Abstract
Background
Low back pain (LBP) is responsible for considerable personal suffering worldwide. Those with persistent disabling symptoms also contribute to substantial costs to society via healthcare expenditure and reduced work productivity. While there are many treatment options, none are universally endorsed. The idea that chronic LBP is a condition best understood with reference to an interaction of physical, psychological and social influences, the 'biopsychosocial model', has received increasing acceptance. This has led to the development of multidisciplinary biopsychosocial rehabilitation (MBR) programs that target factors from the different domains, administered by healthcare professionals from different backgrounds.
Objectives
To review the evidence on the effectiveness of MBR for patients with chronic LBP. The focus was on comparisons with usual care and with physical treatments measuring outcomes of pain, disability and work status, particularly in the long term.
Search methods
We searched the CENTRAL, MEDLINE, EMBASE, PsycINFO and CINAHL databases in January and March 2014 together with carrying out handsearches of the reference lists of included and related studies, forward citation tracking of included studies and screening of studies excluded in the previous version of this review.
Selection criteria
All studies identified in the searches were screened independently by two review authors; disagreements regarding inclusion were resolved by consensus. The inclusion criteria were published randomised controlled trials (RCTs) that included adults with non‐specific LBP of longer than 12 weeks duration; the index intervention targeted at least two of physical, psychological and social or work‐related factors; and the index intervention was delivered by clinicians from at least two different professional backgrounds.
Data collection and analysis
Two review authors extracted and checked information to describe the included studies, assessed risk of bias and performed the analyses. We used the Cochrane risk of bias tool to describe the methodological quality. The primary outcomes were pain, disability and work status, divided into the short, medium and long term. Secondary outcomes were psychological functioning (for example depression, anxiety, catastrophising), healthcare service utilisation, quality of life and adverse events. We categorised the control interventions as usual care, physical treatment, surgery, or wait list for surgery in separate meta‐analyses. The first two comparisons formed our primary focus. We performed meta‐analyses using random‐effects models and assessed the quality of evidence using the GRADE method. We performed sensitivity analyses to assess the influence of the methodological quality, and subgroup analyses to investigate the influence of baseline symptom severity and intervention intensity.
Main results
From 6168 studies identified in the searches, 41 RCTs with a total of 6858 participants were included. Methodological quality ratings ranged from 1 to 9 out 12, and 13 of the 41 included studies were assessed as low risk of bias. Pooled estimates from 16 RCTs provided moderate to low quality evidence that MBR is more effective than usual care in reducing pain and disability, with standardised mean differences (SMDs) in the long term of 0.21 (95% CI 0.04 to 0.37) and 0.23 (95% CI 0.06 to 0.4) respectively. The range across all time points equated to approximately 0.5 to 1.4 units on a 0 to 10 numerical rating scale for pain and 1.4 to 2.5 points on the Roland Morris disability scale (0 to 24). There was moderate to low quality evidence of no difference on work outcomes (odds ratio (OR) at long term 1.04, 95% CI 0.73 to 1.47). Pooled estimates from 19 RCTs provided moderate to low quality evidence that MBR was more effective than physical treatment for pain and disability with SMDs in the long term of 0.51 (95% CI ‐0.01 to 1.04) and 0.68 (95% CI 0.16 to 1.19) respectively. Across all time points this translated to approximately 0.6 to 1.2 units on the pain scale and 1.2 to 4.0 points on the Roland Morris scale. There was moderate to low quality evidence of an effect on work outcomes (OR at long term 1.87, 95% CI 1.39 to 2.53). There was insufficient evidence to assess whether MBR interventions were associated with more adverse events than usual care or physical interventions.
Sensitivity analyses did not suggest that the pooled estimates were unduly influenced by the results from low quality studies. Subgroup analyses were inconclusive regarding the influence of baseline symptom severity and intervention intensity.
Authors' conclusions
Patients with chronic LBP receiving MBR are likely to experience less pain and disability than those receiving usual care or a physical treatment. MBR also has a positive influence on work status compared to physical treatment. Effects are of a modest magnitude and should be balanced against the time and resource requirements of MBR programs. More intensive interventions were not responsible for effects that were substantially different to those of less intensive interventions. While we were not able to determine if symptom intensity at presentation influenced the likelihood of success, it seems appropriate that only those people with indicators of significant psychosocial impact are referred to MBR.
Keywords: Adult, Humans, Back Pain, Back Pain/psychology, Back Pain/rehabilitation, Chronic Pain, Chronic Pain/psychology, Chronic Pain/rehabilitation, Occupational Therapy, Occupational Therapy/methods, Pain Measurement, Psychotherapy, Randomized Controlled Trials as Topic, Social Support, Work
Plain language summary
Multidisciplinary treatment for back pain
Review question
Is treatment involving a team of therapists from several different clinical professions helpful for people with long‐term back pain?
Background
Low back pain (LBP) is a condition that causes a great deal of pain and suffering across the world and also accounts for large costs to society due to healthcare spending and missed work. Previous research has shown that LBP that has persisted for several months or years is often associated with psychological and social problems. Multidisciplinary treatments target physical as well as psychological and social aspects of LBP and involve a team of healthcare providers with different professional backgrounds and training.
Study characteristics
We collected all the published studies up to February 2014; there were 41 studies (with 6858 participants) that compared multidisciplinary treatment to other treatments. Most studies compared a multidisciplinary treatment to usual care (such as care by a general practitioner) or to treatments that only addressed physical factors (such as exercise or physiotherapy). All the people in the studies had LBP for more than three months and most had received some other sort of treatment previously.
Key results
There was moderate quality evidence that multidisciplinary treatment results in larger improvements in pain and daily function than usual care or treatments aimed only at physical factors. The difference was not very large, about 1 point on a 10 point scale for pain, but this may be important for people whose symptoms have not responded to other treatments. There was also moderate evidence that multidisciplinary treatment doubled the likelihood that people were able to work in the next 6 to 12 months compared to treatments aimed at physical factors.
While these programs seem to be more effective than alternatives, the effects needs to be balanced with their costs in terms of money, resources and time. Multidisciplinary treatment programs are often quite intensive and expensive, so they are probably most appropriate for people with quite severe or complex problems.
Summary of findings
Summary of findings for the main comparison. Multidisciplinary compared to usual care for chronic low back pain.
| Multidisciplinary compared to usual care for chronic low back pain | ||||||
| Patient or population: Patients with chronic low back pain Intervention: Multidisciplinary Biopsychosocial Rehabilitation Comparison: Usual care | ||||||
| Outcomes | Baseline | Comparative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Back pain long term 0‐10 Numerical or visual scale, where 0 equals no pain at all and 10 is the worst pain imaginable. Follow‐up: median 12 mth | # The baseline for the most representative study is 5.8 out of 10 | The mean back pain long term in the MBR groups was 0.21 standard deviations lower (0.37 to 0.04 lower) | 821 (7 studies) | ⊕⊕⊕⊝ moderate1 | This is a small effect that may be clinically relevant in this patient group | |
| Disability long term Mostly Roland Morris 24‐point scale where 0 equals no disability at all and 24 is seriously disabled. Follow‐up: median 12 mth | # The baseline for the most representative study is 11.4 out of 24 | The mean disability long term in the MBR groups was 0.23 standard deviations lower (0.4 to 0.06 lower) | 722 (6 studies) | ⊕⊕⊕⊝ moderate1 | This is a small effect that may be clinically relevant in this patient group | |
|
Assumed risk* Usual care |
Corresponding risk MBR |
Relative effect (95% CI) | ||||
| Work long term Proportion working Follow‐up: median 12 mth | 744 per 1000 | 751 per 1000 (679 to 810) | OR 1.04 (0.73 to 1.47) | 1360 (7 studies) | ⊕⊕⊕⊝ moderate1 | This difference is not statistically or clinically relevant |
| Adverse events | not estimable | not estimable | not estimable | 0 | No evidence | |
| #Of the included trials for this outcome, we chose the study that has the largest weighting in the overall result in Revman (Von Korff 2005). This figure represents the baseline mean in the control group of this particular study. *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio |
||||||
1 High risk of bias in included studies
Summary of findings 2. Multidisciplinary compared to physical treatment for chronic low back pain.
| Multidisciplinary compared to physical treatment for chronic low back pain | ||||||
| Patient or population: Patients with chronic low back pain Intervention: Multidisciplinary Biopsychosocial Rehabilitation Comparison: Physical treatment | ||||||
| Outcomes | Baseline | Comparative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Pain long term 0‐10 Numerical or visual scale, where 0 equals no pain at all and 10 is the worst pain imaginable. Follow‐up: median 12 mth | # The baseline for the most representative study is 4.5 out of 10 | The mean pain long term in the MBR groups was 0.51 standard deviations lower (1.04 lower to 0.01 higher) | 872 (9 studies) | ⊕⊕⊝⊝ low1,2 | This is a moderate effect that is probably clinically relevant in this patient group | |
| Disability long term Various Follow‐up: median 12 mth | # The baseline for the most representative study is 51 out of 100 on the Daily Activities subscale of the Dallas Questionnaire; 0 equals no disability and 100 is seriously disabled | The mean disability long term in the MBR groups was 0.68 standard deviations lower (1.19 to 0.16 lower) | 1169 (10 studies) | ⊕⊕⊝⊝ low1,2 | This is a moderate effect that is probably clinically relevant in this patient group | |
|
Assumed risk* Physical treatment |
Corresponding risk MBR |
Relative effect (95% CI) | ||||
|
Work long term
Proportion working Follow‐up: median 12 mth |
659 per 1000 | 783 per 1000 (729 to 830) | OR 1.87 (1.39 to 2.53) | 1006 (8 studies) | ⊕⊕⊕⊝ moderate1 | This is a moderate effect that is probably clinically relevant in this patient group |
| Adverse events | not estimable | not estimable | not estimable | 0 | No evidence | |
| #Of the included trials for this outcome, we chose the study that used a NRS pain scale that has the largest weighting in the overall result in Revman (Roche 2007/2011). This figure represents the baseline mean in the control group of this particular study. *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
1 High risk of bias in included studies 2 Substantial heterogeneity, I2 > 60%
Summary of findings 3. Multidisciplinary compared to surgery for chronic low back pain.
| Multidisciplinary compared to surgery for chronic low back pain | ||||||
| Patient or population: Patients with chronic low back pain Intervention: Multidisciplinary Biopsychosocial Rehabilitation Comparison: Surgery | ||||||
| Outcomes | Baseline | Comparative effects (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Pain long term SF‐36 Pain subscale; where 100 equals pain‐free Follow‐up: median 24 mth | # The baseline for the most representative study is 28.6 out of 100 | The mean pain long term in the MBR groups was 0.25 standard deviations higher (0.04 lower to 0.53 higher) | 385 (2 studies) | ⊕⊕⊝⊝ low1,2 | This difference is not statistically or clinically relevant | |
| Disability long term Oswestry; 100‐point scale where 0 equals no disability and 100 is seriously disabled. Follow‐up: median 24 mth | # The baseline for the most representative study is 46.5 out of 100 | The mean disability long term in the MBR groups was 0.25 standard deviations higher (0.08 lower to 0.57 higher) | 423 (2 studies) | ⊕⊕⊝⊝ low1,3 | This difference is not statistically or clinically relevant | |
|
Assumed risk* Surgery |
Corresponding risk MBR |
Relative effect (95% CI) | ||||
|
Work long term Proportion working Follow‐up: 24 months |
309 per 1000 | 230 per 1000 |
OR 0.67 (0.31 to 1.45) |
133 (1 study) |
⊕⊕⊝⊝ low1,2 | This difference is not statistically or clinically relevant |
| Adverse events Adverse events due to study interventions | 127 per 1000 | 0 per 1000 |
OR 28.25 (3.77 to 211.93) |
385 (2 studies) |
⊕⊕⊝⊝ low1,2 | This difference may be clinically relevant in this patient group |
| #Of the included trials for this outcome, we chose the study that has the largest weighting in the overall result in Revman (Fairbank 2005). This figure represents the baseline mean in the control group of this particular study. *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
1 High risk of bias in included studies 2 Total sample size < 400 3 Substantial heterogeneity, I2 > 60%
Summary of findings 4. Multidisciplinary compared to wait list for chronic low back pain.
| Multidisciplinary compared to wait list for chronic low back pain | ||||||
| Patient or population: Patients with chronic low back pain Intervention: Multidisciplinary Biopsychosocial Rehabilitation Comparison: Wait list | ||||||
| Outcomes | Assumed risk | Comparative effects (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|
Pain long term 0‐100 Visual scale, where 0 equals no pain at all and 100 is the worst pain imaginable |
# The baseline for the most representative study is 51.02 out of 100 | not estimable | 0 | No evidence | Only short‐term results available for this comparison | |
|
Disability long term Mostly Roland Morris 24‐point scale where 0 equals no disability at all and 24 is seriously disabled |
# The baseline for the most representative study is 13.96 out of 24 | not estimable | 0 | No evidence | Only short‐term results available for this comparison | |
|
Assumed risk Wait List |
Corresponding risk MBR |
Relative effect (95% CI) | ||||
| Work long term | not estimable | not estimable | not estimable | 0 | No evidence | |
| Adverse events | not estimable | not estimable | not estimable | 0 | No evidence | |
| #Of the included trials for this outcome, we chose the study that has the largest weighting in the overall result in Revman (Smeets 2006/2008). This figure represents the baseline mean in the control group of this particular study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
Background
Description of the condition
Low back pain (LBP) and the associated disability are responsible for a significant personal burden globally. Recent epidemiological research suggests LBP is the leading cause of years lived with disability (Vos 2012). There is also a substantial societal burden, with costs attributable to healthcare services and to loss of work productivity running into the billions of dollars annually in many western countries (Maetzal 2002). Lifetime prevalence rates are high, approaching 70% to 80% according to some studies, and a significant proportion of patients develop chronic symptoms lasting three months or more (Henschke 2008). Chronic LBP results in ongoing personal suffering for the involved individuals and most of the substantial economic costs associated with the condition (Lambeek 2011; Maetzal 2002). The focus of this review was on patients with chronic LBP.
Description of the intervention
Despite the large volume of clinical research focused on identifying effective treatments for chronic LBP (Artus 2010; Ferreira 2010; Machado 2009) optimal management remains a source of contention (Koes 2010). One treatment approach is founded on the conceptualisation of LBP as a biopsychosocial problem (Waddell 2004). This approach is supported by the observation that LBP, particularly at the chronic stage, is characterised by a combination of physical, psychological and social dysfunctions (Costa 2009). Further, it appears that psychological and social factors may play a role in the development and maintenance of pain and disability (den Hollander 2010; Linton 2011; Nicholas 2011). This has led to the design of interventions to address multiple factors, typically involving a combination of physical, psychological and educational components and often delivered by a team of clinicians with different skills (Guzman 2006; Smeets 2006). Recent decades have seen an increase in research into a multidisciplinary approach due to wider acceptance of the biopsychosocial model (Foster 2011), the ineffectiveness of monotherapies (Artus 2010), and promising reports from clinical practice. Multidisciplinary biopsychosocial rehabilitation (MBR) may be delivered in multidisciplinary pain clinics, rehabilitation centres or outpatient settings.
Recent Cochrane reviews have addressed behavioural treatment for chronic LBP (Henschke 2011), physical conditioning programs for improving work outcomes in workers with back pain (Schaafsma 2013), and individual patient education for LBP (Engers 2008). These reviews generally report small effects that arise from single‐discipline interventions in the population of interest. Karjalainen 2003 investigated the effects of multidisciplinary treatments on subacute back pain, however they identified only two studies that met their inclusion criteria. The previous version of this Cochrane review was published in 2001, with searches performed up to 1998. It has subsequently been withdrawn by The Cochrane Collaboration due to being out of date (Guzman 2006).
How the intervention might work
The theoretical basis of the intervention comes from the biopsychosocial model (Waddell 2004). According to the theory, chronic LBP involves impairments of physical, psychological and social functioning, and effective treatment requires intervention that specifically addresses these problems. Multidisciplinary biopsychosocial rehabilitation includes elements aimed at improving back‐related physical dysfunction as well as addressing psychological issues or targeting social or work‐related behaviours, or both. There is some evidence from systematic reviews to suggest that these interventions may have a positive effect on work participation outcomes in the long term (Norlund 2009; van Geen 2007).
Why it is important to do this review
Although promising, it is notable that MBR often involves investment of substantial staffing and financial resources by the heathcare system. The indirect costs burden employers, insurance companies and patients as well. The value of MBR has often been questioned because data are lacking regarding its effectiveness and cost‐effectiveness (Smeets 2009). While two meta‐analyses on the effectiveness of MBR have been published (Cutler 1994; Flor 1992), they were completed more than 20 years ago and are now clearly out of date. More recently performed reviews have not included a quantitative synthesis of the evidence. The most recent Cochrane review that directly assessed the effectiveness of MBR on patients with chronic LBP was published in 2001, but this review was withdrawn in 2006 because the literature search was out of date (Guzman 2006). Collection and synthesis of the evidence relevant to the effectiveness of MBR for chronic LBP was overdue.
Objectives
To review the evidence on the effectiveness of MBR for patients with chronic LBP. The focus was on comparisons with usual care and with physical treatments measuring outcomes of pain, disability and work status, particularly in the long term.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) published in full in peer‐reviewed journals were included, all other study types were excluded.
Types of participants
RCTs that investigated male or female participants, or both, with non‐specific chronic LBP and who were older than 18 years of age were included. Chronic LBP was defined as back pain that had persisted for 12 weeks or more. If a RCT recruited LBP patients with a mixed duration of symptoms (that is it also included patients with < 12 weeks duration), it was included if data for the chronic LBP patients were presented separately or if greater than 75% of participants had symptoms for more than 12 weeks. Trials that recruited patients with spinal pain at any level were included if > 75% of participants had LBP. Trials including participants with clearly diagnosed radiculopathy or only patients who had back surgery in the previous 12 months were excluded. Trials were also excluded if they included participants with specific LBP caused by infection, neoplasm, metastasis, rheumatoid arthritis or other inflammatory articular conditions (for example ankylosing spondylitis), spinal stenosis or fracture. Diagnoses such as disc degeneration or bulging discs, facet joint dysfunction and sacroiliac joint pain were included in the review.
Types of interventions
MBR was defined as an intervention that involves a physical component (for example an exercise program) and at least one other element from the biopsychosocial model, that is psychological or social and occupational. The intervention program had to have been delivered by clinicians from different disciplines, that is a minimum of two healthcare professionals from different professional backgrounds had to be involved in the intervention delivery. The different components of the intervention had to be offered as an integrated program involving communication between the providers responsible for the different components. We expected clinicians would include physicians, psychologists, physiotherapists, social workers, occupational therapists and exercise therapists.
The authors acknowledge that there is no consensus regarding the definition of multidisciplinary treatment. We chose to align our conceptualisation of multidisciplinary treatment with a biopsychosocial model of LBP and included studies with interventions that addressed at least two parts of the model (Guzman 2006; Ravenek 2010; van Geen 2007). While there is some overlap (in terms of included studies) with the Cochrane review of behavioural treatments (Henschke 2011), the review of physical conditioning as part of a return to work strategy (Schaafsma 2013), and the review of back schools (Heymans 2010), we expected that the total set of included trials would be substantially different.
Any type of control intervention was included, but the following comparisons were evaluated separately. Comparisons 1 and 2 represent the main focus of this review.
MBR versus usual care.
MBR versus physical treatment.
MBR versus surgery.
MBR versus waiting list.
Where there was more than one MBR program assessed against a non‐MBR control in the same trial, the more intensive program was used in the comparison. Studies that compared different MBR programs with each other were included and described but between group differences were not synthesised.
Types of outcome measures
Patient‐centred outcomes formed the principle target for this review. Outcomes were categorised in three groups according to the follow‐up time after randomisation.
Short term: up to three months.
Medium term: > three months and less than 12 months.
Long term: 12 months or more.
Where a study reported multiple follow‐up times, the time points closest to three, six and 12 months were used in the meta‐analyses.
Primary outcomes
Pain
Back‐specific disability or functional status
Work status (return to work, sick leave)
Measures collected at long‐term follow‐up were considered primary outcomes.
Secondary outcomes
Generic health or quality of life (QoL)
Healthcare service ulitilisation
Global improvement
Psychological and cognitive function (depression, anxiety, fear avoidance, coping strategies)
Adverse events
Search methods for identification of studies
Electronic searches
Relevant RCTs meeting our inclusion criteria were identified by a computer‐aided search of CENTRAL (The Cochrane Library), which includes the Cochrane Back Review Group Trials Register; MEDLINE (OvidSP); EMBASE (OvidSP); PsycINFO (OvidSP) and CINAHL (EBSCOhost) databases. Databases were searched from 1998 (the date of the search conducted for the previous version of this review) until January and March 2014. The search strategies can be found in Appendix 1.
The searches were devised and run by a research librarian from the Cochrane Back Review Group according to their guidelines (Furlan 2009). A highly sensitive search strategy for retrieval of controlled trials was run in conjunction with specific searches for LBP and multidisicplinary treatment. We considered RCTs published in any language.
All search results were screened independently by two of three authors (SK, AA, AC). Clearly ineligible studies were excluded based on title and abstract. Full text articles were retrieved for all remaining studies and these were again screened independently by two authors for inclusion. Disagreements regarding inclusion were resolved via consensus or via a third author (RO), where necessary.
Searching other resources
Following the electronic searches, the reference lists of relevant publications were screened; these included systematic reviews relevant to the topic and studies included in this review. Citation tracking of included RCTs was also conducted using Science Citation Index. All articles included in the previous version of this review (Guzman 2006) were included and studies listed as excluded in that review were screened against the inclusion criteria.
Data collection and analysis
Selection of studies
Studies were included in the review according to the following inclusion criteria:
randomised controlled trial (RCT);
included adult patients with chronic LBP;
compared MBR intervention with a control intervention or waiting list;
published as full text in a peer‐reviewed journal.
Data extraction and management
Data were extracted from all included studies by one author (SK) and checked by a second author (AC). Extracted data included the following.
Population characteristics: participant source or setting, mean age, gender proportions, duration of symptoms, baseline pain and disability measures.
Intervention characteristics: description of interventions (index and control), duration and number of sessions, delivery type (e.g. individual or group), clinicians responsible for delivery.
Outcome data (baseline and follow‐up): pain, disability or function, work‐related outcomes, global improvement, healthcare service utilisation, QoL, psychological function, adverse events.
Outcome data were entered into RevMan for analysis.
Assessment of risk of bias in included studies
Risk of bias was assessed using the Cochrane Back Review Group risk of bias tool (Furlan 2009). Assessments were conducted independently by two authors (SK, AA) and disagreements were resolved by consensus. Where necessary, a third author (RO) was involved to resolve disagreements. Sensitivity analyses using the results of the risk of bias assessments are described below.
Measures of treatment effect
Clinical homogeneity regarding the control intervention, outcome measure and timing of measurement was assessed prior to pooling. Random‐effects models were used to quantify pooled treatment effect sizes.
Unit of analysis issues
All included studies randomised participants and analysed results at the individual patient level.
Dealing with missing data
For meta‐analysis of continuous outcomes we extracted and analysed group means, standard deviations and sample sizes at each follow‐up point. For dichotomous outcomes we used event counts and sample sizes. Where medians instead of means were reported, these were substituted into the analysis. Where follow‐up standard deviations were not reported, we used the standard deviation for the same measure at baseline, or follow‐up, as a substitute. Where neither the baseline or follow‐up standard deviation was reported, we calculated an estimate of the standard deviation from the same measure reported in other studies within the comparison. Attempts were made to contact authors of the original studies to supply data where insufficient data were reported in the article. Where no estimate was possible using the aforementioned methods, the data were not used in the meta‐analysis.
Assessment of heterogeneity
I2 statistics were inspected and taken into account when assessing the quality of evidence; they were not used to determine whether or not to perform meta‐analysis. in the GRADE assessment the quality of the evidence for an effect was downgraded by one level for inconsistency where the I2 statistic was greater than 60% (that is substantial heterogeneity as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Assessment of reporting biases
Inspection of funnel plots was conducted to investigate reporting bias where there were sufficient trials in a particular comparison.
Data synthesis
Dichotomous outcomes were analysed by calculating the pooled odds ratio (OR). Continuous outcomes were analysed by calculating the pooled mean difference (MD) when the same instrument was used to measure outcomes, or the standardised mean difference (SMD) when different instruments were used. The uncertainty was expressed with 95% confidence intervals (95% CI). The outcome measures from the individual trials were combined through meta‐analysis where possible (in terms of clinical comparability of population, intervention and outcomes between trials) using random‐effects models.
We assessed the overall quality of the evidence for each outcome using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and by the Cochrane Back Review Group (Furlan 2009). Factors that may decrease the quality of the evidence were: study design and risk of bias, inconsistency of results, indirectness, imprecision and other factors (for example reporting bias). The quality of the evidence for a specific outcome was reduced by a level according to the performance of the studies in a particular comparison against these five factors. The quality of evidence was graded down by one level for risk of bias where any studies included in a comparison did not meet the threshold of six items on the risk of bias scale (Furlan 2009). The quality of the evidence was downgraded for inconsistency of results where the I2 statistic was greater than 60% (substantial heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions), and graded down for precision where there were less than a total of 400 participants in the comparison (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We conducted pre‐planned subgroup analyses based on the following parameters.
Baseline symptom intensity. Studies were categorised according to the mean score for all participants at baseline on a pain scale and a back‐specific disability measure. Where mean scores were 60% or greater of the scale maximum for both pain and disability the studies were categorised as high intensity, all others others were considered low intensity.
Intervention intensity. Interventions that involved more than 100 face‐to‐face hours delivered on a daily basis were categorised as high intensity, and interventions that involved less that 30 hours delivered on a non‐daily basis were categorised as low intensity for the subgroup analyses. Other interventions were categorised as mid‐intensity and were excluded from these subgroup analyses (Guzman 2006).
In cases where insufficient information was reported to categorise a study, the study was excluded from the subgroup analysis.
Sensitivity analysis
We performed sensitivity analyses to see if the overall estimates of effectiveness changed when only evidence from studies with low risk of bias was considered. Two definitions of low risk of bias were defined: 1) fulfilling six or more risk of bias criteria, and 2) reporting adequate concealment of treatment allocation.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 6168 potentially eligible titles, a further 11 articles where identified through checking of reference lists and citation tracking. Following the search and screening and retrieval of 164 full text articles, 31 studies were determined to be eligible (Figure 1). These were added to the 10 studies included in the previous version of the review to make a total of 41 included studies.
1.
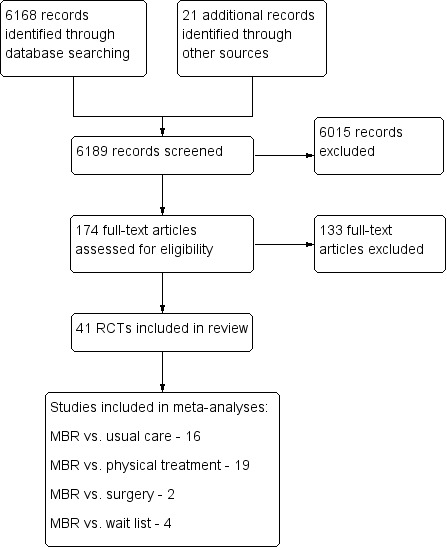
Study flow diagram.
Included studies
Most of the included studies were conducted in Europe (33 studies), three were from Iran, three from North America, and two from Australia. Sample sizes ranged from 20 to 542, with a total of 6858 participants included (Characteristics of included studies). Sixteen studies reported on a comparison of MBR with usual care, 19 with physical treatment, two with surgery, and four with a wait list; 12 studies reported comparisons between two different types of MBR intervention, see below. Participants in the included studies were usually referred to rehabilitation units by primary care practitioners or insurance providers. In most studies the average age of participants was between 40 and 45 years, gender balance was varied, and the average duration of symptoms was usually more than one year. Four studies reported high baseline symptom intensity (> 60% on pain and disability scales), 33 studies were categorised as low baseline symptom intensity, and there were insufficient data reported to categorise four studies. Fifteen studies reported high intervention intensity (> 100 hours contact time delivered on a daily basis), 15 studies were categorised as low intervention intensity (< 30 hours contact time delivered on a non‐daily basis), and 11 studies were neither high nor low intensity according to these criteria.
MBR versus usual care (Abbassi 2012; Basler 1997; Bendix 'A' 1996/1998; Lambeek 2010; Linton 2005; Lukinmaa 1989; Mitchell 1994; Moix 2003; Morone 2011; Morone 2012; Skouen 2002; Strand 2001; Tavafian 2008; Tavafian 2011; Vollenbroek‐Hutten 2004; Von Korff 2005).
MBR versus physical treatment (Alaranta 1994; Bendix 'B' 1995/1998; Bendix 'C' 2000; Coole 2013; Harkapaa 1989; Henchoz 2010; Jousset 2004; Kaapa 2006; Kool 2007; Mangels 2009; Monticone 2013; Morone 2012; Nicholas 1991; Nicholas 1992; Roche 2007/2011; Schweikert 2006; Smeets 2006/2008; Streibelt 2009; Turner 1990).
MBR versus surgery (Fairbank 2005; Hellum 2011).
MBR versus waiting list (Jackel 1990; Kole‐Snijders 1999; Smeets 2006/2008; Turner 1990).
Studies that compared two MBR programs: Abbassi 2012; Bendix 'B' 1995/1998; Harkapaa 1989; Kole‐Snijders 1999; Leeuw 2008; Linton 2005; Mangels 2009; Meng 2011; Nicholas 1991; Skouen 2002; Smeets 2006/2008; Van den Hout 2003.
Excluded studies
There were 133 studies retrieved in full text format and eventually excluded (Characteristics of excluded studies). The most common reasons for exclusion were: study design other than RCT, inclusion of participants other than those with chronic LBP, and index interventions that did not include two or more elements of the biopsychosocial model or were not delivered by clinicians of different clinical backgrounds.
Risk of bias in included studies
Included studies met one to nine of the 12 criteria for low risk of bias. Thirteen of the 41 studies (32%) were assessed as low risk of bias since they met six or more criteria (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
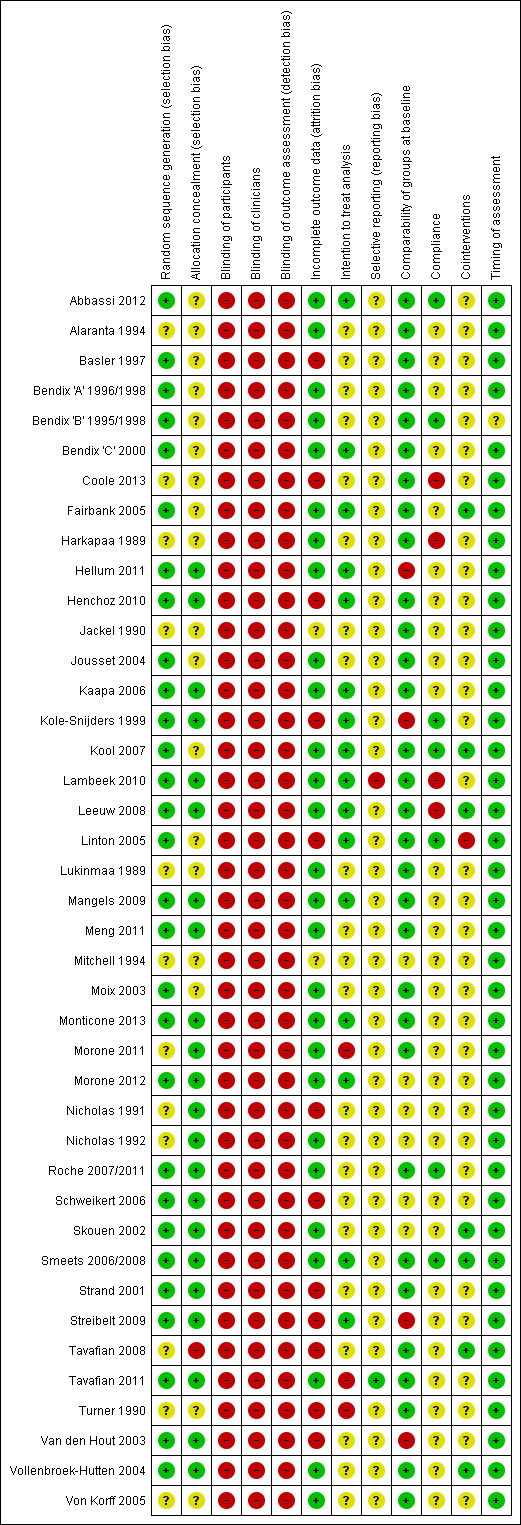
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were described as randomised but only 29 studies clearly described an adequate randomisation procedure, and 23 studies described concealment of allocation.
Blinding
The nature of the interventions and the primary outcomes (pain and disability) meant that blinding of patients, clinicians or assessors was not possible in the included studies.
Incomplete outcome data
A total of 26 studies reported outcome data that met the criteria for completeness, 16 studies reported an intention‐to‐treat analysis.
Selective reporting
The criterion regarding the potential presence of reporting bias was assessed on a strict basis. A study was only listed as low risk of bias if the fact that all collected outcomes were reported was explicitly stated in the manuscript, or all the outcomes listed in a published protocol of the study were reported in the manuscript. Only one study met this criterion.
Other potential sources of bias
Sufficient information to determine that randomised groups were comparable at baseline was reported in 31 studies, treatment compliance was assessed as adequate in seven studies, and risk of bias arising from the use of co‐interventions was assessed as low in six studies. Timing of assessment was clearly the same across groups in 40 studies.
Funnel plots were created for comparisons with at least 10 included studies (Higgins 2011) and they were inspected visually to assess the risk of publication bias (Figure 4; Figure 5; Figure 6). Three analyses (pain and disability in the short term and disability in the long term) in the MBR versus physical treatment comparison met this criterion. None of the plots showed substantial asymmetry aside from one outlying medium‐sized study that reported very large effects in favour of MBR (Monticone 2013).
4.
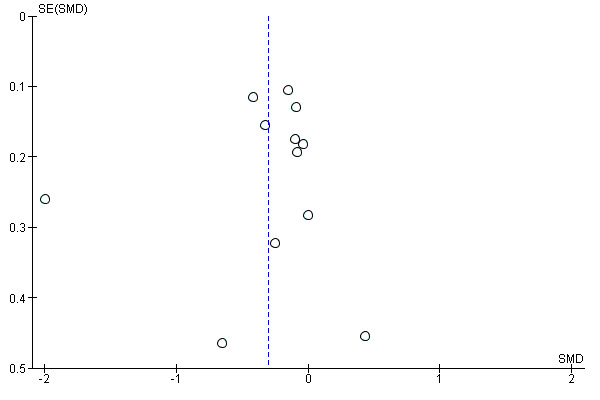
Funnel plot of comparison: MBR versus physical treatment. Pain short term.
5.
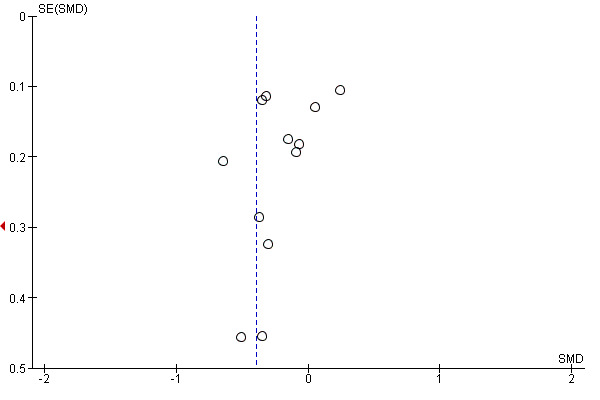
Funnel plot of comparison: MBR versus physical treatment. Disability short term.
6.
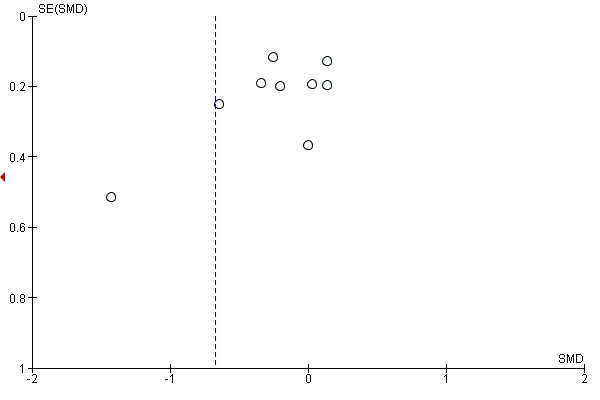
Funnel plot of comparison: MBR versus physical treatment. Disability long term.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
MBR versus usual care
Primary outcomes
Sixteen studies reported on the effect of a MBR intervention versus usual care. More details regarding the content of the interventions are provided in the individual study descriptions (Characteristics of included studies). Between six and nine studies provided data for pain outcomes at each time point (total n = 740 to 879), six to nine studies for disability outcomes (total n = 722 to 939) and two to seven for work outcomes (total n = 373 to 1360).
For pain, point estimates for the pooled between group differences ranged from 0.21 to 0.60 (SMD) in the short, medium and long term (Figure 7; Figure 8; Figure 9); in all cases the 95% CIs did not cross zero, indicating a statistically significant effect in favour of MBR over usual care. For disability, estimates ranged from 0.23 to 0.43 (SMD) and were all significant in favour of MBR (Figure 10; Figure 11; Figure 12). The pooled effects on work outcomes ranged from 1.4 to 1.6 (OR) and were not statistically significant at any time point (Figure 13; Figure 14; Figure 15).
7.
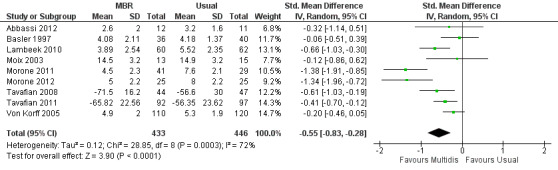
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.1 Back pain short term.
8.
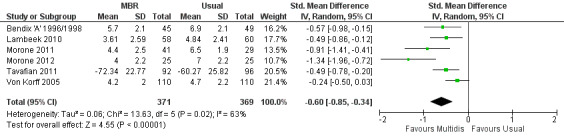
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.2 Back pain medium term.
9.

Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.3 Back pain long term.
10.

Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.4 Disability short term.
11.
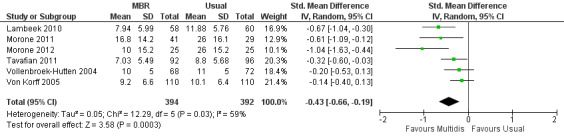
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.5 Disability medium term.
12.
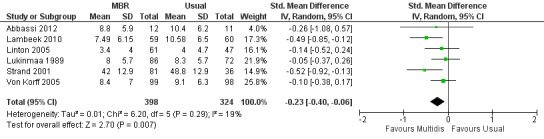
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.6 Disability long term.
13.
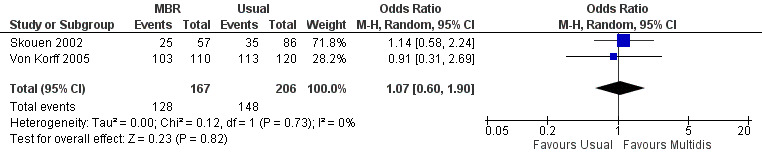
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.7 Work short term.
14.
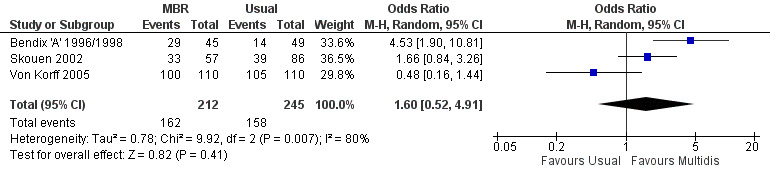
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.8 Work medium term.
15.
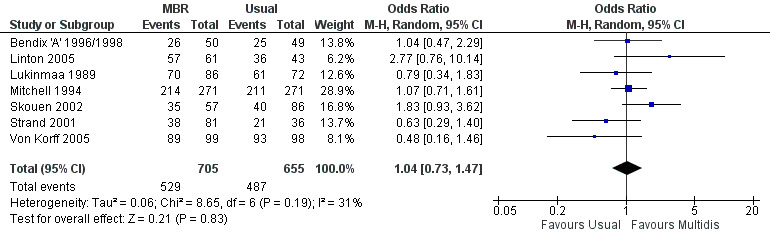
Forest plot of comparison: 1 Multidisciplinary versus usual care, outcome: 1.9 Work long term.
The effects on pain and disability translated to approximately 0.5 to 1.4 points on a 0 to 10 numerical rating scale (NRS) and 1.4 to 2.5 points on a 0 to 24 Roland Morris Disability Questionnaire, respectively. The lower end of the estimate was reported for long‐term outcomes and the upper end for short and medium‐term outcomes.
The included studies provided low quality evidence that MBR was more effective than usual care on pain in the short and medium term, and moderate quality evidence for the effect in the long term. The quality of evidence for the effect on disability was moderate at all time points. The quality of evidence for no effect on work outcomes was low in the short and medium term and moderate in the long term. The quality of evidence was downgraded for risk of bias for all outcomes and further downgraded for inconsistency for some outcomes (Table 1).
Heterogeneity
Pooled estimates should be considered in the light of significant statistical heterogeneity amongst the effect sizes of the included studies; in six (of the nine) instances the I2 statistic was in excess of the 'moderate' threshold of 30%, in three instances it was above the 'substantial' threshold of 60%.
Sensitivity analyses
In general, the pooled effect sizes from the high quality studies were of similar magnitude to those from all included studies, and this was the case regardless of how high quality was defined. However, few studies met the high quality criteria, which resulted in larger CIs around the estimates and meant that some estimates that were significant in the complete analysis were no longer significant in the sensitivity analysis. Overall, inclusion of low quality studies in the meta analyses did not appear to result in a bias towards overestimation of the effect of MBR versus usual care.
Subgroup analyses
While a subgroup analysis for symptom intensity was planned, only one study in the comparison met our a priori determined criteria for high mean baseline pain and disability intensity.
A second subgroup analysis was performed on intervention intensity. In most cases the effect estimates from high and low intensity interventions were quite similar and there was substantial overlap of CIs. There was no pattern of smaller or larger effects for either intervention category. Overall, the intensity of the intervention appeared to have little influence on the effect of MBR versus usual care (Analysis 5.2; Analysis 5.4; Analysis 5.6; Analysis 5.8; Analysis 5.10; Analysis 5.12; Analysis 5.14; Analysis 5.16; Analysis 5.18).
5.2. Analysis.
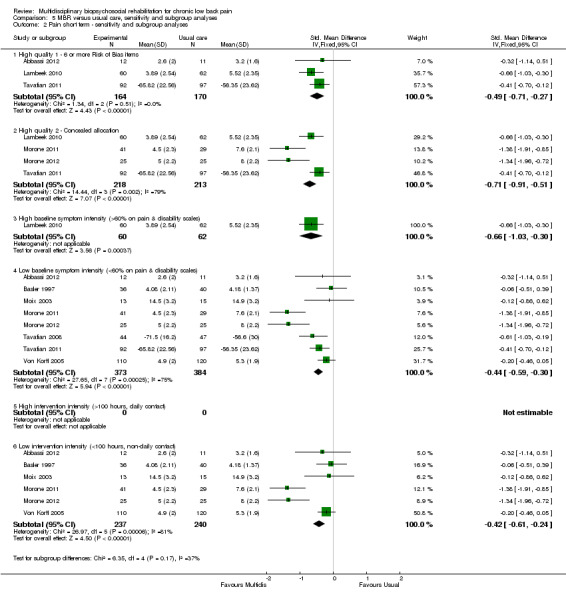
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 2 Pain short term ‐ sensitivity and subgroup analyses.
5.4. Analysis.
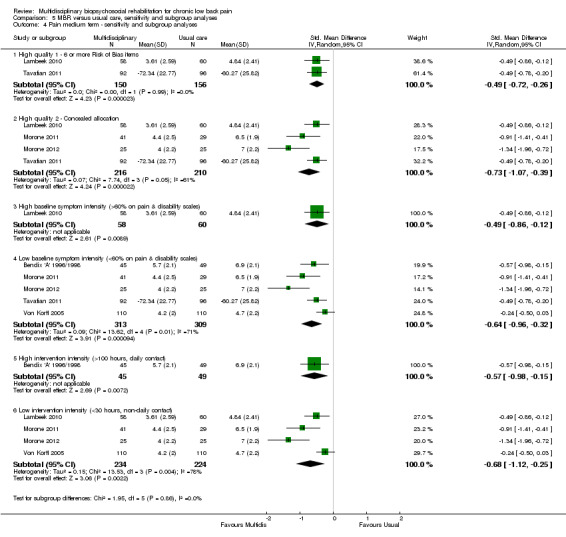
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 4 Pain medium term ‐ sensitivity and subgroup analyses.
5.6. Analysis.
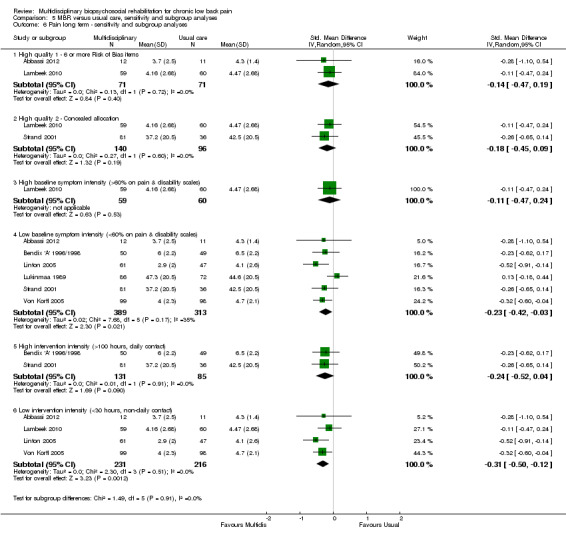
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 6 Pain long term ‐ sensitivity and subgroup analyses.
5.8. Analysis.

Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 8 Disability short term ‐ sensitivity and subgroup analyses.
5.10. Analysis.
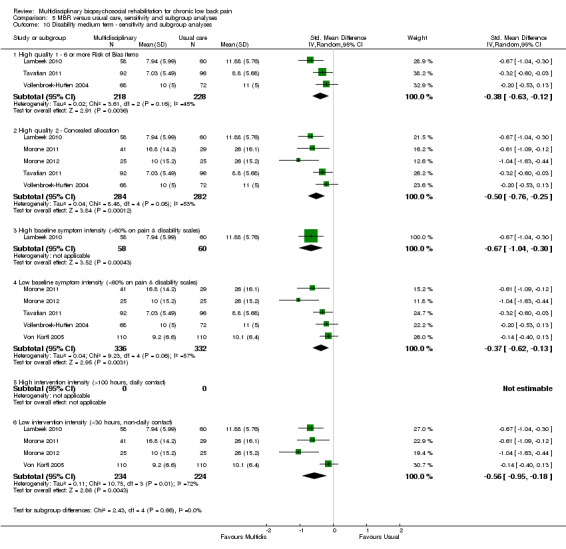
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 10 Disability medium term ‐ sensitivity and subgroup analyses.
5.12. Analysis.
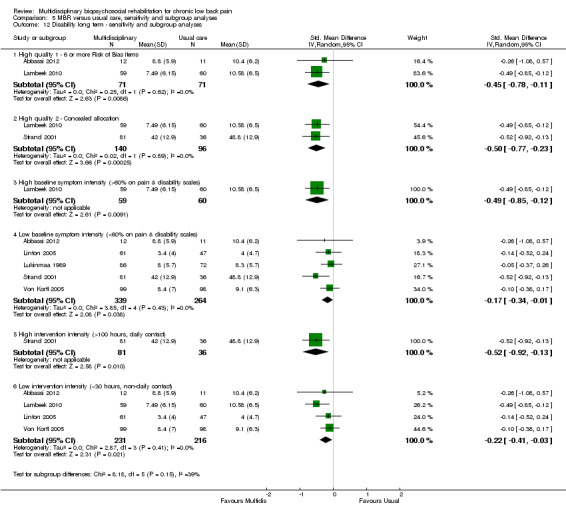
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 12 Disability long term ‐ sensitivity and subgroup analyses.
5.14. Analysis.
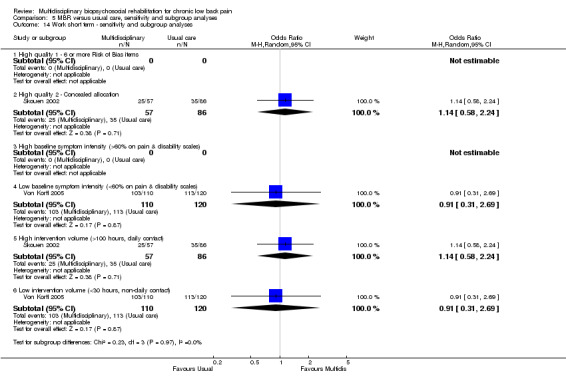
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 14 Work short term ‐ sensitivity and subgroup analyses.
5.16. Analysis.
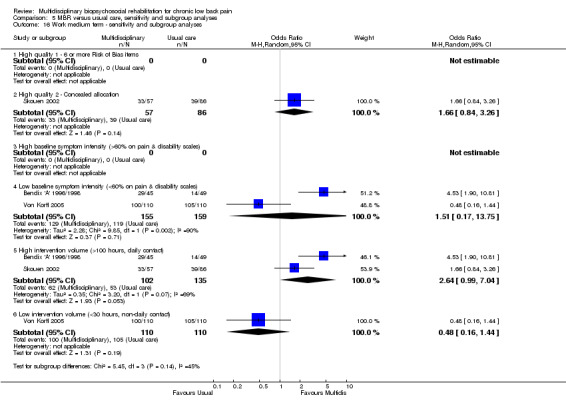
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 16 Work medium term ‐ sensitivity and subgroup analyses.
5.18. Analysis.
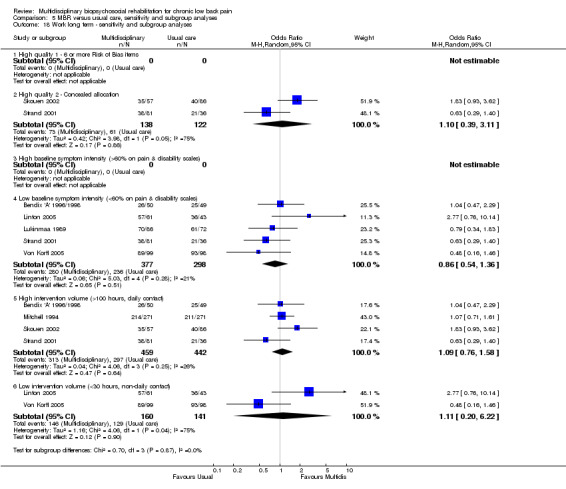
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 18 Work long term ‐ sensitivity and subgroup analyses.
Secondary outcomes
Three studies reported on QoL (Short Form (SF)‐36) outcomes in the short and medium term that could be used to calculate pooled effect sizes. Precision was low but these analyses suggested an effect on the SF‐36 mental components subscale (MD in the short term of 15.25, in the medium term of 7.59) in favour of MBR (Analysis 1.11; Analysis 1.13), but no effect on the physical components subscale (MD in the short term of 13.45, in the medium term of 7.41) (Analysis 1.10; Analysis 1.12). Pooled estimates of effect on psychological outcomes showed a statistically significant effect in favour of MBR on catastrophising in the short term (SMD 0.43) (Analysis 1.14) and long term (SMD 0.40) (Analysis 1.15) and an effect on fear avoidance at the long (SMD of 0.29) (Analysis 1.17), but not the short term (SMD of 0.69) (Analysis 1.16). The only study that mentioned adverse events (Lambeek 2010) reported none in the MBR group, it was unclear whether adverse events in the usual care group were recorded.
1.11. Analysis.
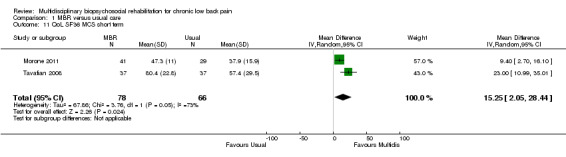
Comparison 1 MBR versus usual care, Outcome 11 QoL SF36 MCS short term.
1.13. Analysis.
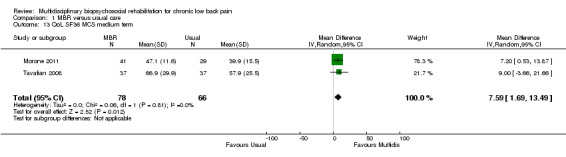
Comparison 1 MBR versus usual care, Outcome 13 QoL SF36 MCS medium term.
1.10. Analysis.
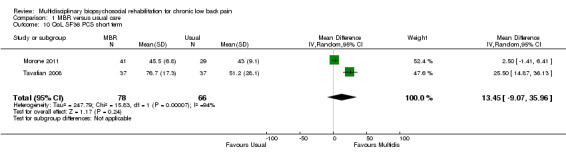
Comparison 1 MBR versus usual care, Outcome 10 QoL SF36 PCS short term.
1.12. Analysis.

Comparison 1 MBR versus usual care, Outcome 12 QoL SF36 PCS medium term.
1.14. Analysis.
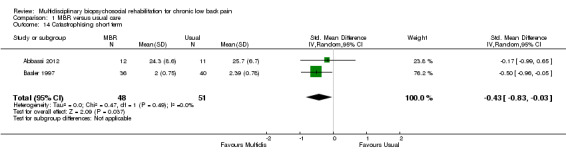
Comparison 1 MBR versus usual care, Outcome 14 Catastrophising short term.
1.15. Analysis.
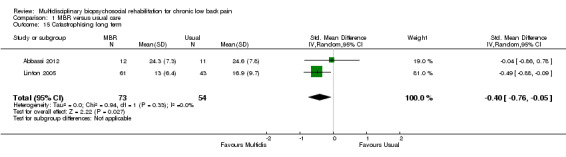
Comparison 1 MBR versus usual care, Outcome 15 Catastrophising long term.
1.17. Analysis.
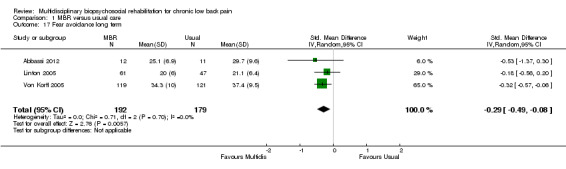
Comparison 1 MBR versus usual care, Outcome 17 Fear avoidance long term.
1.16. Analysis.
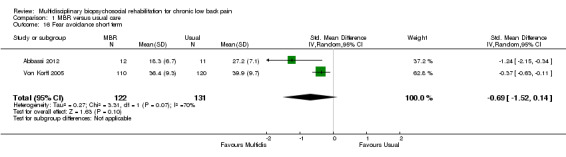
Comparison 1 MBR versus usual care, Outcome 16 Fear avoidance short term.
MBR versus physical treatment
Nineteen studies reported on the effect of an MBR intervention versus physical treatment; more details regarding the content of the interventions are provided in the individual study descriptions (Characteristics of included studies). Between 9 and 12 studies provided data for the pain outcomes at each time point (total n = 531 to 1661), 8 to 13 studies for disability outcomes (total n = 511 to 1878) and 3 to 8 for work outcomes (total n = 221 to 1006).
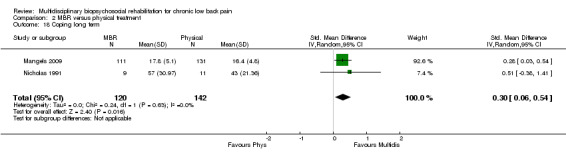
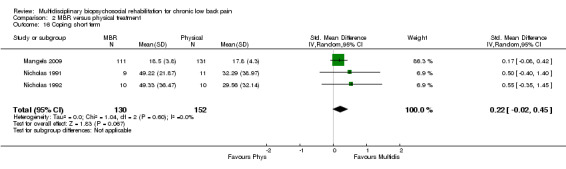
For pain, pooled estimates were in favour of MBR and ranged from 0.28 to 0.51 (SMD) with a statistically significant effect in the short and medium term (Figure 16; Figure 17) but not at long term (Figure 18). For disability, effects ranged from 0.21 to 0.68 (SMD) in favour of MBR; they were significant for the short and long term (Figure 19; Figure 20) but not for medium term (Figure 21).
16.
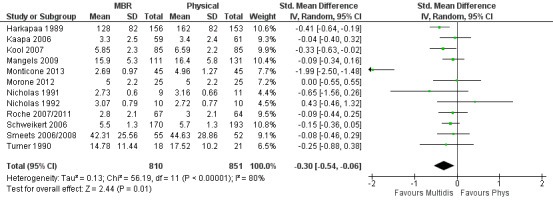
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.1 Pain short term.
17.

Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.2 Pain medium term.
18.
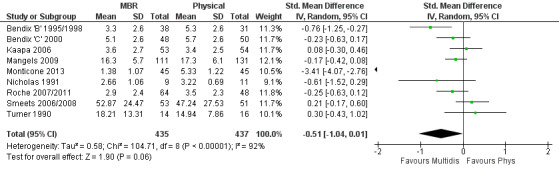
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.3 Pain long term.
19.
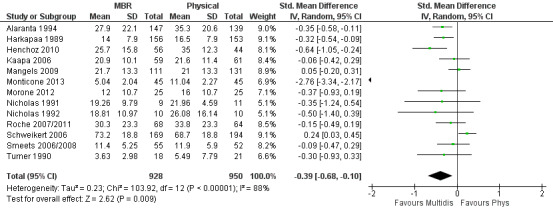
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.4 Disability short term.
20.
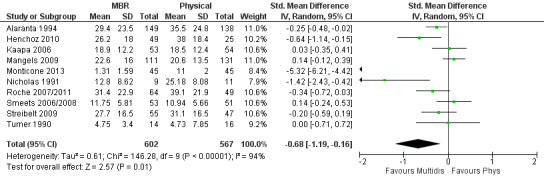
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.6 Disability long term.
21.

Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.5 Disability medium term.
Pooled effect sizes on pain and disability in the short and long term were heavily influenced by one low risk of bias study that reported a very large effect, three to five times the size of the effects reported by the other studies (SMD 1.99 to 5.32) (Monticone 2013). Inclusion of this study introduced substantial heterogeneity into the meta‐analyses. Removal from the pooled analyses reduced the I2 values substantially, from 81% to 92% to 0% to 49% for pain, and from 88% to 94% to 60% to 61% for disability. If this study was removed from the meta‐analyses, the pooled effect estimates for pain ranged from 0.14 to 0.28 (SMD) and were statistically significant in the short and medium term but not long term, and for disability the estimates ranged from 0.18 to 0.21 (SMD) and they were statistically significant in the short but not medium or long term.
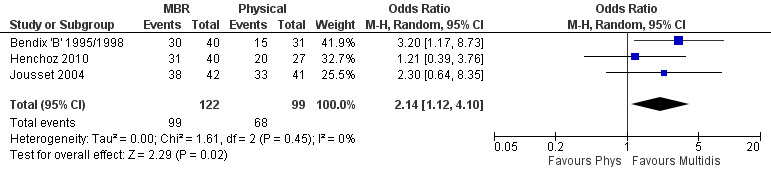
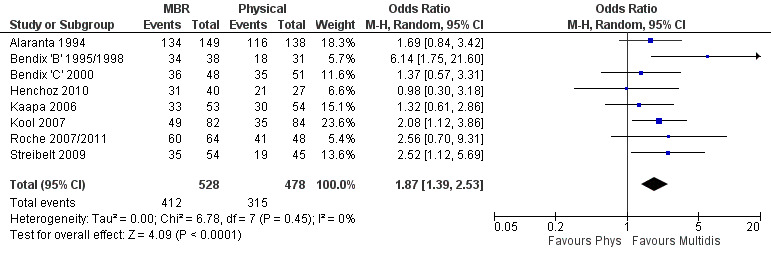
For work outcomes, the between group differences at short term were not significant (Figure 22) but there were significant ORs of 1.87 to 2.14 in favour of MBR for the medium and long term (Figure 23; Figure 24).
22.
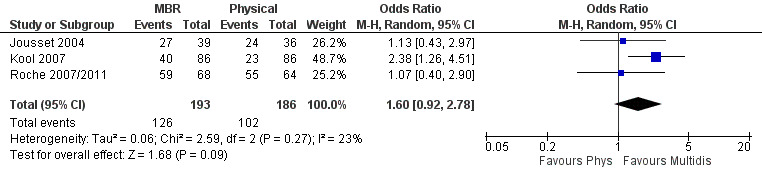
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.7 Work short term.
23.
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.8 Work medium term.
24.
Forest plot of comparison: 2 Multidisciplinary versus physical treatment, outcome: 2.9 Work long term.
The effects on pain and disability translated to approximately 0.6 to 1.2 points on a 0 to 10 NRS and 1.2 to 4.0 points on a 0 to 24 Roland Morris Disability Questionnaire, respectively. The upper end of the estimates was reported for the outcomes at long term. Regarding work outcomes, the estimates indicated that people receiving a MBR intervention had approximately twice the odds of those receiving a purely physical treatment of being at work six and 12 months after the intervention.
The included studies provided low quality evidence that MBR was more effective than physical treatment on pain and disability in the short and long term, and moderate quality evidence for the effect in the medium term. For work outcomes, there was low quality evidence of no effect at short term, low quality evidence of a positive effect at medium term, and moderate quality evidence of an effect at long term (Table 2).
Heterogeneity
Pooled estimates should be considered in the light of significant statistical heterogeneity amongst the effect sizes of the included studies. For all six pain and disability comparisons the I2 statistic was in excess of the 'moderate' threshold of 30%, and in four instances it was above the 'substantial' threshold of 60% (Higgins 2011). Removal of the outlier study from the analyses generally reduced inconsistency from substantial to moderate levels. Statistical heterogeneity was minor for work outcomes.
Sensitivity analyses
For pain and disability outcomes the effect sizes for the short and long‐term analyses from the high quality studies were comparable to those from the complete analyses. In many cases, however, the estimate was no longer statistically significant, likely because of the reduced precision due to fewer included studies. For pain and disability in the medium term, the estimates were substantially lower for the first sensitivity analysis but comparable for the second as compared to the complete analyses. In all cases, both the sensitivity analyses and the complete analysis effect estimates were non‐significant. For work outcomes at short and medium term, sensitivity analyses had only one or two studies included, making interpretation difficult. For long‐term work outcomes, estimates from the high quality studies were very similar to those from the complete analyses. Overall, inclusion of low quality studies in the meta‐analysis did not appear to result in a bias towards overestimation of the effect of MBR versus physical treatment.
Subgroup analyses
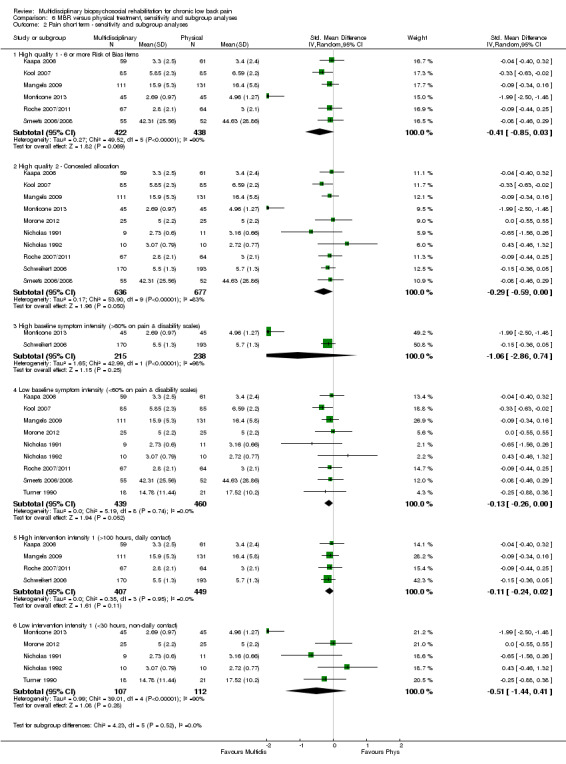
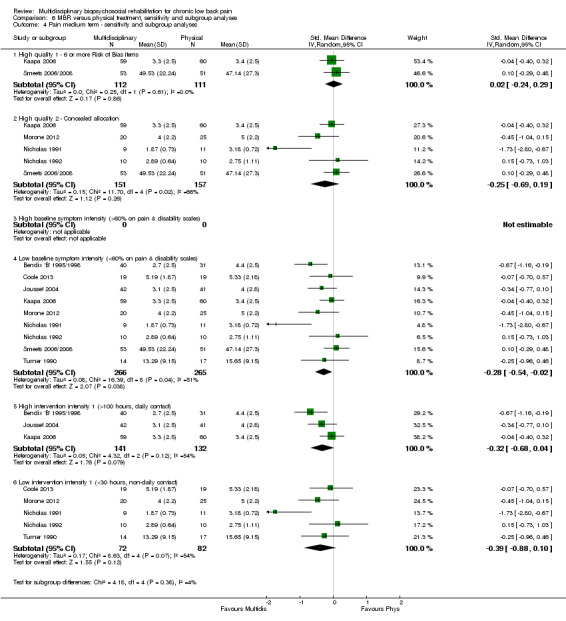
The participants in two studies were categorised as having high baseline symptom intensity according to our criteria. One of these studies (Monticone 2013) reported a very large effect in favour of MBR, three to five times the size of the effects reported by other studies in the comparison. Due to the influence of this study, the estimates of effect for high baseline symptom intensity were substantially larger than those for low intensity. Given these circumstances, the influence of baseline symptom intensity on the effect of MBR versus physical rehabilitation was unclear.
The low intensity interventions had substantially larger effect estimates for pain and disability at all time points, although only one estimate (for pain at short term) was statistically significant; there was substantial overlap of CIs around the estimates for the two intervention types. No low intensity intervention studies reported work outcomes, hence the influence of this variable could not be assessed. The influence of intervention intensity on the effect of MBR versus physical rehabilitation was unclear (Analysis 6.2; Analysis 6.4; Analysis 6.6; Analysis 6.8; Analysis 6.10; Analysis 6.12; Analysis 6.14; Analysis 6.16; Analysis 6.18).
6.2. Analysis.
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 2 Pain short term ‐ sensitivity and subgroup analyses.
6.4. Analysis.
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 4 Pain medium term ‐ sensitivity and subgroup analyses.
6.6. Analysis.
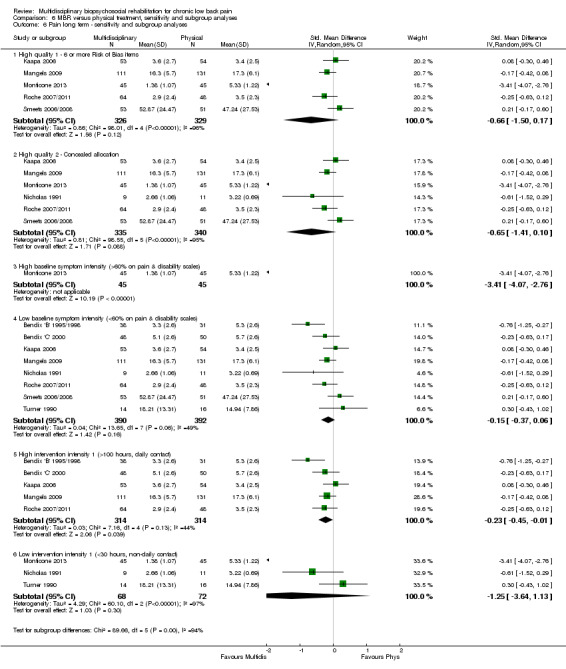
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 6 Pain long term ‐ sensitivity and subgroup analyses.
6.8. Analysis.

Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 8 Disability short term ‐ sensitivity and subgroup analyses.
6.10. Analysis.
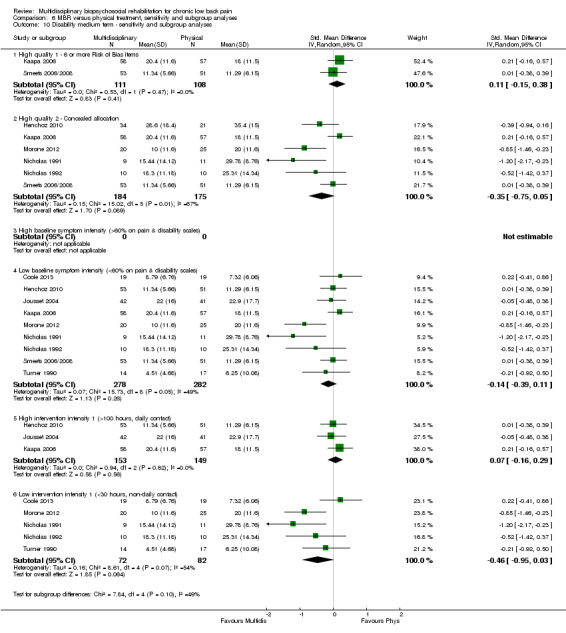
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 10 Disability medium term ‐ sensitivity and subgroup analyses.
6.12. Analysis.
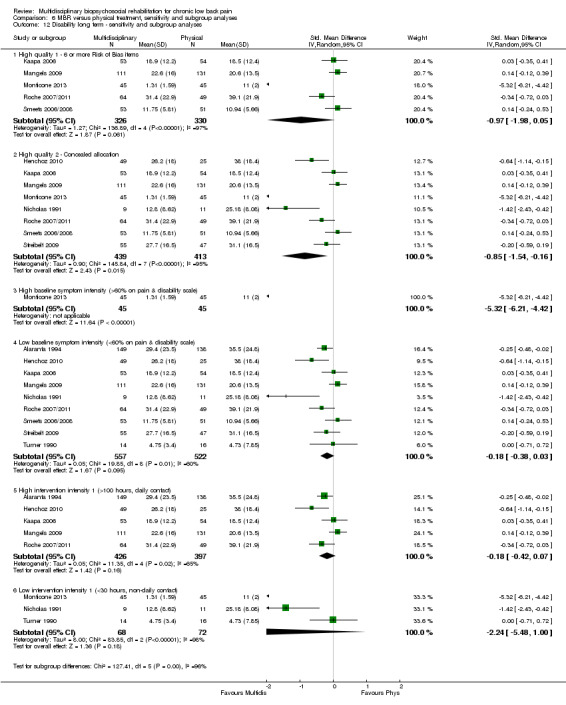
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 12 Disability long term ‐ sensitivity and subgroup analyses.
6.14. Analysis.

Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 14 Work short term ‐ sensitivity and subgroup analyses.
6.16. Analysis.
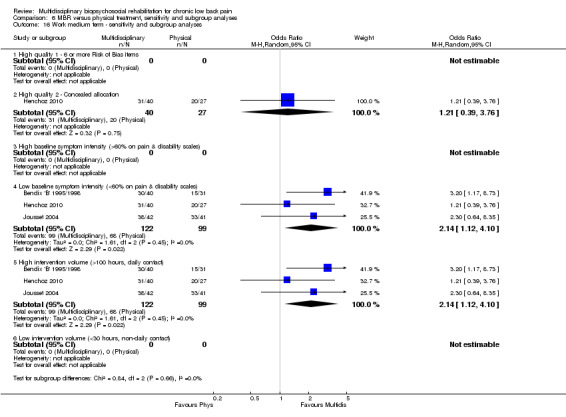
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 16 Work medium term ‐ sensitivity and subgroup analyses.
6.18. Analysis.
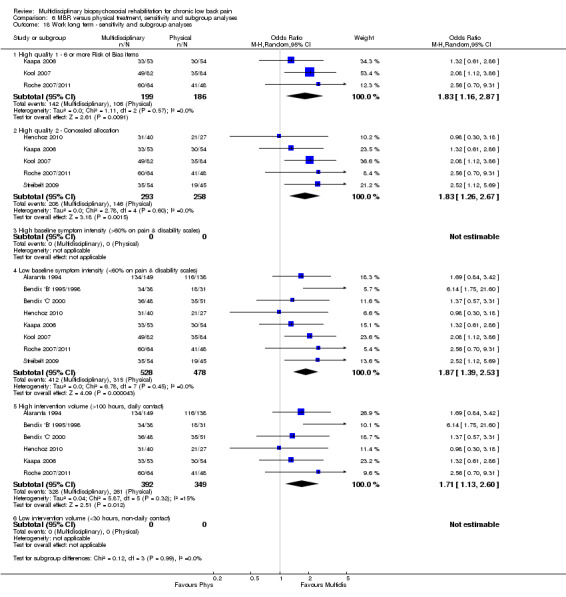
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 18 Work long term ‐ sensitivity and subgroup analyses.
Secondary outcomes
Three studies reported QoL (SF‐36) outcomes in the short and medium term that could be used to calculate pooled effect sizes (Analysis 2.10; Analysis 2.11). Precision was low and the results showed no difference between the groups. Two studies contributed to a pooled estimate of the effect on the number of healthcare visits in the long term (Analysis 2.12), which showed no difference between groups. Seven studies reported on depression (Analysis 2.13; Analysis 2.14; Analysis 2.15), anxiety (Analysis 2.21; Analysis 2.22) and self‐efficacy (Analysis 2.19; Analysis 2.20); the pooled estimates showed no effect in the short, medium or long term. Pooled effects of MBR on coping were statistically significant in the medium and long term (Analysis 2.17; Analysis 2.18) but not in the short term (Analysis 2.16). No included studies reported adverse events specifically associated with the study interventions. One study reported 'side effects' (Smeets 2006/2008), although it was not clear that these were actually adverse events associated with the study interventions.
2.10. Analysis.
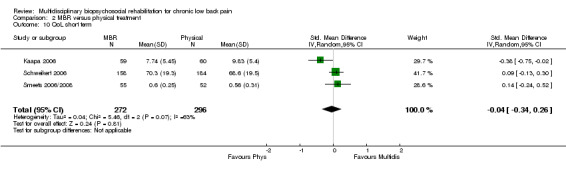
Comparison 2 MBR versus physical treatment, Outcome 10 QoL short term.
2.11. Analysis.
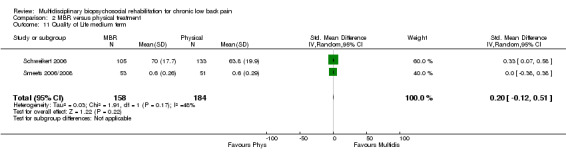
Comparison 2 MBR versus physical treatment, Outcome 11 Quality of Life medium term.
2.12. Analysis.
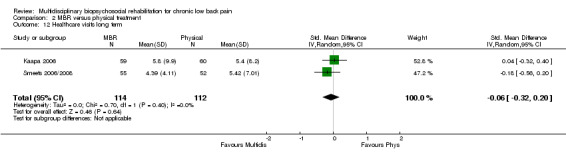
Comparison 2 MBR versus physical treatment, Outcome 12 Healthcare visits long term.
2.13. Analysis.
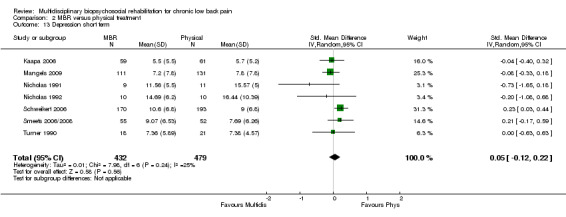
Comparison 2 MBR versus physical treatment, Outcome 13 Depression short term.
2.14. Analysis.

Comparison 2 MBR versus physical treatment, Outcome 14 Depression medium term.
2.15. Analysis.

Comparison 2 MBR versus physical treatment, Outcome 15 Depression long term.
2.21. Analysis.

Comparison 2 MBR versus physical treatment, Outcome 21 Anxiety short term.
2.22. Analysis.
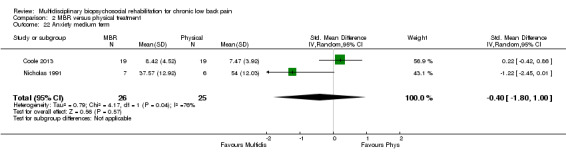
Comparison 2 MBR versus physical treatment, Outcome 22 Anxiety medium term.
2.19. Analysis.
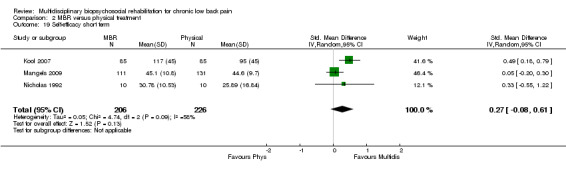
Comparison 2 MBR versus physical treatment, Outcome 19 Self‐efficacy short term.
2.20. Analysis.

Comparison 2 MBR versus physical treatment, Outcome 20 Self‐efficacy medium term.
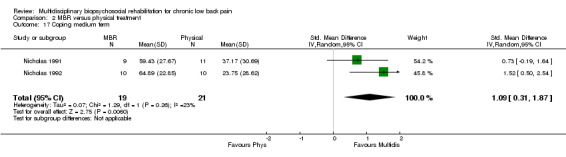
2.17. Analysis.
Comparison 2 MBR versus physical treatment, Outcome 17 Coping medium term.
2.18. Analysis.
Comparison 2 MBR versus physical treatment, Outcome 18 Coping long term.
2.16. Analysis.
Comparison 2 MBR versus physical treatment, Outcome 16 Coping short term.
MBR versus surgery
Two studies reported on the effect of an MBR intervention versus surgery, both studies reported pain and disability in the long term (total n = 423) and one study reported on work outcome in the long term (n = 133).
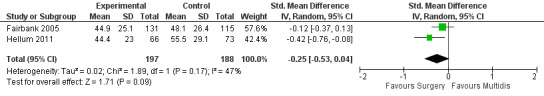
The pooled effect estimates were not significantly different between MBR and surgery for pain (SMD of 0.25) (Figure 25), disability (SMD of 0.25) (Figure 26) or work; the quality of the evidence was low (Table 3). In both studies adverse events associated with the surgical interventions were reported: 19 complications in Fairbank 2005 and six complications in Hellum 2011 with no complications reported in the MBR group in either study (Figure 27).
25.
Forest plot of comparison: 3 Multidisciplinary versus surgery, outcome: 3.1 Pain long term.
26.

Forest plot of comparison: 3 Multidisciplinary versus surgery, outcome: 3.2 Disability long term.
27.
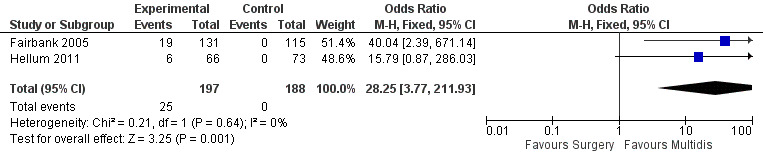
Forest plot of comparison: 3 MBR versus surgery, outcome: 3.4 Adverse events/complications.
Sensitivity and subgroup analyses were not conducted for this comparison due to the small number of included studies.
MBR versus waiting list
Four studies reported on the effect of an MBR intervention versus a waiting list control, and three studies (total n = 213) reported on pain and disability in the short term.
For pain there was a statistically significant difference of 0.73 (SMD) (Figure 28), and for disability a statistically significant difference of 0.49 (SMD) (Figure 29) in the short term. These estimates translated to a difference of approximately 1.7 points on a 0 to 10 pain NRS and 2.9 on a 0 to 24 Roland Morris Disability Questionnaire. The quality of the evidence was very low for pain and low for disability.
28.
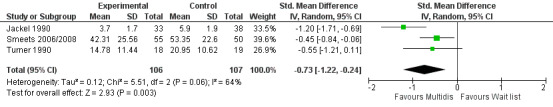
Forest plot of comparison: 4 Multidisciplinary versus wait list, outcome: 4.1 Pain short term.
29.

Forest plot of comparison: 4 Multidisciplinary versus wait list, outcome: 4.2 Disability short term.
Sensitivity and subgroup analyses were not conducted for this comparison due to the small number of included studies.
Other included studies
Twelve studies compared outcomes from two different MBR interventions. A description of these individual studies is provided (Characteristics of included studies) but pooled between group analyses were not conducted. We made this decision because such comparisons did not address the question of whether MBR is more effective that alternative interventions.
Discussion
Summary of main results
We set out to conduct an updated review on the impact of multidisciplinary rehabilitation on people with chronic low back pain (LBP). We found 31 recently published randomised clinical trials which added to the 10 included in the previous review to form a substantial evidence base, with data on close to 7000 people. Overall we found that when compared with usual care, MBR decreased pain and disability to a moderate degree but had little to no effect on work outcomes. When compared with physical rehabilitation, MBR showed moderate effects on pain, disability and work outcomes. Although the quality of the evidence was moderate or low depending on the comparison, the overall size of the effects of MBR was quite consistent. They translate to an average difference in pain of about 1 to 2 points on a 10‐point scale, and an average difference in disability of 2 to 4 points on the 24‐point Roland Morris questionnaire. The improvement of work outcomes with MBR when compared to physical rehabilitation translates to about double the odds of being at work 12 months later. It would seem unlikely that conducting further RCTs will substantially change our view of the mean effect that can be expected from MBR programs.
Several factors need to be taken into account when interpreting our findings to formulate recommendations regarding clinical implementation. The resources and costs associated with delivering MBR programs should be considered and weighed against those of usual care or physical training regimens. For example, MBR in 15 of the included studies required more than 100 face‐to‐face hours of training. Cost‐effectiveness data were not extracted as part of this review. The proportion of people that experienced a clinically relevant improvement was not typically reported in the RCTs and did not form a part of this review; as such we cannot be sure the extent to which the between group difference reflects an important change on behalf of the participants. On the other hand, the people referred to these programs had long‐standing symptoms which had not responded to previous treatments. As a longer duration of symptoms is an indicator of poor prognosis, a modest improvement in symptom severity compared to another treatment may be significant for this population.
For our main comparisons, pooled effects do not appear to have been overestimated due to inclusion of low quality studies. The influence of baseline symptom intensity on the effectiveness of MBR is unclear because the subgroup analyses were hampered by the small numbers of studies that included samples with symptom intensity that met our a priori threshold. Samples recruited to the included studies typically reported moderate levels of pain intensity (4 to 6 points on the NRS) and disability (8 to 12 points on the Roland Morris Questionnaire). When we divided the included studies according to the hours of face‐to‐face intervention we did not find a consistent pattern in favour of either high or low intensity interventions.
The inconsistent nature of data collection and reporting made drawing conclusions regarding the secondary outcomes of quality of life, healthcare utilisation and adverse events difficult. Comparable estimates for these outcomes were reported by too few studies to estimate the effect of MBR. MBR did not appear to have any additional significant effect on symptoms of depression compared to physical rehabilitation.
Only two and four RCTs, respectively, were included in comparisons of MBR with surgery or waiting list controls. From each comparison a pooled estimate was generated for pain and disability at one time point. There was low quality evidence of no significant difference between MBR and surgery. The effects in favour of MBR versus waiting list controls were of moderate size, but the quality of the evidence was very low to low. While 12 studies included a comparison of one MBR intervention versus another, we did not perform a synthesis of these data. Synthesis was not undertaken as it does not directly inform decisions as to whether MBR or some other intervention should be administered in this patient group.
Overall completeness and applicability of evidence
Work outcomes and healthcare utilisation are key considerations for assessing the effects of MBR in this population, since they are primary determinants of the societal burden of the condition (Maetzal 2002). Many of the included studies did not report these outcomes, and when reported they were measured in different ways. The lack of standardisation of measurement in these areas makes quantitative synthesis of the body of evidence problematic. For example, in the MBR versus physical treatment comparison 13 of the 19 studies reported a work‐related outcome measure yet only three, three and eight studies (short, medium, long term) could be included in the meta‐analyses. The fact that these data were not reported in a comparable manner limits our ability to estimate the true effect of MBR for this critical outcome.
The subgroup analysis that investigated the influence of high baseline symptom intensity proved inconclusive, largely because so few studies recruited a sample with high enough intensity. The threshold we chose to indicate high intensity (greater than 60% of the maximum possible score on a pain and a disability measure) is admittedly arbitrary, but it is surprising that only three of the 41 included studies met this criterion. While there is evidence that higher symptom severity at presentation is a prognostic indicator of poor outcome following MBR (van de Hulst 2005; van Hooff 2014; Verkerk 2013), direct evidence that it is a modifier of the effect of MBR is lacking. It could be argued that only those with severe physical symptoms and psychological dysfunction are likely to require, and therefore preferentially benefit from, a comprehensive MBR program. Matching of a more comprehensive and complex intervention with more clinically complex patients makes intuitive sense, and this is supported by recent evidence from the primary care setting (Hill 2011).
Quality of the evidence
Only 32% of the included studies met our threshold for low risk of bias (fulfilling at least six items from the CBRG risk of bias tool) and not surprisingly all meta‐analyses included studies with high risk of bias. We applied a stringent rule that inclusion of any (one or more) studies at high risk of bias in a meta‐analysis meant downgrading the quality of the evidence by one level within the GRADE system. Such decisions involve a degree of subjective judgement and a more relaxed interpretation of the risk of bias may have resulted in the conclusion that the quality of the evidence in support of the effectiveness of MBR was stronger. To explore this issue we conducted sensitivity analyses. Although not conclusive, the sensitivity analyses did not indicate that inclusion of lower quality studies resulted in overestimation of the effect. This, along with the consistency of the size of the pooled effects on pain and disability, gives confidence that the reported estimates for the primary outcomes are robust. Quality of evidence was also commonly downgraded for inconsistency, this was particularly evident when one outlying study was included in the meta analyses. Exclusion of this study resulted in more consistent and precise pooled estimates for pain and disability across the time points in the MBR versus physical treatment comparison, and provides further evidence that the estimates are robust.
Potential biases in the review process
There is no universally accepted definition of what constitutes MBR. The authors have chosen a definition based on their interpretation of the biopsychosocial model and reflective of the different expertise within the various clinical professions. Presumably it is possible that selection of a different definition could result in inclusion of different studies and hence different effect estimates.
The MBR interventions evaluated in the included studies differed from each other in a number of ways. There were differences in the number of face‐to‐face sessions and the intensity of the treatment; differences in the settings; differences in the balance of the interventions in terms of focus on physical, psychological and social factors; and differences in the backgrounds of the clinicians that administered the interventions. This clinical heterogeneity is likely due to varying conceptualisations of MBR and also to uncertainty regarding the pathological cause of non‐specific LBP. Further heterogeneity is also introduced by differences in the control interventions. By using a random‐effects model for generating the pooled estimates and incorporating the I2 statistic into the evidence quality assessment we have attempted to account for this heterogeneity.
While most studies measured pain intensity in a similar manner, there was great variability in the measures used for other domains. Despite the efforts of initiatives such as COMET (Williamson 2012) and IMMPACT (Dworkin 2005), the findings indicate that there is little consensus on the choice of measurement instruments. This renders meaningful synthesis of the body of evidence difficult and may also introduce bias as decisions must be made regarding which outcome measures should be included in the pooled effect estimates. In addition, the lack of a core outcome measurement set increases the chances of selective reporting of results of trials (Williamson 2012). Only one study in this review met the criterion for absence of reporting bias (Tavafian 2011). It is to be hoped that current efforts to increase the registration of trials (Costa 2012) and publication of detailed protocols will improve this situation in the future.
The influence of publication bias on the results is difficult to assess due to the number of studies contributing to each pooled estimate. Only three estimates included the minimum 10 studies recommended by The Cochrane Collaboration for formal assessment of publication bias. The funnel plots for these estimates indicate the possibility of small study bias, a possibility also reported in the review conducted by Norlund 2009.
Agreements and disagreements with other studies or reviews
Guzman 2006 reviewed the literature up to 1998 and found that intensive MBR programs had important effects on disability outcomes and small effects on pain. Evidence regarding work outcomes was equivocal. A 'levels of evidence' synthesis performed by van Geen 2007 reported positive effects on work participation but not on pain or disability; their study performed searches up until 2003 and included 10 studies, of which seven are in the current review. Ravenek 2010 included 12 studies from 1998 onwards, of which seven were common to this review, and the authors reported conflicting evidence from low quality studies on work outcomes, no effect on pain, and no effect on function. Norlund 2009 conducted a systematic review of studies involving people with subacute or chronic LBP and included three studies with chronic LBP, all are in this review. Their meta‐analysis showed no effect on return to work for MBR interventions in the chronic LBP studies. It is somewhat surprising that the present review showed no impact of MBR on work outcomes when compared to usual care but a moderate impact when compared to physical treatment. One possibility is that studies comparing MBR to usual care included populations with less severe occupational impairment (thus harder to show an impact). Another possibility is that physical treatment when not paired with concomitant psychological or social interventions may promote a sick role and interfere with attainment of occupational goals.
Few included studies involved an explicitly designed and focused workplace intervention, an issue also identified by Ravenek 2010. They also observed that occupational therapists were rarely involved in multidisciplinary rehabilitation interventions despite the fact that improvement of work‐related outcomes is an often stated goal. They and others (Ektor‐Andersen 2008) also pointed out the need for a greater degree of cooperation between workers, employers and insurers to facilitate these outcomes.
No clear conclusions can be drawn regarding whether the intensity of the intervention had an influence on the size of the treatment effect. While the previous version of this review suggested that this factor may be important, a recent systematic review (Waterschoot 2014) was inconclusive and found that dose factors could not be disentangled from content factors when determining their influence on the effect size. Individual RCTs conducted by Rose 1997 and Skouen 2002 reported no substantial differences in effect between multidisciplinary programs of differing intensity. At this point in time it is unclear how much face‐to‐face time is optimal for MBR interventions. This is a critical question given the role of face‐to‐face time in driving the cost of MBR.
Authors' conclusions
Implications for practice.
Choosing an MBR intervention over usual care or a physical treatment program for chronic low back pain is likely to result in a positive effect on pain and disability outcomes. It is also likely that MBR will have a beneficial effect on work outcomes compared to physical treatment. However, given the moderate size of these effects and the potentially high cost of an intensive intervention, in terms of both the monetary and time burden, the decision to refer to MBR requires some consideration. While our subgroup analyses were inconclusive regarding the influence of higher or lower symptom intensity at baseline, it would appear there is little to gain by referring those without substantial physical and psychosocial impacts of their condition to such an intervention. Clinical practice guidelines (Dagenais 2010) commonly recommend assessment and treatment of physical and psychosocial factors and referral to appropriately trained clinicians for management of these factors where present. This recommendation would seem more appropriate than a recommendation of MBR simply based on chronicity of symptoms.
Implications for research.
The quality of the evidence regarding the primary outcomes is at best moderate, although consistent in terms of effect size. Despite this, the volume of evidence is substantial and conducting further, similar studies is unlikely to greatly change the estimate of the effectiveness of MBR versus usual care or physical treatment. This being the case, it is important to consider whether the effect is clinically worthwhile. The ideal methods for determining whether an effect is clinically worthwhile are far from settled (Ferreira 2012) but the point has been made that such an estimation needs to take the cost of the intervention into account. In this case costs include not only those of the intervention in terms of time, inconvenience and money but also costs of other healthcare utilisation (for example medications, visits to healthcare providers) and costs of productivity losses due to low back pain, as compared to those associated with other options.
The results of this review could be said to mirror those of others in the low back pain field in that small effects are observed between the index and control interventions (Hayden 2005; Henschke 2011; Rubinstein 2011). This situation is largely due to the fact that the pathology underlying non‐specific low back pain is, at best, unclear. Given that this is the case, it is unsurprising that the mechanism of effect of the different intervention options is also unknown. Studies that investigate the mechanism of effect of different treatments (Mansell 2013; Smeets 2006) and investigate the effectiveness of treatments in subgroups (Foster 2013; Hancock 2009; Kamper 2010) of patients have the potential to improve our understanding of the condition.
Recent studies that have conducted cost‐effectiveness analyses of MBR for chronic pain have produced conflicting results. Lambeek 2010 found that their MBR program was cost‐effective compared to usual care in the Netherlands; Smeets 2009 reported that while graded activity plus problem solving was cost‐effective compared to a physical program, the combination of graded activity, problem solving and physical therapy was not cost‐effective compared to physical therapy only. Interpretation of cost‐effectiveness studies requires some sophistication, not least of all consideration of to whom the costs and benefits are apportioned. Substantial benefits may be evident at a societal level despite a modest mean clinical effect at an individual level, but the issue is complicated by the question of whether the costs are borne by the state, by insurers, or by the individual. Most of the societal costs associated with back pain are indirect costs, primarily work productivity losses (Lambeek 2011; Maetzal 2002). Of interest then is whether research should focus on improving our capacity to identify those people at greatest risk of work disability (prognostic studies) and treat them distinctly from those whose back pain typically does not result in work absences or reduced productivity. Further, incorporation into MBR of treatment modalities that specifically focus on re‐integration to the work place would be of value. It is recognised that in order to do so communication and collaboration barriers between employers, the healthcare system and insurance companies must be addressed.
What's new
| Date | Event | Description |
|---|---|---|
| 21 May 2014 | New search has been performed | Inclusion of 31 new RCTs since the previous version, this enabled performance of several meta‐analyses rather than narrative descriptions of results only. Updated methods included up‐to‐date risk of bias assessment and summary synthesis of quality of evidence using the GRADE system. |
| 21 May 2014 | New citation required and conclusions have changed | The broad conclusions are in agreement with the previous version in that MBR is effective compared to usual care and non‐MBR interventions for chronic LBP. This review provides quantification of the mean effect size and does not confirm the finding that more intensive interventions resulted in larger effects. It is unlikely that conducting further RCTs comparing MBR to usual care or physical treatments will change our estimate of the effect of these interventions. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 24 June 2008 | Amended | Converted to new review format |
| 3 February 2006 | Amended | Feb 3/06 ‐ contact author informed that review will be tagged as 'withdrawn' in this month's submission. It will be re‐instated once the update has been submitted and approved for publication. Literature search last done in 1998. VEP |
| 30 October 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Teresa Marin, Rachel Couban and Shireen Harbin from the Cochrane Back Review group for support and for developing and conducting the electronic searches.
Appendices
Appendix 1. Search strategies
MEDLINE
Ovid MEDLINE(R) <1946 to January Week 4 2014>, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations <January 30, 2014>
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
exp Patient Care Team/
exp Patient Care Management/
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinics/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centr$).mp.
exp Social Work/
exp Occupational Therapy/
exp Rehabilitation/ or exp Rehabilitation Centers/ or exp Rehabilitation, Vocational/
exp Treatment Outcome/
exp Behavior Therapy/
"Recovery of Function"/
functional restoration.mp.
*Pain/rh
or/12‐30
exp Arthritis, Rheumatoid/
exp Neoplasms/
exp Musculoskeletal Diseases/cn, su [Congenital, Surgery]
exp Central Nervous System/
exp Central Nervous System Diseases/
exp Dentistry/
exp Tooth Diseases/
or/32‐38
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/40‐50
51 and 11 and 31
52 not 39
EMBASE
Embase <1980 to 2014 Week 10>
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 or 30
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
32 and 33
32 not 34
31 not 35
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
patient care team.mp.
exp Patient Care/
patient care management.mp.
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinic/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centre$).mp.
exp Occupational Therapy/
exp Social Work/
exp Vocational Rehabilitation/
exp Rehabilitation Center/
rehabilitation clinic$.mp.
exp REHABILITATION/
exp Treatment Outcome/
behavior therapy.mp. or exp Behavior Therapy/
or/37‐56
exp Rheumatoid Arthritis/
exp NEOPLASM/
exp Musculoskeletal Disease/cn, su [Congenital Disorder, Surgery]
exp Central Nervous System/
exp Central Nervous System Disease/
exp Tooth Disease/
exp Musculoskeletal System Inflammation/
exp Musculoskeletal System Malformation/
exp HEADACHE/
exp Osteoarthritis/
or/58‐67
36 and 57
69 not 68
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low Back Pain/
or/71‐81
70 and 82
CENTRAL
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumbar next pain OR coccyx OR coccydynia OR sciatica OR spondylosis
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor: [Intervertebral Disk] explode all trees
#13 postlaminectomy
#14 arachnoiditis
#15 failed near back
#16 MeSH descriptor: [Cauda Equina] explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 MeSH descriptor: [Patient Care Team] this term only
#30 MeSH descriptor: [Patient Care Management] explode all trees
#31 MeSH descriptor: [Comprehensive Health Care] explode all trees
#32 MeSH descriptor: [Pain Clinics] explode all trees
#33 multidisciplinary
#34 interdisciplinary
#35 multiprofessional
#36 multi‐professional
#37 multimodal
#38 multi‐modal
#39 pain clinic
#40 functional restoration
#41 biopsychosocial
#42 MeSH descriptor: [Patient Education as Topic] explode all trees
#43 MeSH descriptor: [Social Support] explode all trees
#44 MeSH descriptor: [Social Environment] explode all trees
#45 (pain clinic* or pain center* or pain service* or pain relief unit* or pain centr*)
#46 MeSH descriptor: [Social Work] explode all trees
#47 MeSH descriptor: [Occupational Therapy] explode all trees
#48 MeSH descriptor: [Rehabilitation, Vocational] explode all trees
#49 MeSH descriptor: [Rehabilitation Centers] explode all trees
#50 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49
#51 #28 and #50, in Trials
CINAHL
S86 S85 NOT S84
S85 S49 and S76
S84 S77 or S78 or S79 or S80 or S81 or S82 or S83
S83 (MH "Tooth Diseases+")
S82 (MH "Dentistry+")
S81 (MH "Central Nervous System Diseases+")
S80 (MH "Central Nervous System+")
S79 (MH "Musculoskeletal Diseases/FG/SU")
S78 (MH "Neoplasms+")
S77 (MH "Arthritis, Rheumatoid+")
S76 S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58
or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68
or S69 or S70 or S71 or S72 or S73 or S74 or S75
S75 (MH "Behavior Therapy+")
S74 (MH "Treatment Outcomes+")
S73 "rehabilitation clinic*"
S72 (MH "Rehabilitation Centers+")
S71 (MH "Rehabilitation+")
S70 (MH "Rehabilitation, Vocational+")
S69 (MH "Occupational Therapy+")
S68 (MH "Social Work+")
S67 "pain relief unit*" 5
S66 "pain service*" 161
S65 "pain centre*" 21
S64 "pain center*" 141
S63 (MH "Pain Clinics") 368
S62 (MH "Social Environment+") 24,968
S61 (MH "Support, Psychosocial+") 36,808
S60 (MH "Patient Education") 37,325
S59 "patient care management" 81
S58 (MH "Patient Centered Care") 11,891
S57 "patient care team" 63
S56 (MH "Combined Modality Therapy+") 16,090
S55 "multimodal" 1,509
S54 multiprofessional 561
S53 (MH "Collaboration")
S52 "interdisciplinary"
S51 "multidisciplinary"
S50 (MH "Multidisciplinary Care Team+")
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
PsycINFO
PsycINFO <2002 to January Week 3 2014>
1. clinical trials/
2. controlled trial.mp.
3. RCT.mp.
4. (Random* adj3 trial).mp.
5. (clin* adj3 trial).mp.
6. (sing* adj2 blind*).mp.
7. (doub* adj2 blind*).mp.
8. placebo.mp. or exp Placebo/
9. latin square.mp.
10. (random* adj2 assign*).mp.
11. prospective studies/
12. (prospective adj stud*).mp.
13. (comparative adj stud*).mp.
14. treatment effectiveness evaluation/
15. treatment effectiveness evaluation/
16. (evaluation adj stud*).mp.
17. exp Posttreatment Followup/
18. follow?up stud*.mp.
19. or/1‐18
20. back pain/
21. lumbar spinal cord/
22. (low adj back adj pain).mp.
23. (back adj pain).mp.
24. spinal column/
25. (lumbar adj2 vertebra*).mp.
26. coccyx.mp.
27. sciatica.mp.
28. lumbago.mp.
29. dorsalgia.mp.
30. back disorder*.mp.
31. "back (anatomy)"/
32. ((disc or disk) adj degenerat*).mp.
33. ((disc or disk) adj herniat*).mp.
34. ((disc or disk) adj prolapse*).mp.
35. (failed adj back).mp.
36. or/20‐35
37. 19 and 36
38. interdisciplinary treatment approach/
39. multimodal treatment approach/
40. multidisciplinary.mp.
41. patient care team.mp.
42. patient care management.mp.
43. client education/
44. Patient Education.mp.
45. social support/
46. Social Environments/
47. biopsychosocial approach/
48. pain clinic.mp.
49. pain center.mp.
50. pain centre.mp.
51. social casework/
52. exp case management/
53. occupational therapy/
54. rehabilitation centers/
55. exp vocational rehabilitation/
56. interdisciplinary.mp.
57. multiprofessional.mp.
58. or/38‐57
59. 37 and 58
Data and analyses
Comparison 1. MBR versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Back pain short term | 9 | 879 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.83, ‐0.28] |
| 2 Back pain medium term | 6 | 740 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.85, ‐0.34] |
| 3 Back pain long term | 7 | 821 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.04] |
| 4 Disability short term | 9 | 939 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.62, ‐0.19] |
| 5 Disability medium term | 6 | 786 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.66, ‐0.19] |
| 6 Disability long term | 6 | 722 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.40, ‐0.06] |
| 7 Work short term | 2 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.90] |
| 8 Work medium term | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.52, 4.91] |
| 9 Work long term | 7 | 1360 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 10 QoL SF36 PCS short term | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 13.45 [‐9.07, 35.96] |
| 11 QoL SF36 MCS short term | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 15.25 [2.05, 28.44] |
| 12 QoL SF36 PCS medium term | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 7.41 [‐4.99, 19.81] |
| 13 QoL SF36 MCS medium term | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 7.59 [1.69, 13.49] |
| 14 Catastrophising short term | 2 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.83, ‐0.03] |
| 15 Catastrophising long term | 2 | 127 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.76, ‐0.05] |
| 16 Fear avoidance short term | 2 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.52, 0.14] |
| 17 Fear avoidance long term | 3 | 371 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.49, ‐0.08] |
1.1. Analysis.
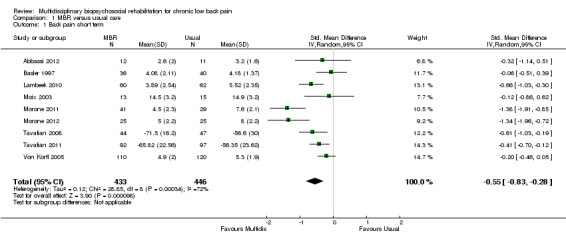
Comparison 1 MBR versus usual care, Outcome 1 Back pain short term.
1.2. Analysis.
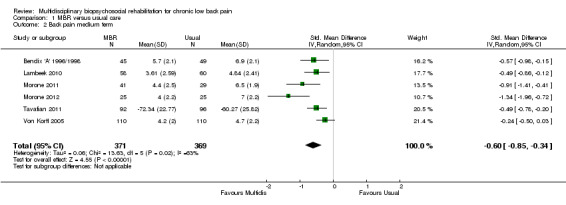
Comparison 1 MBR versus usual care, Outcome 2 Back pain medium term.
1.3. Analysis.

Comparison 1 MBR versus usual care, Outcome 3 Back pain long term.
1.4. Analysis.

Comparison 1 MBR versus usual care, Outcome 4 Disability short term.
1.5. Analysis.
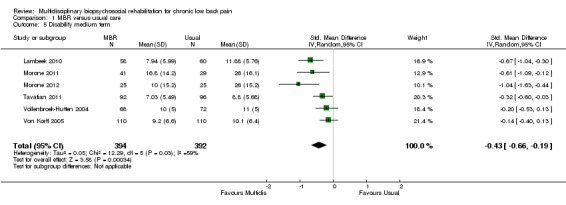
Comparison 1 MBR versus usual care, Outcome 5 Disability medium term.
1.6. Analysis.
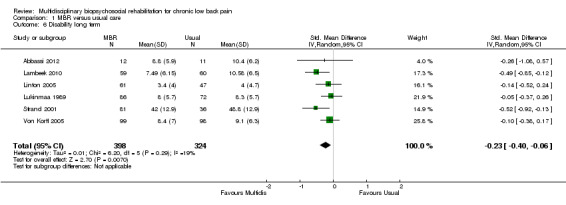
Comparison 1 MBR versus usual care, Outcome 6 Disability long term.
1.7. Analysis.
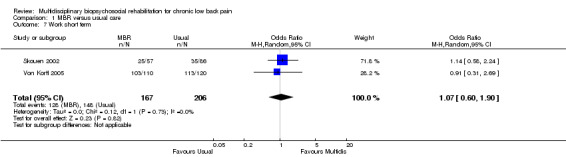
Comparison 1 MBR versus usual care, Outcome 7 Work short term.
1.8. Analysis.
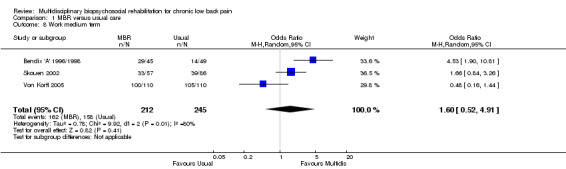
Comparison 1 MBR versus usual care, Outcome 8 Work medium term.
1.9. Analysis.
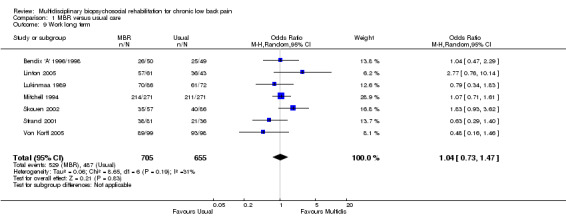
Comparison 1 MBR versus usual care, Outcome 9 Work long term.
Comparison 2. MBR versus physical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short term | 12 | 1661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.54, ‐0.06] |
| 2 Pain medium term | 9 | 531 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.54, ‐0.02] |
| 3 Pain long term | 9 | 872 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.04, 0.01] |
| 4 Disability short term | 13 | 1878 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.68, ‐0.10] |
| 5 Disability medium term | 9 | 511 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.48, 0.06] |
| 6 Disability long term | 10 | 1169 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.19, ‐0.16] |
| 7 Work short term | 3 | 379 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.92, 2.78] |
| 8 Work medium term | 3 | 221 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.12, 4.10] |
| 9 Work long term | 8 | 1006 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.39, 2.53] |
| 10 QoL short term | 3 | 568 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.34, 0.26] |
| 11 Quality of Life medium term | 2 | 342 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.12, 0.51] |
| 12 Healthcare visits long term | 2 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.32, 0.20] |
| 13 Depression short term | 7 | 911 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
| 14 Depression medium term | 7 | 411 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.42, 0.09] |
| 15 Depression long term | 5 | 506 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.40, 0.30] |
| 16 Coping short term | 3 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.45] |
| 17 Coping medium term | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.09 [0.31, 1.87] |
| 18 Coping long term | 2 | 262 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.06, 0.54] |
| 19 Self‐efficacy short term | 3 | 432 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.08, 0.61] |
| 20 Self‐efficacy medium term | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.40, 0.92] |
| 21 Anxiety short term | 2 | 377 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.67, 0.47] |
| 22 Anxiety medium term | 2 | 51 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.80, 1.00] |
2.1. Analysis.
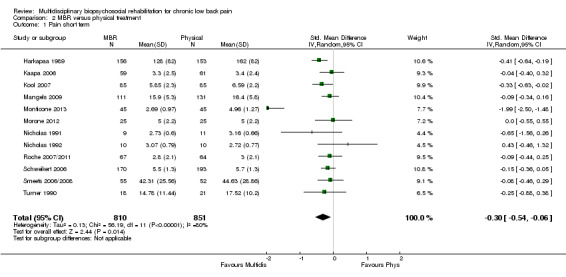
Comparison 2 MBR versus physical treatment, Outcome 1 Pain short term.
2.2. Analysis.
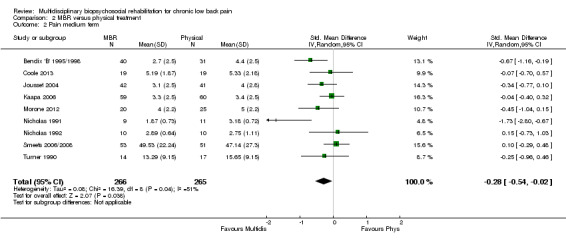
Comparison 2 MBR versus physical treatment, Outcome 2 Pain medium term.
2.3. Analysis.
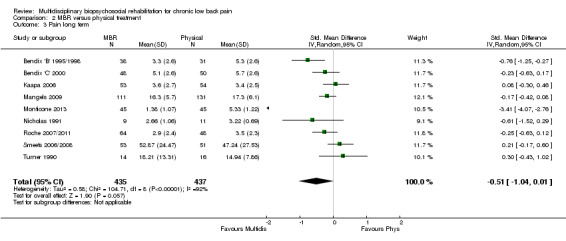
Comparison 2 MBR versus physical treatment, Outcome 3 Pain long term.
2.4. Analysis.
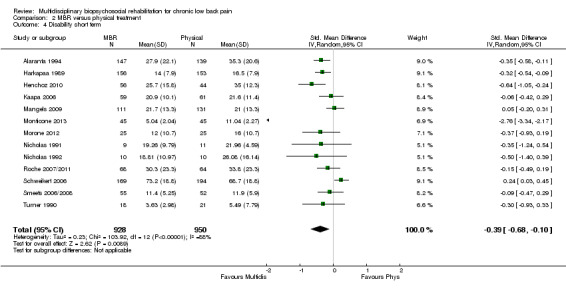
Comparison 2 MBR versus physical treatment, Outcome 4 Disability short term.
2.5. Analysis.
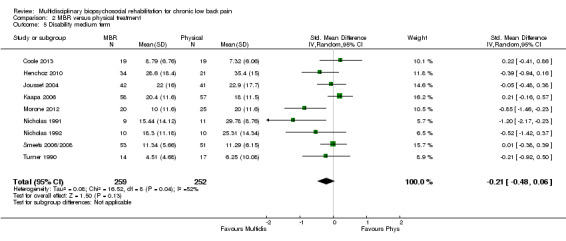
Comparison 2 MBR versus physical treatment, Outcome 5 Disability medium term.
2.6. Analysis.
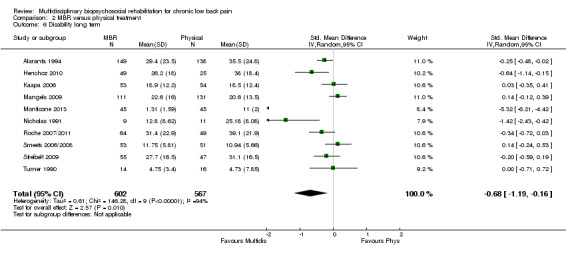
Comparison 2 MBR versus physical treatment, Outcome 6 Disability long term.
2.7. Analysis.
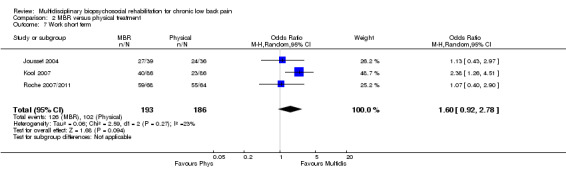
Comparison 2 MBR versus physical treatment, Outcome 7 Work short term.
2.8. Analysis.

Comparison 2 MBR versus physical treatment, Outcome 8 Work medium term.
2.9. Analysis.
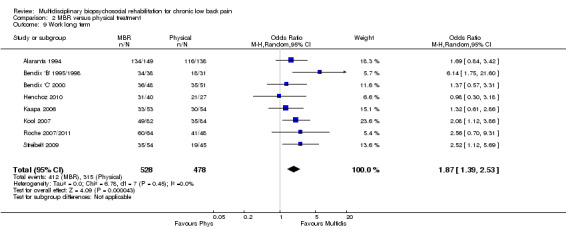
Comparison 2 MBR versus physical treatment, Outcome 9 Work long term.
Comparison 3. MBR versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain long term | 2 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.53, 0.04] |
| 2 Disability long term | 2 | 423 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.08, 0.57] |
| 3 Work long term | 1 | 133 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.31, 1.45] |
| 4 Adverse events/complications | 2 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.25 [3.77, 211.93] |
| 5 QoL SF36 PCS long term | 2 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.70, 0.14] |
| 6 QoL SF36 MCS long term | 2 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.19] |
3.1. Analysis.

Comparison 3 MBR versus surgery, Outcome 1 Pain long term.
3.2. Analysis.
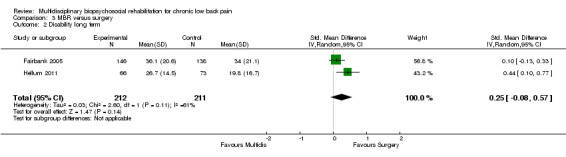
Comparison 3 MBR versus surgery, Outcome 2 Disability long term.
3.3. Analysis.
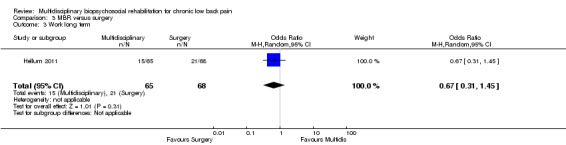
Comparison 3 MBR versus surgery, Outcome 3 Work long term.
3.4. Analysis.
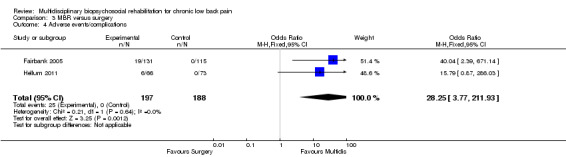
Comparison 3 MBR versus surgery, Outcome 4 Adverse events/complications.
3.5. Analysis.

Comparison 3 MBR versus surgery, Outcome 5 QoL SF36 PCS long term.
3.6. Analysis.
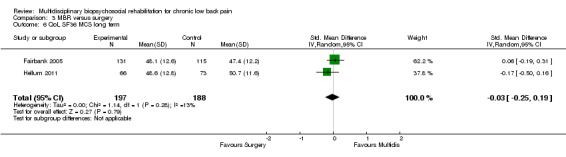
Comparison 3 MBR versus surgery, Outcome 6 QoL SF36 MCS long term.
Comparison 4. MBR versus wait list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short term | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.22, ‐0.24] |
| 2 Disability short term | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.76, ‐0.22] |
| 3 Depression short term | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.59, 0.18] |
4.1. Analysis.
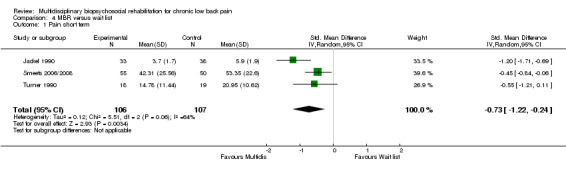
Comparison 4 MBR versus wait list, Outcome 1 Pain short term.
4.2. Analysis.
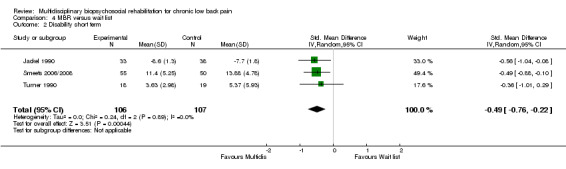
Comparison 4 MBR versus wait list, Outcome 2 Disability short term.
4.3. Analysis.

Comparison 4 MBR versus wait list, Outcome 3 Depression short term.
Comparison 5. MBR versus usual care, sensitivity and subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short term ‐ all studies | 9 | 879 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.83, ‐0.28] |
| 2 Pain short term ‐ sensitivity and subgroup analyses | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 High quality 1 ‐ 6 or more Risk of Bias items | 3 | 334 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.71, ‐0.27] |
| 2.2 High quality 2 ‐ Concealed allocation | 4 | 431 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐0.91, ‐0.51] |
| 2.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.03, ‐0.30] |
| 2.4 Low baseline symptom intensity (<60% on pain & disability scales) | 8 | 757 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.59, ‐0.30] |
| 2.5 High intervention intensity (>100 hours, daily contact) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Low intervention intensity (<100 hours, non‐daily contact) | 6 | 477 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.61, ‐0.24] |
| 3 Pain medium term ‐ all studies | 6 | 740 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.85, ‐0.34] |
| 4 Pain medium term ‐ sensitivity and subgroup analyses | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 High quality 1 ‐ 6 or more Risk of Bias items | 2 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] |
| 4.2 High quality 2 ‐ Concealed allocation | 4 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.07, ‐0.39] |
| 4.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 118 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.86, ‐0.12] |
| 4.4 Low baseline symptom intensity (<60% on pain & disability scales) | 5 | 622 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.96, ‐0.32] |
| 4.5 High intervention intensity (>100 hours, daily contact) | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.98, ‐0.15] |
| 4.6 Low intervention intensity (<30 hours, non‐daily contact) | 4 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.12, ‐0.25] |
| 5 Pain long term ‐ all studies | 7 | 821 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.04] |
| 6 Pain long term ‐ sensitivity and subgroup analyses | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 High quality 1 ‐ 6 or more Risk of Bias items | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.47, 0.19] |
| 6.2 High quality 2 ‐ Concealed allocation | 2 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.45, 0.09] |
| 6.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.47, 0.24] |
| 6.4 Low baseline symptom intensity (<60% on pain & disability scales) | 6 | 702 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.42, ‐0.03] |
| 6.5 High intervention intensity (>100 hours, daily contact) | 2 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.52, 0.04] |
| 6.6 Low intervention intensity (<30 hours, non‐daily contact) | 4 | 447 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.50, ‐0.12] |
| 7 Disability short term ‐ all analyses | 9 | 939 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.62, ‐0.19] |
| 8 Disability short term ‐ sensitivity and subgroup analyses | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 High quality 1 ‐ 6 more or Risk of Bias items | 4 | 485 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.71, 0.08] |
| 8.2 High quality 2 ‐ Concealed allocation | 5 | 582 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.80, ‐0.30] |
| 8.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.16, ‐0.42] |
| 8.4 Low baseline symptom intensity (<60% on pain & disability scales) | 8 | 817 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.56, ‐0.13] |
| 8.5 High intervention intensity (>100 hours, daily contact) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Low intervention intensity (<30 hours, non‐daily contact) | 7 | 599 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.75, ‐0.11] |
| 9 Disability medium term ‐ all studies | 6 | 786 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.66, ‐0.19] |
| 10 Disability medium term ‐ sensitivity and subgroup analyses | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 High quality 1 ‐ 6 or more Risk of Bias items | 3 | 446 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.63, ‐0.12] |
| 10.2 High quality 2 ‐ Concealed allocation | 5 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.76, ‐0.25] |
| 10.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 118 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.04, ‐0.30] |
| 10.4 Low baseline symptom intensity (<60% on pain & disability scales) | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.62, ‐0.13] |
| 10.5 High intervention intensity (>100 hours, daily contact) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Low intervention intensity (<30 hours, non‐daily contact) | 4 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.95, ‐0.18] |
| 11 Disability long term ‐ all studies | 6 | 722 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.40, ‐0.06] |
| 12 Disability long term ‐ sensitivity and subgroup analyses | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 High quality 1 ‐ 6 or more Risk of Bias items | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.78, ‐0.11] |
| 12.2 High quality 2 ‐ Concealed allocation | 2 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.77, ‐0.23] |
| 12.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.85, ‐0.12] |
| 12.4 Low baseline symptom intensity (<60% on pain & disability scales) | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.34, ‐0.01] |
| 12.5 High intervention intensity (>100 hours, daily contact) | 1 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.92, ‐0.13] |
| 12.6 Low intervention intensity (<30 hours, non‐daily contact) | 4 | 447 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.41, ‐0.03] |
| 13 Work short term ‐ all studies | 2 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.60, 1.90] |
| 14 Work short term ‐ sensitivity and subgroup analyses | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 High quality 1 ‐ 6 or more Risk of Bias items | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 High quality 2 ‐ Concealed allocation | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.58, 2.24] |
| 14.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Low baseline symptom intensity (<60% on pain & disability scales) | 1 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.31, 2.69] |
| 14.5 High intervention volume (>100 hours, daily contact) | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.58, 2.24] |
| 14.6 Low intervention volume (<30 hours, non‐daily contact) | 1 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.31, 2.69] |
| 15 Work medium term all studies | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.52, 4.91] |
| 16 Work medium term ‐ sensitivity and subgroup analyses | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 High quality 1 ‐ 6 or more Risk of Bias items | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 High quality 2 ‐ Concealed allocation | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.84, 3.26] |
| 16.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Low baseline symptom intensity (<60% on pain & disability scales) | 2 | 314 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.17, 13.75] |
| 16.5 High intervention volume (>100 hours, daily contact) | 2 | 237 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.99, 7.04] |
| 16.6 Low intervention volume (<30 hours, non‐daily contact) | 1 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.16, 1.44] |
| 17 Work long term ‐ all studies | 7 | 1360 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 18 Work long term ‐ sensitivity and subgroup analyses | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 High quality 1 ‐ 6 or more Risk of Bias items | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 High quality 2 ‐ Concealed allocation | 2 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.39, 3.11] |
| 18.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Low baseline symptom intensity (<60% on pain & disability scales) | 5 | 675 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.54, 1.36] |
| 18.5 High intervention volume (>100 hours, daily contact) | 4 | 901 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.76, 1.58] |
| 18.6 Low intervention volume (<30 hours, non‐daily contact) | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.20, 6.22] |
5.1. Analysis.
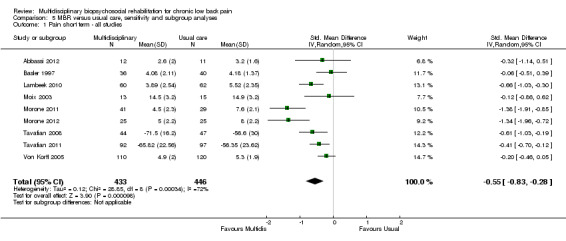
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 1 Pain short term ‐ all studies.
5.3. Analysis.
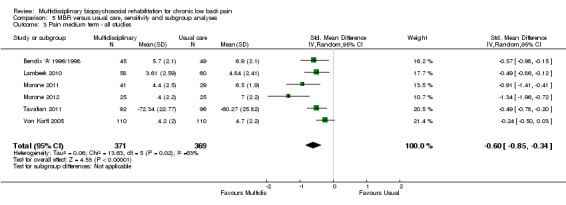
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 3 Pain medium term ‐ all studies.
5.5. Analysis.
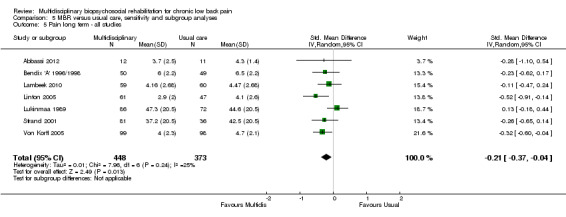
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 5 Pain long term ‐ all studies.
5.7. Analysis.
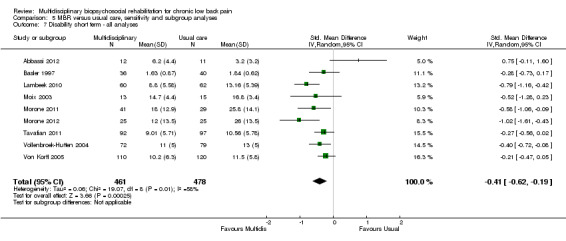
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 7 Disability short term ‐ all analyses.
5.9. Analysis.
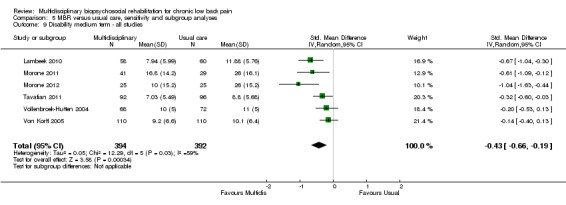
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 9 Disability medium term ‐ all studies.
5.11. Analysis.
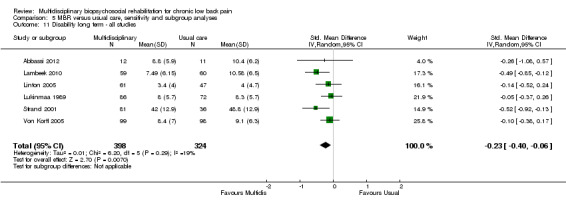
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 11 Disability long term ‐ all studies.
5.13. Analysis.
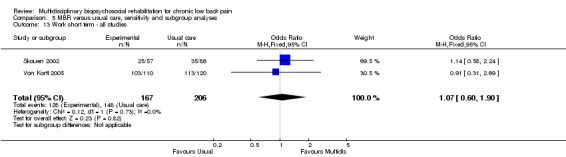
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 13 Work short term ‐ all studies.
5.15. Analysis.
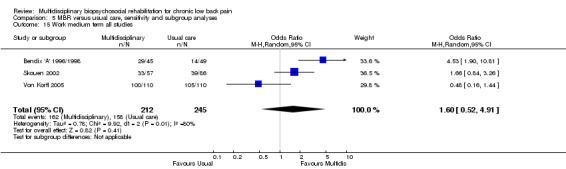
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 15 Work medium term all studies.
5.17. Analysis.
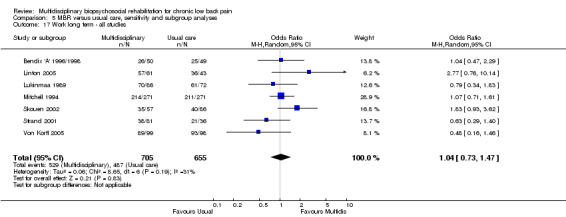
Comparison 5 MBR versus usual care, sensitivity and subgroup analyses, Outcome 17 Work long term ‐ all studies.
Comparison 6. MBR versus physical treatment, sensitivity and subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short term ‐ all studies | 11 | 1352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.57, ‐0.01] |
| 2 Pain short term ‐ sensitivity and subgroup analyses | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 High quality 1 ‐ 6 or more Risk of Bias items | 6 | 860 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.85, 0.03] |
| 2.2 High quality 2 ‐ Concealed allocation | 10 | 1313 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.59, ‐0.00] |
| 2.3 High baseline symptom intensity (>60% on pain & disability scales) | 2 | 453 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.86, 0.74] |
| 2.4 Low baseline symptom intensity (<60% on pain & disability scales) | 9 | 899 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, 0.00] |
| 2.5 High intervention intensity 1 (>100 hours, daily contact) | 4 | 856 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 2.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.44, 0.41] |
| 3 Pain medium term ‐ all studies | 9 | 531 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.54, ‐0.02] |
| 4 Pain medium term ‐ sensitivity and subgroup analyses | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 High quality 1 ‐ 6 or more Risk of Bias items | 2 | 223 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.24, 0.29] |
| 4.2 High quality 2 ‐ Concealed allocation | 5 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.69, 0.19] |
| 4.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Low baseline symptom intensity (<60% on pain & disability scales) | 9 | 531 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.54, ‐0.02] |
| 4.5 High intervention intensity 1 (>100 hours, daily contact) | 3 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.68, 0.04] |
| 4.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 5 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.88, 0.10] |
| 5 Pain long term ‐ all studies | 9 | 872 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.04, 0.01] |
| 6 Pain long term ‐ sensitivity and subgroup analyses | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 High quality 1 ‐ 6 or more Risk of Bias items | 5 | 655 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.50, 0.17] |
| 6.2 High quality 2 ‐ Concealed allocation | 6 | 675 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.41, 0.10] |
| 6.3 High baseline symptom intensity (>60% on pain & disability scales) | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐4.07, ‐2.76] |
| 6.4 Low baseline symptom intensity (<60% on pain & disability scales) | 8 | 782 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.37, 0.06] |
| 6.5 High intervention intensity 1 (>100 hours, daily contact) | 5 | 628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.45, ‐0.01] |
| 6.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 3 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐3.64, 1.13] |
| 7 Disability short term ‐ all studies | 13 | 1878 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.68, ‐0.10] |
| 8 Disability short term ‐ sensitivity and subgroup analyses | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 High quality 1 ‐ 6 or more Risk of Bias items | 5 | 691 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.27, 0.15] |
| 8.2 High quality 2 ‐ Concealed allocation | 10 | 1244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.84, ‐0.02] |
| 8.3 High baseline symptom intensity (>60% on pain & disability scales) | 2 | 453 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐4.18, 1.69] |
| 8.4 Low baseline symptom intensity (<60% on pain & disability scales) | 11 | 1425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.36, ‐0.11] |
| 8.5 High intervention intensity 1 (>100 hours, daily contact) | 7 | 1552 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.38, 0.06] |
| 8.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.93, 0.19] |
| 9 Disability medium term ‐ all studies | 9 | 511 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.48, 0.06] |
| 10 Disability medium term ‐ sensitivity and subgroup analyses | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 High quality 1 ‐ 6 or more Risk of Bias items | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.15, 0.38] |
| 10.2 High quality 2 ‐ Concealed allocation | 6 | 359 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.75, 0.05] |
| 10.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Low baseline symptom intensity (<60% on pain & disability scales) | 9 | 560 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 10.5 High intervention intensity 1 (>100 hours, daily contact) | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.16, 0.29] |
| 10.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 5 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.95, 0.03] |
| 11 Disability long term ‐ all studies | 10 | 1169 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.19, ‐0.16] |
| 12 Disability long term ‐ sensitivity and subgroup analyses | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 High quality 1 ‐ 6 or more Risk of Bias items | 5 | 656 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.98, 0.05] |
| 12.2 High quality 2 ‐ Concealed allocation | 8 | 852 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.54, ‐0.16] |
| 12.3 High baseline symptom intensity (>60% on pain & disability scale) | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.32 [‐6.21, ‐4.42] |
| 12.4 Low baseline symptom intensity (<60% on pain & disability scale) | 9 | 1079 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.38, 0.03] |
| 12.5 High intervention intensity 1 (>100 hours, daily contact) | 5 | 823 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.42, 0.07] |
| 12.6 Low intervention intensity 1 (<30 hours, non‐daily contact) | 3 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐5.48, 1.00] |
| 13 Work short term ‐ all studies | 3 | 379 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.92, 2.78] |
| 14 Work short term ‐ sensitivity and subgroup analyses | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 High quality 1 ‐ 6 or more Risk of bias items | 2 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.82, 3.76] |
| 14.2 High quality 2 ‐ Concealed allocation | 2 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.82, 3.76] |
| 14.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Low baseline symptom intensity (<60% on pain & disability scales) | 3 | 379 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.92, 2.78] |
| 14.5 High intervention volume (>100 hours, daily contact) | 2 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.55, 2.20] |
| 14.6 Low intervention volume (<30 hours, non‐daily contact) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Work medium term ‐ all studies | 3 | 221 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.12, 4.10] |
| 16 Work medium term ‐ sensitivity and subgroup analyses | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 High quality 1 ‐ 6 or more Risk of Bias items | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 High quality 2 ‐ Concealed allocation | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.39, 3.76] |
| 16.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Low baseline symptom intensity (<60% on pain & disability scales) | 3 | 221 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.12, 4.10] |
| 16.5 High intervention volume (>100 hours, daily contact) | 3 | 221 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.12, 4.10] |
| 16.6 Low intervention volume (<30 hours, non‐daily contact) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Work long term ‐ all studies | 8 | 1006 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.39, 2.53] |
| 18 Work long term ‐ sensitivity and subgroup analyses | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 High quality 1 ‐ 6 or more Risk of Bias items | 3 | 385 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [1.16, 2.87] |
| 18.2 High quality 2 ‐ Concealed allocation | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [1.26, 2.67] |
| 18.3 High baseline symptom intensity (>60% on pain & disability scales) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Low baseline symptom intensity (<60% on pain & disability scales) | 8 | 1006 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.39, 2.53] |
| 18.5 High intervention volume (>100 hours, daily contact) | 6 | 741 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [1.13, 2.60] |
| 18.6 Low intervention volume (<30 hours, non‐daily contact) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 1 Pain short term ‐ all studies.
6.3. Analysis.
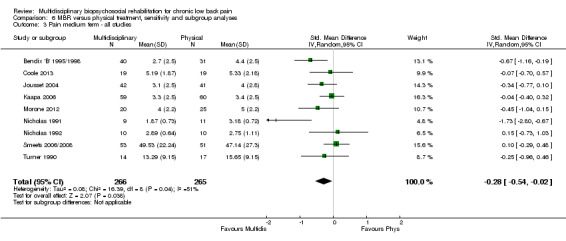
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 3 Pain medium term ‐ all studies.
6.5. Analysis.
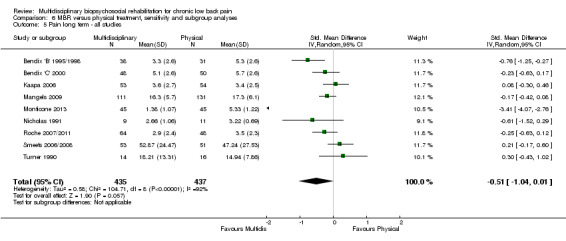
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 5 Pain long term ‐ all studies.
6.7. Analysis.

Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 7 Disability short term ‐ all studies.
6.9. Analysis.
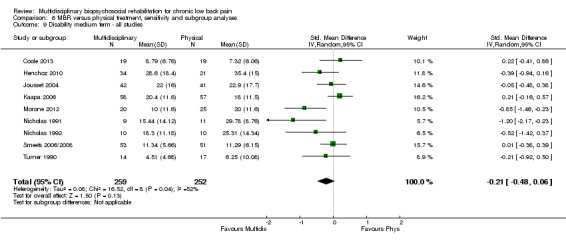
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 9 Disability medium term ‐ all studies.
6.11. Analysis.
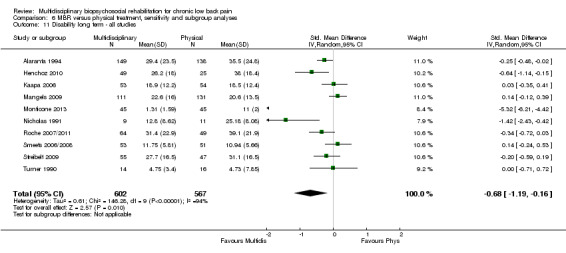
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 11 Disability long term ‐ all studies.
6.13. Analysis.
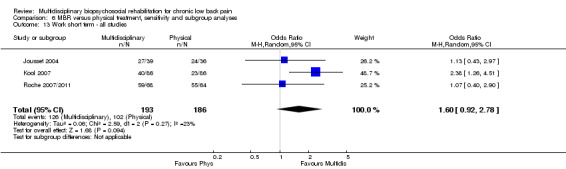
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 13 Work short term ‐ all studies.
6.15. Analysis.
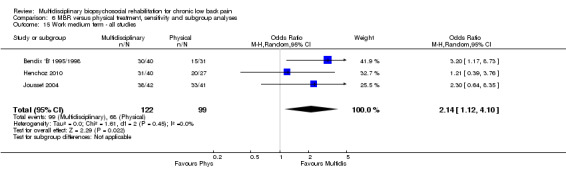
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 15 Work medium term ‐ all studies.
6.17. Analysis.
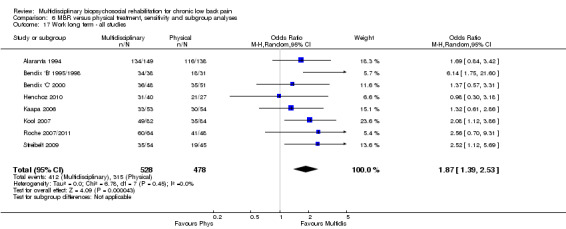
Comparison 6 MBR versus physical treatment, sensitivity and subgroup analyses, Outcome 17 Work long term ‐ all studies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbassi 2012.
| Methods | RCT conducted in Iran | |
| Participants | Patients referred to a pain clinic at a university medical centre with LBP >6 months, age 18‐70, married. 33 patients randomised, 88% female, average age 45 years, median duration of pain 74 months | |
| Interventions |
MBR (P‐MPMP): Group Rx (6/group) 7x weekly sessions 2 hours each session, + 1 session with doctor, + 1 session with physiotherapist. Light mobilisation, coping skills training, education regarding anatomy, physiology, medication, exercise session Usual (SMC): standard medical care, pain medication MBR‐2 (SA‐MPMP): As per P‐MPMP with involvement of spouse |
|
| Outcomes | Pain (VAS), disability (RMDQ), catastrophising (PCS), fear avoidance (TSK) Follow‐ups: ST (7 weeks), LT (12 months) |
|
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2.3. "Patients were randomized to the three groups in blocks of twelve using a software‐generated ramdomization plan" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 33 participants assessed at baseline, 29 assessed at follow‐up |
| Intention to treat analysis | Low risk | 2.6 Statistical analysis. "The results presented are based on intention to treat analyses" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | 3.1 Baseline characteristics. Groups comparable across demographic and important clinical characteristics |
| Compliance | Low risk | 3.2 Adherence. High levels of attendance across all groups |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | 2.4 Assessment. Measurement at baseline, end of treatment and 12 month follow‐up |
Alaranta 1994.
| Methods | RCT conducted in Finland | |
| Participants | Workers on social insurance with back pain > 6 months, age 30‐47, less than 2 back surgeries, no contraindications to exercise. 293 patients randomised, 56% female, average age 40.5 years, mean duration of pain not reported | |
| Interventions |
MBR (Akseli): 3 weeks daily HEP then 3 weeks inpatient program (42 hours per week). Program: strength training, aerobic training, relaxation, stretching, CBT, discussion groups Physical (control): 3 weeks inpatient program: passive physiotherapy (electrotherapies, massage, traction), muscle training, pool exercises, back school |
|
| Outcomes | Disability (Million Pain Disability questionnaire), Work (WHO occupational handicap scale, sick leave days), Utilisation (reduction in physician visits, reduction in physiotherapist visits) Follow‐ups: ST (3 months), LT (12 months) |
|
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. Acceptable 12‐month follow‐up rate 287/293 |
| Intention to treat analysis | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups similar on symtpom severity, age and work characteristics |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Pg.1341 1st column. "The follow‐up examinations were carried out 3 months and 12 months after the clinical baseline examination" |
Basler 1997.
| Methods | RCT conducted in Germany | |
| Participants | Patients referred to a pain treatment centre with a diagnosis of LBP. 76 patients randomised, 75.6% female, average age 49.3 years, mean duration of pain 10.8 years | |
| Interventions |
MBR (CBT+): 1 session /week for 12 weeks, 150min each session, group format (5‐8 people per group), plus homework assignments. CBT (pain education, relaxation, modifying beliefs, pleasant activity scheduling), posture training, strengthening, stretching + Pain meds, nerve blocks, TENS, physiotherapy. Usual (control): Pain meds, nerve blocks, TENS, physiotherapy |
|
| Outcomes | Pain (NRS), Disability (Dusseldorf Disability scale ‐ physical function subscale), Medication use (medication use in days per week), catastrophising. Follow‐ups: ST (3 months) |
|
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.115 1st column. "Through assignment of random numbers, patienst were allocated to an experimental or control group" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.118 Sample. 76 of 94 randomized patients completed follow‐up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | pg.118 Sample. "No signififcant differences between experimental and control subjects in these (baseline) variables" |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.115 1st column. "Three assessments were taken: pre‐treatment, post‐treatment and at a 2.2. Assessment instruments 6‐month follow‐up" |
Bendix 'A' 1996/1998.
| Methods | RCT conducted in Denmark | |
| Participants | Patients referred to a back centre with disabling LBP >6 months, threatened job situation. 94 patients randomised, 70.2% female, median age 40 years, mean duration of pain not reported | |
| Interventions |
MBR (FR): 39 hours/week for 3 weeks inpatient, in groups (7/group), plus 1x 6 hour session/week for 3 weeks). Aerobic exercise, strength, stretching, simulated work tasks, biofeedback, pan coping, goal setting, cognitive appraisal, relaxation, job seeking skills, recreation, ball games, running, swimming Usual (control): Usual care in Denmark, patients free to seek any treatment |
|
| Outcomes | Pain (NRS), disability (LBP rating scale), work (working or able to return to work, days of sick leave), improvement (global rating of change), utilisation (contacts with health care system, admission to hospital due to LBP, LBP surgery), medication (amount and type of prescription medication) Follow‐ups: MT (4 months), LT (2 and 5 years) |
|
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.63 Methods. "patients were randomly assigned to a treatment group or a control group according to thminimisation principle" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 94/106 randomized participants analysed |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg. 66 Results. Follow‐up assessment at 4 months and 12 months |
Bendix 'B' 1995/1998.
| Methods | RCT conducted in Denmark | |
| Participants | Patients referred to a back centre with disabling LBP >6 months, threatened job situation. 106 patients randomised, 75.4% female, median age 42 years, mean duration of pain not reported | |
| Interventions |
MBR (FR): 39 hours/week for 3 weeks inpatient, in groups (7/group), plus 1x 6 hour session/week for 3 weeks). Aerobic exercise, strength, stretching, simulated work tasks, biofeedback, pan coping, goal setting, cognitive appraisal, relaxation, job seeking skills, recreation, ball games, running, swimming Physical (control): 2x 2 hour sessions/week for 6 weeks, in groups (7‐8/group). Aerobics, progressive strengthening, back school MBR‐2: 2x 2 hour sessions/week for 6 weeks, in groups (7‐8/group). Psychological pain management, warm‐up exercises, progressive strengthening |
|
| Outcomes | Pain (NRS), disability (LBP rating scale), work (working or able to return to work, days of sick leave), improvement (global rating of change), utilisation (contacts with health care system, admission to hospital due to LBP, LBP surgery), medication (amount and type of prescription medication) Follow‐ups: MT (4 months), LT (1, 2 and 5 years) |
|
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.149 Material and Methods. Block randomization, following the minimization principle |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.150 1st column. 9% dropout rate |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Fig 2. Groups comparable on relevant demographic and clinical variables |
| Compliance | Low risk | Table 3. Adequate compliance in all groups |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Unclear risk | pg. 150 Results. Follow‐up at 4 months, 1 year and 5 years |
Bendix 'C' 2000.
| Methods | RCT conducted in Denmark | |
| Participants | Patients referred to a back centre with threatened job situation due to LBP. 127 patients randomised, 65.4% female, median age 41 years, mean duration of pain not reported | |
| Interventions |
MBR (FR): 39 hours/week for 3 weeks inpatient, in groups (7/group), plus 1x 6 hour session/week for 3 weeks). Aerobic exercise, strength, stretching, simulated work tasks, biofeedback, pan coping, goal setting, cognitive appraisal, relaxation, job seeking skills, recreation, ball games, running, swimming. Physical (OIT): 1.5 hour sessions, 3x/week for 8 weeks; aerobic and strengthening exercises |
|
| Outcomes | Pain (NRS), disability (LBP rating scale), work (working or able to return to work, days of sick leave), improvement (global rating of change), utilisation (contacts with health care system, admission to hospital due to LBP, LBP surgery) Follow‐up: LT (5 years) |
|
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pg.2495 "Randomization, or rather stratification by minimization, 24 was intended to equalize age, gender, days of sick leave in 3 years, Manniche’s rating scale score19 (reflecting pain, disability, and physical measures), and smoking across the two treatments" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. "For the participants starting FR and OIT, the dropout rate during treatment was 14% and 19%, respectively" |
| Intention to treat analysis | Low risk | pg.2497 Statistical Methods. "intention‐to‐treat analyses were performed to account for dropouts at different phases of the study" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.2497 Outcome Evaluation. "The 1‐year follow‐up evaluation was carried out at a meeting in the Back Cente" |
Coole 2013.
| Methods | RCT conducted in UK | |
| Participants | Patients referred to group rehabilitation with LBP >6 weeks, employed, concerned about work ability. 51 patients randomised, 52.9% female, average age 44 years, mean duration of pain 88 months | |
| Interventions |
MBR (Group and Individual Work): Maximum of 10 weeks of 2‐3 hours/week. Group education and physical activity program with CBT approach. Possible referral to psychologist. Individual work support, max. 8 face‐to‐face contacts of 90min; workplace assessment, barrier to LBP managements, communication with employer, work‐focused interventions Physical (Control): Maximum of 10 weeks of 2‐3 hours/week. Group education and physical activity program with CBT approach. Possible referral to psychologist |
|
| Outcomes | Pain (NRS), Disability (RDQ), Work Ability (Work Ability Index Question), Anxiety (HADS subscale), Depression (HADS subscale), Fear Avoidance (FAB‐Qwork). Follow‐ups: MT (6 months) | |
| Notes | Subgroup analyses: Low intervention intensity, Baseline symptom intensity unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fig 1. 38/59 randomised subjects analysed |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | High risk | Table 5. 35% and 21% of the two groups did not attend intervention at all |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. Follow‐up at 6 months |
Fairbank 2005.
| Methods | RCT conducted in the UK | |
| Participants | Patients referred to surgical departments of 15 hospitals with age 18‐55, LBP >1 year, surgeon unsure if surgery or rehab more suitable. 349 patients randomised, 50.7% female, average age not reported, mean duration of pain 8 years | |
| Interventions |
MBR (Rehabilitation): 5 days/week for 3/52 plus 1 follow‐up session. Stretching, strengthening, stabilisation, cardiovascular endurance, hydrotherapy. CBT approach; pacing, addressing unhelpful beliefs and fears Surgery (Surgery): Spinal stabilisation surgery |
|
| Outcomes | Pain (SF‐36 bodily pain), Disability (ODI), General Health (SF‐36) Follow‐ups: LT (2 years) | |
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pg.2 Treatment allocation. "Randomisation was generated centrally by computer program" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart. 12‐month follow‐up rate 89% |
| Intention to treat analysis | Low risk | pg.2 Statistical Methods. "We carried out an intention to treat analysis" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1 |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Low risk | Table 3 (Rivero‐Arias). Other resource usage comparable between groups |
| Timing of assessment | Low risk | pg.2 Outcome Measures. "We assessed outcomes at baseline and 6, 12, and 24 months from randomisation" |
Harkapaa 1989.
| Methods | RCT conducted in Finland | |
| Participants | Blue collar workers recruited by mail conducting physically strenuous work, with chronic or recurrent LBP for >2 years, LBP reduces physical capacity and caused sick leave. 459 patients randomised, 37% female, average age 44.9 years, mean duration of pain not reported | |
| Interventions |
MBR (Inpatient): 3 weeks inpatient program, sessions in groups (6‐8/group). Swedish back school, back exercises, relaxation exercises, heat/electrotherapy, discussion groups on coping, discussion on back care. HEP stretching and stretching + massage and strengthening and physical exercises. 2nd part (1.5 yr later), 2/52 inpatient program rehearse and refresh back, self‐care skills Physical (control): Written and oral instructions; back exercises and ergonomics MBR‐2 (outpatient, control): 2 session/week for 2 months (15 sessions). Swedish back school, back exercises, relaxation exercises, heat/electrotherapy, discussion groups on coping, discussion on back care. HEP stretching and stretching. 2nd part (1.5 yr later), 8 sessions inpatient program rehearse and refresh back, self‐care skills |
|
| Outcomes | Pain (Pain Index), Disability (LBP Disability Index). Follow‐ups: ST (3 months). | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.82 Materials and Methods. 459/476 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | High risk | Table 4. Compliance different between groups |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.82 Procedure. "Follow‐ups were carried out 3, 8 and 18 months after the first treatment" |
Hellum 2011.
| Methods | RCT conducted in Norway | |
| Participants | Patients referred to local hospitals and primary care with age 25‐55 years, LBP >1 year, unsuccessful physio or chiro for 6 months, Oswestry >30%, degenerative disc changes. 173 patients randomised, 50.8% female, average age 41 years, mean duration of pain 81 months | |
| Interventions |
MBR (Rehabilitation): Outpatient treatment in groups, 60 hours over 2 to 5 weeks. Education (anatomy, psychology, imaging, coping, medication, family, work and social life), daily exercises (endurance, strength, coordination), challenging beliefs Surgery (Surgery): Disc replacement with artificial disc (ProDisc) |
|
| Outcomes | Pain (SF‐36 bodily pain), Disability (ODI), General Health (SF‐36 and EQ5D), Work (% return to work), Satisfaction (% satisfied with outcome), Fear Avoidance (FABQ), Self‐Efficacy Follow‐ups: ST (6 weeks and 3 months), MT (6 months), LT (1 and 2 years) |
|
| Notes | Subgroup analyses: Mid intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 Study Design. "They were randomised in blocks with a website hosted by the medical faculty" |
| Allocation concealment (selection bias) | Low risk | pg.2 Study Design. "Allocation was concealed for all people involved in the trial" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 172/179 patients followed up |
| Intention to treat analysis | Low risk | pg.4 Planned analyses. "The main statistical analysis was in the intention to treat population" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | High risk | pg.5 Patient Characteristics. "Low back pain score and SF‐36 mental health subscores, however, were significantly worse in the rehabilitation group than in the surgery group" |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. Follow‐up at 6 weeks, 3 months, 6 months, 12 months, 24 months |
Henchoz 2010.
| Methods | RCT conducted in Switzerland | |
| Participants | Patients referred to a hospital rheumatology outpatient clinic with age 18‐60 years, LBP >6 weeks. 109 patients randomised, 32% female, average age 39.8 yers, mean duration of pain not reported | |
| Interventions |
MBR (Multidisciplinary Rehabilitation): 3 weeks with sessions 5 days/week, 5‐7 hours/day, in groups (n=5) and individual. Intensive physical and ergonomic training, psychological pain management, back school, social and work‐related education, tailored medication programme Physical (Control): 18 physiotherapy sessions (45min) over 9 weeks. Active exercise and passive modalities to manage pain, improve mobility and increase activity level |
|
| Outcomes | Disability (ODI), Work (% working) Follow‐ups: LT (3 weeks), MT (6 weeks), LT (1 year) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 Design. "allocated by a secretary not involved in the study to a functional multidisciplinary rehabilitation programme (FMR) or outpatient physiotherapy (OP) according to computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | pg.2 Design. "...sealed in opaque envelopes with consecutive numbering" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fig 1. 67/109 randomized patients followed up |
| Intention to treat analysis | Low risk | Fig 1. "Included in ITT analysis" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Tables 1/2. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.3 Outcomes. "ODI was recorded at the beginning of treatment (T0), at 3‐week (T3w), 9‐week (T9w), 6‐month (T6m), 9‐month (T9m) and 12‐month (T12m) follow‐up" |
Jackel 1990.
| Methods | RCT conducted in Germany | |
| Participants | 71 patients randomised, 62% female, average age 48.7 years, mean duration of pain 12.8 years | |
| Interventions |
MBR (Therapy): 4‐6 weeks of daily therapy. Physical therapy 2x/ day in pool, 1x/ day in gym. Education: anatomy, lifting instructions. 8‐10x mudbaths, 8x massage, 8x electro therapy. Psychology: pain beliefs, coping, depression, impact of pain on life Waiting‐list (control) |
|
| Outcomes | Pain, Disability (0‐10 Activities of daily living scale) Follow‐up: ST (4 weeks) |
|
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 2/3. Comparable on clinical characteristics |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Tables 2/3. 4 weeks |
Jousset 2004.
| Methods | RCT conducted in France | |
| Participants | Patients referred to a hospital multidisciplinary LBP clinic, by GP, specialists, industrial physicians, insurance advisors. Age 18‐50 years, LBP not relieved by conventional treatment, threatened job situation. 84 patients randomised, 33.3% female, average age 40.3 years, mean duration of pain not reported | |
| Interventions |
MBR (FRP): 5 weeks duration, 6 hours/day, 5 day/week in groups (n=6‐8). Exercise with physiotherapist; warm‐up, stretching, flexibility, aerobic exercises (walking, running, cycling), strengthening, muscular endurance, coordination exercises. OT; work simulations. Psychologist; counselling Physical (AIT): 5 weeks duration, 3 sessions/week of 1 hour, plus HEP; 2 session/week of 50 minutes. Active exercise directed by physiotherapist, flexibility, stretching, strengthening, proprioception exercises, endurance training, HEP; jogging, swimming, stretching |
|
| Outcomes | Pain (NRS), Disability (Quebec Disability Scale), Work (% return to work, days of sick leave, ability to work), Medication, Anxiety/Depression (HAD, Dallas) Follow‐ups: MT (6 months) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.488 Materials. "Block randomization was performed using an eightelement permutation table" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 42/86 randomized patients followed‐up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Tables 1/2. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.489, last sentence. "Evaluation at 6 months was performed for all patients at the rehabilitation center" |
Kaapa 2006.
| Methods | RCT conducted in Finland | |
| Participants | Patients referred to two Occupational Health centers with age 22‐57 years, female health care workers, daily or nearly daily LBP for the past 1 year. 102 patients randomised, 100% female, average age 46.3 years, mean duration of pain 15 months | |
| Interventions |
MBR (Multidisciplinary Rehabilitation): 2 weeks for 5 days/week, 6 hours/day, then 5 weeks of 2 sessions of 4 hours/week, 2 weeks HEP. CBT stress management, relaxation, Swedish Back School (education), aerobic fitness, flexibility, coordination, strengthening, progressive relaxation Physical (Control): 10 sessions of 1 hour over 6‐8 weeks. Passive treatment (massage, electro modlities, traction, mobiilsation), active (stretching, mobility, coordination exercises), general increase in physical activity was recommended (walking, swimming, daily activities) |
|
| Outcomes | Pain (NRS), Disability (ODI), General Health, Work (Subjective Work Capacity), Health Care Utilization (Number of Visits), Depression (DEPS) Follow‐ups: ST (post‐treatment), MT (6 months), LT (1 and 2 years) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.372 Randomization. "The randomization list was generated by an independent biostatistician using a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | pg.372 Randomization. "The physiotherapist then randomized each patient into one of the two groups by opening an opaque sealed envelope...and the randomization results were kept in sealed envelopes, one for each patient" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 89% and 90% at 12 month follow‐up |
| Intention to treat analysis | Low risk | pg.373 Statistical analysis. "All patients were included in the analysis on the basis of their intervention allocation" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Tables 2/3. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. Post‐intervention, 6, 12, 24 months |
Kole‐Snijders 1999.
| Methods | RCT conducted in the Netherlands | |
| Participants | Patients referred to rehabilitation centre by GP or specialist with age 18‐65 years, LBP >6 months, discrepancy between objective findings and pain complaints, partner willing to participate in treatment. 148 patients randomised, 64% female, average age 39.8 years, mean duration of pain 10 years | |
| Interventions |
MBR (OPCO): Individual and group, 5 weeks inpatient plus 3 weeks outpatient. Operant behavioural treatment, quota‐based activities, standing and sitting tolerance, daily activity schedule for home, spouse group training (education and discussion). Cognitive coping skills, increasing pain control and self‐efficacy, education, biofeedback MBR‐2 (OPDI, Control 1): Individual and group, 5 weeks inpatient plus 3 weeks outpatient. Operant behavioural treatment, quota‐based activities, standing and sitting tolerance, daily activity schedule for home, spouse group training (education and discussion). Group discussion (attention control for cognitive coping training) Waiting list (Control 2) |
|
| Outcomes | Recovery (% improved) Follow‐ups: ST (2 months), MT (6 months), LT (12 months) | |
| Notes | Subgroup analyses: High intensity intervention, Baseline symptom intensity unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.933 Study design. "Allocation to the three conditions occurred following a randomizationm procedure" |
| Allocation concealment (selection bias) | Low risk | pg.933 Study design. "a number that an indpendent researcher blindly drew and assigned" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.936 Attrition. 107/148 patients available at 12 month follow‐up |
| Intention to treat analysis | Low risk | pg.936 Intention to treat analysis. "intention to treat analysis was done" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | High risk | pg.937 Baseline comparisons. Between group differences on dependent variables |
| Compliance | Low risk | pg.937 Compliance. Similar compliance across conditions |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 3. Post‐treatment, 6, 12 months |
Kool 2007.
| Methods | RCT conducted in Switzerland | |
| Participants | Patients referred to work rehabilitation centre with age 20‐55 years, non acute NSLBP, >6 weeks sick leave in the last 6 months. 174 patients randomised, 21.3% female, average age 42.1 years, mean duration of pain not reported | |
| Interventions |
MBR (FCT): 6 days/week for 3 weeks, 4 hours/day. Time contingent: work simulation, endurance training, strengthening, aerobic training. Counselling, education, self‐efficacy, analgesic medication Physical (PCT): 6 days/week for 3 weeks, 2.5 hours/day. All activity was pain‐contingent: Passive and active mobilisation, stretching, strengthening, min‐back school (education), heat, electrotherapy, massage, progressive relaxation, analgesic medication |
|
| Outcomes | Pain (NRS), Work (% at work), Overall Improvement (Likert Scale), Medication (% taking medication), Self‐Efficacy (PACT) Follow‐ups: ST (3 months), LT (12 months) | |
| Notes | Subgroup analyses: Mid intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.1080 Design. "Randomization was concealed" |
| Allocation concealment (selection bias) | Unclear risk | pg.1080 Design. "Randomization was concealed" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 166/174 randomized patients followed up |
| Intention to treat analysis | Low risk | pg.1091 Statistics. "Analysis was based on the intention‐to‐treat principle" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Low risk | pg.1091 Protocol compliance. "All patients attended at least 90% of the scheduled treatments, and treatment duration was comparable" |
| Cointerventions | Low risk | pg.1093 Health Care use. "Interventions after rehabilitation were comparable in the FCT and PCT group" |
| Timing of assessment | Low risk | pg.1090 Outcome measurement. Follow‐up 1 year |
Lambeek 2010.
| Methods | RCT conducted in the Netherlands | |
| Participants | Patients referred to a hospital outpatient clinic with age 18‐65 years, LBP >3 months, in paid work. 134 patients randomised, 42% female, average age 46.2 years, mean duration of pain not reported | |
| Interventions |
MBR (Integrated Care): up to 12 weeks (26 sessions). CBT‐based graded activity, education, ergonomics, workplace intervention (including employer). Usual (Control): usual care in the Netherlands as directed by medical specialist |
|
| Outcomes | Pain (VAS), Disability (RMDQ), Work (days of sick leave) Follow‐ups: ST (3 months), MT (6 months), LT (12 months) | |
| Notes | Subgroup analyses: Low intensity intervention, High baseline symptom intensity (>60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.3 Randomisation. "For every stratum, an independent statistician carried out block randomisation of four allocations, using a computer generated random sequence table. A research assistant prepared opaque, sequentially numbered and sealed coded envelopes for each stratum" |
| Allocation concealment (selection bias) | Low risk | pg.3 Randomisation. "For every stratum, an independent statistician carried out block randomisation of four allocations, using a computer generated random sequence table. A research assistant prepared opaque, sequentially numbered and sealed coded envelopes for each stratum" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.3 Loss to follow‐up. "Data on sick leave were complete for all patients at baseline, and for 93% of the patients during the 12 months of follow‐up" |
| Intention to treat analysis | Low risk | pg.3 Statistical analysis. "All analyses were done according to the intention to treat principle" |
| Selective reporting (reporting bias) | High risk | Pain coping and QoL not reported |
| Comparability of groups at baseline | Low risk | Table 1 & pg.4 Patient characteristics. Groups comparable on relevant demographic and clinical variables |
| Compliance | High risk | pg.3 Non‐compliance. Several cases of non‐compliance |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.2 Outcome measures. "Questionnaires were administered to the patients at baseline and after 3, 6, 9, and 12 months" |
Leeuw 2008.
| Methods | RCT conducted in the Netherlands | |
| Participants | Adults referred by physicians from outpatient rehabilitation centres or responded to advertisements with LBP >3 months with RMDQ score >3 and Tampa Scale for Kinesiophobia score >33. 85 patients randomised, 48% female, average age 45 years, mean duration of pain 9 years | |
| Interventions |
MBR (EXP): 16x 2 session/week, 1 hours/session. Information re: diagnosis, imaging, continued active approach, treatment rationale. Establishment of heirachy of feared activities, explanation of fear avoidance model, gradual, systematic exposure to feared activities. Behvioural experiments to test consequences of engagement in feared activities MBR‐2 (GA): 26x 2 session/week, 1 hoursr/session. Information re: diagnosis, imaging, continued active approach, treatment rationale. Identification of functional treatment goals, quota‐based gradual increase in perfomance of functional activities |
|
| Outcomes | Pain (McGill), Disability (Quebec), main complaints (Patient specific complaints scale), harmfulness of activities (PHODA), pain Catastrophizing (PCS), daily activity (acceleromoeter) Follow‐ups: LT (12 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization procedure. "Patients were randomized to EXP or GA within each treatment centre, following a predetermined and computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Randomization procedure. "After the second pre‐treatment measurement, patients received a sealed envelop from the research assistant containing a sheet of coloured paper indicating treatment assignment" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 73/85 patients completed follow‐up |
| Intention to treat analysis | Low risk | 2.7.1 Treatment outcomes ‐ Intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | High risk | 3.1 Flow of participants. "Treatment was prematurely terminated either by the patient or the therapist in 12 patients (29%) of the EXP condition, and in 14 patients (33%) of the GA condition for various reasons" |
| Cointerventions | Low risk | 2.2 Participants. "Patients were requested not to seek diagnostic or therapeutic procedures during therapy other than their allocated treatment" |
| Timing of assessment | Low risk | Table 2. Post treatment, 6 month follow‐up |
Linton 2005.
| Methods | RCT conducted in Sweden | |
| Participants | Patients referred to primary care facilities at risk of developing long‐term disability, employed, with back or neck pain (back pain 85%), <4 months of sick leave over the past year. 185 patients randomised, 83% female, average age 49 years, mean duration of pain not reported | |
| Interventions |
MBR (PT+CBT): Physical Therapy (unconstrained volume of treatment); information on prevention, cause of LBP, activity advice, functional training, personalised programs. 6 weeks, 1 session/week, 2 hours/session plus homework. CBT intervention (groups n=6‐10); pain education, problem solving, coping skills, increase in function, graded activity, stress management, relaxation, dealing with exacerbations. Minimal intervention (1 session), education, actvity advice, booklet, patients free to seek any medical care MBR‐2 (CBT, Control 1): 6 weeks, 1 session/week, 2 hoursr/session plus homework, CBT intervention (groups = 6‐10 persons); pain education, problem solving, coping skills, increase in function, graded activity, stress management, relaxation, dealing with exacerbations. Minimal intervention (1 session), education, actvity advice, booklet, patients free to seek any medical care Usual (Control 2): Minimal intervention (1 session), education, actvity advice, booklet, patients free to seek any medical care |
|
| Outcomes | Pain (NRS), Disability (RMDQ), Work (% sick leave), Health Care Utilisation (number of visits), Medication (number of days of consumption), Fear Avoidance (TSK), Anxiety (HADS subscale), Depression (HADS subscale), Pain Catastrophising (PCS) Follow‐ups: LT (12 months) | |
| Notes | Subgroup analyses: Low intensity Intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants. "randomly assigned to 1 of the 3 groups using a computer‐generated block randomization" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completion and participation rates. Differential dropout between groups |
| Intention to treat analysis | Low risk | Completion and participation rates. "An “intention to treat” approach was used" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Tables 1/2. Groups comparable on relevant demographic and clinical variables |
| Compliance | Low risk | Completion and participation rates. High attanedance rates across the groups |
| Cointerventions | High risk | Minimal treatment group. Patients free to seek any care |
| Timing of assessment | Low risk | Measures and Procedures. 1 year follow‐up |
Lukinmaa 1989.
| Methods | RCT conducted in Finland | |
| Participants | Patients with LBP referred to a regional hospital. 203 patients randomised, 52.7% female, average age 43.6 years, mean duration of pain 15.3 months | |
| Interventions |
MBR (Biopsychosocial): 5 days inpatient, treatment according to the biopsychosocial model Usual (Biomedical): orthopaedic outpatient treatment according to the biomedical model |
|
| Outcomes | Pain (VAS), Disability (RMDQ), General Health (global perceived effect), Work (% retired) Follow‐ups: LT (12 months) |
|
| Notes | Subgroup analyses: Mid intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.137. 78% follow‐up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | pg.136 Table. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.136 Methods of collecting data. One year follow‐up |
Mangels 2009.
| Methods | RCT conducted in Germany | |
| Participants | Patients referred to an orthopaedic rehabilitation hospital with a musculoskeletal disease, approximately 85% with dorsalgia (ICD M54). 363 patients randomised, 77.7% female, average age 48.8 years, mean duration of pain not reported | |
| Interventions |
MBR (BP + booster): 4 weeks inpatient intervention, individual or group sessions. Analgesic medication as required, aerobic exercises, coordination exercise, ergonomic advice, ADL physical capacity training, back school (education), massage, electrotherapy, hydrotherapy, thermotherapy, nutrition and social advice. CBT‐based psychological pain management (group sessions, n=11), BPS model education, pain coping, progressive muscle relaxation. 7 booster sessions on telephone over 12/12 to reinnforce inpatient topics, problem‐solving, goal setting, relaxation, coping etc MBR‐2 (BP, Control 1): 4 weeks inpatient intervention, individual or group sessions. Analgesic medication as required, aerobic exercises, coordination exercise, ergonomic advice, ADL physical capacity training, back school (education), massage, electrotherapy, hydrotherapy, thermotherapy, nutrition and social advice. CBT‐based psychological pain management (group sessions, n=11), BPS model education, pain coping, progressive muscle relaxation Physical (Control 2): 3 weeks of mostly physical/orthopedic treatment including active physiotherapy, passive modalities and occupational therapy |
|
| Outcomes | Sensory Pain (SES), Disability (PDI), General Health (SF‐12), Depression (BDI), Action‐oriented Coping, Self‐Efficacy (PSEQ) Follow‐ups: ST (1 month), LT (12 months) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.357 Study design and procedure. "Randomization was carried out by an administration secretary of the rehabilitation hospital who received random numbers from the study center" |
| Allocation concealment (selection bias) | Low risk | pg.357 Study design and procedure. "treatment was allocated in a blind way" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 340/363 randomized patients followed up |
| Intention to treat analysis | Low risk | pg.360 Statistical analysis. "all of the patients were further analyzed as intended to treat" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | pg.360 Sample characteristics. "The groups did not differ on sex, age, marital status, education, medical diagnoses, and all pretreatment scores such as disability, depression, self‐efficacy, pain perception, life satisfaction, health status, and pain coping strategies" |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. 12‐month follow‐up |
Meng 2011.
| Methods | RCT conducted in Germany | |
| Participants | Patients referred to orthopaedic rehabilitation hospital with age 18‐65 years, musculoskeletal disease, approximately 98% with dorsalgia (ICD M54). 382 patients randomised, 64% female, average age 49 years, mean duration of pain was > 5 years for 46% of patients | |
| Interventions |
MBR (BPS Back School): 7 sessions of 55 minutes, in groups (n<16). Back school, education, (anatomy, epidemiology, risk factors, therapy), BPS model, pain education, fear avoidance, coping, social aspects, muscle training and stabilisation exercises, recommendation for increased physical activity MBR‐2 (Back School, Control): 6 sessions of 55 minutes, in groups (up to 60). Back school, posture and movement exercises, pain education, coping, education |
|
| Outcomes | Action‐oriented coping Follow‐ups: MT (6 months), LT (12 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.249. "Patients were randomly assigned to the 2 groups using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | pg.249. "Randomization was performed by a scientific assistant of the research institute (central randomization per phone or e‐mail)" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.249 2nd column. "Follow‐up rates exceeded 75% at all time points (Fig. 1)" |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | pg.250 1st column. "No systematic differences existed between the IG and the CG concerning sociodemographic and medical data as well as length of stay. Thus, randomization proved successful." |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.249 Study design and participants. "Data were assessed at admission, discharge, as well as 6 and 12 months follow‐ups" |
Mitchell 1994.
| Methods | RCT conducted in Canada | |
| Participants | Workers on Worker's Compensation Board list referred to 2 work rehabilitation clinics. Injured workers who had not recovered and returned to work after 3 months, with inappropriate illness behaviour. 542 patients randomised, 28.5% female, average age not reported, mean duration of pain not reported | |
| Interventions |
MBR (FR): 8 weeks, 7 hours/day, 5 days/week (total 280 hours), group sessions (n=10‐12). Physical exercise; mobility, strengthening, flexibility, endurance, stretching, ice, circuit training, work simulation exercises (lifting). Behavioural and cognitive treatment, correction of unhelpful beliefs, education, relaxation, biofeedback, personal responsibility Usual (Control): Usual care, variable including; physio, medication, manipulation, acupuncture, work hardening, back schools, active exercise |
|
| Outcomes | Work (% full‐time work, days of sick leave) Follow‐ups: LT (1 year) | |
| Notes | Subgroup analyses: High intensity intervention, Baseline symptom intensity unclear. Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Unclear risk | Not reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg. 635 Results. 12‐month follow‐up |
Moix 2003.
| Methods | RCT conducted in Spain | |
| Participants | Adult patients in a hospital pain clinic with LBP/radiculopathy or cervical pain. 30 patients randomised, 53.3% female, average age 54.3 years, mean duration of pain not reported | |
| Interventions |
MBR (Interdisc): Usual care + interdisciplinary program (11 sessions/week, about 60 minutes/session). Interdisciplinary program: psychology Usual (Control): Usual care, pain control through the treatment of the anaesthesiology team |
|
| Outcomes | Pain, Disability, Medication (% reduced use), Depression (BDI), Anxiety (STAI) Follow‐ups: ST (post‐treatment) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.5 last sentence. "Los sujetos se repartieron al azar en dos grupos: 1) grupo experimental al que además del tratamiento estandar se le aplicó el programa interdisciplinar y 2) grupo control que recibió el tratamiento estándar que consistía en el control del dolor por parte del equipo de anestesiologia" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 2. Clinical and demographic variables comparable |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. Post‐treatment |
Monticone 2013.
| Methods | RCT conducted in Italy | |
| Participants | Patients referred to a rehabilitation centre with LBP >3 months and >18 years. 90 patients randomised, 57.8% female, average age 49.8 years, mean duration of pain 25.8 months | |
| Interventions |
MBR (CBT + Physical): 5 weeks program plus 1 year reinforcement. 1 individual CBT session/week of 60 minutes, then 1 session/month for 12 months. Sessions included fear avoidance beliefs, catastrophising, inappropriate beliefs, negative thoughts, transferring attention, graded exposure, motivation, goal‐setting. Physical program, individually delivered: active and passive mobilisations, stretching, strengthening, postural/motor control exercises. Up to 10 sessions, 2 sessions/week for 5 weeks, plus home exercise program Physical (Control): physical program, individually delivered, active and passive mobilisations, stretching, strengthening, postural/motor control exercises. Up to 10 sessions, 2 sessions/week for 5 weeks, plus home exercise program |
|
| Outcomes | Pain (NRS), Disability (RMDQ), General Health (SF‐36), Fear Avoidance (TSK) Follow‐ups: ST (post‐treatment), LT (1 and 2 years) | |
| Notes | Subgroup analyses: Low intensity intervention, High baseline symptom intensity (>60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 Randomization. "randomized the patients to one of the 2 treatment programs using a list previously generated by a biostatistician (SAS PROC PLAN)" |
| Allocation concealment (selection bias) | Low risk | pg.2 Randomization. "...delivered to the Principal Investigator with blinded treatment codes" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.4 Participant flow. "No patients dropped out during the course of the study" |
| Intention to treat analysis | Low risk | Flow chart. No dropout, all patients treated as per protocol |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 3. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.3 Outcome measures. "questionnaires were completed before treatment (T1), 5 weeks later (at the end of the instructive phase, T2), and then 12 months (post‐treatment analysis, T3) and 24 months after the end of the instructive phase (1‐year follow‐up, T4)" |
Morone 2011.
| Methods | RCT conducted in Italy | |
| Participants | Patients referred to a rehabilitation centre with age 18‐80 years and NSLBP >3 months. 73 patients randomised, 64.3% female, average age 60.2 years, mean duration of pain not reported | |
| Interventions |
MBR (Back School): 4 weeks, 10 sessions in groups (4‐5), each session 1 hour. Education (anatomy, pain, stress management, workplace, sporting activities, posture), exercise prescription (ergonomics, ADLs, HEP), stretching, strengthening, core stability Usual (Control): medical care, mostly pharmacological |
|
| Outcomes | Pain (VAS), Disability (ODI), General Health (SF‐36) Follow‐ups: ST (post‐treatment and 3 months), MT (6 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | pg.535 last sentence. "The othet physiatrist was involved in patients' randomization and was unaware of clinical features" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.538 Results. 70/74 ranodmized patients followed up |
| Intention to treat analysis | High risk | pg.537 last sentence. "A per‐protocol analysis was performed on primary and secondary outcome measures" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Tables 1/3/4. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.536 Outcome measures. Baseline, end of treatment, 3, 6 months |
Morone 2012.
| Methods | RCT conducted in Italy | |
| Participants | Italy. Patients referred to an acadeimc hospital with age 18‐75 years and NSLBP >3 months. 75 patients randomised, 72% female, average age 55.4 years, mean duration of pain not reported | |
| Interventions |
MBR (Back School): 4 weeks, 10 sessions in groups (4‐5), each session 1 hour. Education (anatomy, pain, stress management, workplace, sporting activities, posture), exercise prescription (ergonomics, ADLs, HEP), stretching, strengthening, core stability Physical (Control 1): 4/52, 3 sessions per week, proprioceptive and perception tasks while lying on deformable latex cones Usual (Control 2): medical care, mostly pharmacological |
|
| Outcomes | Pain (VAS), Disability (ODI) Follow‐ups: ST (post‐treatment and 3 months), MT (6 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.342 1st sentence. "Specifically, patients were asked to take a sealed envelope from a box, containing a piece of paper with the assignment, which was, concealed until the envelope was opened" |
| Allocation concealment (selection bias) | Low risk | pg.342 1st sentence. "Specifically, patients were asked to take a sealed envelope from a box, containing a piece of paper with the assignment, which was, concealed until the envelope was opened" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 3. 70/75 randomized patients analyzed |
| Intention to treat analysis | Low risk | pg 344, 1st column, last paragraph. "An intention‐to‐treat analysis was performed" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Unclear risk | Insufficient information reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.342 1st paragraph. "Each patient was assessed before and at the end of treatment and also at the 12‐ and 24‐week follow‐up" |
Nicholas 1991.
| Methods | RCT conducted in Australia | |
| Participants | Patients referred from pain clinic, GPs or specialist with age 20‐60 years, LBP >6 months. 58 patients randomised, 51.7% female, average age 41.2 years, mean duration of pain 7 years | |
| Interventions |
MBR (Behavioural+Relaxation): 5 weeks, 2 sessions/week. Education (anatomy, back care, lifting, medication, diet/weight),Strengthening exercises, mobilisation exercises, hydrotherapy, HEP. Behavioural treatment; goal‐setting (social, home, work) and advice, medication reduction, pacing, given positive reinforcement plus relaxation treatment; progressive muscle relaxation Physical (Physiotherapy): 5 weeks, 2 sessions/week. Education (anatomy, back care, lifting, medication, diet/weight), strengthening exercises, mobilisation exercises, hydrotherapy, HEP Note: 6 treatment groups in total, only the above groups used for this review |
|
| Outcomes | Pain (PRC), Disability (SIP), Medication (number of types), Anxiety (STAI), Depression (BDI), Pain Beliefs (PBQ), Coping (CSQ) Follow‐ups: ST (post‐treatment), MT (6 months), LT (12 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | pg.226 Experimental design. "The random assignment was performed after the pretreatment assessment. Thus, the experiments did not know to which condition a subject would be assigned at the time the pretreatment assessments were conducted" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.232 Attrition. 39/58 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Unclear risk | Insufficient information reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.226 Experimental design. "repeated measurements were conducted on four occasions‐pretreatment, post‐treatment, 6 months and 12 months after the end of treatment" |
Nicholas 1992.
| Methods | RCT conducted in Australia | |
| Participants | Patients referred from pain clinic, GPs or specialist with age 20‐60 years, LBP >6 months. 20 patients randomised, 45% female, average age 43.7 years, mean duration of pain 5.5 years | |
| Interventions |
MBR (CBT): 5 weeks, 2 sessions/week. Education (anatomy, back care, lifting, medication, diet/weight), strengthening exercises, mobilisation exercises, hydrotherapy, HEP, chronic pain education, coping, attentional porcesses, challenging and altering unhelpful cognitions, distraction techniques. Relaxation treatment: progressive muscle relaxation Physical (Control): 5 weeks, 2 sessions/week. Education (anatomy, back care, lifting, medication, diet/weight), strengthening exercises, mobilisation exercises, hydrotherapy, HEP. Attention control, general group discussion about living with back pain, no advice or information provided |
|
| Outcomes | Pain (PRC), Disability (SIP), Medication (% using), Depression (BDI), Coping (CSQ), Self‐Efficacy (PSEQ) Follow‐ups: ST (post‐treatment), MT (6 months) | |
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score). Included in Guzman 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | pg.341 1st paragraph. "The random assignment was conducted after the pretreatment assessment and before the program started. Thus, neither the psychologist nor the physiotherapist knew to which condition a subject would be assigned at the time the pretreatment assessments were conducted" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pg.340 Subjects. 18/20 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Unclear risk | Insufficient information reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Table 1. Post‐treatment, 6 months |
Roche 2007/2011.
| Methods | RCT conducted in France | |
| Participants | Patients referred to a hospital multidisciplinary LBP clinic with age 18‐50 years, LBP >3 months, on sick leave or at risk of work disability, not in temporary employment. 132 patients randomised, 35% female, average age 39.8 years, mean duration of pain not reported | |
| Interventions |
MBR (FR): 5 weeks, 5 days/week in groups (n=6‐8), 6 hours/day. Exercise with physiotherapist: warm‐up, stretching, flexibility, aerobic exercises (walking, running, cycling), strengthening, muscular endurance, coordination exercises. OT: work simulations. Psychologist: counselling Physical (AIT, Control): 5 weeks, 3 sessions/week of 1 hour each; plus HEP, 2 sessions/week of 50 minutes each. Active exercise directed by physiotherapist, flexibility, stretching, strengthening, proprioception exercises, endurance training. HEP: jogging, swimming, stretching |
|
| Outcomes | Pain (VAS), Disability (Dallas PQ), Work (% return to work, % working full‐time, days of sick leave), Anxiety/Depression (Dallas PQ) Follow‐ups: ST (post‐treatment), LT (1 year) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.1230 Population. "Block randomization was undertaken with an 8‐element permutation table established by an independent methodologist" |
| Allocation concealment (selection bias) | Low risk | pg.1230 Population. "Block randomization was undertaken with an 8‐element permutation table established by an independent methodologist" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 119/132 randomized patients followed‐up completely |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Low risk | Fig 1. 131/132 received complete interventions |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.1230 last sentence. "patients were off work during the 5 weeks of treatment. At the beginning (t0) and the end of the treatment (t5), they were assessed in the rehabilitation center" |
Schweikert 2006.
| Methods | RCT conducted in Germany | |
| Participants | Patients with NSLBP >6 months recruited from lists of a work insurer for referral to a rehabilitation hospital. 409 patients randomised, 17.1% female, average age 46.7 years, mean duration of pain not reported | |
| Interventions |
MBR (CBT): 3 weeks inpatient program, small groups. Daily physiotherapy: 2 times/day exercises, massage, electrotherapies, education, advice about risk factors, back care, etc. CBT: coping, motivation, pain management, relaxation, distraction, cognitive reappraisal Physical (control): 3 weeks inpatient program, small groups. Daily physiotherapy: 2 times/day exercises, massage, electrotherapies, education, advice about risk factors, back care, etc |
|
| Outcomes | Pain (Likert Scale), Disability (Hannover Scale), General Health (EuroQoL), Work (days off‐work), Depression (STAI) Follow‐ups: ST (post‐treatment), MT (6 months) | |
| Notes | Subgroup analyses: High intensity intervention, High baseline symptom intensity (>60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2520 Materials and methods. "randomization was performed by an external biometrical unit using Rancode Professional 3.6" |
| Allocation concealment (selection bias) | Low risk | pg.2520 Materials and methods. "randomization was performed by an external biometrical unit using Rancode Professional 3.6" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.2521 Results 1st column. Most of the dropouts in the treatment group |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Comparability of groups at baseline | Unclear risk | Insufficient information reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. 6 month follow‐up |
Skouen 2002.
| Methods | RCT conducted in Norway | |
| Participants | Employees on National Health Insurance list were invited. Patients with LBP sick‐listed for >8 weeks or at least 2 months/year over the last 2 years. 195 patients randomised, 43.5% female, average age 43.6 years, mean duration of pain not reported | |
| Interventions |
MBR (Extensive MD): 4 weeks, 5 days/week, 6 hours/day. CBT (group sessions): fear avoidance, coping strategies. Education: pain mechanisms, anatomy, exercise advice. Workplace interventions. Graded exercise program: stretching, strengthening, mobility, coordination exercises, aerobic trainnig. Relaxation, body awareness training. HEP at the end of the intervention MBR‐2 (Light MD, Control 1): Unspecified number of consultations (average 3) with physiotherapist and sometimes nurse or psychologist if necessary. Education; exercise, lifestyle, fear avoidance, advice to reduce illness behaviours and anxiety. HEP program and advice to stay active and gradually increase activity levels Usual (Control 2): usual care under GP, involving pain medication, referral to physiotherapist or chiropractic |
|
| Outcomes | Work (% return to work) Follow‐ups: LT (1, 1.5 and 2 years) | |
| Notes | Subgroup analyses: High intensity intervention, Baseline symptom intensity unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.902 2nd column. "patients were allocated at random by means of a sequence of prelabeled cards contained in sealed envelopes. The allocation sequence was prepared beforehand by a physician outside the clinic" |
| Allocation concealment (selection bias) | Low risk | pg.902 2nd column. "patients were allocated at random by means of a sequence of prelabeled cards contained in sealed envelopes. The allocation sequence was prepared beforehand by a physician outside the clinic" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1A. 195/211 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Unclear risk | Insufficient information reported |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Low risk | pg 903. Acceptable levels across groups |
| Timing of assessment | Low risk | Fig 1B. 3, 6, 12 months follow‐up |
Smeets 2006/2008.
| Methods | RCT conducted in the Netherlands | |
| Participants | Patients referred by GPs and specialists to 3 rehabilitation centers. Patients with age 18‐65 years, LBP >3 months, RMDQ score >3, ability to walk at least 100 meters. 212 patients randomised, 41.6% female, average age 47.2 years, mean duration of pain 4.7 years | |
| Interventions |
MBR (CT): 10 weeks, active physical training (3 times/week for 1 hour 45 minutes sessions): aerobic training, strengthening, stretching. CBT: operant behavioural graded activity (18 sessions), problem‐solving (10 sessions), modification of dysfunctional beliefs, HEP increasing activity Physical (APT, Control 1): 10/52 Rx. Active physical training (3 times/week for 1 hour 45 minutes): aerobic training, strengthening, stretching MBR‐2 (CBT, Control 2): operant behavioural graded activity (18 sessions), problem‐solving (10 sessions), modification of dysfunctional beliefs, HEP increasing activity Waiting list (control 3) |
|
| Outcomes | Pain (VAS), Disability (RMDQ), Main Complaints, Self‐Perceived Improvement, Work, Health Care Utilization (number of visits), Depression (BDI), Catastrophising (PCL) Follow‐ups: ST (post‐treatment), MT (6 months), LT (1 year) | |
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.264 Randomization. "a randomization list was generated under the supervision of an independent statistician" |
| Allocation concealment (selection bias) | Low risk | pg.264 Randomization. "Opaque, sequentially numbered, sealed envelopes were prepared for each rehabilitation centre before enrolment started" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.267 Results. 156/172 randomized patients followed‐up |
| Intention to treat analysis | Low risk | pg.267 Statistical analyses. "All statistical analyses were performed according to the intention‐to‐treat principle" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Low risk | Fig 1. Adequate compliance across groups |
| Cointerventions | Low risk | Fig 1. Few additional treatments across groups |
| Timing of assessment | Low risk | pg.266 Outcome measures. "Outcome measures were recorded at baseline, immediately post‐treatment and 6 and 12 months post‐treatment" |
Strand 2001.
| Methods | RCT conducted in Norway | |
| Participants | Patients sick‐listed >8 weeks due to LBP. 117 patients randomised, 61% female, average age 43.6 years, mean duration of pain 10 years | |
| Interventions |
MBR (Multidisciplinary Rehabilitation): 4 weeks, 5 days/week, 6 hours/day. Physical treatment (strengthening, body awareness, aerobic fitness, relaxation), education, CBT (coping, responsibility for Rx, focus away from pain), workplace intervention Usual (Control): usual care in the community, most had physiotherapy, 1/3 have alternative interventions |
|
| Outcomes | Work (% return to work) Follow‐ups: ST (post‐treatment), MT (8 months), LT (1 year) | |
| Notes | Subgroup analyses: High intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In Haldorsen 1998. "individuals were allocated at random to one of two groups, the Treatment group or the Control group, by means of a sequence of pre labeled cards contained in sealed envelopes. The allocation sequence was prepared beforehand by a physician" |
| Allocation concealment (selection bias) | Low risk | In Haldorsen 1998. "individuals were allocated at random to one of two groups, the Treatment group or the Control group, by means of a sequence of pre labeled cards contained in sealed envelopes. The allocation sequence was prepared beforehand by a physician" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.801 Patients. 117/162 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.801 Procedure. "The same physiotherapist assessed patients at baseline (before randomization) and at the 1‐year follow‐up" |
Streibelt 2009.
| Methods | RCT conducted in Germany | |
| Participants | Patients referred to rehabilitation centre from work insurance provider, limited work ability due to chronic musculoskeletal disorder. 222 patients randomised, 16.7% female, average age 45.8 years, mean duration of pain not reported | |
| Interventions |
MBR (FCEMR): 3 weeks inpatient program, 3‐4 hours treatment/day. Physical therapy, exercises, massage, education, relaxation. Focus on work‐specific skills and functional capacity with operant behavioural approach. Coping skills training Physical (MR, Control): 3 weeks inpatient program, 3‐4 hours treatment/day. Physical therapy, exercises, massage, education, relaxation |
|
| Outcomes | Disability (PDI), Work (weeks off‐work, % return to work) Follow‐ups: LT (1 year) | |
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.4 Study design. "A computer‐generated randomization list was created by a statistician" |
| Allocation concealment (selection bias) | Low risk | pg.4 Study design. "Randomized allocation of the patients in either the treatment or the control group was done by an external institute" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.7 Sample. >50% dropout rate |
| Intention to treat analysis | Low risk | pg.6 Analysis. "Cases were analysed as intended to treat" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | High risk | Baseline characteristics. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | Fig 1. 1 year follow‐up |
Tavafian 2008.
| Methods | RCT conducted in Iran | |
| Participants | Adult women recruited from outpatient rheumatology clinics with age >18 years, LBP >3 months. 102 patients randomised, 100% female, average age 42.9 years, mean duration of pain 9.1 months | |
| Interventions |
MBR (Education): 4 days, 5 sessions, based on Back school. Education: anatomy, physiology, pathology of LBP, self‐care, health behaviours, biomechanics, lifestyle factors, prevention. Psychologist: coping skills, anger management, relaxation. Physiotherapist: stretching, strengthening, posture, functional movement advice (HEP) Usual (Control): medical management, mostly medication prescription (analgesics, muscle relaxants, NSAIDs, anti‐depressants) |
|
| Outcomes | Pain (SF‐36 bodily pain), General Health (SF‐36) Follow‐ups: ST (3 months), MT (6 months), LT (12 months) |
|
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | High risk | pg.1618 1st column. "The treatment allocation was not concealed" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.1618 Fig 1. 74/102 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | No stated |
| Cointerventions | Low risk | pg.1618 1st column. "cointerventions were avoided for both group" |
| Timing of assessment | Low risk | pg 1617 Study Design. "This was a blind randomized controlled trial with a 3, 6, and 12 months follow up" |
Tavafian 2011.
| Methods | RCT conducted in Iran | |
| Participants | Patients referred to rheumatology clinics in 3 teaching hospitals and 1 private clinic with age > 18 years, LBP >3 months. 197 patients randomised, 22% female, average age 45.3 years, mean duration of pain 6.8 years | |
| Interventions |
MBR (Education): 5 sessions of 2 hours in one week, plus monthly booster sessions and monthly telephone counselling, group setting. Education (anatomy, risk factors, lifestyle advice, posture, diagnosis, pain education). Streching, strengthening, relaxation exercises (HEP). Psychologist: coping, stress, perceptions of stress and control, emotional reactions, problem solving, relaxation. CBT: maladaptive cognitions, fear avoidance, activity participation, adjustment to pain. Motivational counselling. Medications prescribe as needed (analgesics, muscle relaxants, NSAIDs, anti‐depressants) Usual (Control): Medical management, mostly medication prescription (analgesics, muscle relaxants, NSAIDs, anti‐depressants) |
|
| Outcomes | Pain (SF‐36 bodily pain), Disability (RMDQ), General Health (SF‐36) Follow‐ups: ST (3 months), MT (6 months) |
|
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.812 Study design. "Participants were randomly assigned into the intervention or control group through random permutation blocking of every 6 participants" |
| Allocation concealment (selection bias) | Low risk | pg.812 Study design. "The sequence of allocation was concealed to the rheumatologist who selected the eligible patients" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 188/197 randomized patients followed up |
| Intention to treat analysis | High risk | pg.814 Statistical analysis. "no intention‐to‐treat analysis was performed" |
| Selective reporting (reporting bias) | Low risk | pg.814 Statistical analysis. "All data analyses were carried out according to the preestablished analysis plan" |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Unclear |
| Timing of assessment | Low risk | pg.812 Study design. "Data were collected at the time of randomization (baseline), 3, and 6‐month follow‐ups" |
Turner 1990.
| Methods | RCT conducted in the USA | |
| Participants | Patients responded to advertisements or referred by community physicians with age > 20‐65 years, LBP >6 months. 96 patients randomised, 49% female, average age 44 years, mean duration of pain 12.9 years | |
| Interventions |
MBR (Behav/Exerc): 8 sessions, 1x/ week for 2 hours, in groups of 5‐10. Behavioral: Education about pain behaviours (with spouse), communication training, goal‐setting for behavioural activities, group discussions, homework. Exercise (10‐20min 5x /week HEP): gradually progressed walking/jogging on a quota system, warm‐up and cool‐down stretches Physical (Exercise): 8 sessions, 1x/ week for 2 hours, in groups of 5‐10. Exercise (10‐20min 5x /week HEP): gradually progressed walking/jogging on a quota system, warm‐up and cool‐down stretches Waiting list (Control) |
|
| Outcomes | Pain (McGill), Disability (Sickness Impact Profile), Depression (CES‐D) Follow‐ups: ST (2 months), MT (6 months) ST (12 months) |
|
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.575 Immediate treatment effects. Dropout >20% at post‐treatment assessment |
| Intention to treat analysis | High risk | pg.575 Immediate treatment effects. Only compliers analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | pg.575 Analysis of pre‐treatment differences. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.574 Outcome measures. A comprehensive set of measures, described below, was administered immediately before and after treatment and 6 and 12 months following treatment" |
Van den Hout 2003.
| Methods | RCT conducted in the Netherlands | |
| Participants | Patients with recent work absence due to LBP, referred by GP, occupational or rehabilitation physician with age 18‐65 years, LBP >6 months, on sick leave for LBP >6 weeks. 84 patients randomised, 33.7% female, average age 40.5 years, mean duration of pain 1.6 years | |
| Interventions |
MBR (GAPS): 19 half day sessions over 8 weeks, in groups (n=5). Graded activity: gradual increase in physical activities, including work‐specific tasks, as directed by occupational therapist included a work visit, back education and lifting instructions, ADLs, leisure activities, housework. Problem solving: CBT approach to problem solving skills, training and application of skills to daily life, included homework assignments. Group education sessions related to back and back pain MBR‐2 (CAGE): 19 half day sessions over 8 weeks, in groups (n=5). Graded activity: gradual increase in physical activities, including work‐specific tasks, as directed by occupational therapist included a work visit, back education and lifting instructions, ADLs, leisure activities, houeswork. Group education sessions related to back and back pain |
|
| Outcomes | Work (% working). Follow‐ups: MT (6 months), LT (1 year) |
|
| Notes | Subgroup analyses: Mid intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.88 Study design. "The randomization scheme was computer‐generated and was known only to the logistics planner of the rehabilitation center" |
| Allocation concealment (selection bias) | Low risk | pg.88 Study design. "The randomization scheme was computer‐generated and was known only to the logistics planner of the rehabilitation center" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pg.91 Baseline characteristics. 84/108 randomized patients followed up |
| Intention to treat analysis | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | High risk | Table 1. Baseline difference in RMDQ |
| Compliance | Unclear risk | Not stated |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.90 Statistical analysis. "Differences in work status were assessed by means of 2 tests regarding work status one week before the intervention, and 6 and 12 months after the intervention" |
Vollenbroek‐Hutten 2004.
| Methods | RCT conducted in the Netherlands | |
| Participants | Patients admitted to a multidisciplinary rehabilitation program for LBP with age 18‐60 years, pain >6 months, no surgery in the last 3 months. 163 patients randomised, % female not reported, average age 39 years, mean duration of pain 5 years | |
| Interventions |
MBR (RRP): 9 hours/week for 7 weeks in groups of 8 patients. Education: back pain, chronicity, interaction of reduced physical activity and pain. Physical training: aerobic training, swimming, physiotherapy. Occupational therapy Usual (Control): unconstrained, usual care in the Netherlands |
|
| Outcomes | Disability (RMDQ), General Health (EQ‐5D), Fear Avoidance (TSK) Follow‐ups: ST (post‐treatment), MT (6 months) | |
| Notes | Subgroup analyses: Mid‐intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.568 Protocol. "To enable an adequate assignment procedure a computer programme was used" |
| Allocation concealment (selection bias) | Low risk | pg.568 Protocol. "Randomization was performed by a person not involved in either the treatment or this study" |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1. 142/163 randomized patients followed up |
| Intention to treat analysis | Unclear risk | pg.570 1st column. "For all analyses an intention‐to‐treat analysis, including patients with protocol deviations, was performed" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | pg.570 Compliance. Insufficient information reported |
| Cointerventions | Low risk | pg.571 Co‐interventions. Negligible visits to other practitioners |
| Timing of assessment | Low risk | pg.569 1st paragraph. "Measurements were performed before randomization (T0), in the week after treatment or eight weeks after T0 (T1) and six months after T0 (T5)" |
Von Korff 2005.
| Methods | RCT conducted in USA | |
| Participants | Members of an insurance scheme receiving primary care for LBP with age 25‐60 years, score >7/23 on RMDQ. 240 patients randomised, 62.5% female, average age 49.8 years, mean duration of pain not reported | |
| Interventions |
MBR (Intervention): 4 visits of 90 minutes. Psychologist: identify fears, relationship b/w activity and pain, goal setting, relaxation, managing flare‐ups. Physiotherapist: discussed concerns and identify barriers to increasing activity, stretches, exercises to achieve activity goals (HEP). Self‐management book Usual (Control): usual care in the community, usually included medication |
|
| Outcomes | Pain (NRS), Disability (RMDQ), General Health (SF‐36), Work (% able to work), Fear Avoidance (TSK) Follow‐ups: ST (2 months), MT (6 months), LT (1 and 2 years) |
|
| Notes | Subgroup analyses: Low intensity intervention, Low baseline symptom intensity (<60% of maximum scale score) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants | High risk | Not possible |
| Blinding of clinicians | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible; patient reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pg.325 Results. 197/240 randomised patients followed up |
| Intention to treat analysis | Unclear risk | pg.325 Analysis. "Intent to treat analyses included all randomized participants for whom follow‐up data were available" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Comparability of groups at baseline | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Compliance | Unclear risk | pg.325 Results. Compliance only reported in intervention group |
| Cointerventions | Unclear risk | Not stated |
| Timing of assessment | Low risk | pg.324 Masking. "At 2, 6, 12 and 24 months after randomization, follow‐up telephone interviews were conducted by an interviewer" |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhmadeeva 2009 | Not a full paper, conference abstract |
| Albaladejo 2010 | Intervention does not contain two or more elements from the biopsychosocial model |
| Andrade 2008 | Intervention does not contain two or more elements from the biopsychosocial model |
| Anema 2007 | Participants do not all have chronic LBP |
| Angst 2009 | Study is not a RCT |
| Bachmann 2009 | Intervention does not contain two or more elements from the biopsychosocial model |
| Bahrke 2006 | Study is not a RCT |
| Bandemeer‐Greulich 2006 | Study is not a RCT |
| Bandemeer‐Greulich 2008 | Study is not a RCT |
| Basler 2007 | Intervention not delivered by a multidisciplinary team |
| Bastiaenen 2004 | Study is not a RCT |
| Becker 2000 | Participants do not all have chronic LBP |
| Becker 2008 | Intervention does not contain two or more elements from the biopsychosocial model |
| Bendix 1998a | Study does not compare treatment effects |
| Bethge 2011 | Participants do not all have chronic LBP |
| Binder 2007 | Study is not a RCT |
| Bliokas 2007 | Participants do not all have chronic LBP |
| Brox 2003 | Intervention not delivered by a multidisciplinary team |
| Buhrman 2011 | Intervention not delivered by a multidisciplinary team |
| Bultman 2009 | Participants do not all have chronic LBP |
| Busch 2011 | Participants do not all have chronic LBP |
| Campello 2012 | Participants do not all have chronic LBP |
| Cecchi 2012 | Intervention not delivered by a multidisciplinary team |
| Christiansen 2010 | Intervention not delivered as an integrated program |
| Demoulin 2006 | Study is not a RCT |
| Dibbelt 2006 | Study is not a RCT |
| Dobscha 2009 | Participants do not all have chronic LBP |
| Donzelli 2006 | Intervention does not contain two or more elements from the biopsychosocial model |
| Driessen 2011a | Participants do not all have chronic LBP |
| Driessen 2011b | Participants do not all have chronic LBP |
| Dufour 2010 | Intervention does not contain two or more elements from the biopsychosocial model |
| Dysvik 2005 | Study is not a RCT |
| Ektor‐Andersen 2008 | No defined MBR intervention reported |
| Esmer 2010 | Intervention not delivered by a multidisciplinary team |
| Ewert 2009 | Participants do not all have chronic LBP |
| Ferrari 2006 | Participants do not all have chronic LBP |
| Friedberg 2010 | Appraisal of another study |
| Friedrich 1998 | Intervention not delivered by a multidisciplinary team |
| Friedrich 2005 | Intervention not delivered by a multidisciplinary team |
| Froholdt 2011 | Intervention does not contain two or more elements from the biopsychosocial model |
| Froholdt 2012 | Intervention not delivered by a multidisciplinary team |
| Frost 1998 | Intervention does not contain two or more elements from the biopsychosocial model |
| Gatchel 2003 | Participants do not all have chronic LBP |
| George 2009 | Not a full paper, conference abstract |
| George 2010a | Not a full paper, conference abstract |
| George 2010b | Study is not a RCT |
| George 2011 | Participants do not all have chronic LBP |
| Glattacker 2012 | Study is not a RCT |
| Glombiewski 2010 | Intervention not delivered by a multidisciplinary team |
| Glomsrod 2001 | Participants do not all have chronic LBP |
| Gohner 2006 | Participants do not all have chronic LBP |
| Greitemann 2006 | Study is not a RCT |
| Hagen 2006 | Participants do not all have chronic LBP |
| Hagen 2010 | Participants do not all have chronic LBP |
| Hallegraeff 2009 | Participants do not all have chronic LBP |
| Hampel 2009 | Study is not a RCT |
| Henchoz 2010a | Intervention does not contain two or more elements from the biopsychosocial model |
| Henchoz 2010b | Intervention does not contain two or more elements from the biopsychosocial model |
| Heymans 2006 | Participants do not all have chronic LBP |
| Hlobil 2005 | Participants do not all have chronic LBP |
| Hodselmans 2001 | Intervention does not contain two or more elements from the biopsychosocial model |
| Huge 2006 | Study is not a RCT |
| Jensen 2005 | Study is not a RCT |
| Jensen 2007 | Study is not a RCT |
| Jensen 2009 | Study is not a RCT |
| Jensen 2011 | Participants do not all have chronic LBP |
| Jensen 2012 | Participants do not all have chronic LBP |
| Jensen 2013 | Participants do not all have chronic LBP |
| Johnson 2013 | Reported in Hellum 2011 |
| Kainz 2006 | Study is not a RCT |
| Kolip 2001 | Study is not a RCT |
| Kumar 2010 | Intervention does not contain two or more elements from the biopsychosocial model |
| Lamb 2010 | Participants do not all have chronic LBP |
| Lang 2003 | Study is not a RCT |
| Le Gall 2001 | Study is not a RCT |
| Lee 2013 | Participants do not all have chronic LBP |
| Leon 2009 | Participants do not all have chronic LBP |
| Lindell 2008 | Participants do not all have chronic LBP |
| Linton 2000 | Study is not a RCT |
| Ljungkvist 2000 | Study is not a RCT |
| Loisel 2002 | Participants do not all have chronic LBP |
| Lonn 1999 | Intervention does not contain two or more elements from the biopsychosocial model |
| Mannion 2001 | Intervention does not contain two or more elements from the biopsychosocial model |
| Mannion 2013 | Intervention not delivered by a multidisciplinary team |
| Martin 2000 | Participants do not all have chronic LBP |
| Mattila 2007 | Participants do not all have chronic LBP |
| Meyer 2005 | Participants do not all have chronic LBP |
| Mohr 2009 | Study is not a RCT |
| Molde 2003 | Participants do not all have chronic LBP |
| Nazzal 2013 | Intervention not delivered by a multidisciplinary team |
| Nicholas 2013 | Participants do not all have chronic LBP |
| Niemisto 2005 | Intervention does not contain two or more elements from the biopsychosocial model |
| Padua 2009 | Study is not a RCT |
| Paolucci 2012 | Reported in Morone 2011 |
| Rainville 2002 | Intervention does not contain two or more elements from the biopsychosocial model |
| Rantonen 2012 | Participants do not all have chronic LBP |
| Rossignol 2000 | Participants do not all have chronic LBP |
| Rothman 2013 | Participants do not all have chronic LBP |
| Sahin 2011 | Intervention not delivered by a multidisciplinary team |
| Shete 2012 | Participants do not all have chronic LBP |
| Siemonsma 2013 | Intervention not delivered by a multidisciplinary team |
| Sjostrum 2010 | Study is not a RCT |
| Sleptsova 2013 | Participants do not all have chronic LBP |
| Sorensen 2010 | Intervention does not contain two or more elements from the biopsychosocial model |
| Staal 2004 | Intervention does not contain two or more elements from the biopsychosocial model |
| Stapelfeldt 2011 | Participants do not all have chronic LBP |
| Steenstra 2006 | Intervention does not contain two or more elements from the biopsychosocial model |
| Stier 2001 | Study is not a RCT |
| Storheim 2003 | Intervention does not contain two or more elements from the biopsychosocial model |
| Storro 2004 | Study is not a RCT |
| Strong 2006 | Intervention not delivered by a multidisciplinary team |
| Sundberg 2009 | Participants do not all have chronic LBP |
| Tlach 2011 | Study is not a RCT |
| Torstensen 1998 | Intervention does not contain two or more elements from the biopsychosocial model |
| Trapp 2009 | Not a full paper, conference abstract |
| Tsauo 2009 | Intervention does not contain two or more elements from the biopsychosocial model |
| Turk 1998 | Study is not a RCT |
| Underwood 2004 | Intervention does not contain two or more elements from the biopsychosocial model |
| van Beurden 2012 | Participants do not all have chronic LBP |
| van der Roer 2008 | Intervention not delivered by a multidisciplinary team |
| van Hoof 2010 | Study is not a RCT |
| Verbeek 2002 | Participants do not all have chronic LBP |
| Vermeulen 2011 | Participants do not all have chronic LBP |
| Verra 2012 | Study is not a RCT |
| Vibe Fersum 2013 | Intervention not delivered by a multidisciplinary team |
| Vlaeyen 1995 | Intervention does not contain two or more elements from the biopsychosocial model |
| Vong 2011 | Intervention not delivered by a multidisciplinary team |
| Wagner 2007 | Study is not a RCT |
| Wand 2004 | Participants do not all have chronic LBP |
| Wessels 2007 | Participants do not all have chronic LBP |
| Whitfill 2010 | Participants do not all have chronic LBP |
| Wilkey 2008 | Intervention does not contain two or more elements from the biopsychosocial model |
| Yang 2010 | Intervention not delivered by a multidisciplinary team |
Differences between protocol and review
Small changes were made to the inclusion criteria between this review and the previous version. This involved clarifying that interventions must be delivered by a truly multidisciplinary team of practitioners with different clinical backgrounds relevant to the portion of the intervention they delivered. The sensitivity and subgroup analyses described in the 'Methods' section were devised after publication of the previous version of the review but before commencement of searches for this review.
Contributions of authors
SK, MvT, RO, JG and RS planned the review and developed the protocol. SK, AA and AC screened titles and abstracts, SK and AA performed the RoB assessments. SK and AC conducted the handsearches and extracted and checked the data. SK wrote the initial draft of the manuscript and all authors critically reviewed successive drafts.
Sources of support
Internal sources
-
National Health and Medical Research Council, Australia.
Fellowship for SK
External sources
No sources of support supplied
Declarations of interest
MvT was involved in the conduct of one of the included studies (Lambeek 2010), and RS was involved in one of the included studies (Smeets 2006/2008). They were not involved in the risk of bias assessment or data extraction.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abbassi 2012 {published data only}
- Abbasi M, Dehghani M, Keefe FJ, Jafari H, Behtash H, Shams J. Spouse‐assisted training in pain coping skills and the outcome of multidisciplinary pain management for chronic low back pain treatment: A 1‐year randomized controlled trial. European Journal of Pain 2012;16(7):1033‐43. [DOI] [PubMed] [Google Scholar]
Alaranta 1994 {published data only}
- Alaranta H, Rytökoski U, Rissanen A, Talo S, Rönnemaa T, Puukka P, et al. Intensive physical and psychosocial training program for patients with chronic low back pain. A controlled clinical trial. Spine 1994;19:1339‐49. [DOI] [PubMed] [Google Scholar]
Basler 1997 {published data only}
- Basler H, Jakle C, Kroner‐Herwig B. Incorporation of cognitive behavioral treatment into the medical care of chronic low back patients: A controlled randomized study in German pain treatment centers. Patient Education & Counseling 1997;31:113‐24. [DOI] [PubMed] [Google Scholar]
Bendix 'A' 1996/1998 {published data only}
- Bendix AE, Bendix T, Haestrup C, Busch E. A prospective, randomized 5‐year follow‐up study of functional restoration in chronic low back pain patients. European Spine Journal 1998;7(2):111‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix AF, Bendix T, Vaegter K, Lund C, Frolund L, Holm L. Multidisciplinary intensive treatment for chronic low back pain: a randomized, prospective study. Cleveland Clinic Journal of Medicine 1996;63:62‐9. [DOI] [PubMed] [Google Scholar]
Bendix 'B' 1995/1998 {published data only}
- Bendix AE, Bendix T, Haestrup C, Busch E. A prospective, randomized 5‐year follow‐up study of functional restoration in chronic low back pain patients. European Spine Journal 1998;7(2):111‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix AF, Bendix T, Ostenfeld S, Bush E, Andersen A. Active treatment programs for patients with chronic low back pain: a prospective, randomized, observer blinded study. European Spine Journal 1995;4:149‐52. [DOI] [PubMed] [Google Scholar]
Bendix 'C' 2000 {published data only}
- Bendix T, Bendix A, Labriola M, Haestrup C, Ebbehoj N. Functional restoration versus outpatient physical training in chronic low back pain: a randomized comparative study. Spine 2000;25(19):2494‐500. [DOI] [PubMed] [Google Scholar]
Coole 2013 {published data only}
- Coole C, Drummond A, Watson P. Individual work support for employed patients with low back pain: a randomized controlled pilot trial. Clinical Rehabilitation 2013;27(1):40‐50. [DOI] [PubMed] [Google Scholar]
Fairbank 2005 {published data only}
- Fairbank J, Frost H, Wilson‐MacDonald J, Yu L‐M, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ 2005;330(7502):1233‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero‐Arias O, Campbell H, Gray A, Fairbank J, Frost H. Surgical stabilisation of the spine compared with a programme of intensive rehabilitation for the management of patients with chronic low back pain: cost utility analysis based on a randomised controlled trial. BMJ 2005;330(7502):1239‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harkapaa 1989 {published data only}
- Harkapaa K, Jarvikoski A, Mellin G, Hurri H. Pain, disability, compliance, and reported treatment benefits three months after treatment. Scandinavian Journal of Rehabilitation Medicine 1989;21:81‐9. [PubMed] [Google Scholar]
Hellum 2011 {published data only}
- Hellum C, Johnsen LG, Storheim K, Nygaard O P, Brox JI, Rossvoll I, et al. Surgery with disc prosthesis versus rehabilitation in patients with low back pain and degenerative disc: two year follow‐up of randomised study. BMJ 2011;342:d2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henchoz 2010 {published data only}
- Henchoz Y, Goumoens P, So AK, Paillex R. Functional multidisciplinary rehabilitation versus outpatient physiotherapy for non specific low back pain: randomized controlled trial. Swiss Medical Weekly 2010;140(w13133):1‐7. [DOI] [PubMed] [Google Scholar]
Jackel 1990 {published data only}
- Jackel WH, Cziske R, Gerdes N, Jacobi E. Assessment of the effectiveness of inpatient rehabilitation measures in patients with chronic low back pain: a prospective, randomized, controlled study [German]. Rehabilitation 1990;29:129‐33. [PubMed] [Google Scholar]
Jousset 2004 {published data only}
- Jousset N, Fanello S, Bontoux L, Dubus V, Billabert C, Vielle B. Effects of functional restoration versus 3 hours per week physical therapy: a randomized controlled study. Spine 2004;29(5):487‐94. [DOI] [PubMed] [Google Scholar]
Kaapa 2006 {published data only}
- Kaapa EH, Frantsi K, Sarna S, Malmivaara A. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain: a randomized trial. Spine 2006;31(4):371‐6. [DOI] [PubMed] [Google Scholar]
Kole‐Snijders 1999 {published data only}
- Goossens ME, Rutten‐Van Molken MP, Kole‐Snijders AM, Vlaeyen JW, Breukelen G, Leidl R. Health economic assessment of behavioural rehabilitation in chronic low back pain: a randomized clinical trial. Health Economics 1998;7:39‐51. [DOI] [PubMed] [Google Scholar]
- Kole‐Snijders AMJ, Vlaeyen JWS, Goossens MEJB, Rutten‐Van Molken MPMH, Heuts PHTG, Breukelen G, Eek H. Results of a randomized clinical trial. Journal of Consulting and Clinical Psychology 1999;67(6):931‐44. [DOI] [PubMed] [Google Scholar]
Kool 2007 {published data only}
- Kool J, Bachmann S, Oesch P, Knuesel O, Ambergen T, Bie R. Function‐centered rehabilitation increases work days in patients with nonacute nonspecific low back pain: 1‐year results from a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(9):1089‐94. [DOI] [PubMed] [Google Scholar]
Lambeek 2010 {published data only}
- Lambeek LC, Bosmans JE, Royen BJ, Tulder MW, Mechelen W, Anema JR. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010;341:6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeek LC, Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ 2010;340(7749):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeuw 2008 {published data only}
- Leeuw M, Goossens MEJB, Breukelen GJP, Jong JR, Heuts PHTG, Smeets RJEM, et al. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain 2008;138:192‐207. [DOI] [PubMed] [Google Scholar]
Linton 2005 {published data only}
- Linton SJ, Boersma K, Jansson M, Svärd L, Botvalde M. The effects of cognitive‐behavioral and physical therapy preventive interventions on pain‐related sick leave: a randomized controlled trial. Clinical Journal of Pain 2005;21(2):109‐19. [DOI] [PubMed] [Google Scholar]
Lukinmaa 1989 {published data only}
- Lukinmaa A. Low back pain as a biopsychosocial problem. A controlled clinical trial and a costeffectiveness analysis [Finnish]. Kansanelakelaitoksen Julkaisuja 1989;ML:1‐90. [Google Scholar]
Mangels 2009 {published data only}
- Mangels M, Schwarz S, Worringen U, Holme M, Rief W. Evaluation of a behavioral‐medical inpatient rehabilitation treatment including booster sessions: a randomized controlled study. Clinical Journal of Pain 2009;25(5):356‐64. [DOI] [PubMed] [Google Scholar]
Meng 2011 {published data only}
- Meng K, Seekatz B, Roband H, Worringen U, Vogel H, Faller H. Intermediate and long‐term effects of a standardized back school for inpatient orthopedic rehabilitation on illness knowledge and self‐management behaviors: a randomized controlled trial. Clinical Journal of Pain 2011;27(3):248‐57. [DOI] [PubMed] [Google Scholar]
Mitchell 1994 {published data only}
- Mitchell RI, Carmen GM. The functional restoration approach to the treatment of chronic pain in patients with soft tissue and back injuries. Spine 1994;19:633‐42. [DOI] [PubMed] [Google Scholar]
Moix 2003 {published data only}
- Moix J, Canellas M, Osorio C, Bel X, Girvent F, Martos A. Efficacy of an interdisciplinary educational program in patients with chronic back pain [Spanish]. Dolor 2003;18(3):149‐57. [Google Scholar]
Monticone 2013 {published data only}
- Monticone M, Ferrante S, Rocca B, Baiardi P, Dal Farra F, Foti C. Effect of a long‐lasting multidisciplinary program on disability and fear‐avoidance behaviors in patients with chronic low back pain: results of a randomized controlled trial. Clinical Journal of Pain 2013;29(11):929‐38. [DOI] [PubMed] [Google Scholar]
Morone 2011 {published data only}
- Morone G, Paolucci T, Alcuri MR, Vulpiani MC, Matano A, Bureca I. Quality of life improved by multidisciplinary back school program in patıents with chronic non‐specific low back pain: a single blind randomized controlled trial. Europan Journal of Physical and Rehabilitation Medicine 2011;47(4):533‐41. [PubMed] [Google Scholar]
Morone 2012 {published data only}
- Morone G, Iosa M, Paolucci T, Fusco A, Alcuri R, Spadini E. Efficacy of perceptive rehabilitation in the treatment of chronic nonspecific low back pain through a new tool: a randomized clinical study. Clinical Rehabilitation 2012;26(4):339‐50. [DOI] [PubMed] [Google Scholar]
Nicholas 1991 {published data only}
- Nicholas MK, Wilson PH, Goyen J. Operant‐behavioural and cognitive behavioural treatment for chronic low back pain. Behavioural Research and Therapy 1991;29:225‐38. [DOI] [PubMed] [Google Scholar]
Nicholas 1992 {published data only}
- Nicholas MK, Wilson PH, Goyen J. Comparison of cognitive behavioral group treatment and an alternative non‐psychological treatment for chronic low back pain. Pain 1992;48:339‐47. [DOI] [PubMed] [Google Scholar]
Roche 2007/2011 {published data only}
- Roche G, Ponthieux A, Parot‐Shinkel E, Jousset N, Bontoux L, Dubus V. Comparison of a functional restoration program with active individual physical therapy for patients with chronic low back pain: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(10):1229‐35. [DOI] [PubMed] [Google Scholar]
- Roche‐Leboucher G, Petit‐Lemanach A, Bontoux L, Dubus‐Bausiere V, Parot‐Shinkel E, Fanello S. Multidisciplinary intensive functional restoration versus outpatient active physiotherapy in chronic low back pain: A randomized controlled trial. Spine 2011;36(26):2235‐42. [DOI] [PubMed] [Google Scholar]
Schweikert 2006 {published data only}
- Schweikert B, Jacobi E, Seitz R, Cziske R, Ehlert A, Knab J. Effectiveness and cost‐effectiveness of adding a cognitive behavioral treatment to the rehabilitation of chronic low back pain. Journal of Rheumatology 2006;33(12):2519‐26. [PubMed] [Google Scholar]
Skouen 2002 {published data only}
- Skouen JS, Grasdal AL, Haldorsen EM, Ursin H. Relative cost‐effectiveness of extensive and light multidisciplinary treatment programs versus treatment as usual for patients with chronic low back pain on long‐term sick leave: randomized controlled study. Spine 2002;27(9):901‐9. [DOI] [PubMed] [Google Scholar]
Smeets 2006/2008 {published data only}
- Smeets RJ, Severens JL, Beelen S, Vlaeyen JW, Knottnerus JA. More is not always better: cost‐effectiveness analysis of combined, single behavioral and single physical rehabilitation programs for chronic low back pain. European Journal of Pain 2009;13(1):71‐81. [DOI] [PubMed] [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Hidding A, Kester ADM, Heijden GJMG, Knottnerus JA. Chronic low back pain: Physical training, graded activity with problem solving training, or both? The one‐year post‐treatment results of a randomized controlled trial. Pain 2008;134(8):263‐76. [DOI] [PubMed] [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Hidding A, Kester ADM, Heijden GJMG, Geel ACM, Knottnerus JA. Active rehabilitation for chronic low back pain: Cognitive‐behavioral, physical, or both? First direct post‐treatment results from a randomized controlled trial. BMC Musculoskeletal Disorders 2006;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2001 {published data only}
- Strand LI, Ljunggren AE, Haldorsen EMH, Espehaug B. The impact of physical function and pain on work status at 1‐year follow‐up in patients with back pain. Spine 2001;26(7):800‐8. [DOI] [PubMed] [Google Scholar]
Streibelt 2009 {published data only}
- Streibelt M, Thren K, Muller‐Fahrnow W. Effects of FCE‐based multidisciplinary rehabilitation in patients with chronic musculoskeletal disorders ‐ results of a randomized controlled trial [German]. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin 2009;19(1):34‐41. [Google Scholar]
Tavafian 2008 {published data only}
- Tavafian SS, Jamshidi AR, Montazeri A. A randomized study of back school in women with chronic low back pain: quality of life at three, six, and twelve months follow‐up. Spine 2008;33(15):1617‐21. [DOI] [PubMed] [Google Scholar]
Tavafian 2011 {published data only}
- Tavafian SS, Jamshidi AR, Mohammad K. Treatment of chronic low back pain: a randomized clinical trial comparing multidisciplinary group‐based rehabilitation program and oral drug treatment with oral drug treatment alone. Clinical Journal of Pain 2011;27(9):811‐8. [DOI] [PubMed] [Google Scholar]
Turner 1990 {published data only}
- Turner JA, Clancy S, McQuade KJ, Cardenas DD. Effectiveness of behavioral therapy for chronic low back pain: a component analysis. Journal of Consulting and Clinical Psychology 1990;58(5):573‐9. [DOI] [PubMed] [Google Scholar]
Van den Hout 2003 {published data only}
- Hout JHC, Vlaeyen JWS, Heuts PH, Zijlema JH, Wijnen JA. Secondary prevention of work‐related disability in nonspecific low back pain: does problem‐solving therapy help? A randomized clinical trial. Clinical Journal of Pain 2003;19(2):87‐96. [DOI] [PubMed] [Google Scholar]
Vollenbroek‐Hutten 2004 {published data only}
- Vollenbroek‐Hutten MMR, Hermens HJ, Wever D, Gorter M, Rinket J, IJerman MJ. Differences in outcome of a multidisciplinary treatment between subgroups of chronic low back pain patients defined using two multiaxial assessment instruments: the Multidimensional Pain Inventory and lumbar dynamometry. Clinical Rehabilitation 2004;18(5):566‐79. [DOI] [PubMed] [Google Scholar]
Von Korff 2005 {published data only}
- Korff M, Balderson BH, Saunders K, Miglioretti DL, Lin EH, Berry S. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain 2005;113(3):323‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhmadeeva 2009 {published data only}
- Akhmadeeva L, Setchenkova N, Magzhanov R. Effectiveness of transcutaneous dynamic electrical nerve stimulation in low back pain: A pilot randomized controlled trial. Journal of Neurological Sciences 2009;285:S320. [Google Scholar]
Albaladejo 2010 {published data only}
- Albaladejo C, Kovacs FM, Royuela A, Pino R, Zamora J. The efficacy of a short education program and a short physiotherapy program for treating low back pain in primary care: a cluster randomized trial. Spine 2010;35(5):483‐96. [DOI] [PubMed] [Google Scholar]
Andrade 2008 {published data only}
- Andrade SC, Araujo AG, Vilar MJ. [Back school for patients with non‐specific chronic low‐back pain: benefits from the association of an exercise program with patient's education]. [Portuguese]. Acta Reumatologica Portuguesa 2008;33(4):443‐50. [PubMed] [Google Scholar]
Anema 2007 {published data only}
- Anema JR, Steenstra IA, Bongers PM, Vet HC, Knol DL, Loisel P, Mechelen W. Multidisciplinary rehabilitation for subacute low back pain: graded activity or workplace intervention or both? A randomized controlled trial. Spine 2007;32(3):291‐300. [DOI] [PubMed] [Google Scholar]
Angst 2009 {published data only}
- Angst F, Verra ML, Lehmann S, Brioschi R, Aeschlimann A. Clinical effectiveness of an interdisciplinary pain management programme compared with standard inpatient rehabilitation in chronic pain: a naturalistic, prospective controlled cohort study. Journal of Rehabilitation Medicine 2009;41(7):569‐75. [DOI] [PubMed] [Google Scholar]
Bachmann 2009 {published data only}
- Bachmann S, Wieser S, Oesch P, Schmidhauser S, Knusel O, Kool J. Three‐year cost analysis of function‐centred versus pain‐centred inpatient rehabilitation in patients with chronic non‐specific low back pain. Journal of Rehabilitation Medicine 2009;41(11):919‐23. [DOI] [PubMed] [Google Scholar]
Bahrke 2006 {published data only}
- Bahrke U, Bandemer‐Greulich U, Fikentscher E, Muller K, Schreiber B, Konzag TA. [Chronic low back pain with endurant pain coping‐‐optimizing rehabilitation success in a hitherto neglected patient group]. [German]. Rehabilitation 2006;45(6):336‐44. [DOI] [PubMed] [Google Scholar]
Bandemeer‐Greulich 2006 {published data only}
- Bandemer‐Greulich U, Schreiber B, Fikentscher E, Bahrke U. Protective and repressive factors for success in rehabilitation of chronic low back pain. [German]. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin 2006;16(5):297‐302. [Google Scholar]
Bandemeer‐Greulich 2008 {published data only}
- Bandemer‐Greulich U, Bosse B, Fikentscher E, Konzag TA, Bahrke U. [Efficacy of psychological interventions on pain coping strategies in orthopedic rehabilitation of chronic low back pain]. [German]. Psychotherapie, Psychosomatik, Medizinische Psychologie 2008;58(1):32‐7. [DOI] [PubMed] [Google Scholar]
Basler 2007 {published data only}
- Basler HD, Bertalanffy H, Quint S, Wilke A, Wolf U. TTM‐based counselling in physiotherapy does not contribute to an increase of adherence to activity recommendations in older adults with chronic low back pain ‐ A randomised controlled trial. European Journal of Pain 11;1:31‐7. [DOI] [PubMed] [Google Scholar]
Bastiaenen 2004 {published data only}
- Bastiaenen CH, Bie RA, Wolters PM, Vlaeyen JW, Bastiaanssen JM, Klabbers AB, et al. Treatment of pregnancy‐related pelvic girdle and/or low back pain after delivery design of a randomized clinical trial within a comprehensive prognostic cohort study. BMC Public Health 2004;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2000 {published data only}
- Becker N, Sjogren P, Bech P, Olsen AK, Eriksen J. Treatment outcome of chronic non‐malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Pain 2000;84(2‐3):203‐11. [DOI] [PubMed] [Google Scholar]
Becker 2008 {published data only}
- Becker A, Leonhardt C, Kochen MM, Keller S, Wegscheider K, Baum E, et al. Effects of two guideline implementation strategies on patient outcomes in primary care: a cluster randomized controlled trial. Spine 2008;33(5):473‐80. [DOI] [PubMed] [Google Scholar]
Bendix 1998a {published data only}
- Bendix AF, Bendix T, Haestrup C. Can it be predicted which patients with chronic low back pain should be offered tertiary rehabilitation in a functional restoration program? A search for demographic, socioeconomic, and physical predictors. Spine 1998;23(16):1775‐84. [DOI] [PubMed] [Google Scholar]
Bethge 2011 {published data only}
- Bethge M, Herbold D, Trowitzsch L, Jacobi C. Work status and health‐related quality of life following multimodal work hardening: a cluster randomised trial. Journal of Back and Musculoskeletal Rehabilitation 2011;24(3):161‐72. [DOI] [PubMed] [Google Scholar]
Binder 2007 {published data only}
- Binder A. [Chronic backache with neuropathic component. Individual basic therapy plus multimodal approach]. [German]. MMW Fortschritte der Medizin 2007;149(20):49. [PubMed] [Google Scholar]
Bliokas 2007 {published data only}
- Bliokas VV, Cartmill TK, Nagy BJ. Does systematic graded exposure in vivo enhance outcomes in multidisciplinary chronic pain management groups?. Clinical Journal of Pain 2007;23(4):361‐74. [DOI] [PubMed] [Google Scholar]
Brox 2003 {published data only}
- Brox JI, Sorensen R, Friis A, Nygaard O, Indahl A, Keller A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine 2003;17:1913‐21. [DOI] [PubMed] [Google Scholar]
Buhrman 2011 {published data only}
- Buhrman M, Nilsson‐Ihrfeldt E, Jannert M, Strom L, Andersson G. Guided internet‐based cognitive behavioural treatment for chronic back pain reduces pain catastrophizing: a randomized controlled trial. Journal of Rehabilitation Medicine 2011;43(6):500‐5. [DOI] [PubMed] [Google Scholar]
Bultman 2009 {published data only}
- Bultmann U, Sherson D, Olsen J, Hansen CL, Lund T, Kilsgaard J. Coordinated and tailored work rehabilitation: a randomized controlled trial with economic evaluation undertaken with workers on sick leave due to musculoskeletal disorders. Journal of Occupational Rehabilitation 2009;19(1):81‐93. [DOI] [PubMed] [Google Scholar]
Busch 2011 {published data only}
- Busch H, Bodin L, Bergstrom G, Jensen IB. Patterns of sickness absence a decade after pain‐related multidisciplinary rehabilitation. Pain 2011;152(8):1727‐33. [DOI] [PubMed] [Google Scholar]
Campello 2012 {published data only}
- Campello M, Ziemke G, Hiebert R, Weiser S, Brinkmeyer M, Fox B, et al. Implementation of a multidisciplinary program for active duty personnel seeking care for low back pain in a U.S. Navy Medical Center: a feasibility study. Military Medicine 2012;177(9):1075‐80. [DOI] [PubMed] [Google Scholar]
Cecchi 2012 {published data only}
- Cecchi F, Negrini S, Pasquini G, Paperini A, Conti AA, Chiti M, et al. Predictors of functional outcome in patients with chronic low back pain undergoing back school, individual physiotherapy or spinal manipulation. European Journal of Physical and Rehabilitation Medicine 2012;48(3):371‐8. [PubMed] [Google Scholar]
Christiansen 2010 {published data only}
- Christiansen S, Oettingen G, Dahme B, Klinger R. A short goal‐pursuit intervention to improve physical capacity: a randomized clinical trial in chronic back pain patients. Pain 2010;149(3):444‐52. [DOI] [PubMed] [Google Scholar]
Demoulin 2006 {published data only}
- Demoulin C, Maquet D, Tomasella M, Croisier J, Crielaard J, Vanderthommen M. Benefits of a physical training program after back to school for chronic low back pain patients. Journal of Musculoskeletal Pain 2006;14(2):21‐31. [Google Scholar]
Dibbelt 2006 {published data only}
- Dibbelt S, Greitemann B, Schel C. Long‐term efficiency of orthopedic rehabilitation in chronic back pain ‐‐ the integrative orthopedic psychosomatic concept (IopKo) [German]. Rehabilitation 2006;45(6):324‐35. [DOI] [PubMed] [Google Scholar]
Dobscha 2009 {published data only}
- Dobscha SK, Corson K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA 2009;301(12):1242‐52. [DOI] [PubMed] [Google Scholar]
Donzelli 2006 {published data only}
- Donzelli S, Domenica F, Cova AM, Galletti R, Giunta N. Two different techniques in the rehabilitation treatment of low back pain: A randomized controlled trial. Europa Medicophysica 2006;42(3):205‐10. [PubMed] [Google Scholar]
Driessen 2011a {published data only}
- Driessen MT, Proper KI, Anema JR, Knol DL, Bongers PM, Beek AJ. Participatory ergonomics to reduce exposure to psychosocial and physical risk factors for low back pain and neck pain: results of a cluster randomised controlled trial. Occupational and Environmental Medicine 2011;68(9):674‐81. [DOI] [PubMed] [Google Scholar]
Driessen 2011b {published data only}
- Driessen MT, Proper KI, Anema JR, Knol DL, Bongers PM, Beek AJ. The effectiveness of participatory ergonomics to prevent low‐back and neck pain ‐ results of a cluster randomized controlled trial. Scandinavian Journal of Work, Environment and Health 2011;37(5):383‐93. [DOI] [PubMed] [Google Scholar]
Dufour 2010 {published data only}
- Dufour N, Thamsborg G, Oefeldt A, Lundsgaard C, Stender S. Treatment of chronic low back pain: a randomized, clinical trial comparing group‐based multidisciplinary biopsychosocial rehabilitation and intensive individual therapist‐assisted back muscle strengthening exercises. Spine 35;5:469‐76. [DOI] [PubMed] [Google Scholar]
Dysvik 2005 {published data only}
- Dysvik E, Natvig GK, Eikeland OJ, Brattberg G. Results of a multidisciplinary pain management program: A 6‐ and 12‐month follow‐up study. Rehabilitation Nursing 2005;30(5):198‐206. [DOI] [PubMed] [Google Scholar]
Ektor‐Andersen 2008 {published data only}
- Ektor‐Andersen J, Ingvarsson E, Kullendorff M, Orbaek P. High cost‐benefit of early team‐based biomedical and cognitive‐behaviour intervention for long‐term pain‐related sickness absence. Journal of Rehabilitation Medicine 40;1:1‐8. [DOI] [PubMed] [Google Scholar]
Esmer 2010 {published data only}
- Esmer G, Blum J, Rulf J, Pier J. Mindfulness‐based stress reduction for failed back surgery syndrome: a randomized controlled trial. Journal of the American Osteopathic Association 2010;110(11):646‐52. [PubMed] [Google Scholar]
Ewert 2009 {published data only}
- Ewert T, Limm H, Wessels T, Rackwitz B, Garnier K, Freumuth R, Stucki G. The comparative effectiveness of a multimodal program versus exercise alone for the secondary prevention of chronic low back pain and disability. Physical Medicine and Rehabilitation 2009;1(9):798‐808. [DOI] [PubMed] [Google Scholar]
Ferrari 2006 {published data only}
- Ferrari R, Fipaldini E, Birbaumer N. Individual characteristics and results of biofeedback training and operant treatment in patients with chronic pain. [Italian]. Psicoterapia Cognitiva e Comportamentale 2006;12(2):161‐79. [Google Scholar]
Friedberg 2010 {published data only}
- Friedberg MW. Group cognitive behavioral treatment improves chronic low back pain in a cost‐effective manner. Journal of Clinical Outcomes Management 2010;17(6):7‐9. [Google Scholar]
Friedrich 1998 {published data only}
- Friedrich M, Gittler G, Halberstadt Y, Cermak T, Heiller I. Combined exercise and motivation program: effect on the compliance and level of disability of patients with chronic low back pain: A randomized controlled trial. Archives of Physical Medical Rehabilitation 1998;79:475‐87. [DOI] [PubMed] [Google Scholar]
Friedrich 2005 {published data only}
- Friedrich M, Gittler G, Arendasy M, Friedrich KM. Long‐term effect of a combined exercise and motivational program on the level of disability of patients with chronic low back pain. Spine 2005;30(9):995‐1000. [DOI] [PubMed] [Google Scholar]
Froholdt 2011 {published data only}
- Froholdt A, Holm I, Keller A, Gunderson RB, Reikeraas O, Brox JI. No difference in long‐term trunk muscle strength, cross‐sectional area, and density in patients with chronic low back pain 7 to 11 years after lumbar fusion versus cognitive intervention and exercises. Spine Journal 2011;11(8):718‐25. [DOI] [PubMed] [Google Scholar]
Froholdt 2012 {published data only}
- Froholdt A, Reikeraas O, Holm I, Keller A, Brox JI. No difference in 9‐year outcome in CLBP patients randomized to lumbar fusion versus cognitive intervention and exercises. European Spine Journal 2012;21(12):2531‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frost 1998 {published data only}
- Frost H, Lamb SE, Klaber Moffett JA, Fairbank JC, Moser JS. A fitness programme for patients with chronic low back pain: 2‐year follow‐up of a randomised controlled trial. Pain 1998;75(2‐3):273‐9. [DOI] [PubMed] [Google Scholar]
Gatchel 2003 {published data only}
- Gatchel RJ, Polatin PB, Noe C, Gardea M, Pulliam C, Thompson J. Treatment‐ and cost‐effectiveness of early intervention for acute low‐back pain patients: a one‐year prospective study. Journal of Occupational Rehabilitation 2003;13(1):1‐9. [DOI] [PubMed] [Google Scholar]
George 2009 {published data only}
- George SZ, Teyhen DS, Wu SS, Wright A, Dugan JL, Yang G, et al. Psychosocial education improves low back pain beliefs: results from a cluster randomized clinical trial (NCT00373009). The Journal of Orthopaedic and Sports Physical Therapy 2009;39(1):A30‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2010a {published data only}
- George SZ, Robinson ME. Patient satisfaction with behavioral physical therapy interventions: secondary analysis from a randomized clinical trial. The Journal of Orthopaedic and Sports Physical Therapy 2010;40(1):A22. [Google Scholar]
George 2010b {published data only}
- George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. The Journal of Orthopaedic and Sports Physical Therapy 2010;40(11):694‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2011 {published data only}
- George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, Robinson ME. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: Results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Medicine 2011;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glattacker 2012 {published data only}
- Glattacker M, Heyduck K, Meffert C. Illness beliefs, treatment beliefs and information needs as starting points for patient information‐Evaluation of an intervention for patients with chronic back pain. Patient Education & Counseling 2012;86(3):378‐89. [DOI] [PubMed] [Google Scholar]
Glombiewski 2010 {published data only}
- Glombiewski JA, Hartwich‐Tersek J, Rief W. Two psychological interventions are effective in severely disabled, chronic back pain patients: a randomised controlled trial. International Journal of Behavioral Medicine 2010;17(2):97‐107. [DOI] [PubMed] [Google Scholar]
Glomsrod 2001 {published data only}
- Glomsrod B, Lonn JH, Soukup MG, Bo K, Larsen S. "Active back school", prophylactic management for low back pain: Three‐year follow‐up of a randomized, controlled trial. Journal of Rehabilitation Medicine 2001;33(1):26‐30. [DOI] [PubMed] [Google Scholar]
Gohner 2006 {published data only}
- Gohner W, Schlicht W. Preventing chronic back pain: evaluation of a theory‐based cognitive‐behavioural training programme for patients with subacute back pain. Patient Education & Counseling 2006;64(1‐3):87‐95. [DOI] [PubMed] [Google Scholar]
Greitemann 2006 {published data only}
- Greitemann B, Dibbelt S, Buschel C. [Multidisciplinary orthopedic rehabilitation program in patients with chronic back pain and need for changing job situation ‐‐ long‐term effects of a multimodal, multidisciplinary program with activation and job development]. [German]. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2006;144(3):255‐66. [DOI] [PubMed] [Google Scholar]
Hagen 2006 {published data only}
- Hagen EM. [Does light mobilization treatment reduce long‐term sick leave for low back pain? ] [Norwegian]. Norsk Epidemiologi 2006;16(2):137‐44. [Google Scholar]
Hagen 2010 {published data only}
- Hagen EM, Odelien KH, Lie SA, Eriksen HR. Adding a physical exercise programme to brief intervention for low back pain patients did not increase return to work. Scandinavian Journal of Public Health 2010;38(7):731‐8. [DOI] [PubMed] [Google Scholar]
Hallegraeff 2009 {published data only}
- Hallegraeff JM, Greef M, Winters JC, Lucas C. Manipulative therapy and clinical prediction criteria in treatment of acute nonspecific low back pain. Perceptual and Motor Skills 2009;108(1):196‐208. [DOI] [PubMed] [Google Scholar]
Hampel 2009 {published data only}
- Hampel P, Graef T, Krohn‐Grimberghe B, Tlach L. Effects of gender and cognitive‐behavioral management of depressive symptoms on rehabilitation outcome among inpatient orthopedic patients with chronic low back pain: A 1 year longitudinal study. European Spine Journal 2009;18(12):1867‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henchoz 2010a {published data only}
- Henchoz Y, Goumoens P, Norberg M, Paillex R, So AK. Role of physical exercise in low back pain rehabilitation: a randomized controlled trial of a three‐month exercise program in patients who have completed multidisciplinary rehabilitation. Spine 2010;35(12):1192‐9. [DOI] [PubMed] [Google Scholar]
Henchoz 2010b {published data only}
- Henchoz Y, Pinget C, Wasserfallen JB, Paillex R, Goumoens P, Norberg M, Kai‐Lik So A. Cost‐utility analysis of a three‐month exercise programme vs usual care following multidisciplinary rehabilitation for chronic low back pain. Journal of Rehabilitation Medicine 2010;42(9):846‐52. [DOI] [PubMed] [Google Scholar]
Heymans 2006 {published data only}
- Heymans MW, Vet HC, Bongers PM, Knol DL, Koes BW, Mechelen W. The effectiveness of high‐intensity versus low‐intensity back schools in an occupational setting: a pragmatic randomized controlled trial. Spine 2006;31(10):1075‐82. [DOI] [PubMed] [Google Scholar]
Hlobil 2005 {published data only}
- Hlobil H, Staal JB, Twisk J, Koke A, Ariens G, Smid T, Mechelen W. The effects of a graded activity intervention for low back pain in occupational health on sick leave, functional status and pain: 12‐Month results of a randomized controlled trial. Journal of Occupational Rehabilitation 2005;15(4):569‐80. [DOI] [PubMed] [Google Scholar]
Hodselmans 2001 {published data only}
- Hodselmans AP, Jaegers SM, Goeken LN. Short‐term outcomes of a back school program for chronic low back pain. Archives of Physical and Medical Rehabilitation 2001;82:1099‐105. [DOI] [PubMed] [Google Scholar]
Huge 2006 {published data only}
- Huge V, Schloderer U, Steinberger M, Wuenschmann B, Schops P, Beyer A, Azad SC. Impact of a functional restoration program on pain and health‐related quality of life in patients with chronic low back pain. Pain Medicine 2006;7(6):501‐8. [DOI] [PubMed] [Google Scholar]
Jensen 2005 {published data only}
- Jensen IB, Bergstrom G, Ljungquist T, Bodin L. A 3‐year follow‐up of a multidisciplinary rehabilitation programme for back and neck pain. Pain 2005;115(3):273‐83. [DOI] [PubMed] [Google Scholar]
Jensen 2007 {published data only}
- Jensen I, Bergstrom G, Bodin L, Ljungquist T, Nygren A. [Effects of rehabilitation after seven years. Evaluation of two rehabilitation programs in Sweden]. [Swedish]. Lakartidningen 2007;103(23):1829‐30. [PubMed] [Google Scholar]
Jensen 2009 {published data only}
- Jensen IB, Busch H, Bodin L, Hagberg J, Nygren A, Bergstrom G. Cost effectiveness of two rehabilitation programmes for neck and back pain patients: A seven year follow‐up. Pain 2009;152(3):202‐8. [DOI] [PubMed] [Google Scholar]
Jensen 2011 {published data only}
- Jensen C, Jensen OK, Christiansen DH, Nielsen CV. One‐year follow‐up in employees sick‐listed because of low back pain: randomized clinical trial comparing multidisciplinary and brief intervention. Spine 2011;36(15):1180‐9. [DOI] [PubMed] [Google Scholar]
Jensen 2012 {published data only}
- Jensen, C.Jensen, O. K.Nielsen, C. V. Sustainability of return to work in sick‐listed employees with low‐back pain. Two‐year follow‐up in a randomized clinical trial comparing multidisciplinary and brief intervention. BMC Musculoskeletal Disorders 2012;13:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2013 {published data only}
- Jensen C, Nielsen CV, Jensen O, Petersen K. Cost‐effectiveness and cost‐benefit analyses of a multidisciplinary intervention compared with a brief intervention to facilitate return to work in sick‐listed patients with low back pain. Spine 2013;38(13):1059‐67. [DOI] [PubMed] [Google Scholar]
Johnson 2013 {published data only}
- Johnsen LG, Brinckmann P, Hellum C, Rossvoll I, Leivseth G. Segmental mobility, disc height and patient‐reported outcomes after surgery for degenerative disc disease: a prospective randomised trial comparing disc replacement and multidisciplinary rehabilitation. Bone & Joint Journal 2013;95B(1):81‐9. [DOI] [PubMed] [Google Scholar]
Kainz 2006 {published data only}
- Kainz B, Lich M, Engel E, Ckel WH. Comparison of three outpatient therapy forms for treatment of chronic low back pain ‐‐ findings of a multicentre, cluster randomized study [German]. Rehabilitation 2006;45(2):65‐77. [DOI] [PubMed] [Google Scholar]
Kolip 2001 {published data only}
- Kolip P, Czujek J, Greitemann B, Rosowski E, Schmidt B, Slangen K. ["Zest for life instead of strain of illness" ‐ implementation and evaluation of a programme activating chronic back pain patients in a rehabilitation clinic] [German]. Die Rehabilitation 2001;40(5):267‐74. [DOI] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar S, Sharma VP, Shukla R, Dev R. Comparative efficacy of two multimodal treatments on male and female sub‐groups with low back pain. Journal of Back and Musculoskeletal Rehabilitation 2010;23(1):1‐9. [DOI] [PubMed] [Google Scholar]
Lamb 2010 {published data only}
- Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al. Group cognitive behavioural treatment for low‐back pain in primary care: a randomised controlled trial and cost‐effectiveness analysis. Lancet 2010;75(9718):916‐23. [DOI] [PubMed] [Google Scholar]
Lang 2003 {published data only}
- Lang E, Liebig K, Kastner S, Neundorfer B, Heuschmann P. Multidisciplinary rehabilitation versus usual care for chronic low back pain in the community: effects on quality of life. Spine Journal 2003;3(4):270‐6. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee WYA, Lee WCE, Law SW, Lau WKA, Leung SM, Sieh KM, et al. Managing psychosocial contributors in low back pain patients‐‐a randomised controlled trial. Journal of Orthopaedics, Trauma and Rehabilitation 2013;17:46‐51. [Google Scholar]
Le Gall 2001 {published data only}
- Gall S, Ozguler A, Piciotti M, Morel‐Fatio M, Leclerc A, Boureau F. Predictive factors of chronic low‐back pain and evaluation of a multidisciplinary pain management program in a population still in activity. [French]. Archives des Maladies Professionnelles et de Medecine du Travail 2001;62(7):575. [Google Scholar]
Leon 2009 {published data only}
- Leon Mateos L, Jover Jover JA, Abasolo Alcazar L, Loza Santamaria E, Perez Nieto MA, Redondo Delgado MM. Functional recovery in low back pain: Efficacy of an early cognitive behavioral intervention. Arthritis and Rheumatism (Arthritis Care and Research) 2009;61(7):996‐1003. [DOI] [PubMed] [Google Scholar]
Lindell 2008 {published data only}
- Lindell O, Johansson SE, Strender LE. Subacute and chronic, non‐specific back and neck pain: cognitive‐behavioural rehabilitation versus primary care. A randomized controlled trial. BMC Musculoskeletal Disorders 2008;9:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linton 2000 {published data only}
- Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive‐behavior intervention and two forms of information for patients with spinal pain. Spine 2000;25(21):2825‐31. [DOI] [PubMed] [Google Scholar]
Ljungkvist 2000 {published data only}
- Ljungkvist I. Short‐ and long‐term effects of a 12‐week intensive functional restoration programme in individuals work‐disabled by chronic spinal pain. Scandinavian Journal of Rehabilitation Medicine. Supplement 2000;40:1‐14. [PubMed] [Google Scholar]
Loisel 2002 {published data only}
- Loisel P, Lemaire J, Poitras S, Durand MJ, Champagne F, Stock S, et al. Cost‐benefit and cost‐effectiveness analysis of a disability prevention model for back pain management: a six year follow up study. Occupational and Environmental Medicine 2002;59(12):807‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lonn 1999 {published data only}
- Lønn JH, Glomsrød B, Soukup MG, Bø K, Larsen S. Active back school: prophylactic management for low back pain. A randomized, controlled, 1‐year follow‐up study. Spine 1999;24(9):865‐71. [DOI] [PubMed] [Google Scholar]
Mannion 2001 {published data only}
- Mannion AF, Müntener M, Taimela S, Dvorak J. Comparison of three active therapies for chronic low back pain: results of a randomized clinical trial with one‐year follow‐up. Rheumatology 2001;40(7):772‐8. [DOI] [PubMed] [Google Scholar]
Mannion 2013 {published data only}
- Mannion AF, Brox JI, Fairbank JCT. Comparison of spinal fusion and nonoperative treatment in patients with chronic low back pain: Long‐term follow‐up of three randomized controlled trials. Spine Journal 2013;11:1438‐48. [DOI] [PubMed] [Google Scholar]
Martin 2000 {published data only}
- Martin C, Carney T, Obonyo T, Lamont L. Setting up a pain management programme. The Ayrshire experience. Scottish Medical Journal 2000;45(2):45‐8. [DOI] [PubMed] [Google Scholar]
Mattila 2007 {published data only}
- Mattila R, Malmivaara A, Kastarinen M, Kivel SL, Nissinen A. The effects of lifestyle intervention for hypertension on low back pain: a randomized controlled trial. Spine 2007;32(26):2943‐7. [DOI] [PubMed] [Google Scholar]
Meyer 2005 {published data only}
- Meyer K, Fransen J, Huwiler H, Uebelhart D, Klipstein A. Feasibility and results of a randomised pilot‐study of a work rehabilitation programme. Journal of Back and Musculoskeletal Rehabilitation 2005;18:67‐78. [Google Scholar]
Mohr 2009 {published data only}
- Mohr B, Krohn‐Grimberghe B, Graf T, Schulze J, Petermann F, Hampel P. [Patients with chronic low back pain: the impact of psychosocial features]. [German]. Rehabilitation 2009;48(5):288‐97. [DOI] [PubMed] [Google Scholar]
Molde 2003 {published data only}
- Molde Hagen E, Grasdal A, Eriksen HR. Does early intervention with a light mobilization program reduce long‐term sick leave for low back pain: a 3‐year follow‐up study. Spine 2003;28(20):2309‐15. [DOI] [PubMed] [Google Scholar]
Nazzal 2013 {published data only}
- Nazzal ME, Saadah MA, Saadah LM, Al‐Omari MA, Al‐Oudat ZA, Nazzal MS, et al. Management options of chronic low back pain. A randomized blinded clinical trial. Neurosciences 2013;18(2):152‐9. [PubMed] [Google Scholar]
Nicholas 2013 {published data only}
- Nicholas MK, Asghari A, Blyth FM, Wood BM, Murray R, McCabe R, et al. Self‐management intervention for chronic pain in older adults: A randomised controlled trial. Pain 2013;154(6):824‐35. [DOI] [PubMed] [Google Scholar]
Niemisto 2005 {published data only}
- Niemisto YL, Rissanen P, Sarna S, Lahtinen‐Suopanki T, Lindgren K, Hurri H. Cost‐effectiveness of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain: a prospective randomized trial with 2‐year follow‐up. Spine 2005;30(10):1109‐15. [DOI] [PubMed] [Google Scholar]
Padua 2009 {published data only}
- Padua R, Bondi R, Ceccarelli E, Alviti F. A randomized study of back school in women with chronic low back pain. Quality of life at three, six, and twelve months follow‐up. Spine 2009;34(12):1336. [DOI] [PubMed] [Google Scholar]
Paolucci 2012 {published data only}
- Paolucci T, Morone G, Iosa M, Fusco A, Alcuri R, Matano A, Bureca I, Saraceni VM, Paolucci S. Psychological features and outcomes of the Back School treatment in patients with chronic non‐specific low back pain. A randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2012;48(2):245‐53. [PubMed] [Google Scholar]
Rainville 2002 {published data only}
- Rainville J, Jouve CA, Hartigan C, Martinez E, Hipona MJ. Comparison of short‐ and long‐term outcomes for aggressive spine rehabilitation delivered two versus three times per week. Spine 2002;2(6):402‐7. [DOI] [PubMed] [Google Scholar]
Rantonen 2012 {published data only}
- Rantonen J, Luoto S, Vehtari A, Hupli M, Karppinen J, Malmivaara A, Taimela S. The effectiveness of two active interventions compared to self‐care advice in employees with non‐acute low back symptoms: a randomised, controlled trial with a 4‐year follow‐up in the occupational health setting. Occupational and Environmental Medicine 2012;69(1):12‐20. [DOI] [PubMed] [Google Scholar]
Rossignol 2000 {published data only}
- Rossignol M, Abenhaim L, Seguin P, Neveu A, Collet JP, Ducruet T, Shapiro S. Coordination of primary health care for back pain. A randomized controlled trial. Spine 2000;25(2):251‐8. [DOI] [PubMed] [Google Scholar]
Rothman 2013 {published data only}
- Rothman MG, Ortendahl M, Rosenblad A, Johansson AC. Improved quality of life, working ability, and patient satisfaction after a pretreatment multimodal assessment method in patients with mixed chronic muscular pain: a randomized‐controlled study. Clinical Journal of Pain 2013;29(3):195‐204. [DOI] [PubMed] [Google Scholar]
Sahin 2011 {published data only}
- Sahin N, Albayrak I, Durmus B, Ugurlu H. Effectiveness of back school for treatment of pain and functional disability in patients with chronic low back pain: a randomized controlled trial. Journal of Rehabilitation Medicine 2011;43(3):224‐9. [DOI] [PubMed] [Google Scholar]
Shete 2012 {published data only}
- Shete KM, Suryawanshi P, Gandhi N. Management of low back pain in computer users: A multidisciplinary approach. Journal of Craniovertebral Junction and Spine 2012;3(1):7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siemonsma 2013 {published data only}
- Siemonsma P, Stuive I, Roorda L, Vollebregt J, Walker M, Lankhorst G, Lettinga A. Cognitive treatment of illness perceptions in patients with chronic low back pain: a randomized controlled trial. Physical Therapy 2013;93(4):435‐48. [DOI] [PubMed] [Google Scholar]
Sjostrum 2010 {published data only}
- Sjostrom R, Asplund R, Alricsson M. Two‐year outcome of a multidisciplinary vocational rehabilitation programme focused on range of motion of the neck and back. Work 2010;37(4):341‐8. [DOI] [PubMed] [Google Scholar]
Sleptsova 2013 {published data only}
- Sleptsova M, Wossmer B, Grossman P, Langewitz W. Culturally sensitive group therapy for Turkish patients suffering from chronic pain: A randomised controlled intervention trial. Swiss Medical Weekly 2013;Nov:143. [DOI] [PubMed] [Google Scholar]
Sorensen 2010 {published data only}
- Sorensen PH, Bendix T, Manniche C, Korsholm L, Lemvigh D, Indahl A. An educational approach based on a non‐injury model compared with individual symptom‐based physical training in chronic LBP. A pragmatic, randomised trial with a one‐year follow‐up. BMC Musculoskeletal Disorders 2010;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staal 2004 {published data only}
- Staal JB. Graded activity for low back pain in occupational health care: a randomized, controlled trial. Annals of Internal Medicine 2004;140(2):77‐84. [DOI] [PubMed] [Google Scholar]
Stapelfeldt 2011 {published data only}
- Stapelfeldt CM, Christiansen DH, Jensen OK, Nielsen CV, Petersen KD, Jensen C. Subgroup analyses on return to work in sick‐listed employees with low back pain in a randomised trial comparing brief and multidisciplinary intervention. BMC Musculoskeletal Disorders 2011;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steenstra 2006 {published data only}
- Steenstra IA, Anema JR, Bongers PM, Vet HC, Knol DL, Mechelen W. The effectiveness of graded activity for low back pain in occupational healthcare. Occupational and Environmental Medicine 2006;63(11):718‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stier 2001 {published data only}
- Stier R, Gerdes N, Buhrlen B, Haaf HG, Jackel WH. [Reconditioning in groups: an effective programme for the rehabilitation of patients with low back pain?] [German]. Rehabilitation 2001;40(6):321‐31. [DOI] [PubMed] [Google Scholar]
Storheim 2003 {published data only}
- Storheim K, Brox JI, Holm I, Koller AK, Bø K. Intensive group training versus cognitive intervention in sub‐acute low back pain: short‐term results of a single‐blind randomized controlled trial. Journal of Rehabilitation Medicine 2003;35(3):132‐40. [DOI] [PubMed] [Google Scholar]
Storro 2004 {published data only}
- Storro S, Moen J, Svebak S. Effects on sick‐leave of a multidisciplinary rehabilitation programme for chronic low back, neck or shoulder pain: comparison with usual treatment. Journal of Rehabilitation Medicine 2004;36(1):12‐6. [DOI] [PubMed] [Google Scholar]
Strong 2006 {published data only}
- Strong LL, Korff M, Saunders K, Moore JE. Cost‐effectiveness of two self‐care interventions to reduce disability associated with back pain. Spine 2006;31(15):1639‐45. [DOI] [PubMed] [Google Scholar]
Sundberg 2009 {published data only}
- Sundberg T, Petzold M, Wandell P, Ryden A, Falkenberg T. Exploring integrative medicine for back and neck pain ‐ a pragmatic randomised clinical pilot trial. BMC Complementary and Alternative Medicine 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tlach 2011 {published data only}
- Tlach L, Hampel P. Long‐term effects of a cognitive‐behavioral training program for the management of depressive symptoms among patients in orthopedic inpatient rehabilitation of chronic low back pain: A 2‐year follow‐up. European Spine Journal 2011;20(12):2143‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torstensen 1998 {published data only}
- Torstensen TA, Ljunggren AE, Meen HD, Odland E, Mowinckel P, af Geijerstam S. Efficiency and costs of medical exercise therapy, conventional physiotherapy, and self‐exercise in patients with chronic low back pain ‐ A pragmatic, randomized, single‐blinded, controlled trial with 1‐year follow‐up. Spine 1998;23(23):2616‐24. [DOI] [PubMed] [Google Scholar]
Trapp 2009 {published data only}
- Trapp K, Glombiewski JA, Hartwich‐Tersek J, Rief W. Chronic back pain: What does biofeedback add to cognitive‐behavioral treatment? A randomized controlled trial. Pain Practice 2009;9:114. [Google Scholar]
Tsauo 2009 {published data only}
- Tsauo J, Chen W, Liang H, Jang Y. The effectiveness of a functional training programme for patients with chronic low back pain ‐‐ a pilot study. Disability & Rehabilitation 2009;31(13):1100‐6. [DOI] [PubMed] [Google Scholar]
Turk 1998 {published data only}
- Turk DC, Okifuji A. Treatment of chronic pain patients: clinical outcomes, cost‐effectiveness, and cost‐benefits of multidisciplinary pain centers. Critical Reviews in Physical and Rehabilitation Medicine 1998;10(2):181‐208. [Google Scholar]
Underwood 2004 {published data only}
- Underwood M. United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: cost effectiveness of physical treatments for back pain in primary care. BMJ 2004;329(7479):1381‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Beurden 2012 {published data only}
- Beurden KM, Vermeulen SJ, Anema JR, Beek AJ. A participatory return‐to‐work program for temporary agency workers and unemployed workers sick‐listed due to musculoskeletal disorders: a process evaluation alongside a randomized controlled trial. Journal of Occupational Rehabilitation 2012;22(1):217‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Roer 2008 {published data only}
- Roer N, Tulder M, Barendse J, Knol D, Mechelen W, Vet H. Intensive group training protocol versus guideline physiotherapy for patients with chronic low back pain: A randomised controlled trial. European Spine Journal 2008;17(9):1193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hoof 2010 {published data only}
- Hooff ML, Merwe JD, O'Dowd J, Pavlov PW, Spruit M, Kleuver M, Limbeek J. Daily functioning and self‐management in patients with chronic low back pain after an intensive cognitive behavioral programme for pain management. European Spine Journal 2010;19(9):1517‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2002 {published data only}
- Verbeek JH, Weide WE, Dijk FJ. Early occupational health management of patients with back pain: a randomized controlled trial. Spine 2002;27(17):1844‐51. [DOI] [PubMed] [Google Scholar]
Vermeulen 2011 {published data only}
- Vermeulen SJ, Anema JR, Schellart AJM, Knol DL, Mechelen W, Beek AJ. A participatory return‐to‐work intervention for temporary agency workers and unemployed workers sick‐listed due to musculoskeletal disorders: results of a randomized controlled trial. Journal of Occupational Rehabilitation 2011;21(3):313‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verra 2012 {published data only}
- Verra ML, Angst F, Beck T, Lehmann S, Brioschi R, Schneiter R, Aeschlimann A. Horticultural therapy for patients with chronic musculoskeletal pain: results of a pilot study. Alternative Therapies in Health and Medicine 2011;18(2):44‐50. [PubMed] [Google Scholar]
Vibe Fersum 2013 {published data only}
- Vibe Fersum K, O'Sullivan P, Skouen JS, Smith A, Kvale A. Efficacy of classification‐based cognitive functional therapy in patients with non‐specific chronic low back pain: A randomized controlled trial. European Journal of Pain 2013;17(6):916‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlaeyen 1995 {published data only}
- Vlaeyen JWS, Haazen IWCJ, Schuerman JA, Kole‐Snijders AMJ, Eek H. Behavioural rehabilitation of chronic low back pain: Comparison of an operant treatment, an operant‐cognitive treatment and an operant‐respondent treatment. British Journal of Clinical Psychology 1995;34(1):95‐118. [DOI] [PubMed] [Google Scholar]
Vong 2011 {published data only}
- Vong SK, Cheing GL, Chan F, So EM, Chan CC. Motivational enhancement therapy in addition to physical therapy improves motivational factors and treatment outcomes in people with low back pain: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2011;92(2):176‐83. [DOI] [PubMed] [Google Scholar]
Wagner 2007 {published data only}
- Wagner E, Ehrenhofer B, Lackerbauer E, Pawelak U, Siegmeth W. Rehabilitation of non‐specific low back pain: Results of a multidisciplinary in‐patient program. [German]. Schmerz 2007;21(3):226‐33. [DOI] [PubMed] [Google Scholar]
Wand 2004 {published data only}
- Wand BM, Bird C, McAuley JH, Dore CJ, MacDowell M, Souza LH. Early intervention for the management of acute low back pain: A single‐blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine 2004;29(21):2350‐6. [DOI] [PubMed] [Google Scholar]
Wessels 2007 {published data only}
- Wessels T, Ewert T, Limm H, Rackwitz B, Stucki G. Change factors explaining reductions of "interference" in a multidisciplinary and an exercise prevention program for low back pain. Clinical Journal of Pain 2007;23(7):629‐34. [DOI] [PubMed] [Google Scholar]
Whitfill 2010 {published data only}
- Whitfill T, Haggard R, Bierner SM, Pransky G, Hassett RG, Gatchel RJ. Early intervention options for acute low back pain patients: a randomized clinical trial with one‐year follow‐up outcomes. Journal of Occupational Rehabilitation 2010;20(2):256‐63. [DOI] [PubMed] [Google Scholar]
Wilkey 2008 {published data only}
- Wilkey A, Gregory M, Byfield D, McCarthy PW. A comparison between chiropractic management and pain clinic management for chronic low‐back pain in a national health service outpatient clinic. Journal of Alternative and Complementary Medicine 2008;14(5):465‐73. [DOI] [PubMed] [Google Scholar]
Yang 2010 {published data only}
- Yang EJ, Park WB, Shin HI, Lim JY. The effect of back school integrated with core strengthening in patients with chronic low‐back pain. American Journal of Physical Medicine & Rehabilitation 2010;89(9):744‐54. [DOI] [PubMed] [Google Scholar]
Additional references
Artus 2010
- Artus M, Windt DA, Jordan KP, Hay EM. Low back pain symptoms show a similar patternof improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology 2010;49:2346‐56. [DOI] [PubMed] [Google Scholar]
Costa 2009
- Costa Lda C, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, Henschke N. Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339:3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2012
- Costa LO, Lin CW, Grossi DB, Mancini MC, Swisher AK, Cook C. Clinical trial registration in physiotherapy journals: recommendations from the International Society of Physiotherapy Journal Editors. Journal of Physiotherapy 2012;58(4):211‐3. [DOI] [PubMed] [Google Scholar]
Cutler 1994
- Cutler RB, Fishbain DA, Rosomoff HL, Abdel‐Moty E, Khalil TM, Rosomoff RS. Does nonsurgical pain center treatment ofchronic pain return patients to work? A review and meta‐analysis of the literature. Spine 1994;19:643‐52. [DOI] [PubMed] [Google Scholar]
Dagenais 2010
- Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. The Spine Journal 2010;10:514‐29. [DOI] [PubMed] [Google Scholar]
den Hollander 2010
- Hollander M, Jong JR, Volders S, Goossens ME, Smeets RJ, Vlaeyen JW. Fear reduction in patients with chronic pain: a learning theory perspective. Expert Reviews in Neurotherapy 2010;10(11):1733‐45. [DOI] [PubMed] [Google Scholar]
Dworkin 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1‐2):9‐19. [DOI] [PubMed] [Google Scholar]
Engers 2008
- Engers A, Jellema P, Wensing M, Windt DA, Grol R, Tulder MW. Individual patient education for low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004057.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2010
- Ferreira ML, Smeets RJ, Kamper SJ, Ferreira PH, Machado LA. Can we explain heterogeneity among randomized clinical trials of exercise for chronic back pain? A meta‐regression analysis of randomized controlled trials. Physical Therapy 2010;90(10):1383‐403. [DOI] [PubMed] [Google Scholar]
Ferreira 2012
- Ferreira ML, Herbert RD, Ferreira PH, Latimer J, Ostelo RW, Nascimento DP, Smeets RJ. A critical review of methods used to determine the smallest worthwhile effect of interventions for low back pain. Journal of Clinical Epidemiology 2012;65(3):253‐61. [DOI] [PubMed] [Google Scholar]
Flor 1992
- Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: A meta‐analytic review. Pain 1992;48:221‐30. [DOI] [PubMed] [Google Scholar]
Foster 2011
- Foster NE. Barriers and progress in the treatment of low back pain. BMC Medicine 2011;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2013
- Foster NE, Hill JC, O'Sullivan P, Hancock M. Stratified models of care. Best Practice & Research. Clinical Rheumatology 2013;27(5):649‐61. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder MW. Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozeka J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidencedimprecision. Journal of Clinical Epidemiology 2011;64:1283‐93. [DOI] [PubMed] [Google Scholar]
Hancock 2009
- Hancock M, Herbert RD, Maher CG. A guide to interpretation of studies investigating subgroups of responders to physical therapy interventions. Physical Therapy 2009;89(7):698‐704. [DOI] [PubMed] [Google Scholar]
Hayden 2005
- Hayden JA, Tulder MW, Tomlinson G. Systematic review: Strategies for using exercise therapy to improve outcomes in chronic low back pain. Annals of Internal Medicine 2005;142:776‐85. [DOI] [PubMed] [Google Scholar]
Henschke 2008
- Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henschke 2011
- Henschke N, Ostelo RWJG, Tulder MW, Vlaeyen JWS, Morley S, Assendelft WJJ, Main CJ. Behavioural treatment for chronic low‐back pain. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD002014.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heymans 2010
- Heymans MW, Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for acute and subacute non‐specific low‐back pain. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008325] [DOI] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 Updated March 2011; Vol. The Cochrane Collaboration.
Hill 2011
- Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 2011;378(9802):1560‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamper 2010
- Kamper SJ, Maher CG, Hancock MJ, Koes BW, Croft P, Hay EM. Treatment‐based subgroups of low back pain: a guide to appraisal of research studies and a summary of current evidence. Best Practice & Research. Clinical Rheumatology 2010;24:181‐91. [DOI] [PubMed] [Google Scholar]
Karjalainen 2003
- Karjalainen KA, Malmivaara A, Tulder MW, Roine R, Jauhiainen M, Hurri H, Koes BW. Multidisciplinary biopsychosocial rehabilitation for subacute low‐back pain among working age adults. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002193] [DOI] [PubMed] [Google Scholar]
Koes 2010
- Koes BW, Tulder MW, Lin CWC, Macedo LG, McAuley JH, Maher CG. An updated overview of clinical guidelines for the managementof non‐specific low back pain in primary care. European Spine Journal 2010;19:2075‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambeek 2011
- Lambeek LC, Tulder MW, Swinkels ICS, Koppes LLJ, Anema JR, Mechelen W. The trend in total cost of back pain in the Netherlands in the period 2002 to 2007. Spine 2011;36(13):1050‐8. [DOI] [PubMed] [Google Scholar]
Linton 2011
- Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Physical Therapy 2011;91(5):700‐11. [DOI] [PubMed] [Google Scholar]
Machado 2009
- Machado LA, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Analgesic effects of treatments for non‐specific low back pain: a meta‐analysis of placebo‐controlled randomized trials. Rheumatology 2009;48(5):520‐7. [DOI] [PubMed] [Google Scholar]
Maetzal 2002
- Maetzel A, Li L. The economic burden of low back pain:A review of studies published between 1996 and 2001. Best Practice & Research. Clinical Rheumatology 2002;16(1):23‐30. [DOI] [PubMed] [Google Scholar]
Mansell 2013
- Mansell G, Kamper SJ, Kent P. Why and how back pain interventions work: What can we do to find out?. Best Practice & Research. Clinical Rheumatology 2013;27(5):685‐97. [DOI] [PubMed] [Google Scholar]
Nicholas 2011
- Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors ("yellow flags") in patients with low back pain: a reappraisal. Physical Therapy 2011;91(5):737‐53. [DOI] [PubMed] [Google Scholar]
Norlund 2009
- Norlund A, Ropponen A, Alexanderson K. Multidisciplinary interventions: Review of studies of return to work after rehabilitation for low back pain. Journal of Rehabilitation Medicine 2009;41(3):115‐21. [DOI] [PubMed] [Google Scholar]
Ravenek 2010
- Ravenek MJ, Hughes ID, Ivanovich N, Tyrer K, Desrochers C, Klinger L, Shaw L. A systematic review of multidisciplinaryoutcomes in the management of chronic lowback pain. Work 2010;35:349‐67. [DOI] [PubMed] [Google Scholar]
Rose 1997
- Rose MJ, Reilly JP, Pennie B, Bowen‐Jones K, Stanley IM, Slade PD. Chronic low back pain rehabilitation programs: a study of the optimum duration of treatment and a comparison of group and individual therapy. Spine 1997;22:2246‐53. [DOI] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaafsma 2013
- Schaafsma FG, Whelan K, Beek AJ, Es‐Lambeek LC, Ojajärvi A, Verbeek JH. Physical conditioning as part of a return to work strategy to reduce sickness absence for workers with back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001822.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeets 2006
- Smeets RJEM, Vlaeyen JWS, Kester ADM, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive‐behavioral treatment in chronic low back pain. The Journal of Pain 2006;7(4):261‐71. [DOI] [PubMed] [Google Scholar]
Smeets 2009
- Smeets RJ, Severens JL, Beelen S, Vlaeyen JW, Knottnerus JA. More is not always better: cost‐effectiveness analysis of combined, single behavioral and single physical rehabilitation programs for chronic low back pain. European Journal of Pain 2009;13(1):71‐81. [DOI] [PubMed] [Google Scholar]
van de Hulst 2005
- Hulst M, Vollenbroek‐Hutten MMR, IJzerman MJ. A systematic review of sociodemographic, physical, and psychological predictors of multidisciplinary rehabilitation— or, back school treatment outcome in patients with chronic low back pain. Spine 2005;30(7):813‐25. [DOI] [PubMed] [Google Scholar]
van Geen 2007
- Geen JW, Edelaar, MJ Janssen M, Eijk JT. The long‐term effect of multidisciplinary back training: A systematic review. Spine 2007;32(2):249‐55. [DOI] [PubMed] [Google Scholar]
van Hooff 2014
- Hooff ML, Spruit M, O’Dowd JK, Lankveld W, Fairbank JCT, Limbeek J. Predictive factors for successful clinical outcome 1 year after an intensive combined physical and psychologicalprogramme for chronic low back pain. European Spine Journal 2014;23:102‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verkerk 2013
- Verkerk K, Luijsterburg PAJ, Heymans MW, Ronchetti I, Pool‐Goudzwaard AL, Miedema HS, Koes BW. Prognosis and Course of Disability in Patients With Chronic Nonspecific Low Back Pain: A 5‐ and 12‐Month Follow‐up Cohort Study. Physical Therapy 2013;93(12):1603‐14. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzat M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waddell 2004
- Waddell G. The Back Pain Revolution. 2nd Edition. Vol. 1, London: Churchill Livingston, 2004. [Google Scholar]
Waterschoot 2014
- Waterschoot FPC, Dijkstra PU, Hollak NJ, Vries HJ, Geertzen JHB, Reneman M. Dose or content? Effectiveness of pain rehabilitation programs forpatients with chronic low back pain: A systematic review. Pain 2014;155:179‐89. [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, Tugwell P. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Guzman 2006
- Guzman J, Esmail R, Karjalainen KA, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio‐psycho‐social rehabilitation for chronic low‐back pain. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000963.pub2] [DOI] [PubMed] [Google Scholar]


