Abstract
The primary CD8+ T-cell response protected most B-cell-deficient μMT mice against intranasal infection with the HKx31 influenza A virus. Prior exposure did not prevent reinfection upon homologous challenge, and the recall CD8+ T-cell response cleared the virus from the lung within 7 days. Depleting the CD8+ T cells substantially reduced the capacity of these primed mice to deal with the infection, in spite of evidence for established CD4+ T-cell memory. Thus, the control of this relatively mild influenza virus by both primary and secondary CD4+ T-cell responses is relatively inefficient in the absence of B cells and CD8+ T cells.
Influenza virus infection of the murine respiratory tract can be controlled by CD4+ or CD8+ T-cell-mediated processes, although the available evidence indicates that the CD8+ set is more effective (2, 3, 5, 8, 27). Other viruses, such as lymphocytic choriomeningitis virus and the murine gammaherpesvirus 68, can be dealt with only by CD8+ effectors (4, 13, 21). Clearance by the virus-immune CD8+ population has generally been considered to require cognate interaction between cytotoxic T lymphocytes (CTL) and virus-infected target cells (11, 13, 16, 28). However, recent analysis indicates that contact-dependent, perforin-mediated CTL activity is not necessary for the CD8+ T-cell-mediated elimination of some viruses (7, 14). In addition, cytokines secreted (or induced) by CD8+ T cells are apparently sufficient to suppress a hepatitis virus transgene expressed in mouse liver (9, 10). As a consequence, other than for the lymphocytic choriomeningitis virus model (13), there is currently no consensus on the mode of action of virus-immune CD8+ effector T cells.
The same is true for the CD4+ subset. Virus-specific CD4+ CTL can be detected in CD8+ T-cell-deficient mice, although this population is not normally found in intact animals (12, 20). However, adoptive transfer experiments with bone marrow chimeras that express major histocompatibility complex (MHC) class II glycoproteins in the lymphoid compartment (but not on radiation-resistant lung cells) indicate that the immune CD4+ T cells and the virus-infected respiratory epithelium do not need to make direct contact (25). Protection of these chimeric mice might thus be mediated either via cytokines secreted locally (23) as a consequence of the CD4+ T effectors encountering MHC class II+, bone marrow-derived, antigen-presenting cells in the pneumonic lung (25) or by CD4+ T-helper (Th) cells for virus-specific immunoglobulin (Ig) production in the lymphoid tissue (19, 22). The present experiments addressed the issue by analyzing the efficacy of the virus-immune CD4+ T-cell response in Ig −/− μMT (15) mice depleted of CD8+ T cells prior to primary or secondary challenge with the HKx31 (H3N2) influenza A virus (1, 12). Previous experiments have shown that these Ig −/− mice develop a strong HKx31-specific CD4+ T-cell response and that Th precursor (Thp) cells persist in the long term (24).
Primary infection of CD8-depleted μMT mice.
The μMT mice (15) backcrossed to a C57BL/6 (B6) (H-2b) background were supplied from a breeding colony established at St. Jude Children’s Research Hospital. The B6 controls were purchased directly from Jackson Laboratory (Bar Harbor, Maine). Groups of 8- to 12-week-old female mice were anesthetized and infected intranasally (i.n.) with 240 hemagglutinating units (HAU) of the HKx31 influenza A virus (1). Some were depleted of CD8+ T cells by intraperitoneal treatment with monoclonal antibody (MAb) 2.43 commencing 3 days prior to virus challenge and again at 2- to 3-day intervals throughout the course of the experiments (12). Positive and negative controls were given an irrelevant rat Ig MAb instead of 2.43 or were additionally depleted with MAb GK1.5 to CD4 (1). Both the efficacy of the in vivo depletions and the appropriate dilutions of the MAbs were analyzed (26) in two-color mode on a FACScan with Cell Quest software (Becton Dickinson, Mountain View, Calif.) as follows. Single-cell suspensions of lymphocytes were blocked with 10% normal mouse serum and then stained with phycoerythrin (PE)-conjugated anti-CD4 (RM-4-5) or fluorescein isothiocyanate (FITC)-conjugated anti-CD8α (53-6.72). The activation statuses (6, 24) of the CD4+ T cells were assessed by determining the level of CD62L expression (with biotin Mel-14 and then streptavidin red 670). All of the flow cytometry reagents were purchased from Pharmingen (San Diego, Calif.). At the time of sampling, the mice were again anesthetized and bled from the axilla, and single-cell suspensions were made from pooled samples (three or more) or individual mediastinal lymph node (MLN), cervical lymph node (CLN), and spleen samples. Individual lungs were removed, frozen, thawed, homogenized, and clarified by gentle centrifugation prior to determining virus titers by allantoic inoculation into embryonated hen’s eggs (1).
Elimination of the CD8+ T cells greatly increased the susceptibility of the μMT (but not the B6) mice to primary infection with the HKx31 influenza A virus, with only two of eight surviving in a separate group that was left until day 21 after infection (Table 1). Many of the CD8-depleted μMT mice in three other experiments also died before they could be sampled at the later time points. All of those that were still alive at day 21 were moribund, although no virus was detected in three of seven lung homogenates. None of the μMT mice that were depleted of both T-cell subsets were able to terminate the infection, but both the rat Ig-treated and the CD8-depleted B6 mice all cleared the virus within 21 days. Any protection conferred by primary CD4+ T cells acting in the absence of antibody and the CD8+ subset was thus minimal.
TABLE 1.
Virus clearance from the lungs of MAb-depleted μMT and B6 mice
| Daya | No. of animals that were negative/no. sampled after the following treatmentsb:
|
|||||
|---|---|---|---|---|---|---|
| Rat Ig
|
Anti-CD8
|
Anti-CD4 + anti-CD8
|
||||
| μMT | B6 | μMT | B6 | μMT | B6 | |
| 4 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| 7 | 1/7 | 0/3 | 0/7 | 2/7 | ||
| 10 | 1/4 | 3/3 | 1/4 | 2/3 | ||
| 14 | 0/3 | 3/3 | 0/3 | 1/3 | ||
| 21 | 9/9 | 6/6 | 4/7 | 9/9 | 0/2 | 0/3 |
| Mortality | 1/4 | 0/5 | 6/8 | 0/5 | 1/3 | 2/5 |
Days after i.n. infection with 240 HAU of the HKx31 influenza A virus. The presence of virus in homogenized lung samples from individual mice was determined by measuring hemagglutination subsequent to allantoic inoculation into embryonated hen eggs (1). Mortality data are from a separate set of experimental animals that was not sampled prior to day 21.
Virus clearance during the secondary response.
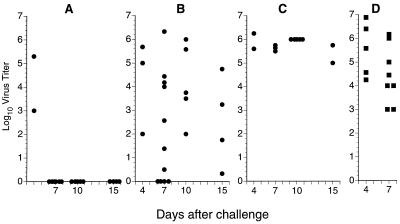
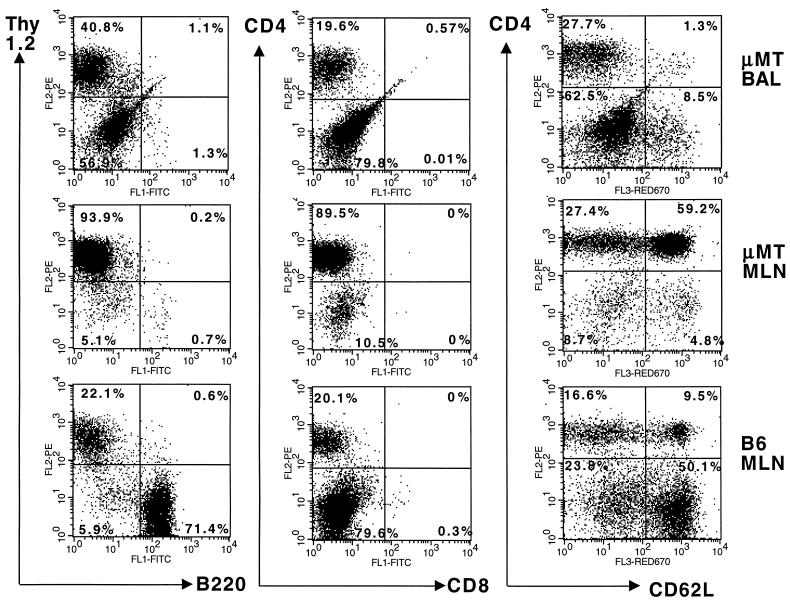
The extreme susceptibility of these CD8-depleted μMT mice to primary HKx31 infection caused us to turn instead to the secondary response. Intact μMT mice were infected i.n. with the HKx31 virus, rested for at least 2 months, and then challenged i.n. with the same dose of virus following the administration of a control rat Ig (Fig. 1A) or MAb treatment (1, 12) to eliminate the CD8+ (Fig. 1B) or CD4+ and CD8+ T cells (Fig. 1C). Naive B6 mice were also depleted of both T-cell populations (Fig. 1D) and challenged at the same time, together with HKx31-primed, CD8-depleted B6 mice. Flow cytometry profiles illustrating both the lack of B220+ B cells in the μMT mice and the efficacy of CD8+ T-cell depletion in the MLN and bronchoalveolar lavage (BAL) populations (1) are shown in the first two panels of Fig. 2.
FIG. 1.
Virus clearance profiles following secondary i.n. challenge with 240 HAU of the HKx31 influenza A virus. The μMT mice were all infected i.n. with the HKx31 virus at 6 weeks of age and then challenged i.n. by the same regimen 60, 180, or 270 days later (see Table 2, experiments 2 to 4). There was no obvious difference between the efficacy of priming for the three groups. The lungs were removed after a further 4 or 7 days and homogenized, and virus titers were determined as log10 50% egg infective doses following endpoint titration in the allantoic cavity of embryonated hen’s eggs (23). The panels present the virus titration results for rat Ig-treated μMT mice (A), CD8-depleted μMT mice (B), and CD4 and CD8 doubly depleted μMT mice (C). As a positive control for virus infection, previously uninfected B6 mice were depleted of both CD4+ and CD8+ T cells (D). Each symbol represents one animal. Two of the four doubly depleted, primed μMT mice depicted in panel C that were to have been assayed at day 15 after infection died before they could be sampled.
FIG. 2.
Flow cytometric analysis of CD8+ T-cell-depleted μMT and B6 mice at 7 days after i.n. challenge with the HKx31 influenza A virus. Mice that had recovered from an identical influenza virus infection 2 months previously were treated with the MAb 2.43 against CD8, given at 2- to 3-day intervals commencing 3 days prior to infection. Pooled BAL samples and single-cell MLN suspensions were obtained from three mice and stained with conjugated MAbs against Thy1.2 (PE–53-2.1) and B220 (FITC–RA3-6B2), CD4 (PE–RM-4-5) and CD8 (FITC–53-6.72), or CD4 and CD62L (biotin–MEL-14 and then streptavidin red 670) prior to two-color flow cytometric analysis in a FACScan. The percentages of stained lymphocytes in the respective quadrants are given. Estimates of virus-specific CD4+ T-cell numbers in the MLN samples from these mice are presented in Table 2 (experiment 4).
The virus titration results for day 4 (Fig. 1A, B, and C) show that the level of infection established in the antibody-negative, immune μMT mice was little different from that found following primary exposure (Fig. 1D). However, while 6 of 6 intact μMT mice cleared the HKx31 virus within a week (Fig. 1A), this was not the case for 16 of 20 CD8-depleted μMT mice assayed between days 7 and 15 after infection (Fig. 1B). Even so, the virus titers in these secondarily challenged μMT mice depleted of the CD8+ effector population (Fig. 1B and 2) were much more variable than those in primed μMT mice and naive B6 mice lacking both T-cell subsets (Fig. 1C and D). The highly activated (6, 24) CD4+ CD62Llow population that could be shown by BAL in the respiratory tracts of the CD8-depleted μMT mice (Fig. 2) may exert some variable measure of control (Fig. 1B).
Assaying the CD4+ T-cell response.
Virus-specific CD4+ Thp cell frequencies were measured by LDA, as described in detail previously (6, 24). Briefly, the CD4+ T cells were enriched by first incubating spleen and lymph node populations with MAbs against MHC class II (TIB120) and CD8 (53-6.72), followed by exposure to sheep anti-mouse or anti-rat Ig-coated Dynabeads (Dynal, Oslo, Norway) to deplete the positive cells with a magnet. Flow cytometric analysis established that 85 to 95% of the remaining cells routinely stained with the PE-conjugated RM-4-5 MAb against CD4 (Pharmingen). These enriched CD4+ T cells were cultured under LDA conditions for 96 h with virus-infected or uninfected antigen-presenting cells (APCs). The APCs were prepared from irradiated B6 spleen cells subsequent to the removal of all T cells by complement lysis following incubation with the MAb AT83 against Thy1.2. Microcultures were considered positive when the level of stimulation for the indicator CTLL line (by [3H]thymidine incorporation) was more than three times the standard deviation of the mean for CTLL cells maintained in medium alone.
The absence of B cells and associated germinal centers resulted in a 10-fold decrease in the size of the μMT spleen (24), which in turn reduced the total virus-specific Thp cell numbers compared with those of the B6 controls (Table 2, experiments 1 and 4). This effect has been described previously and is associated with a tendency for HKx31-specific CD4+ memory T cells to localize to the μMT regional lymph nodes rather than (as is normally the case) to the spleen (24). Depleting the CD8+ subset (Fig. 1) did not obviously modify either the primary (Table 2, experiment 1) or the secondary (experiments 2 and 3) CD4+ T-cell response. Furthermore, there was no evidence for any transient exhaustion (18) of the virus-specific Thp set comparable to that observed previously for the diminished CD8+ CTL precursor cell population in CD4+ T-cell-deficient, MHC class II −/− mice (17). Even when the CD8+ subset is absent, the CD4+ T cells in the lymph nodes are not consumed (17, 18) in an attempt to provide an alternative effector population. Thus, the failure of the CD4+ T-cell effector mechanism(s) to deal reproducibly with influenza virus in the CD8-depleted μMT mice (Table 1; Fig. 1B) was not due to a defect in total Thp cell numbers (Table 2) or to any lack of activation of the CD4+ set that had localized to the lung (Fig. 2).
TABLE 2.
Quantitation of the CD4+ T-cell response
| Expt no. and mouse strain | Days after virus challengea
|
CD8 depletionc | No. of virus-specific Thp cellsb
|
||||
|---|---|---|---|---|---|---|---|
| Primary | Secondary | CLN | MLN | Spleen | Total | ||
| 1 | |||||||
| μMT | 7 | + | ND | 3,018 | 2,571 | 5,589 | |
| − | ND | 1,484 | 2,813 | 4,297 | |||
| 10 | + | ND | 7,106 | 14,336 | 21,442 | ||
| − | ND | 1,778 | 4,507 | 6,285 | |||
| B6 | 7 | + | ND | 632 | 22,308 | 22,940 | |
| 10 | + | ND | 1,026 | 46,683 | 47,709 | ||
| 2 | |||||||
| μMT | 180 | − | 336 | 6,161 | 8,486 | 14,983 | |
| 180 | 3 | − | 5,967 | 2,022 | 6,218 | 14,207 | |
| 180 | 4 | − | 4,563 | 2,620 | 4,501 | 11,684 | |
| 3 | |||||||
| μMT | 270 | − | 4,294 | 7,119 | 1,303 | 12,716 | |
| 270 | 4 | − | 841 | 2,197 | 2,510 | 5,548 | |
| 270 | 4 | + | 778 | 214 | 1,762 | 2,754 | |
| 270 | 7 | − | 1,741 | 1,580 | 12,296 | 15,617 | |
| 270 | 7 | + | 3,334 | 1,828 | 1,617 | 6,779 | |
| 4 | |||||||
| μMT | 60 | 7 | + | 9,376 | 8,985 | 12,748 | 31,109 |
| B6d | 60 | 7 | + | 7,154 | 297 | 118,406 | 125,857 |
All mice were infected i.n. with 240 HAU of the HKx31 influenza virus. This was repeated for the secondary challenge.
The numbers of CD4+ T cells responding to uninfected and influenza virus-infected APCs were calculated by dividing the CD4+ T-cell counts for each organ by the respective frequencies determined by LDA (6, 24). The values for uninfected APCs were subtracted from the values for influenza virus-infected APC. ND, not done.
Prior to and during primary (experiment 1) or secondary (experiments 2, 3, and 4) challenge with HKx31, the mice received intraperitoneal injections of MAb 2.43 against CD8 or a rat Ig control 3 days prior to infection, at the time of infection, and every 2 to 3 days thereafter until the conclusion of the experiment (1).
The present analysis establishes that the capacity of clonally expanded CD4+ T cells (Table 2) to deal with an influenza A virus in the absence of CD8+ T cells and Ig-secreting B cells is both limited and variable (Table 1; Fig. 1B). This may reflect the fact that the various components of the immune system generally work in concert to terminate respiratory virus infections (17, 19, 22). Activated CD8+ T cells are normally the predominant lymphocytes in the virus-infected lung (1, 26). The major role of the CD4+ subset in the murine influenza virus model may be to provide the lymphokines that promote both Ig production (19, 22) and the proliferation of the virus-specific CD8+ effectors (17, 23, 27).
Acknowledgments
We thank Kristen Branum for technical assistance and Vicki Henderson for help with the manuscript.
These experiments were supported by NIH grants AI 29579, AI 07372, and CA 21765 and by the American Lebanese Syrian Associated Charities.
REFERENCES
- 1.Allan W, Tabi Z, Cleary A, Doherty P C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 2.Askonas B A, Taylor P M, Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- 3.Bender B S, Croghan T, Zhang L, Small P A., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty P C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewing C, Topham D J, Doherty P C. Prevalence and activation phenotype of Sendai virus-specific CD4+ T cells. Virology. 1995;210:179–185. doi: 10.1006/viro.1995.1329. [DOI] [PubMed] [Google Scholar]
- 7.Franco M A, Tin C, Rott L S, Vancott J L, McGhee J R, Greenberg H B. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, Fas, and gamma interferon. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham M B, Braciale V L, Braciale T J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidotti L G, Ando K, Hobbs M V, Ishikawa T, Runkel L, Schreiber R D, Chisari F V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti L G, Borrow P, Hobbs M V, Matzke B, Gresser I, Oldstone M B A, Chisari F V. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou S, Doherty P C. Clearance of Sendai virus by CD8+ T cells requires direct targeting to virus-infected epithelium. Eur J Immunol. 1995;25:111–116. doi: 10.1002/eji.1830250120. [DOI] [PubMed] [Google Scholar]
- 12.Hou S, Doherty P C, Zijlstra M, Jaenisch R, Katz J M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 13.Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 14.Kägi D, Seiler P, Pavlovic J, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 16.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo X Y, Tripp R A, Sangster M Y, Doherty P C. The cytotoxic T-lymphocyte response to Sendai virus is unimpaired in the absence of gamma interferon. J Virol. 1997;71:1906–1910. doi: 10.1128/jvi.71.3.1906-1910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 19.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–4355. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller D, Koller B H, Whitton J L, LaPan K E, Brigman K K, Frelinger J A. LCMV-specific, class II-restricted cytotoxic T cells in β2-microglobulin-deficient mice. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 21.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 22.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarawar S R, Carding S R, Allan W, McMickle A, Fujihashi K, Kiyono H, McGhee J R, Doherty P C. Cytokine profiles of bronchoalveolar lavage cells from mice with influenza pneumonia: consequences of CD4+ and CD8+ T cell depletion. Reg Immunol. 1993;5:142–150. [PubMed] [Google Scholar]
- 24.Topham D J, Tripp R A, Hamilton-Easton A M, Sarawar S R, Doherty P C. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 25.Topham D J, Tripp R A, Sarawar S R, Sangster M Y, Doherty P C. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II −/− respiratory epithelium. J Virol. 1996;70:1288–1291. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 27.Tripp R A, Sarawar S R, Doherty P C. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2 IAb gene. J Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- 28.Zinkernagel R M, Doherty P C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]