Abstract
Background
Allogeneic hematopoietic stem cell transplantation (allo‐HCT) is associated with improved outcomes for people with various hematologic diseases; however, the morbidity and mortality resulting from acute and subsequently chronic graft‐versus‐host disease (GVHD) pose a serious challenge to wider applicability of allo‐HCT. Intravenous methotrexate in combination with a calcineurin inhibitor, cyclosporine or tacrolimus, is a widely used regimen for the prophylaxis of acute GVHD, but the administration of methotrexate is associated with a number of adverse events. Mycophenolate mofetil, in combination with a calcineurin inhibitor, has been used extensively in people undergoing allo‐HCT. Conflicting results regarding various clinical outcomes following allo‐HCT have been observed when comparing mycophenolate mofetil‐based regimens against methotrexate‐based regimens for acute GVHD prophylaxis.
Objectives
Primary objective: to assess the effect of mycophenolate mofetil versus methotrexate for prevention of acute GVHD in people undergoing allo‐HCT.
Secondary objectives: to evaluate the effect of mycophenolate mofetil versus methotrexate for overall survival, prevention of chronic GVHD, incidence of relapse, treatment‐related harms, nonrelapse mortality, and quality of life.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE from inception to March 2014. We handsearched conference abstracts from the last two meetings (2011 and 2012) of relevant societies in the field. We searched ClinicalTrials.gov, Novartis clinical trials database (www.novctrd.com), Roche clinical trial protocol registry (www.roche‐trials.com), Australian New Zealand Clinical Trials Registry (ANZCTR), and the metaRegister of Controlled Trials for ongoing trials.
Selection criteria
Two review authors independently reviewed all titles/abstracts and selected full‐text articles for inclusion. We included all references that reported results of randomized controlled trials (RCTs) of mycophenolate mofetil versus methotrexate for the prophylaxis of GVHD among people undergoing allo‐HCT in this review.
Data collection and analysis
Two review authors independently extracted data on outcomes from all studies and compared prior to data entry and analysis. We expressed results as risk ratios (RR) and 95% confidence intervals (CI) for dichotomous outcomes and hazard ratios (HR) and 95% CIs for time‐to‐event outcomes. We pooled the individual study effects using the random‐effects model. Estimates lower than one indicate that mycophenolate mofetil was favored over methotrexate.
Main results
We included three trials enrolling 177 participants (174 participants analyzed). All participants in the trials by Keihl et al. and Bolwell et al. received cyclosporine while all participants enrolled in the trial by Perkins et al. received tacrolimus. However, the results did not differ by the type of calcineurin inhibitor employed (cyclosporine versus tacrolimus). There was no evidence for a difference between mycophenolate mofetil versus methotrexate for the outcomes of incidence of acute GVHD (RR 1.25; 95% CI 0.75 to 2.09; P value = 0.39, very low quality evidence), overall survival (HR 0.73; 95% CI 0.45 to 1.17; P value = 0.19, low‐quality evidence), median days to neutrophil engraftment (HR 0.77; 95% CI 0.51 to 1.17; P value = 0.23, low‐quality evidence), incidence of relapse (RR 0.84; 95% CI 0.52 to 1.38; P value = 0.50, low‐quality evidence), non‐relapse mortality (RR 1.21; 95% CI 0.62 to 2.36; P value = 0.57, low‐quality evidence), and incidence of chronic GVHD (RR 0.92; 95% CI 0.65 to 1.30; P value = 0.62, low‐quality evidence). There was low‐quality evidence that mycophenolate mofetil compared with methotrexate improved platelet engraftment period (HR 0.87; 95% CI 0.81 to 0.93; P value < 0.0001, low‐quality evidence). There was low‐quality evidence that mycophenolate mofetil compared with methotrexate resulted in decreased incidence of severe mucositis (RR 0.48; 95% CI 0.32 to 0.73; P value = 0.0006, low‐quality evidence), use of parenteral nutrition (RR 0.48; 95% CI 0.26 to 0.91; P value = 0.02, low‐quality evidence), and medication for pain control (RR 0.76; 95% CI 0.63 to 0.91; P value = 0.002, low‐quality evidence). Overall heterogeneity was not detected in the analysis except for the outcome of neutrophil engraftment. None of the included studies reported any outcomes related to quality of life. Overall quality of evidence was low.
Authors' conclusions
The use of mycophenolate mofetil compared with methotrexate for primary prevention of GVHD seems to be associated with a more favorable toxicity profile, without an apparent compromise on disease relapse, transplant‐associated mortality, or overall survival. The effects on incidence of GVHD between people receiving mycophenolate mofetil compared with people receiving methotrexate were uncertain. There is a need for additional high‐quality RCTs to determine the optimal GVHD prevention strategy. Future studies should take into account a comprehensive view of clinical benefit, including measures of morbidity, symptom burden, and healthcare resource utilization associated with interventions.
Keywords: Humans, Allografts, Calcineurin Inhibitors, Cyclosporine, Cyclosporine/therapeutic use, Graft vs Host Disease, Graft vs Host Disease/mortality, Graft vs Host Disease/prevention & control, Hematopoietic Stem Cell Transplantation, Hematopoietic Stem Cell Transplantation/adverse effects, Immunosuppressive Agents, Immunosuppressive Agents/adverse effects, Immunosuppressive Agents/therapeutic use, Methotrexate, Methotrexate/adverse effects, Methotrexate/therapeutic use, Mycophenolic Acid, Mycophenolic Acid/adverse effects, Mycophenolic Acid/analogs & derivatives, Mycophenolic Acid/therapeutic use, Randomized Controlled Trials as Topic, Recurrence, Tacrolimus, Tacrolimus/therapeutic use
Plain language summary
Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease following allogeneic hematopoietic stem cell transplantation
Background
Allogeneic hematopoietic stem cell transplantation is a procedure in which a portion of a healthy donor's stem cells (cells that can develop into various types of blood cells) or bone marrow is obtained and prepared for intravenous infusion. Hematopoietic stem cells are taken from a healthy donor and transplanted into the patient (recipient). People undergoing allogeneic hematopoietic stem cell transplantation are at risk of developing graft‐versus‐host disease (GVHD). GVHD results when the transplanted cells from the donor (graft) attack the recipient's (host) body cells because they perceive the recipient's body as foreign. Mycophenolate mofetil and methotrexate are two drugs often used to suppress the human body's reaction against the graft (immune response) and prevent GVHD. We conducted a systematic review of three randomized controlled trials (RCTs, which are clinical studies where people are randomly put into one of two or more treatment groups) that compared mycophenolate mofetil versus methotrexate for use in preventing GVHD among 174 participants. We searched for the relevant studies in March 2014.
Study characteristics
All participants in these RCTs received a drug aimed at suppressing the immune response (cyclosporine or tacrolimus). The study by Perkins and coworkers was funded by public and industry sources. The study by Kiehl and coworkers was funded by public sources. The funding source for the study by Bolwell and coworkers was not specified.
Key results
Our results show no clinically meaningful difference between mycophenolate mofetil and methotrexate on length of survival, incidence of GVHD, disease relapse, or treatment‐related death. People treated with mycophenolate mofetil had a shorter time to make new platelets (cells that help the blood to clot) from the donor cells compared with people treated with methotrexate. In addition, in terms of side effects, people treated with mycophenolate mofetil were less likely to have severe mucositis (inflammation of the mucus membranes), require parenteral nutrition (feeding through a vein), or pain medication.
None of the included studies reported any data related to quality of life.
In summary, mycophenolate mofetil and methotrexate both remain acceptable medications for the prevention of GVHD; however, mycophenolate mofetil seems to be associated with a smaller incidence of harms such as severe mucositis and related supportive care.
Quality of evidence
The overall quality of evidence was low.
Summary of findings
Summary of findings for the main comparison. Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation.
| Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Patient or population: people receiving allogeneic hematopoietic stem cell transplantation Settings: inpatients/hospital Intervention: mycophenolate mofetil versus methotrexate | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Methotrexate | Mycophenolate mofetil | ||||
| Overall survival |
HR 0.73 (0.45 to 1.17) |
129 (2 studies) | ⊕⊕⊝⊝ low1,3 | ||
| Prevention of acute GVHD grade II to IV | Study population | RR 1.25 (0.75 to 2.09) | 174 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| 595 per 1000 | 744 per 1000 (446 to 1000) | ||||
| Moderate5 | |||||
| 368 per 1000 | 460 per 1000 (276 to 769) | ||||
| Incidence of relapse | Study population | RR 0.84 (0.52 to 1.38) | 129 (2 studies) | ⊕⊕⊝⊝ low1,3 | |
| 348 per 1000 | 293 per 1000 (181 to 481) | ||||
| Moderate5 | |||||
| 386 per 1000 | 324 per 1000 (201 to 533) | ||||
| Nonrelapse mortality | Study population | RR 1.21 (0.62 to 2.36) | 89 (1 study) | ⊕⊕⊝⊝ low1,4 | |
| 255 per 1000 | 309 per 1000 (158 to 603) | ||||
| Moderate5 | |||||
| 255 per 1000 | 309 per 1000 (158 to 602) | ||||
| Prevention of chronic GVHD | Study population | RR 0.92 (0.65 to 1.3) | 129 (2 studies) | ⊕⊕⊝⊝ low1,3 | |
| 500 per 1000 | 460 per 1000 (325 to 650) | ||||
| Moderate5 | |||||
| 539 per 1000 | 496 per 1000 (350 to 701) | ||||
| Incidence of severe mucositis | Study population | RR 0.48 (0.32 to 0.73) | 174 (3 studies) | ⊕⊕⊝⊝ low1,3 | |
| 557 per 1000 | 267 per 1000 (178 to 407) | ||||
| Moderate5 | |||||
| 579 per 1000 | 278 per 1000 (185 to 423) | ||||
| Use of total parenteral nutrition | Study population | RR 0.48 (0.26 to 0.91) | 129 (2 studies) | ⊕⊕⊝⊝ low1,3 | |
| 606 per 1000 | 291 per 1000 (158 to 552) | ||||
| Moderate5 | |||||
| 614 per 1000 | 295 per 1000 (160 to 559) | ||||
| Incidence of narcotic use for pain control | Study population | RR 0.76 (0.63 to 0.91) | 129 (2 studies) | ⊕⊕⊝⊝ low1,3 | |
| 909 per 1000 | 691 per 1000 (573 to 827) | ||||
| Moderate5 | |||||
| 921 per 1000 | 700 per 1000 (580 to 838) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GVHD: graft‐versus‐host disease; HR: hazard ratio; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Only one of the included articles described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). The trials conducted by Kiehl et al. and Perkins et al. were open‐label trials. 2 The pooled estimate has wide confidence intervals, which reflects lower precision of the estimate. 3 Only three RCTs are published comparing mycophenolate mofetil versus methotrexate which reflects potential publication bias. 4 Data were reported in only one RCT.
5 Generated by GRADEpro software based on event rate in the control arm of included studies.
Background
Allogeneic hematopoietic stem cell transplantation (allo‐HCT) is associated with improved outcomes for people with various hematologic diseases (Bensinger 2006; Kharfan‐Dabaja 2012; Koreth 2009). Despite improved understanding of the pathophysiology of acute graft‐versus‐host disease (GVHD) and introduction of newer immunosuppressive agents, the morbidity and mortality resulting from acute and subsequently chronic GVHD pose a serious challenge to wider applicability of allo‐HCT (Ferrara 2009). Effects of allo‐HCT are particularly significant when we consider that an increasing number of allo‐HCTs are being performed in populations with known risk factors for development of acute or chronic GVHD (Flowers 2011; Kollman 2001). Specifically, more people are receiving unrelated donor hematopoietic stem cells, which are either human leukocyte antigen (HLA)‐matched or mismatched, and more older people who generally receive stem cell allografts from older siblings are undergoing allo‐HCT (Flowers 2011; Kollman 2001).
Description of the condition
Acute GVHD is a clinico‐pathologic syndrome that affects a significant proportion of allo‐HCT recipients. Approximately 35% to 50% of people undergoing allo‐HCT are expected to develop grade II to IV acute GVHD (Jacobsohn 2007). This syndrome is driven by alloreactive donor T cells that recognize disparate minor histocompatibility antigens. Organs targeted by acute GVHD are largely the skin, liver, and gastrointestinal tract. Diagnosis is made on a clinical basis, but confirmatory pathologic findings on tissue biopsy can help to confirm clinical diagnosis. Severity of the syndrome is associated with increased risk of mortality. Despite pharmacologic immune suppression prophylaxis, many people will still develop the syndrome and experience the attendant morbidity and mortality. The established primary therapy of high‐dose prednisone offers complete remission in 30% to 50% of cases; however, people with steroid‐refractory acute GVHD have poor long‐term survival (Pidala 2010).
While severe acute GVHD is a major source of early post‐allo‐HCT mortality, chronic GVHD constitutes a major threat in terms of late HCT‐associated morbidity, impaired quality of life, symptom burden, disability, and mortality. The majority of people alive beyond 100 days post‐HCT will develop chronic GVHD. In contrast to acute GVHD, chronic GVHD has protean manifestations, many of which have parallels to allied human immune‐mediated disorders. The most commonly involved organ sites are the skin, mouth, eyes, and liver. Following a 2005 National Institutes of Health (NIH) Consensus Conference, major changes were proposed to the diagnosis, classification, and severity scoring of the syndrome (Filipovich 2005). It is distinguished from acute GVHD by the diagnosis of chronic GVHD manifestations and is not based solely on the time from allo‐HCT. The previously used limited/extensive severity classification (Shulman 1980) has also been replaced by a scoring system that takes into account the number and severity of organs involved to produce a global score of mild, moderate, or severe.(Filipovich 2005)
Currently, the evidence supports high‐dose prednisone as primary therapy, but this has limited effectiveness, with most affected people requiring second‐line immune suppressive therapy to control the syndrome.
As survival rates associated with acute GVHD have increased over the past decades, so also have the costs associated with treatment (Svahn 2006). One review by Khera et al. found the costs of allo‐HCT to range from USD 96,000 to USD 204,000 in 2012 and multiple studies agree that major drivers of these costs are post‐transplantation complications such as acute GVHD (Khera 2012; Svahn 2012). Developing effective regimens for the prevention of both acute and chronic GVHD is of paramount importance due to the risk of morbidity and mortality associated with established GVHD and its adverse impact on patient symptom burden, functional ability, and quality of life.
Description of the intervention
As of 2014, no single acute GVHD prophylaxis regimen is considered the standard of care. Intravenous (IV) methotrexate in combination with a calcineurin inhibitor, cyclosporine or tacrolimus, is a widely used regimen for the prophylaxis of acute GVHD. However, administration of methotrexate is associated with a number of adverse events such as severe mucositis, delayed hematopoietic recovery, and organ toxicity (Bolwell 2004; Cutler 2005; Neumann 2005; Perkins 2010; Pinana 2010).
How the intervention might work
Mycophenolate mofetil is an ester prodrug of mycophenolic acid and a known inhibitor of inosine monophosphate dehydrogenase. By inhibition of de novo purine biosynthesis, mycophenolate mofetil selectively targets activated lymphocytes and suppresses the primary antibody response (Allison 2000). In canine models, investigators have established that stable mixed chimeras, organisms composed of a mixture of two or more genetically distinct cells, are achievable with administration of mycophenolate mofetil plus cyclosporine following a sublethal dose of total body irradiation and dog‐leukocyte antigen compatible marrow transplantation (Storb 1997; Yu 1998).
Mycophenolate mofetil has been useful in preventing graft rejection in the field of organ transplantation. Specifically, it is effective in reducing GVHD among people undergoing kidney transplant, and has been evaluated in people undergoing heart, lung, and liver transplants (Knight 2009; Schmeding 2011; Zuk 2009). In addition, mycophenolate mofetil, in combination with a calcineurin inhibitor, has been used extensively in people undergoing allo‐HCT. Several observational studies have evaluated the combination of mycophenolate mofetil with a calcineurin inhibitor as a possible alternative to methotrexate and shown this combination to be well tolerated in both nonmyeloablative and ablative settings with acceptable rates of GVHD (McSweeney 2003; Nieto 2006; Osunkwo 2004).
Why it is important to do this review
Preference for a particular regimen, mycophenolate mofetil or methotrexate, for acute GVHD prophylaxis is largely based on uncontrolled, observational studies, and physician or transplant center preference. Conflicting results regarding various clinical outcomes following allo‐HCT have been observed when comparing mycophenolate mofetil‐based regimens against methotrexate‐based regimens for acute GVHD prophylaxis. These comparisons are further limited by the heterogeneity of participant, disease, and treatment‐related characteristics among studies. Heterogeneity is also introduced by donor and cell source, ablative intensity of preparative regimens, and dosing and schema of administration of acute GVHD prophylaxis agents. With an increasing number of allo‐HCTs being performed in people at high risk of developing acute GVHD, we believe it is important to evaluate the comparative efficacy of the two commonly used prophylactic agents, methotrexate versus mycophenolate mofetil, in the prevention of acute GVHD.
Objectives
Primary objective: to assess the effect of mycophenolate mofetil versus methotrexate for the prevention of acute GVHD in people undergoing allo‐HCT.
Secondary objectives: to evaluate the effect of mycophenolate mofetil versus methotrexate for overall survival, prevention of chronic GVHD, incidence of relapse, treatment‐related harms, nonrelapse mortality, and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We considered all prospective, randomized controlled trials (RCTs) of mycophenolate mofetil versus methotrexate utilizing a parallel study design for inclusion in this systematic review. We excluded all other study designs.
Types of participants
We included studies that enrolled participants who were at risk of developing GVHD as a result of undergoing allo‐HCT. We excluded studies that enrolled participants with an existing diagnosis of acute or chronic GVHD. We applied no restrictions on participant gender, ethnic group, or age. We described the disease type and stage of the included participants.
Types of interventions
Included studies reported on the direct comparison of any mycophenolate mofetil‐based regimen versus any methotrexate‐based regimen administered as prophylaxis for acute GVHD in people undergoing allo‐HCT. Specifically, we considered a regimen to be used as prophylaxis for acute GVHD if 1. the investigators specifically stated mycophenolate mofetil or methotrexate was used as prophylaxis for acute GVHD or 2. if the study inclusion/exclusion criteria excluded people with an existing diagnosis of acute or chronic GVHD. Supportive care and other GVHD prophylaxis/therapies, if any, were similar in both arms. In addition, since mycophenolate mofetil and methotrexate were commonly administered in combination with a calcineurin inhibitor (e.g. cyclosporine or tacrolimus), we included all regimens containing mycophenolate mofetil versus all regimens containing methotrexate, regardless of co‐therapies, in the review.
Types of outcome measures
Primary outcomes
Incidence of acute GVHD.
Overall survival.
Secondary outcomes
Engraftment kinetics evaluated as median days to neutrophil engraftment and median days to platelet engraftment.
Incidence of relapse.
Incidence of non‐relapse mortality (any death occurring without disease relapse/recurrence).
Incidence of chronic GVHD.
Quality of life (if measured using a validated tool for the assessment of quality of life).
Any grade III or IV adverse events of treatment.
Pain evaluated by incidence of narcotic use for pain control.
Search methods for identification of studies
Electronic searches
We conducted an electronic search of Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) using the search strategy in Appendix 1 and MEDLINE using search strategy in Appendix 2 from inception to 17 March 2014. We applied no date or language limits.
Searching other resources
In order to identify any recently completed studies that had not been published in full, we searched conference abstracts from the last two meetings (2011 and 2012) of the American Society of Clinical Oncology (ASCO), American Society of Hematology (ASH), European Group of Blood and Marrow Transplantation (EBMT), and BMT tandem meetings of the American Society of Blood and Marrow Transplantation (ASABM), Center for International Blood and Marrow Transplant Research (CIBMTR), and European Hematology Association (EHA). We also handsearched references of all identified review articles and included studies. In order to identify unpublished or ongoing studies, we searched ClinicalTrials.gov (www.clinicaltrials.gov/), Novartis Clinical Trial Results Database (www.novctrd.com), Roche Clinical Trial Protocol Registry (www.roche‐trials.com), Australian New Zealand Clinical Trials Registry (ANZCTR), and the metaRegister of Controlled Trials.
Data collection and analysis
Selection of studies
Two review authors (RM and TR) reviewed all titles, abstracts, and full‐text reports independently. We included studies that met the following criteria in the review.
Prospective clinical trial.
Parallel study design.
Participants randomized to prophylaxis with mycophenolate mofetil versus methotrexate.
Participants undergoing allo‐HCT.
We matched references on author names, location and setting, specific intervention details, and participants to avoid inclusion of duplicate publications. We resolved any disagreements between review authors during the study selection by consensus with a third review author (MAK‐D or AK).
Data extraction and management
Two review authors independently extracted data according to Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions using a standardized data extraction form containing the following items (Higgins 2011):
general information: study title, authors, source;
study characteristics: study design, setting, duration of follow‐up;
participant characteristics: number of participants enrolled, number of participants included in the analysis, specific disease diagnosis, donor status (related or unrelated donor), HLA‐mismatch, participant age;
interventions: name, dose, route, administration schedule, and associated therapies;
outcomes: incidence of acute GVHD (grades II to IV and III to IV GVHD), overall survival, median days to neutrophil engraftment, median days to platelet engraftment, incidence of relapse, incidence of chronic GVHD, grade III or IV adverse events, nonrelapse mortality (any death occurring without disease relapse/recurrence), pain;
risk of bias.
For studies that had multiple publications, we used the publication with longest follow‐up for extracting data on outcomes. We used earlier publications to extract data on methodology and baseline characteristics. In cases where the method of analysis was not specified by the investigators and only the number of events was reported, we used the number randomized as the denominator. That is, we recorded results according to intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
Two review authors (RM and TR) independently assessed the risk of bias in the included studies using The Cochrane Collaboration's tool for assessing the risk of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions based on extracted information (Higgins 2011a). A third author (MAK‐D or AK) resolved any disagreements between the two review authors. In addition to risk of bias, we evaluated the risk of random error by extracting data on the investigator's predetermined effect difference, alpha, power, and sample size.
Specifically, for assessment of risk of bias, we graded each component of methodologic quality as low, high, or unclear. We evaluated selection bias by assessing the investigators' description of method of randomization and allocation concealment. The method of randomization was:
low risk if the investigators described a random component in the sequence generation process (i.e. refer to a random number table, use a computer random number generator, coin toss);
high risk if the investigators described a nonrandom component in the sequence generation process (sequence generated by odd or even date of birth, some rule based on date (or day) of admission, or some rule based on hospital or clinic record number); and
unclear risk if there was insufficient information about the sequence generation process to permit judgment of 'low risk' or 'high risk'.
We considered allocation concealment to be:
low risk if participants and investigators enrolling participants could not foresee assignment (i.e. use of central allocation, sequentially numbered identical drug containers or sequentially numbered, opaque, sealed envelopes);
high risk if participants or investigators enrolling participants could possibly foresee assignments (allocation based on date of birth, case record number, using an open random allocation schedule); and
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We evaluated performance bias by assessing the investigators' description of blinding of participants and investigators. Performance bias was:
low risk if no blinding was used, but the outcome was not likely to be influenced by lack of blinding or participants and key study personnel were blinded;
high risk if no blinding or incomplete blinding was used, and the outcome was likely to be influenced by lack of blinding; and
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We judged detection bias due to knowledge of the allocated interventions by outcome assessors to be:
low risk if no blinding of outcome assessment was used, but the outcome measurement was not likely to be influenced by lack of blinding or blinding of outcome assessment was ensured;
high risk if there was no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; and
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We judged attrition bias due to the amount, nature, or handling of incomplete outcome data to be:
low risk if there were no missing outcome data, reasons for missing outcome data were unlikely to be related to true outcome, or missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups;
high risk if the reasons for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate, or uses 'as‐treated' analysis with substantial departure of the intervention received from that assigned at randomization or uses potentially inappropriate application of simple imputation;
unclear risk if there was insufficient reporting of attrition/exclusions to permit judgment of 'low risk' or 'high risk' (e.g. number randomized not stated, no reasons for missing data provided).
We considered reporting bias due to selective outcome reporting to be:
low risk if the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review had been reported in the prespecified way or the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified;
high risk if the study's prespecified primary outcomes had not been reported, primary outcomes were reported using measurements that were not prespecified, primary outcomes were not prespecified, outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis, or the study report did not include results for a key outcome that would be expected to have been reported for such a study; and
unclear if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
For the evaluation of risk or random error, we captured whether investigators report predetermined effect difference, alpha, power, and sample size calculation (yes/no and reported values) and if they were able to enroll the prespecified number of participants (prespecified sample size versus total number enrolled per arm).
Measures of treatment effect
Dichotomous data
We summarized dichotomous data (i.e. incidence of acute/chronic GVHD, incidence of relapse, nonrelapse mortality, adverse events, narcotic use) using risk ratio (RR) pooled using the random‐effects model and reported with 95% confidence intervals (CI).
Time‐to‐event data
In cases of time‐to‐event data (i.e. overall survival and days to neutrophil/platelet engraftment), for each included study we calculated the observed minus expected events (O minus E) and variance from the reported time‐to‐event estimates to obtain the log hazard ratio (LnHR) and standard error (SE) of LnHR for imputation using Review Manager 5 (RevMan 2011). In cases where time‐to‐event estimates were not reported, we extracted data from papers using the methods described by Tierney et al. (Tierney 2007). This method allowed calculation of the hazard ratio (HR) from different parameters using indirect calculation of the variance and the number of O minus E events. We pooled time‐to‐event estimates using the random‐effects model and reported with 95% CI using the generic inverse variance method.
Unit of analysis issues
The unit of analysis for this review was individual study. In the case of repeated follow‐up (e.g. reporting of survival at three and six months), we used the longest follow‐up from each study. We treated recurring events (e.g. adverse events) as a single event occurring in one participant (e.g. we considered four instances of nausea in one participant as one participant with nausea). In the case of multiple intervention arms, we combined arms together to create a single pair‐wise comparison.
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), in the case of missing outcome data, we attempted to contact the principal investigator or corresponding author (or both) of the study. If the corresponding author was unable to provide the missing data for an outcome, we included the study in the systematic review but excluded it from the meta‐analysis for the outcome with missing data. We undertook no imputation of missing individual participant data.
Assessment of heterogeneity
To evaluate heterogeneity between pooled studies, we calculated the Chi2 and I2 statistics (Deeks 2011). We considered an I2 statistic > 50% to indicate substantial heterogeneity or a Chi2 test with a significance level at P value < 0.1 to indicate statistically significant heterogeneity.
Assessment of reporting biases
We planned to assess publication bias using a funnel plot if more than 10 studies were included in the review (Egger 1997; Sterne 2011). We evaluated selective reporting of outcomes within studies by comparing outcomes reported with outcomes specified in protocols, when available.
Data synthesis
We performed pooled analysis using Review Manager 5 (RevMan 2011). We employed a random‐effects model using the DerSimonian‐Laird approach to pool studies for all analyses (DerSimonian 1986).
We constructed a 'Summary of findings' table using the most clinically and participant‐relevant outcomes (Guyatt 2011). These outcomes included: overall survival, incidence of relapse, incidence of grade II to IV acute GVHD, incidence of chronic GVHD, nonrelapse mortality, and incidence of any grade III to IV adverse events. In addition, we evaluated and reported the quality of evidence for each outcome according to GRADE guidelines (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e).
Subgroup analysis and investigation of heterogeneity
Originally we planned to conduct subgroup analyses on prognostically relevant factors including gender, age (adult versus child), disease stage, previous treatment, differences in therapy regimen (co‐therapies), remission status prior to conditioning (complete remission), and type of donor (sibling versus unrelated). However, due to the small number of studies identified and lack of reporting by subgroup, we did not perform any subgroup analyses.
Sensitivity analysis
Originally we planned to conduct a sensitivity analysis on all aspects of methodological quality. Due to the small number of studies identified by this systematic review, we have not performed any sensitivity analyses proposed in the protocol.
Results
Description of studies
Results of the search
The electronic search retrieved 263 references and the abstract search retrieved 609 references. In all, we screened 841 unique references by title and abstract. Of these, we selected 25 references for full‐text review. Three studies met the inclusion criteria. Selection flow diagram and reasons for exclusion are provided in Figure 1.
1.
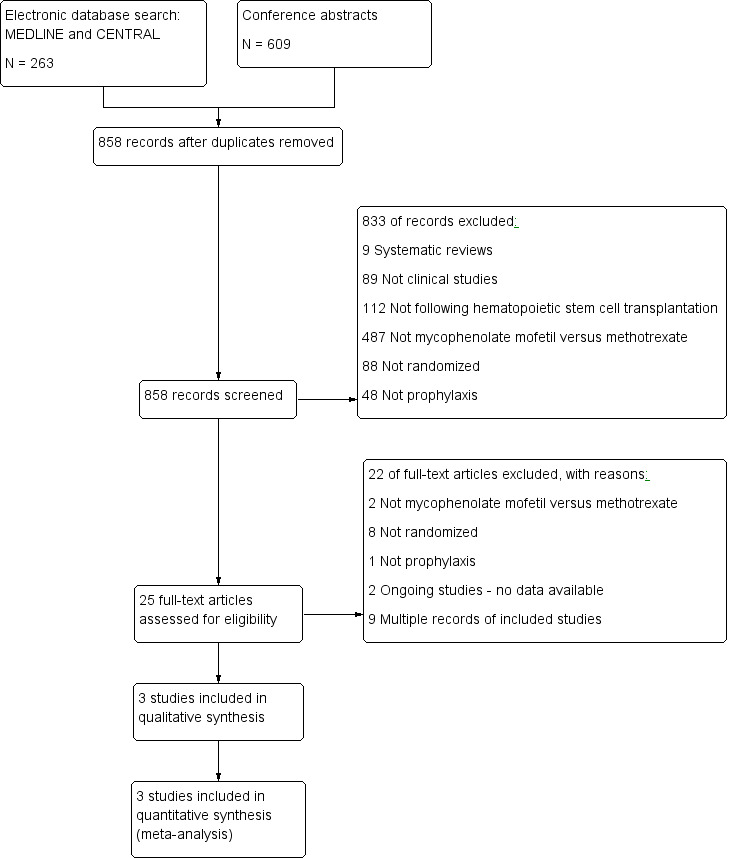
Study flow diagram.
Included studies
The review includes three studies (see Characteristics of included studies) (Bolwell 2004; Kiehl 2002; Perkins 2010).
The study by Kiehl et al. was a multicenter RCT comparing GVHD prophylaxis among three treatment arms; mycophenolate mofetil twice daily at 1 g per dose IV for the duration of the study; mycophenolate mofetil twice daily at 1.5 g per dose IV for the duration of the study; and methotrexate on days one, three, and six (total dose on day one was 15 mg IV, and on days three and six was 10 mg IV for the duration of the study). In addition to randomized treatment, all participants received cyclosporine and prednisolone supportive care. Cyclosporine was begun on day +1 and adjusted to trough plasma levels of 250 to 300 ng/L. At the time of publication of interim findings, 45 participants were randomized to receive either the methotrexate‐containing regimen or mycophenolate mofetil in a dosage of 1 g twice daily or 1.5 g twice daily IV. Eligible participants had received stem cells from a mismatched related or a mismatched or a matched unrelated donor. (Kiehl 2002). The results of the two mycophenolate mofetil arms in this study were pooled for this review.
In the study by Bolwell et al., participants were prospectively randomized 1:1 to receive either cyclosporine plus mycophenolate mofetil or cyclosporine plus methotrexate for GVHD prophylaxis. All participants were required to have a 6/6 HLA‐matched related donor. All donors were required to undergo a bone marrow harvest. Study end points included the incidence of acute GVHD, severity of mucositis, time to engraftment of neutrophils and platelets, and 100‐day survival. In this study, 21 participants received mycophenolate mofetil and 19 participants received methotrexate (Bolwell 2004).
The study by Perkins et al. was a single‐center, randomized phase II trial comparing tacrolimus plus mycophenolate mofetil versus tacrolimus plus methotrexate. ITT analysis included 42 participants randomized to tacrolimus plus mycophenolate mofetil and 47 participants randomized to tacrolimus plus methotrexate (Perkins 2010).
In summary, two RCTs used cyclosporine as the calcineurin inhibitor (Bolwell 2004; Kiehl 2002), and one RCT used tacrolimus as the calcineurin inhibitor (Perkins 2010). The results did not differ based on the type of the calcineurin inhibitor. See Characteristics of included studies table.
Excluded studies
We excluded four observational studies that reported data on mycophenolate mofetil versus methotrexate because they were not RCTs (Neumann 2005; Ostronoff 2009; Piñana 2010; Wang 2002). These four studies were included in the eight non‐randomized trials excluded at the full‐text manuscript phase. We have chosen to specifically report these four since they resemble our included studies more closely than the rest of the excluded studies. See Characteristics of excluded studies table.
Risk of bias in included studies
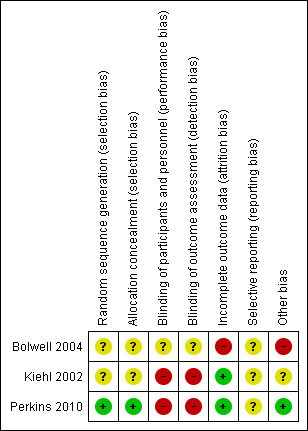
Results of risk of bias assessment are presented in Figure 2.
2.
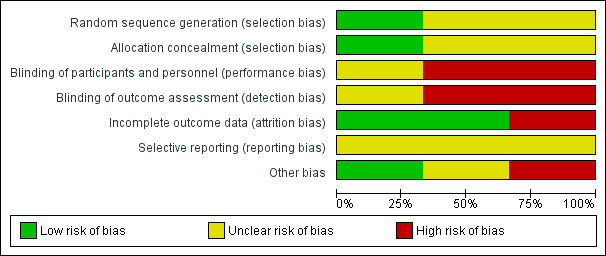
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Only one of the included RCTs described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). We judged the potential risk for selection bias as unclear.
Blinding
The trials conducted by Kiehl et al. and Perkins et al. were open label trials (Kiehl 2002; Perkins 2010). We were unable to determine whether the trial conducted by Bolwell et al. used blinding (Bolwell 2004). We judged the potential risk for detection and performance bias as high.
Incomplete outcome data
An ITT analysis was performed in all trials (Bolwell 2004; Kiehl 2002; Perkins 2010). The trial by Bolwell et al. had less than 50% of planned sample size accrual (Bolwell 2004). We judged the potential risk for attrition bias as low.
Selective reporting
The trial conducted by Perkins et al. reported all major outcomes (Perkins 2010). However, since we did not have access to the trial protocol(s), we could not investigate the potential for selective reporting bias based only on trial publications. We judged the potential risk for selection bias as unclear.
Other potential sources of bias
A sample size was pre‐planned in two trials (Bolwell 2004; Perkins 2010), but the planned number was not reached in one trial (Bolwell 2004). Since the trial by Kiehl et al. was published as an abstract, data regarding a priori sample size calculation, and alpha and beta error were not reported (Kiehl 2002). We judged the potential risk for other sources bias as low.
Effects of interventions
See: Table 1
We included three trials with 174 participants in the analysis. Ninety‐five participants were randomized to the mycophenolate mofetil group and 79 participants to the methotrexate group. The effects of mycophenolate mofetil versus methotrexate are summarized in Table 1.
Benefits
Prevention of acute graft‐versus‐host disease grade II to IV
Data on incidence of acute GVHD grade II to IV were extractable from three RCTs (three comparisons, 174 participants) (Bolwell 2004; Kiehl 2002; Perkins 2010). The pooled analysis found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on prevention of grade II to IV acute GVHD (RR 1.25; 95% CI 0.75 to 2.09; P value = 0.39) (Analysis 1.1). Substantial heterogeneity was detected in the analysis (P value = 0.12, I2 = 52%).
1.1. Analysis.
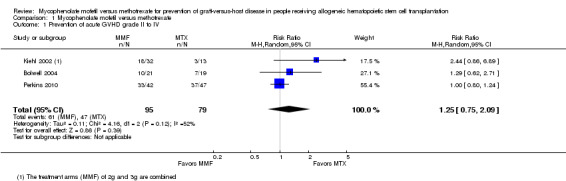
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 1 Prevention of acute GVHD grade II to IV.
Overall survival
Data on overall survival could be extracted from two trials with 129 participants (Bolwell 2004; Perkins 2010). The meta‐analysis found no statistically significant benefit favoring mycophenolate mofetil use (HR 0.73; 95% CI 0.45 to 1.17; P value = 0.19) (Analysis 1.2). No heterogeneity was detected in the analysis (P value = 0.60, I2 = 0%).
1.2. Analysis.
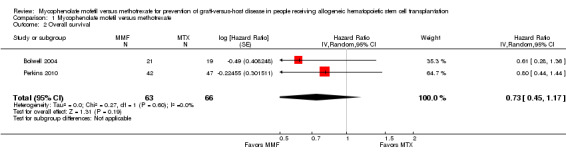
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 2 Overall survival.
Time to neutrophil engraftment
Data on median time to neutrophil engraftment were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no significant difference in the median time to neutrophil engraftment between the mycophenolate mofetil group and the methotrexate group (HR 0.77; 95% CI 0.51 to 1.17; P value = 0.23) (Analysis 1.3). Substantial heterogeneity was detected in the analysis (P value = 0.008, I2 = 86%).
1.3. Analysis.
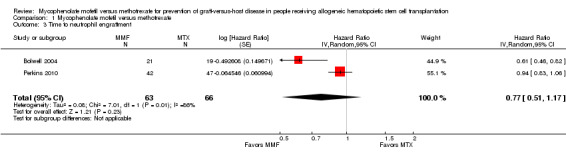
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 3 Time to neutrophil engraftment.
Time to platelet engraftment
Data on median time to platelet engraftment were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found a statistically significant benefit in time to platelet engraftment in the mycophenolate mofetil group compared with the methotrexate group (HR 0.87; 95% CI 0.81 to 0.93; P value < 0.0001) (Analysis 1.4). No heterogeneity was detected in the analysis (P value = 0.43, I2 = 0%).
1.4. Analysis.
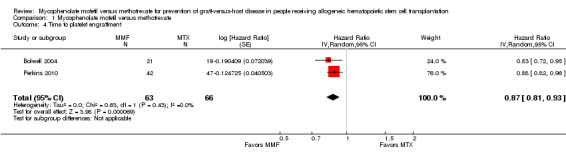
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 4 Time to platelet engraftment.
Incidence of relapse
Data on incidence of relapse were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no benefit with use of mycophenolate mofetil versus methotrexate on incidence of relapse (RR 0.84; 95% CI 0.52 to 1.38; P value = 0.50) (Analysis 1.5). No heterogeneity was detected in the analysis (P value = 0.86, I2 = 0%).
1.5. Analysis.
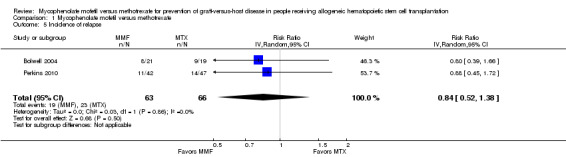
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 5 Incidence of relapse.
Nonrelapse mortality
Data on non‐relapse mortality were extractable from one RCT enrolling 89 participants (Perkins 2010). The data from this RCT found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on nonrelapse mortality (RR 1.21; 95% CI 0.62 to 2.36; P value = 0.57) (Analysis 1.6).
1.6. Analysis.
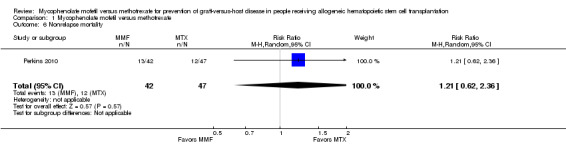
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 6 Nonrelapse mortality.
Prevention of chronic graft‐versus‐host disease
Data on incidence of chronic GVHD were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on prevention of chronic GVHD (RR 0.92; 95% CI 0.65 to 1.30; P value = 0.62) (Analysis 1.7). No heterogeneity was detected in the analysis (P value = 0.69, I2 =0%).
1.7. Analysis.
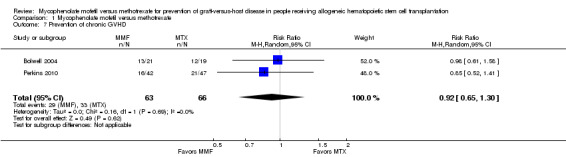
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 7 Prevention of chronic GVHD.
Quality of life
None of the included studies reported any data related to quality of life.
Harms
Incidence of severe mucositis
Data on incidence of severe mucositis were extractable from three RCTs (three comparisons, 174 participants) (Bolwell 2004; Kiehl 2002; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate in reduced incidence of severe mucositis (RR 0.48; 95% CI 0.32 to 0.73; P value = 0.0006) (Analysis 1.8). Low heterogeneity was detected in the analysis (P value = 0.33, I2 = 10%).
1.8. Analysis.
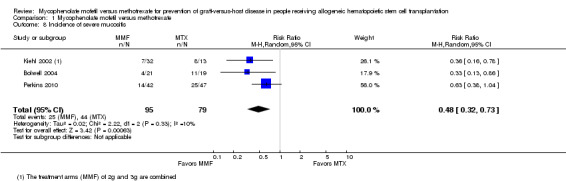
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 8 Incidence of severe mucositis.
Use of total parenteral nutrition
Data on use of total parenteral nutrition were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate suggesting decreased need of total parenteral nutrition with mycophenolate mofetil use (RR 0.48; 95% CI 0.26 to 0.91; P value = 0.02) (Analysis 1.9). Moderate heterogeneity was detected in the analysis (P value = 0.20, I2 = 40%).
1.9. Analysis.
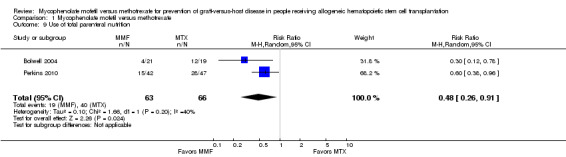
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 9 Use of total parenteral nutrition.
Incidence of narcotic use for pain control
Data on incidence of narcotic use for pain control were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate suggesting a lower narcotic use for pain control with the use of mycophenolate mofetil (RR 0.76; 95% CI 0.63 to 0.91; P value = 0.002) (Analysis 1.10). No heterogeneity was detected in the analysis (P value = 0.53, I2 = 0%).
1.10. Analysis.
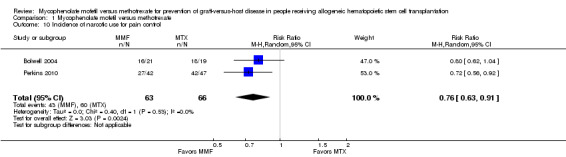
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 10 Incidence of narcotic use for pain control.
Discussion
Summary of main results
The use of mycophenolate mofetil for acute GVHD prophylaxis appears to result in significantly faster platelet engraftment and a lower incidence of severe mucositis compared with methotrexate (Table 1). As a result, hematopoietic allograft recipients receiving mycophenolate mofetil for acute GVHD prophylaxis are likely to require less total parenteral nutrition or narcotics for pain control compared with people treated with methotrexate. This might translate into lower rates of complications that result from use of total parenteral nutrition or narcotics with the use of mycophenolate mofetil instead of methotrexate and, in turn, could reduce the length of hospital stay. However, we did not detect a statistically significant difference between mycophenolate mofetil and methotrexate in regards to risk of relapse, nonrelapse mortality, or overall survival based.
Overall completeness and applicability of evidence
Despite the fact that a better toxicity profile and faster engraftment are always desirable outcomes after allo‐HCT, the absence of a survival advantage or a favorable effect on prevention of acute or chronic GVHD or a lower transplant‐associated mortality with the use of mycophenolate mofetil limits our ability to recommend mycophenolate mofetil over methotrexate. In addition, we found a statistically significant heterogeneity for the outcome of time to neutrophil engraftment and since there were only two trials reporting this outcome, it was not possible to conduct subgroup or sensitivity analyses to explore the heterogeneity. The findings for this outcome need to be interpreted with caution. In addition, it is important to emphasize that the overall number of relevant trials and included participants is small. Accordingly, it is likely that a greater number of studies would be needed to have sufficient power to detect differences in certain outcomes. A major objective in future research will be to incorporate new trials in this analysis if they become available. We are not aware of any currently ongoing trials comparing mycophenolate mofetil versus methotrexate for the prevention of GVHD in people undergoing allo‐HCT. Nevertheless, there is significant diversity in current investigational approaches for prevention of GVHD. However, in our opinion it is highly unlikely that additional high‐quality trials comparing mycophenolate mofetil and methotrexate will be performed in the near future.
Quality of the evidence
We assessed the quality of the included trials according to the previously described quality domains, and these are represented in Figure 2 and Figure 3. Overall methodologic quality of included studies was either low or very low. Two included RCTs had a high risk of performance and detection bias. Two included RCTs reported analyses according to the principle of ITT. None of the included RCTs reported data on quality of life and hence we were not able to perform a meta‐analysis on this outcome. Overall, for majority of the outcomes the quality of evidence was low. For the outcome of prevention of acute GVHD grade II to IV the quality of evidence was very low.
3.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Potential biases in the review process
We did not find any methodologic issues in the preparation of the review that could put it at risk for bias. We are confident that we have identified all eligible studies. We searched multiple electronic databases and conference proceedings. Once we had compiled the final list of included studies, we consulted content experts to ensure no unpublished studies were missed. We obtained all available data from included studies. We contacted study investigators in an attempt to obtain missing information. Two review authors performed study selection and data extraction. All included studies were RCTs. No subgroup analyses, planned in the protocol (see Methods section) were conducted due to lack of individual participant data. Due to the small number of included studies, we decided not to perform any sensitivity analyses.
Authors' conclusions
Implications for practice.
The findings of this analysis suggest that mycophenolate mofetil is an acceptable agent when used in combination with a calcineurin inhibitor for primary prevention of graft‐versus‐host disease (GVHD). Treating clinicians can anticipate a beneficial reduction in severe mucositis and its associated supportive care in comparison with the use of methotrexate plus a calcineurin inhibitor. There was no clinically meaningful difference in the incidence of GVHD, disease relapse, transplant‐associated mortality, or overall survival between people receiving mycophenolate mofetil and people receiving methotrexate.
Implications for research.
This analysis suggests the following implications for future research. First, there is a need for additional high‐quality randomized controlled trials to address the optimal GVHD prevention strategy among people receiving allogeneic hematopoietic stem cell transplantation. Second, this analysis demonstrated that future studies should take into account a comprehensive view of clinical benefit, including measures of morbidity, symptom burden, and healthcare resource utilization associated with interventions. In addition to clinical outcomes, future studies should consider reporting quality of life.
Acknowledgements
We would like to thank the Cochrane Haematological Malignancies Group for critical reading of our protocol and review, and helpful feedback.
Appendices
Appendix 1. Search strategy for CENTRAL
| # | Search history |
| 1 | MeSH descriptor: [Graft vs Host Disease] explode all trees |
| 2 | graft versus host |
| 3 | graft vs host |
| 4 | graft v host |
| 5 | gvhd |
| 6 | runt disease |
| 7 | homologous wasting disease |
| 8 | OR #1‐7 |
| 9 | MeSH descriptor: [Mycophenolic Acid] explode all trees |
| 10 | MYCOPHENOLIC ACID |
| 11 | mycophenolat* or mycophenolat?mofetil* |
| 12 | mykophenolat* or mykophenolat?mofetil* |
| 13 | morpholinoethyl ester |
| 14 | RS 61443 or RS61443 |
| 15 | cellcept* |
| 16 | myfortic* |
| 17 | MMF |
| 18 | OR #9‐17 |
| 19 | #8 AND #18 |
Appendix 2. Search strategy for MEDLINE via PubMed
| # | Search history |
| 1 | GRAFT VS HOST DISEASE |
| 2 | graft versus host*[Title/Abstract] |
| 3 | graft vs host*[Title/Abstract] |
| 4 | graft v host*[Title/Abstract] |
| 5 | gvhd[Title/Abstract] |
| 6 | runt diseas*[Title/Abstract] |
| 7 | homologous wasting diseas*[Title/Abstract] |
| 8 | OR #1‐7 |
| 9 | MYCOPHENOLIC ACID |
| 10 | mycophenolat* OR mycophenolat?mofetil*[Title/Abstract] |
| 11 | mykophenolat* OR mykophenolat mofetil*[Title/Abstract] |
| 12 | mycophenolic* acid*[Title/Abstract] |
| 13 | morpholinoethyl ester |
| 14 | RS 61443[Title/Abstract] OR RS61443[Title/Abstract] |
| 15 | cellcept*[Title/Abstract] |
| 16 | myfortic*[Title/Abstract] |
| 17 | MMF[Title/Abstract] |
| 18 | OR #9‐17 |
| 19 | #8 AND #18 |
| 20 | randomized controlled trial[Publication Type] |
| 21 | controlled clinical trial[Publication Type] |
| 22 | randomi*[Title/Abstract] |
| 23 | placebo[Title/Abstract] |
| 24 | drug therapy[MeSH Subheading] |
| 25 | randomly[Title/Abstract] |
| 26 | trial[Title/Abstract] |
| 27 | groups[Title/Abstract] |
| 28 | OR #20‐27 |
| 29 | "humans"[MeSH Terms] |
| 30 | #28 AND #29 |
| 31 | #19 AND #30 |
Data and analyses
Comparison 1. Mycophenolate mofetil versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prevention of acute GVHD grade II to IV | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.75, 2.09] |
| 2 Overall survival | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.73 [0.45, 1.17] |
| 3 Time to neutrophil engraftment | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.77 [0.51, 1.17] |
| 4 Time to platelet engraftment | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.87 [0.81, 0.93] |
| 5 Incidence of relapse | 2 | 129 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.52, 1.38] |
| 6 Nonrelapse mortality | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.62, 2.36] |
| 7 Prevention of chronic GVHD | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| 8 Incidence of severe mucositis | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.73] |
| 9 Use of total parenteral nutrition | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.91] |
| 10 Incidence of narcotic use for pain control | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bolwell 2004.
| Methods | Randomized controlled trial, single center Sample size calculation: designed to detect an absolute difference of 30% in the incidence of severe mucositis between the 2 study arms (77% incidence with methotrexate, 47% with mycophenolate mofetil) with 80% power using a 2‐sided significance level of 5% Median follow‐up: 23 months |
|
| Participants | Eligible participants included people with hematologic malignancy who were appropriate candidates for a myeloablative allogeneic bone marrow transplant. All participants were required to have a 6/6 HLA matched related donor. All donors were required to undergo a bone marrow harvest. | |
| Interventions |
GVHD prophylaxis Intervention arm (N = 21)
Control arm (N = 19)
Other treatment and supportive care (same for both arms)
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were prospectively randomized 1:1 to receive either [cyclosporine] plus [methotrexate] or [cyclosporine] plus [mycophenolate mofetil] for GVHD prophylaxis" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis data |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | High risk | The study was to enroll 80 participants to "detect an absolute difference of 30% in the incidence of severe mucositis between the two study arms with 80% power using a two‐side significance level of 5%." The study was closed early after enrolling 40 participants |
Kiehl 2002.
| Methods | Randomized controlled trial, multicenter Sample size calculation: not reported Median follow‐up: not reported |
|
| Participants | Underlying disease:
Gender: 29 male and 16 female participants |
|
| Interventions |
GVHD prophylaxis Intervention arm (N = 32)
Control arm (N = 13)
Other treatment and supportive care (same for both arms)
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was conducted |
| Selective reporting (reporting bias) | Unclear risk | Not described (study published as a meeting abstract) |
| Other bias | Unclear risk | Not described (study published as a meeting abstract). No information on sample size calculations was reported. |
Perkins 2010.
| Methods | Randomized controlled trial, single center Sample size calculation: 42 evaluable participants per study arm to detect an absolute difference of 30% (reduction in the incidence of severe mucositis from 60% in the methotrexate arm to 30% in the mycophenolate mofetil arm) (alpha = 0.05, power = 0.80) Median follow‐up: not reported |
|
| Participants | Participants undergoing allogeneic HCT from sibling or unrelated donors matched for 10/10 or 9/10 HLA‐A, ‐B, ‐C, ‐DRB1, and ‐ DQB1 alleles | |
| Interventions |
GVHD prophylaxis Intervention arm (N = 45 randomized and N = 42 analyzed)
Control arm (N = 47)
Other treatment and supportive care (same for both arms)
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified based on predefined conditioning regimen intensity via the Interactive Voice Randomization System |
| Allocation concealment (selection bias) | Low risk | Randomized (1:1) via the Interactive Voice Randomization System coordinated by the Bioinformatics Department of the Moffitt Cancer Center |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treatanalysis used. Withdrawals were described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
GVHD: graft‐versus‐host disease; HCT: hematopoietic stem cell transplantation; HLA: human leukocyte antigen; IV: intravenous.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Neumann 2005 | Not a randomized observational study |
| Ostronoff 2009 | Not a randomized, historical methotrexate control used observational study |
| Piñana 2010 | Not a randomized observational study |
| Wang 2002 | Not a randomized observational study |
Characteristics of ongoing studies [ordered by study ID]
NCT00563589.
| Trial name or title | Mycophenolate Mofetil for the Prophylaxis of Graft‐versus‐host Disease in High Risk Allogeneic Stem Cell Transplantation |
| Methods | Allocation: randomized End point classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: prevention Location: single center |
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | |
| Contact information | Dr. Winnie WW Cheung. Email: cheungww@hotmail.com |
| Notes | Study ongoing. Status unknown. ClinicalTrials.gov identifier: NCT00563589 |
NCT00928018.
| Trial name or title | Tacrolimus/Sirolimus/Methotrexate Versus Tacrolimus/Methotrexate or Cyclosporine/Mycophenolate Mofetil for GVHD Prophylaxis After Reduced Intensity Allogeneic Stem Cell Transplantation for Patients With Lymphoma |
| Methods | Allocation: randomized End point classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment Location: multicenter |
| Participants |
|
| Interventions | Experimental: tacrolimus plus sirolimus plus low‐dose methotrexate Control 1: tacrolimus plus methotrexate Control 2: cyclosporine plus mycophenolate mofetil |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | |
| Contact information | Principal Investigator: Dr. Philippe Armand, Dana‐Farber Cancer Institute |
| Notes | Estimated study completion date: November 2014 It is not clear if random allocation was only for the control versus experimental arm or if random allocation was used to allocate participants to any of the 3 study arms ClinicalTrials.gov identifier: NCT00928018 |
CLL: chronic lymphocytic leukemia; GVHD: graft‐versus‐host disease; HLA: human leukocyte antigen; MCL: mast cell leukemia; NHL: non‐Hodgkin lymphoma; SLL: small lymphocyte lymphoma.
Differences between protocol and review
Due to the small number of studies identified by this systematic review and the lack of data reported by subgroup, we have not performed any subgroup analyses or sensitivity analyses proposed in the protocol.
Contributions of authors
Mohamed Kharfan‐Dabaja (MK‐D), Joseph Pidala (JAP), Janelle B Perkins (JBP), Benjamin Djulbegovic (BD), Ambuj Kumar (AK), and Rahul Mhaskar (RM) contributed to the initiation and design of this review.
Tea Reljic (TR) and RM conducted the search, study selection, and data extraction.
MK‐D, JAP, and AK resolved any disagreements during the conduct of the review.
MK‐D and AK performed a random data check prior to analysis.
TR and RM performed all analyses.
MK‐D, JAP, JBP, and BD contributed clinical expertise.
BD, RM, TR, and AK contributed statistical and methodological expertise.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
The review authors have no conflicts of interest to report.
New
References
References to studies included in this review
Bolwell 2004 {published data only}
- Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplantation 2004;34(7):621‐5. [PUBMED: 15300236] [DOI] [PubMed] [Google Scholar]
- Bolwell BJ, Sobecks R, Pohlman B, Andresen S, Lichtin A, Rybicki L. A prospective, randomized trial comparing cyclosporine + short course methotrexate to cyclosporine + mycophenolate for GVHD prophylaxis in ablative allogeneic BMT. Blood 2003;11(Pt 1):711a. [CENTRAL: CN‐00483283] [DOI] [PubMed] [Google Scholar]
Kiehl 2002 {published data only}
- Fauser A, Linck D S‐EK, Bornhauser M, Blau I, Kroger M WH, Ehninger G, et al. Mycophenolate mofetil in combination with cyclosporine A (CSA) +/‐ prednisolone versus CsA, methotrexate +/‐ prednisolone for the prophylaxis of acute graft‐versus‐host disease (GvHD) after allogeneic stem cell transplantation: results of a randomized phase. Onkologie 2006; Vol. 29, issue Suppl 3:115. [CENTRAL: CN‐00661692]
- Kiehl MG, Linck D, Schaefer‐Eckart K, Kroeger M, Bornhaeuser M, Blau IW. Mycophenolate mofetil in combination with cyclosporine a (CSA) ± prednisolone versus CsA, methotrexate ± prednisolone for the prophylaxis of acute GvHD after allogeneic stem cell transplantation ‐ MMF, a new option in GvHD prophylaxis. Blood 2003;11(Pt 1):716a. [CENTRAL: CN‐00484630] [Google Scholar]
- Kiehl MG, Schafer‐Eckart K, Kroger M, Bornhauser M, Basara N, Blau IW, et al. Mycophenolate mofetil for the prophylaxis of acute graft‐versus‐host disease in stem cell transplant recipients. Transplantation Proceedings 2002;34(7):2922‐4. [PUBMED: 12431658] [DOI] [PubMed] [Google Scholar]
- Kraut L, Bunjes D, Bornhaeuser M, Stockschläder M, Ehninger G, Fauser A, et al. First results of a multicenter randomized trial on the mycophenolate mofetil in the prophylaxis of graft versus host disease. Bone Marrow Transplantation 2000;Suppl 1:S151. [CENTRAL: CN‐00488428] [Google Scholar]
- Kraut L, Guenzelmann S, Shipkova M, Schaefer‐Eckart K, Kienast J, Bornhaeuser M, et al. Randomized multicenter prospective clinical trial on the efficacy of mycophenolate mofetil in comparison to methotrexate for the prophylaxis of acute GvHD in stem cell transplant recipients. Bone Marrow Transplantation 2002;Suppl 2:S177. [CENTRAL: CN‐00488430] [Google Scholar]
Perkins 2010 {published data only}
- Perkins J, Field T, Kim J, Kharfan‐Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft‐versus‐host disease prophylaxis. Biology of Blood and Marrow Transplantation 2010;16(7):937‐47. [PUBMED: 20102746] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Neumann 2005 {published data only}
- Neumann F, Graef T, Tapprich C, Vaupel M, Steidl U, Germing U, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft‐versus‐host disease prophylaxis after stem cell transplantation from HLA‐identical siblings. Bone Marrow Transplantation 2005;35(11):1089‐93. [PUBMED: 15821769] [DOI] [PubMed] [Google Scholar]
Ostronoff 2009 {published data only}
- Ostronoff F, Ostronoff M, Souto‐Maior AP, Domingues M, Sucupira A, Manso DA, et al. Prospective trial of mycophenolate mofetil‐cyclosporine A prophylaxis for acute GVHD after G‐CSF stimulated allogeneic bone marrow transplantation with HLA‐identical sibling donors in patients with severe aplastic anemia and hematological malignancies. Clinical Transplantation 2009;23(1):33‐8. [PUBMED: 18727660] [DOI] [PubMed] [Google Scholar]
Piñana 2010 {published data only}
- Piñana JL, Valcárcel D, Fernández‐Avilés F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced‐intensity conditioning PBSCT from HLA‐identical siblings. Bone Marrow Transplantation 2010;45(9):1449‐56. [PUBMED: 20140024] [DOI] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang J, Song X, Zhang W, Tong S, Hou J, Chen L, et al. Combination of mycophenolate mofetil with cyclosporine A and methotrexate for the prophylaxes of acute graft versus host disease in allogeneic peripheral stem cell transplantation. Zhonghua Yi Xue Za Zhi 2002;82(8):507‐10. [PUBMED: 12133492] [PubMed] [Google Scholar]
References to ongoing studies
NCT00563589 {published data only}
- NCT00563589. Mycophenolate mofetil for the prophylaxis of graft‐versus‐host disease in high risk allogeneic stem cell transplantation. clinicaltrials.gov/ct2/show/NCT00563589 (accessed 28 April 2014).
NCT00928018 {published data only}
- NCT00928018. Tacrolimus/sirolimus/methotrexate versus tacrolimus/methotrexate or cyclosporine/mycophenolate mofetil for GVHD prophylaxis after reduced intensity allogeneic stem cell transplantation for patients with lymphoma. clinicaltrials.gov/show/NCT00928018 (accessed 28 April 2014).
Additional references
Allison 2000
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47(2‐3):85‐118. [PUBMED: 10878285] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bensinger 2006
- Bensinger W. Individual patient data meta‐analysis of allogeneic peripheral blood stem cell transplant vs bone marrow transplant in the management of hematological malignancies: indirect assessment of the effect of day 11 methotrexate administration. Bone Marrow Transplantation 2006;38(8):539‐46. [PUBMED: 16953207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cutler 2005
- Cutler C, Li S, Kim HT, Laglenne P, Szeto KC, Hoffmeister L, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate‐ and non‐methotrexate‐containing graft‐versus‐host disease prophylaxis regimens. Biology of Blood and Marrow Transplantation 2005;11(5):383‐8. [PUBMED: 15846292] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith DG, Schnider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrara 2009
- Ferrara JL, Levine JE, Reddy P, Holler E. Graft‐versus‐host disease. Lancet 2009;373(9674):1550‐61. [PUBMED: 19282026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filipovich 2005
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biology of Blood and Marrow Transplantation 2005;11(12):945‐56. [PUBMED: 16338616] [DOI] [PubMed] [Google Scholar]
Flowers 2011
- Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft‐versus‐host disease and for chronic graft‐versus‐host disease according to National Institutes of Health consensus criteria. Blood 2011;117(11):3214‐9. [PUBMED: 21263156] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jacobsohn 2007
- Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet Journal of Rare Diseases 2007;2:35. [PUBMED: 17784964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kharfan‐Dabaja 2012
- Kharfan‐Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced‐toxicity allogeneic hematopoietic cell transplantation with conventional chemo‐(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplantation 2012;47(9):1164‐70. [PUBMED: 22562081] [DOI] [PubMed] [Google Scholar]
Khera 2012
- Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic stem cell transplantation. Blood 2012;120(8):1545‐51. [PUBMED: 22700725] [DOI] [PubMed] [Google Scholar]
Knight 2009
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009;87(6):785‐94. [PUBMED: 19300178] [DOI] [PubMed] [Google Scholar]
Kollman 2001
- Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 2001;98(7):2043‐51. [PUBMED: 11567988] [DOI] [PubMed] [Google Scholar]
Koreth 2009
- Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta‐analysis of prospective clinical trials. JAMA 2009;301(22):2349‐61. [PUBMED: 19509382] [DOI] [PMC free article] [PubMed] [Google Scholar]
McSweeney 2003
- McSweeney P, Abhyankar S, Petersen F. Tacrolimus and mycophenolate mofetil for GVHD prevention after unrelated donor transplants. Blood 2003;102:2654a. [Google Scholar]
Nieto 2006
- Nieto Y, Patton N, Hawkins T, Spearing R, Bearman SI, Jones RB, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched‐sibling donor allogeneic stem‐cell transplantations conditioned with fludarabine and low‐dose total body irradiation. Biology of Blood and Marrow Transplantation 2006;12(2):217‐25. [PUBMED: 16443519] [DOI] [PubMed] [Google Scholar]
Osunkwo 2004
- Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Ven C, Toro G, et al. A pilot study of tacrolimus and mycophenolate mofetil graft‐versus‐host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biology of Blood and Marrow Transplantation 2004;10(4):246‐58. [PUBMED: 15077223] [DOI] [PubMed] [Google Scholar]
Pidala 2010
- Pidala J, Anasetti C. Glucocorticoid‐refractory acute graft‐versus‐host disease. Biology of Blood and Marrow Transplantation 2010;16(11):1504‐18. [PUBMED: 20096359] [DOI] [PubMed] [Google Scholar]
Pinana 2010
- Pinana JL, Valcarcel D, Fernandez‐Aviles F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced‐intensity conditioning PBSCT from HLA‐identical siblings. Bone Marrow Transplantation 2010;45(9):1449‐56. [PUBMED: 20140024] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schmeding 2011
- Schmeding M, Kiessling A, Neuhaus R, Heidenhain C, Bahra M, Neuhaus P, et al. Mycophenolate mofetil monotherapy in liver transplantation: 5‐year follow‐up of a prospective randomized trial. Transplantation 2011;92(8):923‐9. [PUBMED: 21832958] [DOI] [PubMed] [Google Scholar]
Shulman 1980
- Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft‐versus‐host syndrome in man. A long‐term clinicopathologic study of 20 Seattle patients. American Journal of Medicine 1980;69(2):204‐17. [PUBMED: 6996481] [DOI] [PubMed] [Google Scholar]
Sonis 2001
- Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem‐cell transplantation. Journal of Clinical Oncology 2001;19(8):2201‐5. [PUBMED: 11304772] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Storb 1997
- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP, et al. Stable mixed hematopoietic chimerism in DLA‐identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997;89(8):3048‐54. [PUBMED: 9108426] [PubMed] [Google Scholar]
Svahn 2006
- Svahn BM, Ringden O, Remberger M. Treatment costs and survival in patients with grades III‐IV acute graft‐versus‐host disease after allogenic hematopoietic stem cell transplantation during three decades. Transplantation 2006;81(11):1600‐3. [PUBMED: 16770251] [DOI] [PubMed] [Google Scholar]
Svahn 2012
- Svahn BM, Remberger M, Alvin O, Karlsson H, Ringden O. Increased costs after allogeneic haematopoietic SCT are associated with major complications and re‐transplantation. Bone Marrow Transplantation 2012;47(5):706‐15. [PUBMED: 21874063] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 1998
- Yu C, Seidel K, Nash RA, Deeg HJ, Sandmaier BM, Barsoukov A, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft‐versus‐host disease among lethally irradiated dogs given DLA‐nonidentical unrelated marrow grafts. Blood 1998;91(7):2581‐7. [PUBMED: 9516160] [PubMed] [Google Scholar]
Zuk 2009
- Zuk DM, Pearson GJ. Monitoring of mycophenolate mofetil in orthotopic heart transplant recipients ‐ a systematic review. Transplantation Reviews 2009;23(3):171‐7. [PUBMED: 19345081] [DOI] [PubMed] [Google Scholar]


