 4 Prevents Renal Ischemia-Reperfusion-Induced Cardiac Hypertrophy in Mice. PLoS One. 2015;10:e0139350. doi: 10.1371/journal.pone.0139350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. PharmacolTher. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Chapman FA, Nyimanu D, Maguire JJ, Davenport AP, Newby DE, Dhaun N. The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol. 2021;17:840–53. doi: 10.1038/s41581-021-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, McKinnie SMK, Patel VB, Haddad G, Wang Z, Zhabyeyev P, et al. Loss of Apelin Exacerbates Myocardial Infarction Adverse Remodeling and Ischemia reperfusion Injury: Therapeutic Potential of Synthetic Apelin Analogues. J Am Heart Assoc. 2013;2:e000249. doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gholampour F, Bagheri A, Barati A, Masoudi R, Owji SM. Remote Ischemic Perconditioning Modulates Apelin Expression After Renal Ischemia-Reperfusion Injury. J Surg Res. 2020;247:429–37. doi: 10.1016/j.jss.2019.09.063. [DOI] [PubMed] [Google Scholar]
- 45.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng X, Wei M, Wang D, Qu X, Zhang K, Zhang N, et al. The protective effect of hesperidin against renal ischemia-reperfusion injury involves the TLR-4/NF-κB/iNOS pathway in rats. Physiol Int. 2020;107:82–91. doi: 10.1556/2060.2020.00003. [DOI] [PubMed] [Google Scholar]
- 47.Abogresha NM, Greish SM, Abdelaziz EZ, Khalil WF. Remote effect of kidney ischemia-reperfusion injury on pancreas: role of oxidative stress and mitochondrial apoptosis. Arch Med Sci. 2016;12:252–62. doi: 10.5114/aoms.2015.48130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awad AS, El-Sharif AA. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 2011;11:992–6. doi: 10.1016/j.intimp.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Feng Q, Lu X, Jones DL, Shen J, Arnold JMO. Increased Inducible Nitric Oxide Synthase Expression Contributes to Myocardial Dysfunction and Higher Mortality After Myocardial Infarction in Mice. Circulation. 2001;104:700–4. doi: 10.1161/hc3201.092284. [DOI] [PubMed] [Google Scholar]
- 50.Güvenç M, Cellat M, Uyar A, Özkan H, Gokcek İ, İsler CT, et al. Nobiletin Protects from Renal Ischemia-Reperfusion Injury in Rats by Suppressing Inflammatory Cytokines and Regulating iNOS-eNOS Expressions. Inflammation. 2020;43:336–46. doi: 10.1007/s10753-019-01123-w. [DOI] [PubMed] [Google Scholar]
- 51.Sabry MM, Mahmoud MM, Shoukry HS, Rashed L, Kamar SS, Ahmed MM. Interactive effects of apelin, renin–angiotensin system and nitric oxide in treatment of obesity-induced type 2 diabetes mellitus in male albino rats. Arch PhysiolBiochem. 2019;125:244–54. doi: 10.1080/13813455.2018.1453521. [DOI] [PubMed] [Google Scholar]
- 52.An S, Wang X, Shi H, Zhang X, Meng H, Li W, et al. Apelin protects against ischemia-reperfusion injury in diabetic myocardium via inhibiting apoptosis and oxidative stress through PI3K and p38-MAPK signaling pathways. Aging. 2020;12:25120–37. doi: 10.18632/aging.104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tikellis C, Bernardi S, Burns WC. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. CurrOpin Nephrol Hypertens. 2011;20:62–8. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 54.da Silveira Kátia D, Pompermayer Bosco Kênia S, Diniz Lúcio RL, Carmona Adriana K, Cassali Giovanni D, Bruna-Romero O, et al. ACE2–angiotensin-(1–7)–Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci. 2010;119:385–94. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 55.Sato T, Suzuki T, Watanabe H, Kadowaki A, Fukamizu A, Liu PP, et al. Apelin is a positive regulator of ACE2 in failing hearts. J Clin Invest. 2013;123:5203–11. doi: 10.1172/jci69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato T, Kadowaki A, Suzuki T, Ito H, Watanabe H, Imai Y, et al. Loss of Apelin Augments Angiotensin II-Induced Cardiac Dysfunction and Pathological Remodeling. Int J Mol Sci 2019; 20. 10.3390/ijms20020239. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this manuscript are available upon reasonable request from the corresponding author.