Summary
Background
We sought to identify resistance patterns and key drivers of recent multidrug-resistant tuberculosis (MDR-TB) transmission in a TB-prevalent area in Peru.
Methods
Cross-sectional study including MDR Mycobacterium tuberculosis complex (Mtbc) strains identified in Callao-Peru between April 2017 and February 2019. Mtbc DNA was extracted for whole genome sequencing which was used for phylogenetic inference, clustering, and resistance mutation analyses. Clusters indicative of recent transmission were defined based on a strain-to-strain distance of ≤5 (D5) single nucleotide polymorphisms (SNPs). Epidemiologic factors linked to MDR-TB clustering were analyzed using Poisson regression.
Findings
171 unique MDR-Mtbc strains were included; 22 (13%) had additional fluoroquinolone resistance and were classified as pre-XDR. Six strains (3.5%) harboured bedaquiline (BDQ) resistance mutations and were classified as MDR + BDQ. 158 (92%) Mtbc strains belonged to lineage 4 and 13 (8%) to lineage 2. Using a cluster threshold of ≤5 SNPs, 98 (57%) strains were grouped in one of the 17 D5 clusters indicative of recent transmission, ranging in size from 2 to the largest cluster formed by 53 4.3.3 strains (group_1). Lineage 4.3.3 strains showed the overall highest cluster rate (43%). In multivariate analyses, current or previous imprisonment was independently associated with being part of any MDR-TB transmission clusters (adjusted prevalence ratio [aPR], 1.45; 95% CI, 1.09–1.92).
Interpretation
Pre-XDR-TB emerged in more than 10% of the MDR-TB strains investigated. Transmission of 4.3.3 Mtbc strains especially of the dominant group_1 clone is a major driver of the MDR-TB epidemic in Callao. Current or previous imprisonment was linked to recent MDR-TB transmissions, indicating an important role of prisons in driving the MDR-TB epidemic.
Funding
This work was supported in part by the ERANet-LAC Network of the European Union, Latin America and the Caribbean Countries on Joint Innovation and Research Activities, and FONDECYT. Additional support was received from Leibniz Science Campus Evolutionary Medicine of the Lung, the Deutsche Forschungsgemeinschaft (German Research Foundation, under Germany’s Excellence Strategy—EXC 2167 Precision Medicine in Inflammation), and the Research Training Group 2501 TransEvo.
Keywords: Tuberculosis, Multidrug resistance, Prison, Genome sequencing
Research in context.
Evidence before this study
Tuberculosis (TB) remains a top infectious disease killer worldwide. Globally, about half million new multidrug-resistant (MDR)-TB infections annually result in substantial morbidity and mortality. Nevertheless, our understanding of key drivers of MDR-TB epidemics and secondary transmissions is incomplete, thus limiting our ability to intervene in this global health problem. In the Americas, Peru is especially affected by MDR-TB and is listed among the 30 countries with highest levels for MDR-TB globally. Understanding key drivers of recent MDR-TB transmissions is critical in our region, including the role of prisons. We searched Pubmed, Medline using the search strategy “tuberculosis” and “resistant” and “prison” through September 2023. Prior research in the Americas, particularly coming from Brazil, demonstrated that TB transmissions are common in prisons, and that prison TB strains are interrelated with the wider community. However, few studies have focused on MDR-TB and used whole genome sequencing (WGS).
Added value of this study
We utilized WGS to identify molecular resistance patterns and key drivers of recent MDR-TB transmission in Callao, a TB-prevalent area in Peru. WGS offers the best resolution to identify transmission clusters and ongoing transmission. Our genotypic resistance profile analysis revealed high rates of co-resistance to other first and second line anti-TB drugs. Importantly, we identified a clonal spread of 4.3.3 Latin American-Mediterranean (LAM) MDR-TB strain in our study population. Current or previous imprisonment was linked to recent MDR-TB transmissions, indicating an important role of prisons in driving the MDR-TB epidemic.
Implications of all the available evidence
MDR-TB prevention and control interventions in persons deprived of liberty are urgently needed, not only during incarceration time but also once individuals return to their communities. Further, transmission dynamics and resistance evolution need to be closely monitored in our region ideally with routine WGS surveillance.
Introduction
Tuberculosis (TB), caused by pathogens of the Mycobacterium tuberculosis complex (Mtbc), remains a top infectious disease killer worldwide, accounting for more than 1.5 million deaths each year.1 Globally, approximately 4% of newly developed TB and 19% of previously treated people with TB are rifampicin (RIF)-resistant or multidrug-resistant (resistance to at least RIF and isoniazid [INH]) (RR/MDR-TB), leading to about a half million new MDR-TB infections worldwide annually linked with high treatment failure rates, and increased mortality.1, 2, 3, 4 Nevertheless, our understanding of key drivers of MDR-TB epidemics and secondary transmissions is incomplete, thus limiting our ability to intervene in this global health problem.
The emergence of MDR-TB was traditionally attributed to poor treatment compliance and programmatic failure. However, studies have demonstrated that transmission of resistant Mtbc strains would be the main driver of the MDR-TB epidemic, and particular MDR Mtbc strains can spread for centuries even across borders.5, 6, 7 Prisons are high-congregate settings that often have conditions that favour Mtbc transmission, including overcrowding, poor ventilation, poor sanitation, and a high prevalence of untreated/undiagnosed TB infection and TB disease among inmates.8,9 Incarcerated individuals, correctional facility workers, and people living in areas around prisons are particularly vulnerable to MDR-TB.10, 11, 12 However, the overall contribution of MDR-TB in prisons into the general MDR-TB epidemiology in TB-prevalent areas has not been completely defined. Understanding the influence of prisons and other factors on recent MDR-TB transmissions would be informative to TB control interventions.
Peru is especially affected by MDR-TB in the Americas and is listed among the 30 countries with the highest levels of MDR-TB globally.13 Indeed, Peru had a TB incidence of ∼116 new TB diagnoses per 100,000 inhabitants in 2020, and it is estimated that 6.3% of new TB diagnoses in Peru are MDR-TB.14,15 Callao is one of the cities with the highest rates of MDR-TB in Peru.16 In this study, we utilized whole genome sequencing (WGS), epidemiologic and spatial data to identify drivers of recent MDR-TB transmission and resistance patterns in this region.
Methods
Study design and epidemiologic characteristics
We conducted a cross-sectional study including clinical specimens identified as MDR-TB strains by the Tuberculosis Control Program Laboratory of the Regional Directorate of Health of Callao Region (DIRESA-Callao) in Peru between April 29th 2017 and February 26th 2019. MDR-TB strains were identified based on National TB Program drug susceptibility testing standards.17 Briefly, first-line drug susceptibility testing at the DIRESA-Callao TB laboratory was performed using Microscopic Observation Drug Susceptibility (MODS).18 Second-line drug susceptibility testing (MGIT 960 and Genotype™) was assessed at the National Reference Laboratory of Mycobacteria in Lima, Peru. For this project, MODS liquid cultures of MDR-TB strains were sub-cultured for DNA extraction and quality tested at the Molecular Epidemiology and Genetics Laboratory, Centro de Investigaciones Tecnológicas, Biomedicas y Medioambientales (CITBM) in Callao, Peru. TB is a mandatory-notified diagnosis in Peru. Available demographic and clinical characteristics of MDR-TB cases were extracted from the medical records of the DIRESA-Callao Tuberculosis Control Program Laboratory and TB Program Information System (SIGTB). Data included age, sex, HIV, diabetes mellitus, history of TB contact, prior history of TB, and history of current or prior imprisonment. Data completeness for key variables ranged from 98% to 100% and therefore no imputation of missing values was performed. History of HIV and diabetes mellitus were defined based on routine screening done by the TB Program.
DNA sequencing
WGS was performed at the Research Center Borstel in Germany on the Illumina NextSeq 500 using a modified Nextera workflow.19 WGS data were analyzed using the MTBseq pipeline as described previously.20 The sequencing data have been made public on the European Nucleotide Archive (ENA) and the samples individual accession numbers are listed in Supplementary Table S1.
Mtbc lineage and resistance characterization
Phylogenetic Mtbc lineages and sub-lineages were inferred from signature single nucleotide polymorphisms (SNPs).21,22 For genome-based resistance, prediction polymorphisms in 27 drug resistance associated genes that are involved in drug resistance mechanisms were determined from the WGS data using an updated version (v2022-01-26) of a previously published interpretation catalogue.23 Furthermore, yet uncharacterized INDELS and stop codons in the following genes were also considered as resistance determinants: (a) ethA (ethionamide/prothionamide); (b) pncA (pyrazinamide); (c) rpoB (rifampicin, rifabutin); (d) Rv0678c (bedaquiline, clofazimine); (e) ald (cycloserine); (f) katG (isoniazid); (g) gid (streptomycin); and (h) fbiC and ddn (delamanid). Mutations resulting in a STOP codon were indicated by an underscore ‘_’ sign. For resistance detection variants were called by lowering the default variant detection parameters to read coverage of a minimum of two for each forward and reverse orientation, four reads of a Phred score of at least 20 and 5% allele frequency, to increase the detection limit for not fixed SNP positions. Strains were characterized into resistance categories per World Health Organization (WHO) classifications: MDR (resistance to at least isoniazid [INH] and rifampicin [RIF]); pre extremely drug-resistant (pre-XDR, MDR plus resistance to fluoroquinolones [FQ]).1 MDR Mtbc strains with bedaquiline (BDQ) resistance mutations were classified as MDR + BDQ.
Phylogenetic inference
Phylogenetic trees were calculated based on concatenated SNP alignments. Maximum likelihood (ML) phylogenetic inference was calculated from the concatenated SNP alignment only using polymorphic sites by IQ-TREE2 v1.6.824 with automatic model selection by ModelFinder Plus25 based on the Akaike information criterion (AIC), corrected Akaike information criterion (AAICs), and the Bayesian information criterion (BIC) including ascertainment bias correction (ASC). Branch support was assessed via Ultrafast bootstrap approximation (UFBoot) with 1000 replicates.26 ML trees were midpoint rooted in FigTree 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). Minimum spanning trees were created from the concatenated SNP alignment with GrapeTree 1.5 and the MSTree V2 algorithm.27
Identification of transmission clusters
WGS offers the best resolution to identify transmission clusters and ongoing transmission. The cluster analyses were based on an isolate-to-isolate distance of ≤12 SNPs (D12) and ≤5 SNPs (D5).28,29 With D12 long-term transmission chains can be followed, whereas D5 offers the view on recent and ongoing transmission. Thus, in our study, we defined recent MDR-TB transmission when strains had a distance of ≤5 SNP between each other (D5 clusters). All strains which have at least one other isolate within the distance threshold were assigned to a cluster. A transmission index per isolate was calculated as previously defined.30 Briefly, we determined for every isolate the number of isolates that were in a range of 10 SNPs or less, which is the extend of a putative transmission network. This 10 SNP-threshold was used to infer the number of recently linked cases, as considered within a 10-year time period, based on previous convergent estimates of Mtbc genome evolution rate of ≈0.5 SNPs/genome/year in inter-human transmission chains.31
Data visualization analysis of the distribution of the estimated percentage of strains included in D12 and D5 clusters over all samples sequenced were generated with continuous surface maps of the prevalence distribution of each cluster. These maps were generated using a standard spatial Gaussian kernel interpolation method. Maps were created with ArcGIS Pro version 3.1.
Identification of compensatory mutations
We evaluated rpoA, rpoB, and rpoC genes for the presence of non-synonymous mutations. Detected SNPs were considered as putative compensating, after excluding SNPs in the rifampicin-resistance determining region, other known rifampicin resistance mutations and phylogenetic mutations.
Statistical analyses of the relationship between epidemiologic factors and Mtbc transmission
Project data were analyzed in Stata software (v13.0; StataCorp) and R software. Categorical data were summarized using percentages. Continuous data are presented as median and interquartile range (IQR). We used chi-square and Mann–Whitney tests for bivariate analyses of categorical and continuous variables, respectively. We used Poisson regressions (log link function; with robust estimator of variance) to assess potential factors associated with recent Mtbc transmission (D5 clusters), including age, sex, diabetes mellitus, HIV infection, history of prior TB, TB contact, drug use, and history of current or prior incarceration. Two primary regression models were assessed: in the first model, the outcome variable was defined as being part of any of the MDR-TB transmission clusters (any D5 clusters), whereas in the second model the outcome variable was defined as being part of the dominant D5 cluster. Poisson regression results were reported as prevalence ratios (PR) accompanied by their 95% confidence interval (CI). Spatial data visualization was conducted using spatial interpolation methods to generate continuous surface maps of the distribution of D12 and D5 clusters in the study area. Spatial interpolations were generated using Kernel interpolation methods. Maps were generated using the ArcGIS Pro software.32
Ethics approval
The study protocol was approved by the Ethics Committees of DIRESA-Callao and the Institute of Tropical Medicine Daniel A. Carrion at Universidad Nacional Mayor de San Marcos in Peru (CIEI-2017-014). Individual patient consent was not required for retrospective access to data. The study was registered in the PRISA national research project registry (#EI00000003098).
Role of the funding source
The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Results
Between April 29th 2017 and February 26th 2019, an estimated 2180 cases of TB were reported in the study region, which included 197 MDR-TB case notifications. Of those, 188 were MDR-TB cases with positive culture susceptibility testing. Through our study, we accessed a total of 183 samples. After excluding 8 duplicate or non-Mtbc samples, 175 strains were analyzed for their individual genotypic resistance patterns, phylogenetic lineages, and clustering. Of the 175 Mtbc strains investigated, 171 strains were confirmed as MDR-TB by WGS and used for all downstream analyses (90.9% coverage of culture-positive MDR-TB cases reported in the study region; Supplementary Figure S1). Each strain corresponded to one unique patient.
Epidemiologic characteristics linked to MDR-TB strains
Table 1 shows the epidemiologic characteristics linked to the 171 MDR-TB strains. Median age of the patients was 31 years (IQR, 25–44). Of 171 MDR-TB strains, 114 (66.7%) came from male patients. There were 42 (24.6%) MDR-TB strains from patients who reported a prior history of TB. The rates of diabetes mellitus and HIV co-infection were low in our study population, 8.8% and 5.9%, respectively. Twenty-nine (17%) of strains belonged to individuals with a history of incarceration. Of those, 19 (65.5%) were currently in prison and 10 (34.5%) were previously incarcerated.
Table 1.
Epidemiologic characteristics linked to 171 MDR-TB strains from Callao, Peru.
| Characteristic | Study population |
|---|---|
| Age, median (interquartile range) | 31 (25–44) |
| Age ≥65 years, n (%) | 16 (9.4%) |
| Male sex, n (%) | 114 (66.7%) |
| Diabetes mellitus, n (%) | 15 (8.8%) |
| HIV infection, n (%) | 10 (5.9%) |
| Drug use, n (%) | 39 (22.8%) |
| History of TB contact, n (%) | 22 (12.9%) |
| History of imprisonment, n (%) | 29 (17%) |
| History of prior TB, n (%) | 42 (24.6%) |
Drug-resistance patterns and Mtbc lineage of circulating MDR-TB strains
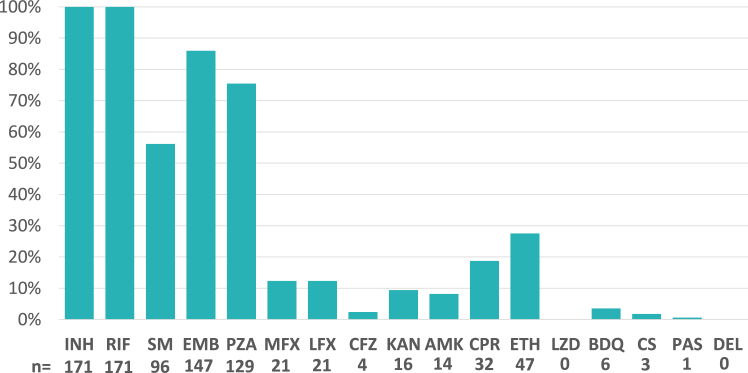
Of the 171 strains confirmed as MDR-TB, 129 (75%) harboured additional resistance mutations against pyrazinamide (PZA) and 147 (86%) against ethambutol (EMB) (Fig. 1, Supplementary Table S1). 22 (13%) also showed markers for fluoroquinolone (FQ) resistance and were classified pre-XDR-TB (Fig. 1, Supplementary Table S1). Interestingly, another six (3.5%) MDR strains had mutations conferring resistance against bedaquiline (BDQ), based on two different mutations in Rv0678 (192_ins_g and N98D), despite BDQ not being available for MDR-TB treatment in this region at the time of the study. Both mutations have been reported before.33,34 Of these six patients only one was treated before for MDR-TB. Five of these patients received clofazimine (CFZ) during their current treatment. Transmitted FQ resistance could be detected for the two clusters group_4 and group_8 where all strains harbored the same resistance conferring mutations. BDQ resistance transmission was found for the two samples of group_13 (Supplementary Figure S3).
Fig. 1.
Percentage of strains from Callao/Peru resistant to a certain antibiotic. INH = isoniazid, RIF = rifampicin, SM = streptomycin, EMB = ethambutol, PZA = pyrazinamide, MFX = moxifloxacin, LFX = levofloxacin, KAN = kanamycin, AMK = amikacin, CPR = capreomycin, ETH = ethionamide, LZD = linezolid, BDQ = bedaquiline, CS = cycloserine, PAS = para-aminosalicylic acid, DEL = delamanid.
Overall, 158 (92%) strains were assigned to lineage 4 (L4), and 13 (8%) strains to lineage 2 (L2). Out of L4, the most prevalent sub-lineage found was lineage 4.3.3 LAM (n = 99, 58%), followed by 4.3.4.2 LAM (n = 18, 11%) and 4.1.2.1 Haarlem (n = 12, 7%) (Table 2). A detailed look at the resistance profiles of strains of the dominant sub-lineage 4.3.3 LAM (n = 99) showed that a high proportion were PZA (91/99; 92%), and EMB (83/99; 84%) resistant. Resistance to ethionamide (ETH) was found in 28% (28/99), and to FQ in 11% (11/99), respectively.
Table 2.
Genome-based DR classification stratified by lineage for the 171 MDR-TB strains from Callao, Peru.
| Total | MDR | MDR + BDQ | preXDR | |
|---|---|---|---|---|
| Dataseta | 171 (100%) | 143 (84%) | 6 (4%) | 22 (13%) |
| Lineage2 | 13 (8%) | 8 (6%) | 0 (0%) | 5 (23%) |
| Lineage4 | 158 (92%) | 135 (94%) | 6 (100%) | 17 (77%) |
| Sub-lineages | ||||
| 2.2.1 Beijing | 6 (4%) | 3 (2%) | 0 (0%) | 3 (14%) |
| 2.2.1 Beijing Asian/Africa 2 | 7 (4%) | 5 (3%) | 0 (0%) | 2 (9%) |
| 4 Euro-American | 4 (2%) | 0 (0%) | 4 (67%) | 0 (0%) |
| 4.3.3 LAM | 99 (58%) | 87 (61%) | 1 (17%) | 11 (50%) |
| 4.3.4.2 LAM | 18 (11%) | 17 (12%) | 0 (0%) | 1 (5%) |
| 4.1.2.1 Haarlem | 12 (7%) | 9 (6%) | 1 (17%) | 2 (9%) |
| 4.3.4.1 LAM | 9 (5%) | 9 (6%) | 0 (0%) | 0 (0%) |
| 4.1.1.3 X-type | 6 (4%) | 5 (3%) | 0 (0%) | 1 (5%) |
| 4.3.2 LAM | 4 (2%) | 4 (3%) | 0 (0%) | 0 (0%) |
| 4.1.1 X-type | 3 (2%) | 1 (1%) | 0 (0%) | 2 (9%) |
| 4.8 mainly T | 3 (2%) | 3 (2%) | 0 (0%) | 0 (0%) |
Denominator is “Total” for the row “Dataset”.
Cluster analysis
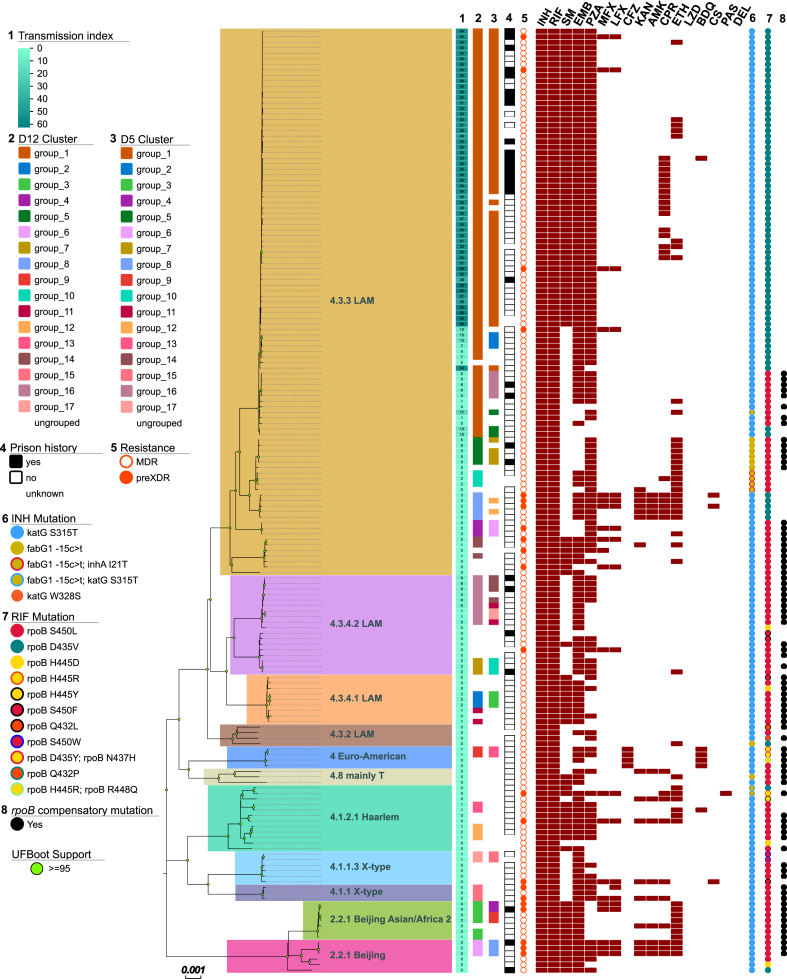
130 (74%) strains were grouped in one of the 17 D12 clusters identified in the study population (Fig. 2). When a stricter threshold of 5 SNPs was used, 98 (56%) strains were grouped in one of the 17 D5 clusters indicative of recent transmission (Fig. 2), ranging in size from 2 to 53 strains (median = 3, average = 5.8, Fig. 2, Supplementary Table S1). Of the 99 strains of the dominant sub-lineage 4.3.3 LAM, 92 (93%) were clustered with D12 and 73 (74%) with D5, indicating a high rate of recent MDR-TB transmission of these strains. The largest detected cluster (D12 group_1), containing 73 (43%) strains, could also be attributed to the sub-lineage 4.3.3 LAM. On D5 level, 53 (31%) strains remained grouped in the largest cluster group_1, which in addition showed much higher transmission indexes compared to the not included strains, indicating a very dense transmission network (Fig. 2). A more detailed analysis considering resistance mutations showed that all strains of the D12 group_1 harboured the katG S315T mutation, however, MDR-TB had evolved at least two times as strains harboured either the rpoB S450L or D435V mutation, respectively. When considering the stricter cluster threshold, all strains of the D5 group_1 harboured rpoB D435V and additional resistances to SM (gidB P84L, 52/53), EMB (embB Y319S, 52/53) and PZA (pncA Q10R, 52/53). Noteworthy the second largest cluster (D12 group_16) only contained 9 (5%) strains. We observed a lower frequency of compensatory mutations in D12 clustered compared to non-clustered strains (39.2 vs. 65.9%; P = 0.003). Similarly, there was a lower frequency of compensatory mutations in strains in D5 clusters compared to strains that did not belong to a cluster (33.7% vs. 61.6%; P < 0.001). This effect was mainly driven by the fact that the strains of the largest cluster group_1 do not contain any known compensatory mutation, e.g., in rpoA, rpoB, or rpoC (Supplementary Table S1).7,30,35
Fig. 2.
Maximum Likelihood phylogeny of 171 MDR TB strains from Callao/Peru. Tracks from left to right: 1: Transmission index, 2: D12 cluster, 3: D5 cluster, 4: prison history, 5: resistance status, resistance to antibiotics, 6: resistance markers for isoniazid and 7: rifampicin, 8: rpoB compensatory mutations.
We were further interested in the relationship of the dominant sub-lineage 4.3.3 MDR strains to a previously published intercontinental transmission chain originating from the Lima region.36 The three strains from the cross-border transmission study (two from Lima, 1 from Madrid) were found to cluster with the five strains of D12 group_8, establishing a direct link to actively transmitted TB in our study population (Supplementary Figure S4).
Factors associated with recent Mtbc transmission
To better understand the factors associated with recent Mtbc transmission, we investigated the relationship between epidemiological characteristics of individuals with MDR-TB and recent transmission clustering (D5 clusters, Table 3). Of note, 26 (27%) of individuals with MDR-TB belonging to D5 transmission clusters had a current or prior history of imprisonment. In unadjusted analyses, we found that male sex (prevalence ratio [PR], 1.53, 95% confidence interval [CI], 1.07–2.17), history of drug use (PR, 1.64; 95% CI, 1.31–2.06), and current or prior history of imprisonment (PR, 1.77; 95% CI, 1.44–2.17) were associated with being in a recent transmission cluster (Table 3). In multivariate Poisson regression modelling, history of imprisonment (current or prior) remained independently associated with being in a recent transmission cluster (adjusted PR [aPR], 1.45; 95% CI, 1.09–1.92). History of imprisonment was also independently associated with being in the dominant group_1 transmission cluster (aPR, 2.33; 95% CI, 1.21–4.48; Supplementary Table S2).
Table 3.
Results of Poisson regression of factors associated with being in any clusters of recent MDR-TB transmission (any D5 clusters).
| Characteristic | Unadjusted PRa | P value | Adjusted PRa | P value |
|---|---|---|---|---|
| Age ≥65 years | 1.1 (0.73–1.65) | 0.642 | 1.32 (0.75–2.33) | 0.343 |
| Male sex | 1.53 (1.07–2.17) | 0.020 | 1.25 (0.84–1.85) | 0.270 |
| Diabetes mellitus | 0.92 (0.56–1.51) | 0.755 | 0.94 (0.56–1.58) | 0.826 |
| HIV infection | 1.24 (0.81–1.9) | 0.329 | 1.3 (0.86–1.98) | 0.216 |
| Drug use | 1.64 (1.31–2.06) | <0.001 | 1.27 (0.95–1.69) | 0.103 |
| History of TB contact | 0.95 (0.63–1.41) | 0.785 | 0.99 (0.67–1.46) | 0.963 |
| History of imprisonment | 1.77 (1.44–2.17) | <0.001 | 1.45 (1.09–1.92) | 0.010 |
| History of prior TB | 1.17 (0.89–1.54) | 0.268 | 1.01 (0.75–1.34) | 0.980 |
Prevalence ratios presented with 95% confidence intervals in parentheses. The adjusted model included the following variables: age ≥65 years, sex, diabetes mellitus, HIV infection, drug use, history of TB contact, history of current or prior imprisonment, history of prior TB.
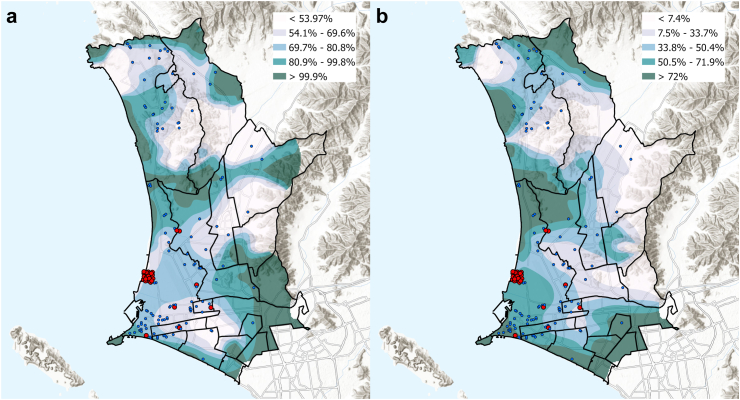
We then analyzed the geospatial distribution of MDR-TB strains in Lima-Callao belonging to D12 (Fig. 3a) and D5 (Fig. 3b) clusters. Data visualization maps show a high percentage of strains grouped in D12 clusters were distributed in the mid and southern part of the study area within adjacent districts to Callao (Fig. 3a; Supplementary Figure S2). Strains belonging to D5 transmission clusters had a similar geospatial distribution, but a larger concentration of strains belonging to this cluster-type was observed in the Callao district, which was also the district with a large concentration of strains linked to current or prior history of imprisonment (Fig. 3b).
Fig. 3.
Spatial distribution of the estimated percentage of (a) strains included in D12 clusters and (b) strains included in D5 clusters. Blue dots illustrate the spatial location of each strain and red dots illustrate the location of strains linked to current or prior history of imprisonment.
Discussion
We found that more than half (57%) of MDR-TB in Callao-Peru was due to recent transmission (SNP distance cutoff ≤5) according to WGS cluster analysis. Recent transmission leading to primary MDR-TB is also supported by the fact that less than 25% of patients had a prior history of TB. Our data indicate that prisons may play a pivotal role in the MDR-TB epidemic in the region as incarceration was found to be associated with clustering, and with being infected with an Mtbc strain of the dominant MDR group_1 clone. Importantly, the genotypic resistance profile of MDR-TB strains investigated, revealed high rates of co-resistance to other first and second-line anti-TB drugs, which could pose challenges for individual treatment plans, as well as preventive strategies among contacts.6 The emergence of BDQ resistance in the study population before the drug was used for MDR-TB treatment in the region is particularly worrying.
High rates of TB, including MDR-TB, have been described in many prison settings globally.37 In Peru, a national survey conducted in 69,890 persons deprived of liberty (PDL) in 2016 found that the self-reported prevalence of TB was 2510 per 100,000, approximately 25 times higher than the overall country TB prevalence.38 Similarly, another report indicated that the incidence of TB in Peru prisons in 2018 was 2810 per 100,000 PDL.39 However, the contribution of prisons (including current or prior incarceration) in local MDR-TB epidemiology has not been well described. In our study, we found that 17% of all MDR-TB strains studied and 27% of MDR-TB strains belonging to a D5 recent transmission cluster were linked to a history of imprisonment. Most of these cases were located within the Callao district, an area that also showed a high percentage of Mtbc strains belonging to a single D5 transmission cluster with high transmission indexes. Our findings are in line with a recent report from the country of Georgia, which showed that almost a third of MDR-TB cases were directly or indirectly linked to prisons.8 In the Americas region, prior research from Brazil and Paraguay has demonstrated that TB transmissions in prisons and the wider community are interrelated, and that migration across neighbour countries has an important role in local and regional TB epidemiology.40 Moreover, studies have shown that MDR-TB from PDL frequently spills over the general population,8,11 as also documented in other South American countries.41, 42, 43 Interestingly, it has been reported that MDR Mtbc strains from prison settings carry increased compensatory mutations that favour Mtbc transmission fitness,8 which may explain the high rate of recent MDR-transmission linked to imprisonment. However, we did not find any compensatory mutations within our large D5 group_1 transmission cluster suggesting that the role of compensatory mutations may not be as important for the successful transmission of MDR Mtbc strains, at least in lineage 4 strains being dominant within the Callao region. Interestingly, strains from an intercontinental cross-border transmission36 were found to cluster with strains from the D12 group_8 cluster, also lacking compensatory mutations. This contrasts our findings to studies in Eastern Europe and India, in which mainly MDR strains of L2 with compensatory mutations have a clear transmission advantage, and also carry more drug resistance mutations.7,30,35 Obviously, the L4 MDR strains found in our study can spread without the previously described compensatory mutations, e.g., in rpoA, rpoB, and rpoC, indicating that either other yet unknown mechanisms lead to enhanced spread, or the importance of compensatory mutations may be variable in strains from different lineages.
This study, which combines WGS and epidemiologic data from an MDR-TB-prevalent setting in the Americas, provides ground evidence that prisons potentially are important drivers of the MDR-TB epidemic in TB endemic areas.44 Likewise, our geospatial data visualization illustrated a striking spatial overlap between the distribution of cases linked to a history of imprisonment and recent transmitted strains. Such overlap might indicate an association between this factor and recent transmission in areas where cases linked to high exposure to prison are circulating, potentially boosting the dispersion of MDR-TB in these areas. Recent geospatial analyses suggest that MDR-TB clustering is associated with small, overlapping activity locations in the community.45 However, further in-depth geographical analyses are needed to disentangle the intriguing spatial associations within and around prisons, and their relationship with MDR-TB transmission in the broader community.
Our data suggest that there is a critical need for cost-effective MDR-TB prevention and control interventions in PDL, not only during incarceration time but also once individuals return to their communities. In addition, a recent study of PDL transferred from Peruvian prisons to Spain revealed new MDR-TB diagnoses detected through a health screening program performed on arrival.46 Such scenarios underscore the regional and global implications of the MDR-TB thread, which extends beyond country and prison borders. Studies to better understand the complex interplay and transmission dynamics between the incarcerated populations and the general population are needed. Over the past two decades, the incarcerated population in Central and South America has increased by 206%, which represents the largest increase globally. More than 10% of all TB cases from Central and South America are reported from prisons, despite prisons accounting for less than 1% of the overall population.47 Our findings are consistent with other studies from the region indicating a pivotal role of prisons in the local MDR-TB epidemic. Control and eventual elimination of TB in the Americas requires urgent interventions in PDL. The National TB Program in Peru continues to make important efforts to intensify TB case finding and follow-up to reduce TB transmissions in prisons, including the implementation of molecular testing (e.g., Xpert®) for rapid diagnosis of TB/resistant TB, timely initiation of appropriate TB treatment, and enhancement of isolation strategies.
If drug resistance rates are considered, we found high first-line resistance, and significant FQ/ETH resistance rates in the strains investigated, especially in strains of the dominant 4.3.3 LAM strains and the group_1 outbreak clone. This is particularly concerning as treatment options in these highly transmitted strains are reduced. This potentially influences the efficacy of newly endorsed MDR-TB treatment regimens, and the resistance evolution, e.g., to FQ should be closely monitored to avoid a further increase as seen in India.35 Besides FQ resistance, resistance to BDQ and CFZ which are core drugs of current MDR-TB treatment regimens globally48, 49, 50, 51 is of particular importance. We identified six Mtbc strains within our cohort that had mutations in Rv0678, which encodes a transcriptional repressor of the MmpS5-MmpL5 efflux system,52 even before bedaquiline was widely used in the region. Although mutations in the Rv0678 gene are not consistently associated with phenotypic resistance,53 an insertion mutation in codon 192, as observed in our population, has been associated with 2- to 16-fold increases in phenotypic minimal inhibitory concentrations (MICs) to BDQ.54 This same mutation may confer cross-resistance to CFZ in an order of 2- to 4-fold MIC increase.54 Notably, 2 out of 4 (50%) strains carrying this Rv0678 insertion mutation belonged to a D5 cluster (group_13), indicating ongoing transmission of MDR-TB strains that harbour BDQ/CFZ resistance-associated mutations. These data are concerning and argue for consequent monitoring of the resistance evolution of MDR-TB strains in the region to allow rapid targeted interventions if resistance is further rising. Of note, our study was conducted in a pre-COVID-19 era. It is estimated that the COVID-19 pandemic has set back global TB control by 10 years.55 Whether transmission and resistance patterns changed in Peru after the peak of the COVID-19 pandemic could be explored in future studies.
This study had limitations. Epidemiologic data were obtained from available TB program records and thus we were unable to include information on individuals’ socioeconomic status nor details on incarceration time or prison conditions. Assessment of co-morbidities was limited to HIV and diabetes mellitus for the study population, as these are reportable conditions within local TB programs. However, these two comorbidities are the most strongly associated with TB among prison inmates according to a recent national survey.56 Our analysis focused on MDR-TB strains and therefore we could not determine whether the history of incarceration was also an important driver of drug-susceptible TB transmissions within the studied population, as reported in other countries in the Latin American region.42,44 Our study sought to identify factors associated with recent MDR-TB transmission, but was not specifically powered to assess the magnitude of association between history of imprisonment and TB transmission—this is reflected in the wide confidence intervals reported. Although a third of MDR-TB diagnoses in our study were in females (which mirrors national TB trends in Peru), our study setting only included a prison system for males. Future studies should include data from female correctional facilities to better understand the contribution of prisons in MDR-TB transmission dynamics across sexes. Callao may not be representative of Peru, and national inquiries would be needed to expand our understanding of the role of prisons in MDR-TB epidemiology in Peru.
In conclusion, we found that MDR-TB transmission is a main driver of the MDR-TB epidemic in Callao-Peru. Importantly, pre-XDR-TB was found in already more than 10% of the MDR-TB strains investigated, and BDQ/CFZ resistance-associated mutations was present in 4% of the strains already. The history of imprisonment was linked to recent MDR-TB transmissions, indicating an important role of prisons in driving the MDR-TB epidemic. MDR-TB prevention and control interventions in PDL, not only during incarceration time but also once individuals return to their communities are urgently needed. Further, transmission dynamics and resistance evolution need to be closely monitored at best by implementing an integrated molecular surveillance based on pathogen WGS. The importance of the clonal spread of MDR 4.3.3 LAM strains in other areas of Peru needs to be investigated.
Contributors
MAH, SN, DB, LA, and PM conceptualized the study. CU, MZ, DR, and DC performed the data analysis. CU and MZ performed the laboratory assays. DR, JP, and MZ collected the data. CU, MZ, SN, and MAH wrote the original draft manuscript. All authors reviewed and edited the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Sequencing data in FASTQ format can be downloaded from the European Nucleotide Archive at https://www.ebi.ac.uk/ena/browser/home. Sample’s individual accession numbers are listed in Supplementary Table S1.
Editor’s note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
M.A.H. reports contracts from Gilead Sciences Inc., Insmed Inc., and AN2 Therapeutics Inc., to the University of Cincinnati, outside the submitted work. All other authors report no potential conflicts.
Acknowledgements
We thank our collaborators at the Direccion Regional de Salud del Callao, Callao, Peru who made this analysis possible. We would like to acknowledge the technical support at Research Center Borstel, especially Vanessa Mohr, and Tanja Niemann, for their aid in the laboratory.
We also acknowledge the technical support of CITBM Laboratory members: Lizbeth Ramirez Cuestas and Rocío Egoavil and the invaluable field support of the technical nurse, Raquel Espinoza.
Funding: This work was supported in part by the ERANet-LAC Network of the European Union, Latin America and the Caribbean Countries on Joint Innovation and Research Activities (ELAC2015/T080940 grant), and FONDECYT (grant # J112-2016). Additional support was received from Leibniz ScienceCampus Evolutionary Medicine of the Lung, the Deutsche Forschungsgemeinschaft (German Research Foundation, under Germany’s Excellence Strategy—EXC 2167 Precision Medicine in Inflammation), and the Research Training Group 2501 TransEvo. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the institutions with which the authors are affiliated.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100674.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2022. Global tuberculosis report 2022. Geneva, Switzerland. [Google Scholar]
- 2.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt, Ahmad N., Ahuja S.D., et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisson G.P., Bastos M., Campbell J.R., et al. Mortality in adults with multidrug-resistant tuberculosis and HIV by antiretroviral therapy and tuberculosis drug use: an individual patient data meta-analysis. Lancet. 2020;396(10248):402–411. doi: 10.1016/S0140-6736(20)31316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zurcher K., Reichmuth M.L., Ballif M., et al. Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: a multicentre cohort study. Lancet Microbe. 2021;2(7):e320–e330. doi: 10.1016/S2666-5247(21)00044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouzouita I., Cabibbe A.M., Trovato A., et al. Whole-genome sequencing of drug-resistant Mycobacterium tuberculosis strains, Tunisia, 2012-2016. Emerg Infect Dis. 2019;25(3):538–546. doi: 10.3201/eid2503.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dheda K., Gumbo T., Maartens G., et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017 doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 7.Merker M., Rasigade J.P., Barbier M., et al. Transcontinental spread and evolution of Mycobacterium tuberculosis W148 European/Russian clade toward extensively drug resistant tuberculosis. Nat Commun. 2022;13(1):5105. doi: 10.1038/s41467-022-32455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gygli S.M., Loiseau C., Jugheli L., et al. Prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis. Nat Med. 2021;27(7):1171–1177. doi: 10.1038/s41591-021-01358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenca M.S., Scaini J.L., Abileira F.S., Goncalves C.V., von Groll A., Silva P.E. Prevalence of tuberculosis in prisons: risk factors and molecular epidemiology. Int J Tuberc Lung Dis. 2015;19(10):1182–1187. doi: 10.5588/ijtld.15.0126. [DOI] [PubMed] [Google Scholar]
- 10.Grenzel M.L., Grande A.J., Paniago A.M.M., Pompilio M.A., de Oliveira S., Trajman A. Tuberculosis among correctional facility workers: a systematic review and meta-analysis. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren J.L., Grandjean L., Moore D.A.J., et al. Investigating spillover of multidrug-resistant tuberculosis from a prison: a spatial and molecular epidemiological analysis. BMC Med. 2018;16(1):122. doi: 10.1186/s12916-018-1111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cords O., Martinez L., Warren J.L., et al. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health. 2021;6(5):e300–e308. doi: 10.1016/S2468-2667(21)00025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2021. WHO global lists of high burden countries for TB, multidrug/rifampicin-resistant TB (MDR/RR-TB) and TB/HIV, 2021–2025. Geneva, Switzerland. [Google Scholar]
- 14.World Health Organization Tuberculosis profile: Peru. https://worldhealthorg.shinyapps.io/tb_profiles/ Available at:
- 15.Otero L., Krapp F., Tomatis C., et al. High prevalence of primary multidrug resistant tuberculosis in persons with no known risk factors. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quispe N., Asencios L., Obregon C., et al. The fourth national anti-tuberculosis drug resistance survey in Peru. Int J Tuberc Lung Dis. 2020;24(2):207–213. doi: 10.5588/ijtld.19.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministerio de Salud, Instituto Nacional de Salud . 2020. Manual de Pruebas de Sensibilidad a Drogas Antituberculosis. Lima, Peru. [Google Scholar]
- 18.Moore D.A., Evans C.A., Gilman R.H., et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355(15):1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baym M., Kryazhimskiy S., Lieberman T.D., Chung H., Desai M.M., Kishony R. Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0128036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl T.A., Utpatel C., Schleusener V., et al. MTBseq: a comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ. 2018;6 doi: 10.7717/peerj.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coll F., McNerney R., Guerra-Assuncao J.A., et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812. doi: 10.1038/ncomms5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merker M., Blin C., Mona S., et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47(3):242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobbel H.P., Merker M., Kohler N., et al. Design of multidrug-resistant tuberculosis treatment regimens based on DNA sequencing. Clin Infect Dis. 2021;73(7):1194–1202. doi: 10.1093/cid/ciab359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018;35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z., Alikhan N.F., Sergeant M.J., et al. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28(9):1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker T.M., Ip C.L., Harrell R.H., et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13(2):137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roetzer A., Schuback S., Diel R., et al. Evaluation of Mycobacterium tuberculosis typing methods in a 4-year study in Schleswig-Holstein, Northern Germany. J Clin Microbiol. 2011;49(12):4173–4178. doi: 10.1128/JCM.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merker M., Barbier M., Cox H., et al. Compensatory evolution drives multidrug-resistant tuberculosis in Central Asia. Elife. 2018;7 doi: 10.7554/eLife.38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker T.M., Lalor M.K., Broda A., et al. Assessment of Mycobacterium tuberculosis transmission in Oxfordshire, UK, 2007-12, with whole pathogen genome sequences: an observational study. Lancet Respir Med. 2014;2(4):285–292. doi: 10.1016/S2213-2600(14)70027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ESRI . ESRI; Redlands, CA, USA: 2020. ArcGIS Pro.x.http://www.esri.com Available at: [Google Scholar]
- 33.Beckert P., Sanchez-Padilla E., Merker M., et al. MDR M. tuberculosis outbreak clone in Eswatini missed by Xpert has elevated bedaquiline resistance dated to the pre-treatment era. Genome Med. 2020;12(1):104. doi: 10.1186/s13073-020-00793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnenkalb L., Carter J.J., Spitaleri A., et al. Bedaquiline and clofazimine resistance in Mycobacterium tuberculosis: an in-vitro and in-silico data analysis. Lancet Microbe. 2023;4(5):e358–e368. doi: 10.1016/S2666-5247(23)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreyer V., Mandal A., Dev P., et al. High fluoroquinolone resistance proportions among multidrug-resistant tuberculosis driven by dominant L2 Mycobacterium tuberculosis clones in the Mumbai Metropolitan Region. Genome Med. 2022;14(1):95. doi: 10.1186/s13073-022-01076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acosta F., Agapito J., Cabibbe A.M., et al. Exportation of MDR TB to Europe from setting with actively transmitted persistent strains in Peru. Emerg Infect Dis. 2019;25(3):596–598. doi: 10.3201/eid2503.180574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira T.R., Passos I.B.J., Bueno J.V.L., Maffacciolli R., Colodette R.M., Miguel P.S. Prevalence of multidrug-resistant tuberculosis in prisons: systematic review and meta-analysis. Indian J Med Microbiol. 2022;40(2):193–199. doi: 10.1016/j.ijmmb.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Salazar-De La Cuba A.L., Ardiles-Paredes D.F., Araujo-Castillo R.V., Maguina J.L. High prevalence of self-reported tuberculosis and associated factors in a nation-wide census among prison inmates in Peru. Trop Med Int Health. 2019;24(3):328–338. doi: 10.1111/tmi.13199. [DOI] [PubMed] [Google Scholar]
- 39.Pan American Health Organization . 2018. Tuberculosis in the Americas.http://iris.paho.org/xmlui/handle/10665.2/49510 Washington, DC. Available at: [Google Scholar]
- 40.Walter K.S., Tatara M.B., Esther da Silva K., et al. Local and travel-associated transmission of tuberculosis at Central Western Border of Brazil, 2014-2017. Emerg Infect Dis. 2021;27(3):905–914. doi: 10.3201/eid2703.203839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busatto C., Possuelo L.G., Bierhals D., et al. Spread of Mycobacterium tuberculosis in Southern Brazilian persons deprived of liberty: a molecular epidemiology study. Eur J Clin Microbiol Infect Dis. 2023;42(3):297–304. doi: 10.1007/s10096-023-04546-4. [DOI] [PubMed] [Google Scholar]
- 42.Sanabria G.E., Sequera G., Aguirre S., et al. Phylogeography and transmission of Mycobacterium tuberculosis spanning prisons and surrounding communities in Paraguay. Nat Commun. 2023;14(1):303. doi: 10.1038/s41467-023-35813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacchi F.P., Praca R.M., Tatara M.B., et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerg Infect Dis. 2015;21(3):452–455. doi: 10.3201/eid2103.140896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter K.S., Dos Santos P.C.P., Goncalves T.O., et al. The role of prisons in disseminating tuberculosis in Brazil: a genomic epidemiology study. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bui D.P., Chandran S.S., Oren E., et al. Community transmission of multidrug-resistant tuberculosis is associated with activity space overlap in Lima, Peru. BMC Infect Dis. 2021;21(1):275. doi: 10.1186/s12879-021-05953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abascal E., Herranz M., Acosta F., et al. Screening of inmates transferred to Spain reveals a Peruvian prison as a reservoir of persistent Mycobacterium tuberculosis MDR strains and mixed infections. Sci Rep. 2020;10(1):2704. doi: 10.1038/s41598-020-59373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter K.S., Martinez L., Arakaki-Sanchez D., et al. The escalating tuberculosis crisis in central and South American prisons. Lancet. 2021;397(10284):1591–1596. doi: 10.1016/S0140-6736(20)32578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahid P., Mase S.R., Migliori G.B., et al. Treatment of drug-resistant tuberculosis. An Official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padmapriyadarsini C., Vohra V., Bhatnagar A., et al. Bedaquiline, delamanid, linezolid and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin Infect Dis. 2022;76(3):e938–e946. doi: 10.1093/cid/ciac528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esmail A., Oelofse S., Lombard C., et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis: a multicenter, randomized controlled clinical trial (the NExT Study) Am J Respir Crit Care Med. 2022;205(10):1214–1227. doi: 10.1164/rccm.202107-1779OC. [DOI] [PubMed] [Google Scholar]
- 51.Conradie F., Bagdasaryan T.R., Borisov S., et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387(9):810–823. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battaglia S., Spitaleri A., Cabibbe A.M., et al. Characterization of genomic variants associated with resistance to bedaquiline and delamanid in naive Mycobacterium tuberculosis clinical strains. J Clin Microbiol. 2020;58(11) doi: 10.1128/JCM.01304-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadura S., King N., Nakhoul M., et al. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother. 2020;75(8):2031–2043. doi: 10.1093/jac/dkaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnenkalb L., Carter J., Spitaleri A., et al. Deciphering bedaquiline and clofazimine resistance in tuberculosis: an evolutionary medicine approach. bioRxiv. 2021 doi: 10.1101/2021.03.19.436148. 2021.03.19.436148. [DOI] [Google Scholar]
- 55.Dheda K., Perumal T., Moultrie H., et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10(6):603–622. doi: 10.1016/S2213-2600(22)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rojas-Bolivar D., Zhu Z., Hurtado Y., et al. 765. Tuberculosis and diabetes mellitus among prison inmates in Peru: results of a national survey, 2016. Open Forum Infect Dis. 2018;5(Suppl 1):S274–S275. doi: 10.1093/ofid/ofy210.772. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.