Abstract
Introduction
Readmission following bronchiectasis exacerbation is a common and challenging clinical problem and few simple predictive tools exist. The COPD Assessment Test (CAT) is an easy-to-use questionnaire. This study aims to evaluate the predictive value of CAT scores in determining the risk of readmission in patients with bronchiectasis exacerbation.
Methods
We conducted a prospective cohort study in 106 bronchiectasis patients admitted with exacerbation. All patients completed the CAT at admission and at discharge. Patients were followed-up for 12 months to collect data on readmission. The area under the curve was used to measure the predictive value of CAT at admission, CAT at discharge and change in CAT for readmission due to bronchiectasis exacerbation.
Results
46 patients were readmitted for bronchiectasis exacerbation within 12 months. High CAT at admission was an independent risk factor for readmission within 12 months in patients with acute exacerbation of bronchiectasis (hazard ratio 3.201, 95% CI 1.065–9.624; p<0.038) after adjustment for confounding variables. The cut-off value of CAT at admission and CAT at discharge to predict 12-month readmission in patients with acute exacerbation of bronchiectasis was 23.5 (sensitivity 62.2%, specificity 83.6%) and 15.5 (sensitivity 52.2%, specificity 87.0%).
Conclusions
CAT at admission is a strong predictor of readmission in patients with bronchiectasis exacerbation.
Shareable abstract
CAT on admission is a strong predictor of readmission in patients with bronchiectasis exacerbation within a 12-month follow-up period https://bit.ly/486h5yb
Introduction
Noncystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis) is a chronic airway disease characterised by abnormal and persistent bronchial dilation [1]. Recent epidemiological studies have shown that the incidence and prevalence of bronchiectasis are rapidly increasing worldwide due to ageing populations and the widespread use of high-resolution computed tomography (HRCT) of the chest [2–9]. Bronchiectasis has a great long-term impact on patient survival and quality of life, which places a huge burden on healthcare systems around the world [10, 11]. Readmissions due to acute exacerbation of bronchiectasis constitute a major clinical problem that is associated with adverse clinical outcomes, including a higher risk of mortality and morbidity [2, 11]. Therefore, prevention of readmissions has become an essential objective of medical care in recent times [8]. Prediction tools have been a hot topic as they help assess patient prognosis, thus helping clinicians provide targeted treatment and healthcare. The Bronchiectasis Severity Index (BSI) [8] and the FACED (forced expiratory volume in 1 s (FEV1), age, chronic colonisation, extension and dyspnoea) [9] and E-FACED [9] scores have been widely recognised as predictors of disease severity and poor clinical outcomes in bronchiectasis. However, all of them do not assess patient quality of life, thus failing to fully address the disease burden. The COPD Assessment Test (CAT), originally used in COPD patients for measurement of health status [12, 13], is a short and simple instrument consisting of eight items covering disease symptoms and restricted activity [14]. Due to symptom overlap between bronchiectasis and COPD, questionnaires such as the CAT and St George's Respiratory Questionnaire (SGRQ) also apply to bronchiectasis patients. Several studies showed that CAT has been validated for use in the evaluation of the quality of life, disease severity and disease progression in bronchiectasis patients [15, 16]. Dudgeon et al. [17] performed a qualitative study in patients with bronchiectasis and found that CAT scores are preferred because of the clear layout and relevant questions. Other studies have shown correlations between CAT and other quality-of-life assessment tools, such as SGRQ and the Bronchiectasis Health Questionnaire (BHQ) [15, 16, 18]. However, there is no publication yet investigating the association between CAT scores and the risk of readmission in patients with acute exacerbation of bronchiectasis. This study aimed to evaluate the predictive ability of CAT to investigate determinants of CAT scores and to predict the risk of readmission in patients with acute exacerbation of bronchiectasis.
Methods
Study design and participants
This prospective cohort study enrolled 106 patients between March 2021 and December 2021. The inclusion criteria were 1) age ≥14 years; 2) diagnosis of bronchiectasis confirmed by HRCT and clinical symptoms [1]; and 3) admission for bronchiectasis exacerbation. Bronchiectasis exacerbation was defined as the worsening of three or more of the six symptoms of cough, changes in sputum volume, purulent sputum, dyspnoea or exercise tolerance, fatigue or discomfort, and haemoptysis for >48 h requiring treatment [11]. The exclusion criteria were: 1) cystic fibrosis bronchiectasis; 2) active tuberculosis or nontuberculous Mycobacterium infection; 3) malignant tumour; 4) severe immunosuppression; 5) bronchiectasis secondary to interstitial lung disease; and 6) unable to complete the questionnaire assessment and unable to complete the follow-up. Patients were classified into three groups based on the baseline CAT scores at admission: low CAT group (CAT scores 0–10), medium CAT group (CAT scores 11–20) and high CAT group (CAT scores 21–40) based on a previous study [15]. The study protocol was approved by the ethics committees of the Beijing Chaoyang Hospital (2020-K-017) and written informed consent was obtained from all patients.
Variables
The following data were recorded: demographic (including sex, age, body mass index (BMI) and smoking status), clinical symptoms (including cough, sputum volume, haemoptysis and modified Medical Research Council (mMRC) dyspnoea scale), comorbidities (hypertension, diabetes, coronary heart disease, COPD and asthma), disease duration, laboratory (serum biomarkers such as white blood cells, neutrophil percentage, lymphocyte percentage, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, D-dimer, albumin, globulin, albumin/globulin ratio and prealbumin), aetiological data (Pseudomonas aeruginosa or other organisms), functional status (forced vital capacity (FVC), FVC % predicted, FEV1, FEV1 % pred) and radiological characteristics (including involvement of lobes and modified Reiff score [19]). The blood and sputum samples were all collected within 24 h of admission. The lung function tests were performed within the 6 months before hospitalisation. All the patients were requested to complete the following questionnaires: CAT [14] (at admission and at discharge), SGRQ [20] (at admission) and BHQ [21] (at admission). All the assessments were performed in accordance with relevant testing standards. Every variable corresponding to a particular score was summed for BSI. The BSI score range was 0–26 points.
Follow-up
Follow-up data were collected via face-to-face interviews or telephone interview every month. Patients were followed-up for 12 months. The primary outcome was readmission due to acute exacerbation at 12 months after discharge.
Statistical analysis
SPSS Statistics 26.0 (IBM Corporation, Armonk, NY, USA) software was used for statistical analysis. Kolmogorov–Smirnov tests were carried out to test for the normality of variables. Continuous variables conforming to normal distribution are presented as mean±sd and compared using ANOVA tests. Those not conforming to normal distribution are presented as median (interquartile range) and compared using the Kruskal–Wallis test. Categorical variables are presented as n (%) and were compared using the Chi-squared test or Fisher's exact test, as appropriate. Bonferroni correction was used for multiple comparisons. Spearman's correlation test was used to evaluate the relationship with CAT scores. Cox proportional hazards regression was used to analysa the association of CAT scores at admission, at discharge and the CAT change with readmission. We performed a collinearity test to rule out multicollinearity between the independent variables. The potential confounding variables were selected in the univariate analysis based on previous research [22–24] and clinical relevance, including age, sex, BMI, mMRC, Charlson Comorbidity Index (CCI), number of exacerbations in previous year, infection with P. aeruginosa, infection with other bacteria, FEV1 % pred, the number of affected lobes and modified Reiff score. Confounding factors with a p-value <0.1 of univariate regression and clinical relevance were included in the multivariate regression model, including age, mMRC, CCI and number of exacerbations in previous year and the number of affected lobes. Associations are presented as hazard ratios (HR) and 95% confidence intervals. We performed a receiver operating characteristic (ROC) analysis to evaluate the potential of CAT scores at admission, at discharge and the change of CAT scores to predict the readmission. The optimal cut-off value was determined when the Youden index (sensitivity + specificity −1) reached the maximum value. p<0.05 was considered to be statistically significant.
Results
Out of 106 eligible participants, six were lost to follow-up during the follow-up period, leaving 100 patients eligible for analysis. Out of 100 patients, the mean±sd age was 56.63±13.24 years and 56 (56%)were female. The mean±sd CAT score at admission was 18.75±8.91. During the 12-month follow-up period, 46 patients required readmission due to bronchiectasis exacerbation, with 84 admissions. Three patients died during the follow-up period; the causes of death were respiratory failure, pulmonary embolism, and coronavirus disease 2019 (COVID-19) infection. Baseline characteristics of patients are presented in table 1. Patients with high CAT were older (60.97±11.98 years versus 51.55±13.33 years, p=0.021) compared with those with low CAT. The high CAT group also had higher significantly more symptoms of dyspnoea, haemoptysis and excessive phlegm production (p<0.001, p=0.021 and p=0.015, respectively). There were no statistically significant differences between the three groups in sex, BMI, smoking status, aetiology and comorbidities. Supplementary figure S1 shows the distribution of number of patients with different change in CAT scores.
TABLE 1.
Baseline clinical characteristics of patients categorised by COPD Assessment Test (CAT) scores at admission
| Total | Low CAT | Medium CAT | High CAT | p-value | Missing data, % | |
| Patients | 100 | 22 | 41 | 37 | ||
| Age, years | 56.63±13.24 | 51.55±13.33 | 55.44±13.35 | 60.97±11.98 | 0.021# | 0.00 |
| Female | 56 (56) | 15 (68.2) | 23 (56.1) | 18 (48.6) | 0.344 | 0.00 |
| BMI, kg·m−2 | 22.7±4.5 | 23.4±2.5 | 22.2±4.2 | 22.8±5.7 | 0.603 | 3.00 |
| Smoking status | 0.321 | 0.00 | ||||
| Never | 74 (74) | 17 (77.3) | 27 (65.9) | 30 (81.1) | ||
| Past | 16 (16) | 3 (13.6) | 7 (17.1) | 6 (16.2) | ||
| Current | 10 (10) | 2 (9.1) | 7 (17.1) | 1 (2.7) | ||
| Aetiology | 0.056 | 0.00 | ||||
| Idiopathic | 57 (57.0) | 13 (59.1) | 26 (63.4) | 18 (48.6) | ||
| Post-infective | 18 (18.0) | 7 (31.8) | 4 (9.8) | 7 (18.9) | ||
| Post-tuberculosis | 16 (16.0) | 0 (0.0) | 9 (22.0) | 7 (18.9) | ||
| Others | 9 (9.0) | 2 (9.1) | 2 (4.9) | 5 (13.5) | ||
| Exacerbations in the preceding year | 1.0 (1.0–2.0) | 0.5 (0–1.5) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.048 | 0.00 |
| Comorbidities | ||||||
| Hypertension | 19 (19.0) | 4 (18.2) | 7 (17.1) | 8 (21.6) | 0.946 | 0.00 |
| Diabetes | 12 (12.0) | 2 (9.1) | 5 (12.2) | 5 (13.5) | 0.927 | 0.00 |
| Coronary heart disease | 10 (10.0) | 2 (9.1) | 2 (4.9) | 6 (16.2) | 0.264 | 0.00 |
| COPD | 3 (3.0) | 1 (4.5) | 0 (0.0) | 2 (5.4) | 0.326 | 0.00 |
| Asthma | 17 (17.0) | 3 (13.6) | 5 (12.2) | 9 (24.3) | 0.324 | 0.00 |
| CCI | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0.146 | 0.00 |
| Clinical symptoms | ||||||
| Cough | 100 (100.0) | 22 (22.0) | 41 (41.0) | 37 (37.0) | 1.000 | 0.00 |
| Sputum volume | 0.015 | |||||
| <20 mL | 62 (62.0) | 15 (68.2) | 30 (73.2) | 17 (45.9) | 0.00 | |
| 20–50 mL | 33 (33.0) | 7 (21.2) | 11 (33.3) | 15 (45.5) | 0.00 | |
| 50–100 mL | 5 (5.0) | 0 (0.0) | 0 (0.0) | 4 (10.8) | 0.00 | |
| >100 mL | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (2.7) | 0.00 | |
| Haemoptysis | 42 (42.0) | 14 (63.6) | 18 (43.9) | 10 (27.0) | 0.021# | 0.00 |
| mMRC ≥2 | 29 (29) | 2 (9.1) | 6 (14.6) | 21 (56.8) | <0.001#,¶ | 0.00 |
| Disease duration, years | 7.0 (1.0–20.0) | 4.0 (0.4–14.3) | 3.0 (0.8–10.5) | 12.0 (2.0–26.0) | 0.115 | 0.00 |
Data are presented as n, mean±sd, n (%) or median (interquartile range), unless otherwise stated. BMI: body mass index; CCI: Charlson Comorbidity Index; mMRC: modified Medical Research Council score. #: p<0.017 (Bonferroni-corrected threshold) for comparison between the low CAT group and the high CAT group; ¶: p<0.017 (Bonferroni-corrected threshold) for comparison between the medium CAT group and the high and very high CAT group.
Laboratory, spirometry, microbiological and radiological characteristics
Table 2 shows the laboratory, spirometry, microbiological and radiological characteristics. Patients in the high CAT group had increased levels of CRP and ESR and decreased levels of albumin and prealbumin compared with those in the medium and low CAT groups (p<0.001, p=0.009, p<0.001 and p=0.006, respectively). 19 (19%) out of 100 patients were infected with P. aeruginosa. Although we found no significant difference in P. aeruginosa infection between the three groups, there was a trend towards an increased proportion of P. aeruginosa infection in patients with high CAT. In our study, 45 patients completed lung function tests and there was no significant difference in lung function indices between the three groups, with the exception of FEV1/FVC. Patients with medium and high CAT had lower FEV1/FVC compared with those with low CAT (p=0.007). With respect to radiological imaging, patients with high CAT had higher numbers of lobe involvement and higher modified Reiff scores than the medium and low CAT groups (p<0.001 and p<0.001, respectively). There were significant differences of the distribution of lobe involvement, with a higher proportion of left upper lobe, lingual, left lower lobe and right middle lobe in the high CAT group. Further, patients with high CAT scores had a higher disease severity (evaluated by BSI score) compared with those with medium and low CAT scores (p<0.001).
TABLE 2.
Laboratory, spirometry, microbiological and radiological variables categorised by COPD Assessment Test (CAT) scores at admission
| Total | Low CAT | Medium CAT | High CAT | p-value | Missing data, % | |
| Patients | 100 | 22 | 41 | 37 | ||
| WBC ×109 cells·mL−1 | 6.05 (4.91–7.58) | 5.65 (4.55–6.71) | 5.83 (4.98–6.97) | 4.67 (6.74–8.41) | 0.217 | 0.00 |
| Neutrophils, % | 59.3 (52.1–69.5) | 57.7 (52.6–66.5) | 57.1 (51.0–67.3) | 66.9 (54.1–74.0) | 0.085 | 0.00 |
| Lymphocytes, % | 30.4 (20.0–37.8) | 33.1 (23.2–38.5) | 30.9 (24.2–39.7) | 23.5 (18.5–35.7) | 0.090 | 0.00 |
| CRP, mg·L−1 | 0.55 (0.27–1.30) | 0.33 (0.21–0.55) | 0.46 (0.27–0.85) | 1.17 (0.44–3.04) | <0.001#,¶ | 8.00 |
| ESR, mm·h−1 | 13.0 (4.0–39.0) | 7.0 (3.0–20.5) | 10.0 (3.0–29.0) | 31.5 (6.0–64.5) | 0.009#,¶ | 0.00 |
| Fibrinogen, g·L−1 | 305.3 (250.2–391.5) | 283.9 (223.2–327.6) | 287.5 (250.9–499.6) | 352.0 (267.7–462.5) | 0.013# | 0.00 |
| D-dimer, ng·mL−1 | 276.3 (144.8–446.8) | 224.2 (150.4–318.4) | 271.2 (107.0–425.9) | 336.9 (139.5–696.2) | 0.232 | 5.00 |
| Albumin, g·L−1 | 39.6±3.8 | 41.9±2.6 | 40.1±3.5 | 37.8±3.9 | <0.001#,¶ | 0.00 |
| Globulin, g·L−1 | 26.7±5.0 | 25.8±4.0 | 25.7±3.6 | 28.5±6.4 | 0.026#,¶ | 0.00 |
| Albumin/globulin ratio | 1.56±0.37 | 1.69±0.31 | 1.61±0.28 | 1.42±0.45 | 0.011#,¶ | 0.00 |
| Prealbumin, mg·dL−1 | 0.20 (0.16–0.24) | 0.23 (0.19–0.25) | 0.21 (0.16–0.25) | 0.16 (0.12–0.22) | 0.006#,¶ | 0.00 |
| Patients with positive bacteria cultures | 41 (41.0) | 5 (22.7) | 19 (46.3) | 17 (45.9) | 0.143 | 0.00 |
| Pseudomonas aeruginosa | 19 (19.0) | 3 (13.6) | 5 (12.2) | 11 (29.7) | 0.110 | 0.00 |
| Klebsiella pneumoniae | 5 (5.0) | 0 (0.0) | 2 (4.9) | 3 (8.1) | 0.445 | 0.00 |
| Haemophilus influenzae | 4 (4.0) | 0 (0.0) | 3 (7.3) | 1 (2.7) | 0.537 | 0.00 |
| Escherichia coli | 5 (5.0) | 1 (4.5) | 3 (7.3) | 1 (2.7) | 0.840 | 0.00 |
| Mycobacterium avium intracellulare | 2 (2.0) | 1 (4.5) | 1 (2.4) | 0 (0.0) | 0.694 | 0.00 |
| Number of affected lobes | 2 (1–4) | 2 (1–2) | 2 (1–4) | 4 (2–5) | <0.001#,¶ | 0.00 |
| Unilateral/bilateral | 69/31 | 12/10 | 13/28 | 6/31 | 0.009# | 0.00 |
| Involvement of lobes | ||||||
| Left upper lobe | 38 (38) | 3 (13.6) | 17 (41.5) | 18 (48.6) | 0.023# | 0.00 |
| Lingual | 50 (50) | 6 (27.3) | 18 (43.9) | 26 (70.3) | 0.004# | 0.00 |
| Left lower lobe | 62 (62.0) | 10 (45.5) | 22 (53.7) | 30 (81.4) | 0.009#,¶ | 0.00 |
| Right upper lobe | 51 (51.0) | 6 (27.3) | 24 (58.5) | 21 (56.8) | 0.041 | 0.00 |
| Right middle lobe | 49 (49.0) | 7 (31.8) | 17 (41.5) | 25 (67.6) | 0.013# | 0.00 |
| Right lower lobe | 42 (42.0) | 6 (27.3) | 16 (39.0) | 20 (54.1) | 0.116 | 0.00 |
| Modified Reiff score | 4 (2–8) | 2 (2–3) | 4 (2–6) | 8 (4–12) | <0.001#,+,¶ | 0.00 |
| BSI | 7 (3–10) | 4 (2–8) | 5 (3–9) | 9 (5–13) | <0.001#,¶ | 0.55 |
| BHQ | 32.1±9.2 | 39.4±7.6 | 32.1±8.5 | 30.6±8.5 | <0.001#,+ | 0.00 |
| SGRQ | ||||||
| Total | 17.2 (5.4–33.4) | 3.3 (2.1–9.7) | 16.6 (7.7–28.9) | 32.8 (16.6–29.4) | <0.001#,¶,+ | 0.00 |
| Symptoms | 15.8 (9.4–38.2) | 10.2 (8.8–20.0) | 15.4 (9.5–28.3) | 37.6 (11.5–61.9) | 0.001# | 0.00 |
| Activity | 17.1 (5.3–35.8) | 0.0 (0.5–3.0) | 17.1 (6.0–29.6) | 35.5 (17.4–47.7) | <0.001#,+ | 0.00 |
| Impact | 17.3 (4.0–32.3) | 1.7 (0.0–10.7) | 16.1 (4.9–28.1) | 31.8 (16.0–46.6) | <0.001#,¶,+ | 0.00 |
| Length of hospital stay, days | 12 (9–14) | 11 (8–14) | 11 (9–13) | 13 (10–16) | 0.096 | 0.00 |
Data are presented as n, median (interquartile range), mean±sd or n (%), unless otherwise stated. WBC: white blood cell; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; BSI: Bronchiectasis Severity Index; BHQ: Bronchiectasis Health Questionnaire; SGRQ: St George's Respiratory Questionnaire. #: p<0.017 (Bonferroni-corrected threshold) for comparison between the low CAT and the high and very high CAT group; ¶: p<0.017 (Bonferroni-corrected threshold) for comparison between the low CAT group and the medium CAT group; +: p<0.017 (Bonferroni-corrected threshold) for comparison between the medium CAT group and the high CAT group.
Correlations between CAT scores at admission and other clinical measures
Table 3 shows the correlation between CAT scores at admission and other demographics, clinical, laboratory, radiological and microbiological variables. CAT score showed a significant and negative correlation with albumin (r= −0.505; p<0.001), prealbumin (r= −0.408; p<0.001), FEV1% pred (r= −0.415; p=0.005) and FVC % pred (r= −0.318; p=0.033). In addition, CAT score was positively associated with the degree of expectoration and dyspnoea. CAT score did not correlate with BMI (r= −0.130; p=0.206). CAT score was positively associated with other quality assessments, including SGRQ and BHQ scores.
TABLE 3.
Correlation between COPD Assessment Test (CAT) scores at admission and clinical measures in patients with acute exacerbation of bronchiectasis
| Correlation | p-value | |
| Age, years | 0.309 | 0.002 |
| BMI, kg·m−2 | −0.130 | 0.206 |
| Exacerbations in past 12 months | 0.213 | 0.035 |
| Expectoration (<20 mL/20–50 mL/50–100 mL/>100 mL) | 0.227 | 0.030 |
| mMRC | 0.630 | <0.001 |
| CRP, mg·L−1 | 0.429 | <0.001 |
| ESR, mm·h−1 | 0.347 | 0.001 |
| Albumin, g·L−1 | −0.505 | <0.001 |
| Prealbumin, mg·day−1 | −0.408 | <0.001 |
| FEV1 % pred | −0.415 | 0.005 |
| FVC % pred | −0.318 | 0.033 |
| Modified Reiff score | 0.542 | <0.001 |
| CCI | 0.233 | 0.020 |
| BSI | 0.690 | <0.001 |
| BHQ | −0.467 | <0.001 |
| SGRQ | ||
| Total | 0.649 | <0.001 |
| Symptoms | 0.521 | <0.001 |
| Activity | 0.617 | <0.001 |
| Impact | 0.609 | <0.001 |
BMI: body mass index; mMRC: modified Medical Research Council score; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; CCI: Charlson Comorbidity Index; BSI: Bronchiectasis Severity Index; BHQ: Bronchiectasis Health Questionnaire; SGRQ: St George's Respiratory Questionnaire.
Outcomes
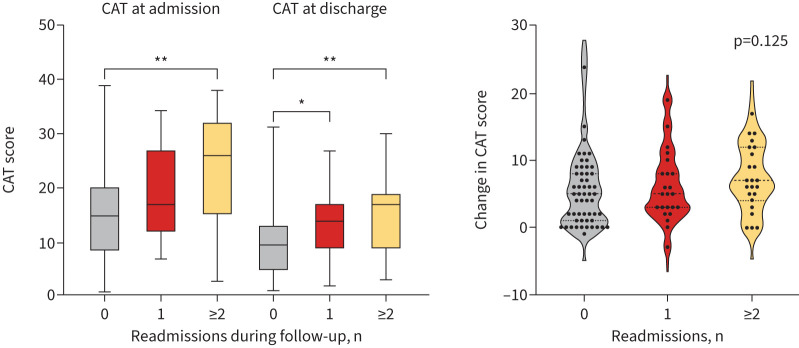
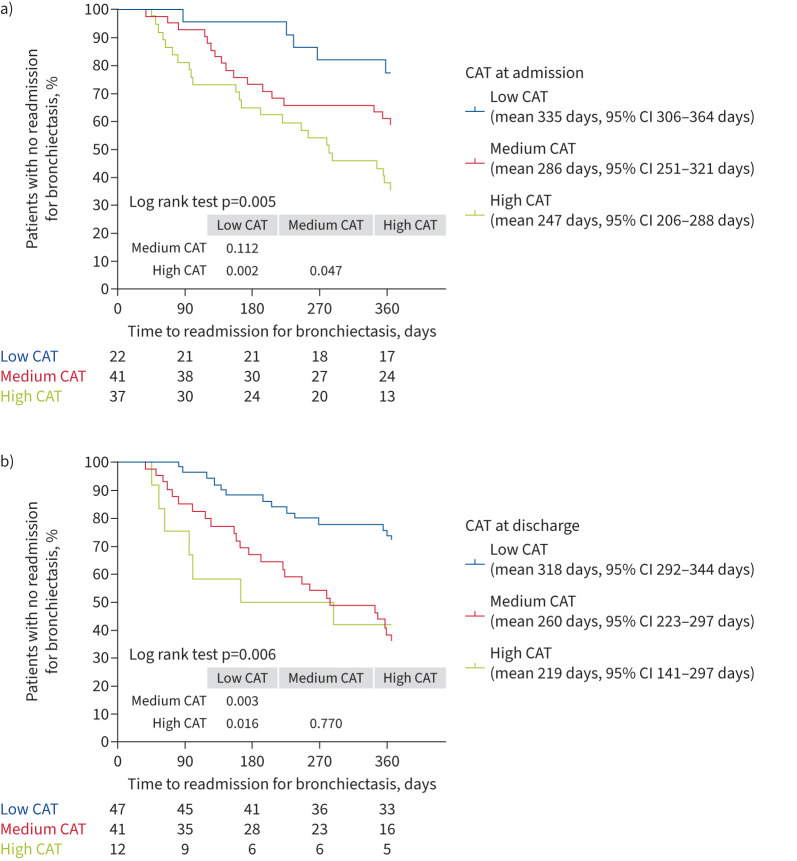
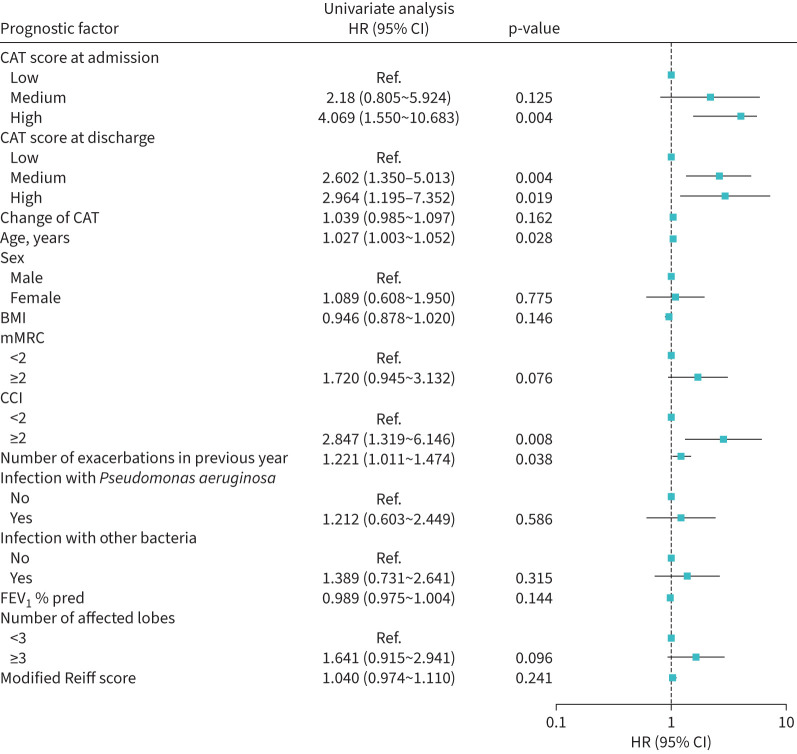
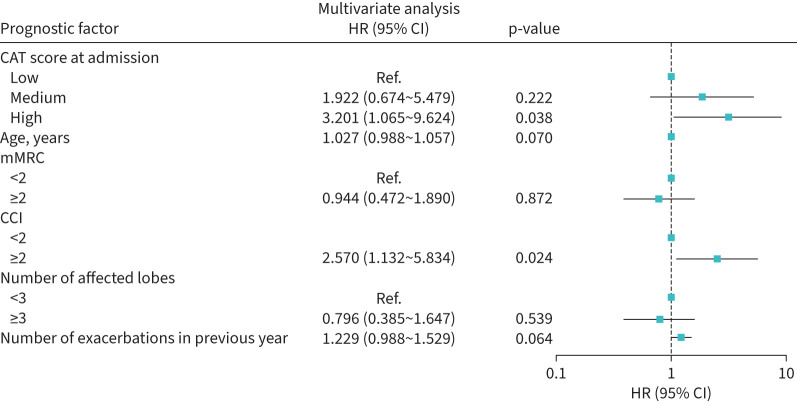
46 (46%) patients had at least one readmission during the 12-month follow-up period. In these patients, the baseline CAT score was significantly higher in those without readmission (p=0.001). CAT score at admission and CAT at discharge was closely associated with the number of readmissions during the 12-month follow-up period (p=0.003 and p=0.001, respectively) (figure 1). However, the change in CAT was similar in patients with no, one and two or more readmissions. Patients with high CAT at admission had a shorter time to first readmission for bronchiectasis compared with those with medium CAT and with low CAT scores at admission (mean estimate 335 days versus 286 days versus 247 days). Patients with high CAT at discharge had a shorter time to first readmission for bronchiectasis compared with those with medium CAT and with low CAT scores at discharge (mean estimate 318 days versus 260 days versus 219 days). The Kaplan–Meier curves showed a significant divergence of readmission for bronchiectasis among patients with different CAT scores at admission and at discharge (log rank test p=0.005 and p=0.006, respectively) (figure 2). Univariate analysis showed that age (HR 1.027, 95% CI 1.003–1.052), high CAT scores at admission (HR 4.069, 95% CI 1.550–10.683), high CAT scores at discharge (HR 2.964, 95% CI 1.195–7.352), medium CAT scores at discharge (HR 2.602, 95% CI 1.350–5.013), CCI ≥2 (HR 2.847, 95% CI 1.319–6.146) and the number of exacerbations in the previous year (HR 1.221, 95% CI 1.011–1.474) were significantly associated with a higher risk of readmission (figure 3). High CAT at admission (HR 3.201, 95% CI 1.065–9.624) remained significant after adjusting for potential confounders compared with low CAT at admission (figure 4). Medium CAT at discharge (HR 2.587, 95% CI 1.248–5.361) was independently associated with readmission for acute exacerbations compared with low CAT at discharge (supplementary figure S2). The change of CAT was not associated with readmission for acute exacerbations in patients with bronchiectasis (supplementary figure S3).
FIGURE 1.
COPD Assessment Test (CAT) scores at admission, CAT scores at discharge and the change of CAT scores in different numbers of readmissions. *: p<0.05; **: p<0.01.
FIGURE 2.
Kaplan–Meier survival analysis grouped by COPD Assessment Test (CAT) scores a) at admission and b) at discharge.
FIGURE 3.
Univariate Cox regression model of readmission in acute exacerbation of bronchiectasis. HR: hazard ratio; CAT: COPD Assessment Test; BMI: body mass index; mMRC: modified Medical Research Council score; CCI: Charlson Comorbidity Index; FEV1: forced expiratory volume in 1 s; Ref: reference.
FIGURE 4.
The impact of COPD Assessment Test (CAT) at admission on readmission in acute exacerbation of bronchiectasis in the multiple Cox regression model. HR: hazard ratio; mMRC: modified Medical Research Council score; CCI: Charlson Comorbidity Index; Ref: reference.
The predictive value of CAT scores at admission, at discharge and the change of CAT
ROC analyses demonstrated that the area under the curve (AUC) value of CAT scores at admission, CAT at discharge and change of CAT was 0.691 (95% CI 0.586–0.797), 0.707 (95% CI 0.603–0.811) and 0.600 (95% CI 0.489–0.711). The cut-off values of CAT at admission and CAT at discharge to predict the 12-month readmission in patients with acute exacerbation of bronchiectasis were 23.5 (with sensitivity of 62.2% and specificity of 83.6%) and 15.5 (with sensitivity of 52.2% and specificity of 87.0%) (figure 5).
FIGURE 5.
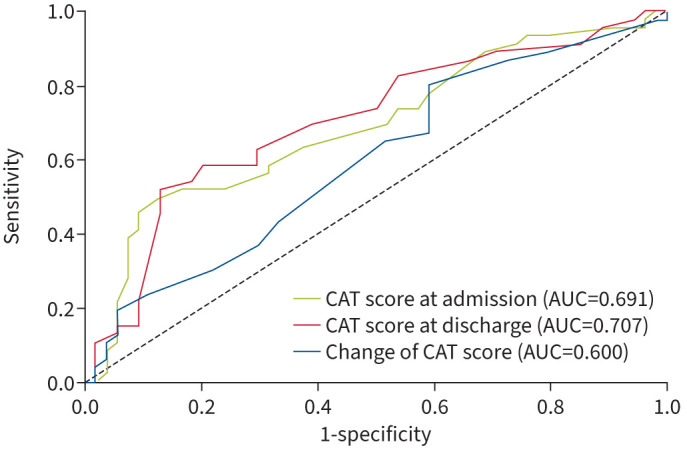
The receiver operating characteristic (ROC) curves of risk of readmission in patients with bronchiectasis exacerbation: ROC of COPD Assessment Test (CAT) at admission, at discharge and the change of CAT.
Discussion
We performed a prospective observational study, aiming to evaluate the predictive value of CAT scores in determining the risk of readmission in patients with acute exacerbation of bronchiectasis. To the best of our knowledge, this was the first prospective study to evaluate the predictive value of the CAT for the risk of readmission after discharge in acute exacerbations of bronchiectasis. The main finding is that patients with high CAT scores at admission (CAT scores ≥20) had a shorter time to the next readmission during the following 12 months compared with those with medium and low CAT scores. At a cut-off score >23, CAT at admission had a sensitivity of 62.2% and a specificity of 83.6% for predicting the risk of readmission.
The CAT, in combination with mMRC, has been a valuable method for classifying COPD patients and guiding treatment. The CAT has now been widely used in the evaluation of symptoms and quality of life in bronchiectasis patients. In this study, patients who were older and presented with more symptoms including dyspnoea, haemoptysis and excessive phlegm production had higher CAT scores. CAT was also associated with mMRC, as reported in previous studies [15]. This is not surprising, as mMRC is calculated using one question about the degree of dyspnoea, which is part of the CAT scale.
We found decreased levels of albumin and prealbumin in patients with high CAT scores compared with those with medium and low CAT scores. There is also a moderate and negative correlation (r= −0.505 for albumin and r= −0.408 for prealbumin) between these indicators and CAT scores. However, the correlation between nutritional indicators and CAT scores has not been described in previous reports. In our study, we found that patients with high CAT scores were older than those with low and moderate CAT scores. Older people were more likely to be malnourished, which might partly explain our findings. Previous studies have shown that patients with low albumin level (<3.5 g·dL−1) had a higher risk of hospitalisations [25], which might partially explain the higher risk of hospitalisations in patients with high CAT scores. Patients with high CAT scores had more severe radiological manifestations than those with medium and low CAT scores. The correlation between CAT scores and FEV1 % pred was −0.415 and increased to 0.542 for modified Reiff scores. The perception of dyspnoea, the extent of bronchiectasis and lung function parameters are clinically interpretable. The pulmonary extent of bronchiectasis has been reported to correlate significantly with symptoms such as daily sputum volume [26] and dyspnoea [27] and lung function parameters such as FEV1 and FVC [26, 28], which might explain our results.
In our study, the correlation between SGRQ and CAT scores was r=0.649. A correlation between the CAT scores and the SGRQ scores has been previously reported to range from 0.75 to 0.90 in bronchiectasis patients [15, 16, 29], which is slightly higher than our results. The reason for the difference is that previous studies mainly focus on patients with stable bronchiectasis and data are included from a different period to the present study. The SGRQ questionnaire asks about the existence of symptoms during the past 3 months, thus weakening the correlation between these two scores. CAT correlated moderately with BHQ (r= −0.467), which have a divergence with prior studies conducted in stable bronchiectasis [15]. We also found significant correlations between CAT and BSI scores (r=0.690). Although the two tools focus on different aspects, the close association between the two indicates that CAT might reflect the severity of the disease.
Patients with high CAT scores were characterised by stronger inflammation responses such as higher levels of CRP, ESR and fibrinogen. Previous studies found that CAT was closely related to some biomarkers related to systemic inflammatory response, such as CRP and plasma fibrinogen [29–31], which are consistent with our findings. Another previous study demonstrated there was a significant decrease in systemic markers of inflammation, including white blood cells and CRP and an increase in quality-of-life scores after treatment compared with the onset of exacerbation in bronchiectasis patients, but found that the change of quality-of-life scores and inflammatory markers is not synchronous [32], which showed that improvements in the quality of life after treatment could not be explained by the improvements in inflammation levels. We also observed that the correlation between CAT scores and CRP was r=0.429, higher than reported in patients with stable bronchiectasis [15]. This disparity could potentially be attributed to the fact that CAT scores in acute exacerbation are driven more by inflammation than in a stable period. There was a positive association between CAT scores at admission and CCI. Previous studies have reported the negative effect of multimorbidity on quality of life [33–35], which was consistent with our findings.
In our study, the 12-month hospital readmission rate was 46%, consistent with what has been reported in previous studies, with hospital readmission rates ranging from 24% to 49.1% [32–35]. After adjusting for those confounding factors, CAT at admission and at discharge were still independent risk factors for readmission within 12 months in patients with bronchiectasis exacerbation. At a cut-off score >23, CAT at admission had a sensitivity of 62.2% and a specificity of 83.6% for predicting the risk of readmission. This suggests that early CAT evaluation in patients with bronchiectasis exacerbation helps predict the risk of readmission within 12 months and provides a reference for the adjustment of clinical treatment plans and the future risk assessment of patients. Additionally, we collected data for CAT score at discharge and found that the CAT score decreased following treatment. The cut-off value of CAT at discharge to predict the 12-month readmission in patients with acute exacerbation of bronchiectasis was 15.5 (with sensitivity of 52.2% and specificity of 87.0%). It shows that the CAT at discharge has a relatively poor sensitivity but high specificity for readmission in bronchiectasis patients. Previous research has demonstrated that CAT scores increase during acute exacerbation but subsequently decrease and remain relatively stable as the condition improves [16, 29]. The change of CAT was not independently associated with the risk of readmission. It had a lower predictive value for readmission, with AUC 0.600. To conclude, CAT at admission is a better tool for evaluating readmission, and the simple tool provides a more robust means of risk stratification. In clinical practice, clinical measurements have been mostly used in the evaluation of the disease severity and disease prognosis and patient feelings are less concerned. As CAT scores are easily or routinely collected in clinical practice, there is potential to produce prognostic indices that are easily implemented into clinical practice.
The present study has several limitations. First, this study was a single-centre study, which will inevitably lead to selection bias. Second, we followed-up patients for only 1 year and lacked the CAT scores after discharge. Third, some clinical data, such as microbiology and lung function parameters, were missing, due to incomplete inspection. Additionally, we were unable to conduct face-to-face interviews with some patients affected by COVID-19 during the follow-up, which could result in recall bias and did not consider the potential influence of medical treatment in patients during the follow-up. Further multicentre studies with larger samples and longer follow-up are warranted to confirm our findings.
In conclusion, we found that high CAT at admission is associated with an increased risk of readmission within 12 months in patients with acute exacerbation of bronchiectasis compared with medium CAT and low CAT.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Figure S1 00867-2023.SUPPLEMENT (29.8KB, png)
Figure S2 00867-2023.SUPPLEMENT2 (364.2KB, png)
Figure S3 00867-2023.SUPPLEMENT3 (94.9KB, png)
Acknowledgements
We would like to express our gratitude to the patients who participated in this research.
Provenance: Submitted article, peer reviewed.
Author contributions: Concept and design of study: X. Bu; acquisition of data: X. Chen, J. Wang, S. He, J. Li, T. Ma, L. Liu and L. Zhang; interpretation and analysis of data: X. Chen, J. Wang and S. He; drafting of manuscript: X. Chen, J. Wang and S. He; revision of manuscript critically for important intellectual content: all authors; approval of final manuscript: all authors. J. Wang acts as the guarantor of the data. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data availability: The data and/or related materials of this study are available from the corresponding author on reasonable request.
Conflict of interest: J. Wang reports no conflicts of interest.
Conflict of interest: X. Chen reports no conflicts of interest.
Conflict of interest: S. He reports no conflicts of interest.
Conflict of interest: J. Li reports no conflicts of interest.
Conflict of interest: T. Ma reports no conflicts of interest.
Conflict of interest: L. Liu reports no conflicts of interest.
Conflict of interest: L. Zhang reports no conflicts of interest.
Conflict of interest: X. Bu reports no conflicts of interest.
Ethics statement: The study protocol was approved by the ethics committees of the Beijing Chaoyang Hospital (2020-K-017) and written informed consent was obtained from all patients.
References
- 1.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. BMJ Open Respir Res 2018; 5: e000348. doi: 10.1136/bmjresp-2018-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013–2017: a nationwide population-based cohort study. Respir Res 2022; 23: 111. doi: 10.1186/s12931-022-02023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goeminne PC, De Soyza A. Bronchiectasis: how to be an orphan with many parents? Eur Respir J 2016; 47: 10–13. doi: 10.1183/13993003.01567-2015 [DOI] [PubMed] [Google Scholar]
- 4.Henkle E, Chan B, Curtis JR, et al. Characteristics and health-care utilization history of patients with bronchiectasis in US Medicare enrollees with prescription drug plans, 2006 to 2014. Chest 2018; 154: 1311–1320. doi: 10.1016/j.chest.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 5.Quint J, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringshausen F, de Roux A, Diel R, et al. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J 2015; 46: 1805–1807. doi: 10.1183/13993003.00954-2015 [DOI] [PubMed] [Google Scholar]
- 7.Monteagudo M, Rodríguez-Blanco T, Barrecheguren M, et al. Prevalence and incidence of bronchiectasis in Catalonia, Spain: a population-based study. Respir Med 2016; 121: 26–31. doi: 10.1016/j.rmed.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Phua H, Lim WY, Ganesan G, et al. Epidemiology and economic burden of bronchiectasis requiring hospitalisation in Singapore. ERJ Open Res 2021; 7: 00334–2021. doi: 10.1183/23120541.00334-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Xu J, Qu JM. Bronchiectasis in China. Ann Am Thorac Soc 2016; 13: 609–616. doi: 10.1513/AnnalsATS.201511-740PS [DOI] [PubMed] [Google Scholar]
- 10.Olveira C, Martínez-García MA. Health-related quality of life questionnaires in bronchiectasis: the simplest way to quantify complexity. Eur Respir J 2017; 49:1700208. doi: 10.1183/13993003.00208-2017 [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell AE. Bronchiectasis – a clinical review. N Engl J Med 2022; 387: 533–545. doi: 10.1056/NEJMra2202819 [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 13.Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2021; 203: 24–36. doi: 10.1164/rccm.202009-3533SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones P, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34: 648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 15.De la Rosa Carrillo D, Olveira C, García-Clemente M, et al. COPD assessment test in bronchiectasis: minimum clinically important difference and psychometric validation: a prospective study. Chest 2020; 157: 824–833. doi: 10.1016/j.chest.2019.08.1916 [DOI] [PubMed] [Google Scholar]
- 16.Finch S, Laska IF, Abo-Leyah H, et al. Validation of the COPD Assessment Test (CAT) as an outcome measure in bronchiectasis. Chest 2020; 157: 815–823. doi: 10.1016/j.chest.2019.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudgeon EK, Crichton M, Chalmers JD. “The missing ingredient”: the patient perspective of health related quality of life in bronchiectasis: a qualitative study. BMC Pulm Med 2018; 18: 81. doi: 10.1186/s12890-018-0631-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Lee S, Lee JS, et al. Validity and reliability of CAT and Dyspnea-12 in bronchiectasis and tuberculous destroyed lung. Tuberc Respir Dis 2012; 72: 467–474. doi: 10.4046/trd.2012.72.6.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W, Gao YH, Xu G, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS One 2014; 9: e113373. doi: 10.1371/journal.pone.0113373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson C, Jones PW, O'Leary CJ, et al. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997; 156: 536–541. doi: 10.1164/ajrccm.156.2.9607083 [DOI] [PubMed] [Google Scholar]
- 21.Spinou A, Siegert RJ, Guan WJ, et al. The development and validation of the Bronchiectasis Health Questionnaire. Eur Respir J 2017; 49: 1601532. doi: 10.1183/13993003.01532-2016 [DOI] [PubMed] [Google Scholar]
- 22.Menéndez R, Méndez R, Polverino E, et al. Factors associated with hospitalization in bronchiectasis exacerbations: a one-year follow-up study. Respir Res 2017; 18: 176. doi: 10.1186/s12931-017-0659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsland I, Sobala R, De Soyza A, et al. Multimorbidity in bronchiectasis: a systematic scoping review. ERJ Open Res 2023; 9: 00296-2022. doi: 10.1183/23120541.00296-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju S, Jeong JH, Heo M, et al. Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis. Chron Respir Dis 2021; 18: 14799731211017548. doi: 10.1177/14799731211017548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch DA, Newell J, Hale V, et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. AJR Am J Roentgenol 1999; 173: 53–58. doi: 10.2214/ajr.173.1.10397099 [DOI] [PubMed] [Google Scholar]
- 27.Martínez-García MA, Perpiñá-Tordera M, Soler-Cataluña JJ, et al. Dissociation of lung function, dyspnea ratings and pulmonary extension in bronchiectasis. Respir Med 2007; 101: 2248–2253. doi: 10.1016/j.rmed.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 28.Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000; 55: 198–204. doi: 10.1136/thorax.55.3.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brill SE, Patel AR, Singh R, et al. Lung function, symptoms and inflammation during exacerbations of non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Res 2015; 16: 16. doi: 10.1186/s12931-015-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanza FC, Castro RAS, de Camargo AA, et al. COPD assessment test (CAT) is a valid and simple tool to measure the impact of bronchiectasis on affected patients. COPD 2018; 15: 512–519. doi: 10.1080/15412555.2018.1540034 [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Qu JM, Xu JF. Bronchiectasis management in China, what we can learn from European Respiratory Society guidelines. Chin Med J 2018; 131: 1891–1893. 10.4103/0366-6999.238134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtney JM, Kelly MG, Watt A, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chron Respir Dis 2008; 5: 161–168. doi: 10.1177/1479972308091823 [DOI] [PubMed] [Google Scholar]
- 33.Butterly EW, Hanlon P, Shah ASV, et al. Comorbidity and health-related quality of life in people with a chronic medical condition in randomised clinical trials: an individual participant data meta-analysis. PLoS Med 2023; 20: e1004154. doi: 10.1371/journal.pmed.1004154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin M, Dubois MF, Hudon C, et al. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes 2007; 5: 52. doi: 10.1186/1477-7525-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pati S, Swain S, Knottnerus JA, et al. Health related quality of life in multimorbidity: a primary-care based study from Odisha, India. Health Qual Life Outcomes 2019; 17: 116. doi: 10.1186/s12955-019-1180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Figure S1 00867-2023.SUPPLEMENT (29.8KB, png)
Figure S2 00867-2023.SUPPLEMENT2 (364.2KB, png)
Figure S3 00867-2023.SUPPLEMENT3 (94.9KB, png)