Abstract
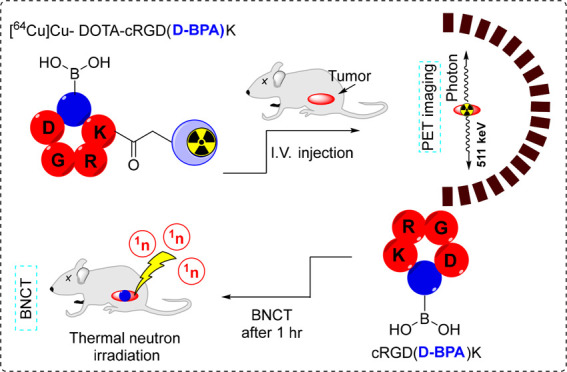
The burgeoning interest in developing boron neutron capture therapy (BNCT) tracers and their accompanying diagnostics for the treatment of recalcitrant tumors has prompted this investigation. Our study aims to devise a tumor treatment strategy utilizing BNCT to target the αvβ3 integrin. To this end, we propose a pioneering boron-infused cyclic Arg-Gly-Asp (RGD) peptide, cRGD(d-BPA)K, designed as an efficacious BNCT tracer. Additionally, we introduce its diagnostic complement, DOTA-cRGD(d-BPA)K, tailored for positron emission tomography (PET) to visualize αvβ3 expressed tumors. Radiolabeling [64Cu]Cu-DOTA-cRGD(d-BPA)K (64Cu-1) resulted in a high radiochemical yield and purity. The radiotracer exhibited exceptional in vitro stability and demonstrated significant uptake in U87MG tumors via PET imaging. Biodistribution analysis using compound 2 showed a 7.0 ppm accumulation of boron in the U87MG tumor 1 h post-intravenous injection. Furthermore, compound 2 displayed superior tumor/blood (2.41) and tumor/muscle (2.46) ratios compared to the clinically approved l-BPA-fructose. Both compound 2 and its diagnostic counterpart 64Cu-1 hold potential for BNCT and cancer diagnosis, respectively, via molecular imaging.
Keywords: BNCT, cyclic RGD peptide, PET, theranostics, boron
Boron neutron capture therapy (BNCT) is among the most promising treatment modalities for challenging tumors such as glioma, skin, head, and neck cancer.1 It relies on the interaction between thermal neutrons (0.025 eV) and boron-10 (10B), which produces lithium-7 (7Li) nuclei, α particles (4He), and γ rays. These nuclei exhibit high linear energy transfer (LET), within the range of 5–9 μm, which is comparable to the average cell diameter.2 The short-range cytotoxicity of these nuclei effectively kills tumor cells while sparing neighboring healthy cells. However, several criteria must be met for a particular 10B containing tracer to provide the best therapeutic outcome. For instance, the tracer should exhibit a high retention time inside the tumor cells and specificity toward cancer tissues to achieve a high concentration inside the tumor cells compared to the healthy cells. Additionally, it should exhibit negligible systemic toxicity and high water solubility. Finally, highly precise thermal neutron irradiation is required when the 10B concentration peaks inside tumors.3,4
Currently, only two BNCT drugs are in clinical trials: l-boronophenylalanine (l-BPA) and disodium mercaptoundecahydro-closo-dodecaborate (BSH). l-BPA, the most frequently used boronated tyrosine derivative, is taken up by the l-type amino acid transporter-1 (LAT-1), which is upregulated in various cancers.5 Because of the low solubility of l-BPA, its complex with fructose has been explored as a solution to enhance its solubility.6 Additionally, 18F-labeled BPA was synthesized to enable in vivo tissue distribution of l-BPA using positron emission tomography (PET).7 Unfortunately, neither boronated precursor was optimal for delivering a therapeutic amount of 10B effectively across the entire targeted tumor. They both suffer from low specificity and are quickly washed away post-injection. Hence, developing more sophisticated tumor-specific 10B-containing agents is crucial.
Recently, significant advancements have been made in cancer diagnosis and treatment, largely through the exploitation of integrins.8 Within this spectrum of integrins, αvβ3 stands out as a prime target due to its overexpression in early endothelial cells during angiogenesis across multiple tumors.9 As a result, a diverse range of Arg-Gly-Asp (RGD) peptides has been extensively investigated for their potential applications in tumor therapy and imaging utilizing diverse imaging techniques such as magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), or PET for tumor imaging.10−13 In a previous study, we enhanced the binding affinity of RGD by incorporating non-natural amino acids bearing cycloalkane groups, making it a highly efficient PET radiopharmaceutical.14 Here, we propose a novel boron-containing cyclic RGD peptide, cRGD(d-BPA)K, as an efficient BNCT tracer, along with its diagnostic counterpart, DOTA-cRGD(d-BPA)K, for PET imaging. To our knowledge, this is the first time that 4-boronophenylalanine amino acids have been incorporated into the cRGD peptide sequence and radiolabeled using 64Cu (t1/2 = 12 h) through a pre-installed DOTA chelator,15 facilitating pharmacokinetic assessment via PET imaging and biodistribution studies.
The DOTA-cRGD(d-BPA)K peptide (1) was synthesized according to Schemes S1 and S2. To incorporate the d-BPA amino acid peptide sequence, the compound Fmoc-d-Phe(4-Bpin)-OH (5) was synthesized by using commercially available (R)-2-amino-3-(4-boronophenyl)propanoic acid. The intermediate compound Fmoc-Phe(4-boronic acid)-OH (6) was obtained after Fmoc protection of the starting material. Compound 6 was obtained in a moderate chemical yield (73%) as a white powder and analyzed using nuclear magnetic resonance (NMR) and electrospray ionization mass spectrometry (ESI-MS). Compound 6 was then treated with pinacol to produce compound 5 in high chemical yield (93%) as a white solid. The intermediate peptide cyclic RGD(d-BPA)K (2) was prepared by conventional solid-phase peptide synthesis (SPPS) (Scheme S1) and analyzed using ESI-MS (Figure S1). Finally, DOTA-cRGD(d-BPA)K peptide (1) was synthesized using peptide 2 and DOTA-NHS ester under basic conditions16 and analyzed using ESI-MS (Figure S4). Furthermore, the chemical purity of purified compounds 2 and 1 was greater than 98%, as confirmed by the analytical HPLC spectra (Figures S3 and S5). Radiolabeling of compound 1 was performed using cyclotron-produced 64CuCl2 at a slightly acidic pH (5.0), as outlined in Scheme 1.
Scheme 1. Radiosynthesis of [64Cu]Cu-DOTA-cRGD(d-BPA)K (64Cu-1).
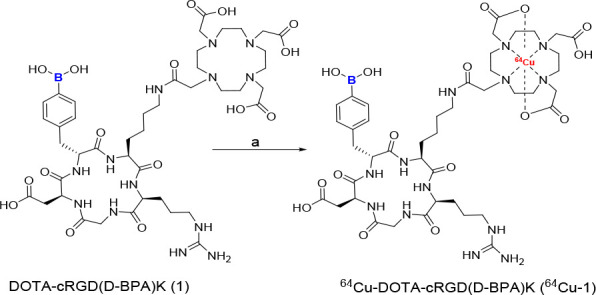
Reagents and conditions: 0.1 M NaOAc aqueous solution (pH 5.0), 64CuCl2 (34 MBq), 80 °C, 900 rpm for 15 min.
The [64Cu]Cu-DOTA-cRGD(d-BPA)K (64Cu-1) was obtained in a high radiochemical yield (>95%) and purity (>99%) within 15 min, as determined by radio thin-layer chromatography (Figure 1a; n = 3). The specific activity of the radiolabeled compound was 37 MBq/μg. A similar protocol was adapted for radiolabeling commercially available DOTA-cRGDyK. 64Cu-1 was subjected to in vitro stability testing before both in vitro and in vivo analyses. For this, 64Cu-1 was incubated in phosphate buffered saline (PBS), human serum (90%), and mouse serum (90%) for over 24 h. Remarkably, 64Cu-1 showed high in vitro stability, recording values of 94.69 ± 1.51% in PBS, 95.87 ± 0.46% in human serum, and 94.93 ± 0.83% in mouse serum over 24 h at 37 °C (Figure 1b). These results confirmed the suitability of the radiolabeled compound for further in vitro and in vivo experiments. U87MG cells were subjected to a 24 h incubation period at 37 °C with compound 2 across a concentration range spanning from 10 to 400 μM. The results, depicted in Figure 1c, demonstrate a viability profile comparable to that observed in the control group. This observation implies the lack of discernible cytotoxic effects resulting from the interaction of compound 2 with boron on U87MG cells. The binding affinity of compound 64Cu-1 toward αvβ3 integrin was assessed through incubation with U87MG cells expressing high levels of αvβ3 integrin for over 2 h. A control experiment was also performed by using [64Cu]Cu-DOTA-cRGDyK. In vitro studies demonstrated that 64Cu-1 effectively targeted the U87MG cells. As shown in Figure 1d, the introduction of d-BPA into the cRGDK peptide did not impair its cancer-targeting ability. The cellular uptakes of 64Cu-1 and [64Cu]Cu-DOTA-cRGDyK were nearly identical and increased over time. These findings align closely with previous reports on c(RGDfK) peptides, indicating comparable affinities for αvβ3 integrin.17 The therapeutic benefits of BNCT stem from the irradiation of areas with a high concentration of 10B within tumors with a thermal neutron beam.18
Figure 1.

In vitro analyses of compound 2 and 64Cu-1: (a) Radio-TLC spectrum of purified 64Cu-1 using 0.1 M citrate buffer as the elution solvent. (b) In vitro stability test of 64Cu-1 in PBS, human serum (90%), and mouse serum (90%) conducted over 24 h. (c) Cell viability study performed with U87MG cells using compound 2. (d) In vitro cell uptake comparison between 64Cu-1 and [64Cu]Cu-DOTA-cRGDyK in U87MG cells.
Noninvasive imaging techniques such as PET play a pivotal role in understanding the in vivo distribution of BNCT tracers prior to thermal neutron irradiation. In this study, the diagnostic companion to compound 2, 64Cu-1, was intravenously administered to mice with U87MG tumors. PET imaging revealed the distribution of the radiotracer across various organs, including the brain, liver, kidneys, muscles, and the tumor itself. The radiotracer exhibited a high radioactivity signal within the tumor just 0.5 h post-injection (3.50 ± 0.56% ID/g) which gradually decreased over time. However, it demonstrated a slow clearance rate, as evidenced by a robust signal observed at 7 h (2.00 ± 0.21% ID/g) and even at 20 h post-injection (1.34 ± 0.25% ID/g). Sufficient accumulation of the tracer was noted at 1 h post-injection, suggesting a potential optimal time point for thermal neutron irradiation. Radioactivity in kidneys peaked at 0.5 h post-injection (6.37 ± 0.10% ID/g) and then rapidly declined over time: 2.94 ± 0.21% ID/g at 1 h, 1.18 ± 0.12% ID/g at 3 h, and 1.16 ± 0.04% ID/g at 5 h. Concurrently, the liver exhibited consistently high radioactivity throughout the experiment. Its peak value occurred at 0.5 h post-injection (2.52 ± 0.17% ID/g), maintaining an almost similar level for up to 20 h post-injection (2.23 ± 0.40% ID/g). A notable observation was the slight increase in liver radioactivity after 3 h, potentially attributed to the dissociation of 64Cu from DOTA in vivo. This dissociation may lead to the subsequent metabolism and transfer of 64Cu to other proteins.19−21
In general, the biodistribution profile of 64Cu-1 exhibited similarities to those of other 64Cu-labeled cyclic RGD peptides.22,23 This similarity highlights its substantial affinity for U87MG tumors, suggesting its potential as a promising BNCT drug. As a result of PET imaging and biodistribution data analysis, 1 h post-injection was identified as the most suitable time for further studies. To ascertain the actual accumulated concentration of boron, U87MG tumor-bearing mice were intravenously injected with compound 2 and the boron concentration was determined using inductively coupled plasma mass spectrometry (n = 4). For comparison, l-BPA-fructose was administered to U87MG tumor-bearing mice under similar conditions.
Compound 2 resulted in a high concentration of boron in the tumor (7.10 ppm). However, the boron concentration in the tumor caused by l-BPA-fructose was nearly twice as high (13.81 ppm) as that caused by cRGD(d-BPA)K (Table 1).Typically, for effective BNCT, the required boron concentration per gram of tumor is ≥20 ppm,24,25 meaning that both compounds displayed insufficient boron concentrations in tumors. The tumor/blood (T/B) or tumor/muscle (T/M) ratio plays a crucial role in safeguarding normal organs during BNCT treatment and is typically required to exceed 2.5.26 The T/B and T/M ratios of compound 2 were measured at 2.46 and 2.41, respectively, approaching the required threshold. Conversely, the T/B and T/M ratios of l-BPA-fructose stood at 1.34 and 1.56, respectively, which were lower than those of compound 2.
Table 1. Biodistribution Results of cRGD(d-BPA)K and l-BPA-Fructose 1 h Post-injection Using U87MG Tumor-Bearing Mice.
| Boron
concentration (μg/g) |
||
|---|---|---|
| Organ | cRGD(d-BPA)K | l-BPA-fructose |
| Blood | 3.40 ± 1.88 | 10.26 ± 0.55 |
| Brain | 0.628 ± 0.02 | 3.93 ± 0.49 |
| Liver | 2.83 ± 0.59 | 7.99 ± 1.43 |
| Kidney | 16.66 ± 2.78 | 28.51 ± 8.33 |
| Muscle | 3.11 ± 0.76 | 9.07 ± 1.60 |
| Tumor | 7.10 ± 1.60 | 13.81 ± 2.50 |
| Tumor/blood | 2.46 ± 1.14 | 1.34 ± 0.22 |
| Tumor/brain | 13.40 ± 0.53 | 3.57 ± 0.82 |
| Tumor/liver | 2.50 ± 0.12 | 1.77 ± 0.45 |
| Tumor/kidney | 0.43 ± 0.08 | 0.52 ± 0.19 |
| Tumor/muscle | 2.41 ± 0.80 | 1.56 ± 0.41 |
According to existing literature, low-molecular-weight l-BPA prompts rapid drug excretion from the tumor and body. To achieve optimal BNCT conditions, continuous drug infusion using the infusion method becomes necessary.27,28 Furthermore, because of its low solubility in water, l-BPA requires an appropriate complexing agent such as fructose for administration.29 However, compound 2, a peptide-based boron compound, exhibited favorable solubility and an adequate retention time in the body, rendering it well-suited for neutron capture therapy. Furthermore, as shown in Figure 2, when labeled with a long-half-life radioisotope such as 64Cu, its pharmacokinetics can be tracked up to 20 h post-injection, facilitating the strategic planning of BNCT. However, achieving a therapeutic dose within the tumor necessitates further enhancement by administering a larger quantity of the drug.
Figure 2.

PET imaging and biodistribution data of 64Cu-1. (a) Coronal and (b) transverse PET images depicting U87MG tumor mice at 0.5, 1, 3, 5, 7, and 20 h post-intravenous injection of 64Cu-1. Tumor locations are highlighted with white arrows. (c) Quantitative analysis of 64Cu-1 uptake in the brain, liver, kidney, muscle, and tumor derived from small-animal PET imaging.
In conclusion, we synthesized two novel αvβ3 tumor-targeting peptides, cRGD(d-BPA)K 2 and 64Cu-1, designed for BNCT and tumor diagnostics, respectively. These peptides incorporated the d-BPA amino acid into their sequences. 64Cu-1 exhibited a notably high tumor uptake, demonstrating its potential as a diagnostic PET imaging agent. Compound 2 displayed an efficient tumor-targeting capability along with a moderate boron concentration within the tumor. Importantly, the T/B and T/M boron concentration ratios surpassed those of clinically approved l-BPA-fructose. Both peptides exhibited significant boron buildup within the tumor just 1 h after being injected intravenously. These reported tracers display promise as tools for PET and BNCT in treating αvβ3 tumors. However, further investigation through neutron irradiation is necessary to explore the full therapeutic potential.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.4c00007.
Materials and methods; synthesis of compounds 1–6, including Schemes S1 and S2; ESI-MS, 1H NMR, and HPLC analyses, including Figures S1–S5; radiochemistry, including Figure S6; and general experimental details (PDF)
This work was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by MSIT, Republic of Korea (No. 50462-2023), and the National Research Foundation of Korea (NRF 2021R1A6A3A01086709).
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Medicinal Chemistry Lettersvirtual special issue “Exploring Covalent Modulators in Drug Discovery and Chemical Biology”.
Supplementary Material
References
- Li J.; Sun Q.; Lu C.; Xiao H.; Guo Z.; Duan D.; Zhang Z.; Liu T.; Liu Z. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nat. Commun. 2022, 13, 2143 10.1038/s41467-022-29780-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R. F.; Coderre J. A.; Vicente M. G. H.; Blue T. E. Boron neutron capture therapy of cancer: current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Mushtaq S.; Ae P. J.; Kim J. Y.; Lee K. C.; Kim K. I. The role of radiolabeling in BNCT tracers for enhanced dosimetry and treatment planning. Theranostics 2023, 13, 5247–5265. 10.7150/thno.88998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba M.; Goswami A.; Bandyopadhyay A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. 10.1039/D0CC06557A. [DOI] [PubMed] [Google Scholar]
- Coghi P.; Li J.; Hosmane N. S.; Zhu Y. Next generation of boron neutron capture therapy (BNCT) agents for cancer treatment. Med. Res.Rev. 2023, 43, 1809–1830. 10.1002/med.21964. [DOI] [PubMed] [Google Scholar]
- Bendel P.; Wittig A.; Basilico F.; Mauri P. L.; Sauerwein W. Metabolism of borono-phenylalanine-fructose complex (BPA-fr) and borocaptate sodium (BSH) in cancer patients-Results from EORTC trial 11001. J. Pharm. Biomed. Anal 2010, 51, 284–287. 10.1016/j.jpba.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Hanaoka K.; Watabe T.; Naka S.; Kanai Y.; Ikeda H.; Horitsugi G.; Kato H.; Isohashi K.; Shimosegawa E.; Hatazawa J. FBPA PET in boron neutron capture therapy for cancer: prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res. 2014, 4, 70. 10.1186/s13550-014-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi H.; Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier J. S.; Barnes L. A.; Shields D. J.; Huang M.; Lau S. K.; Prévost N.; Tarin D.; Shattil S. J.; Cheresh D. A. An integrin αvβ3-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 2009, 15, 1163–1169. 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Lee T. S.; Ryu J.; Hong S.; Kang M.; Im K.; Kang J. H.; Lim S. M.; Park S.; Song R. RGD peptide-conjugated multimodal NaGdF4: Yb3+/Er3+ nanophosphors for upconversion luminescence, MR, and PET imaging of tumor angiogenesis. J. Nucl. Med. 2013, 54, 96–103. 10.2967/jnumed.112.108043. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Guo N.; Li Q.; Ma Y.; Jacboson O.; Lee S.; Choi H. S.; Mansfield J. R.; Niu G.; Chen X. Dynamic PET and optical imaging and compartment modeling using a dual-labeled cyclic RGD peptide probe. Theranostics 2012, 2, 746–756. 10.7150/thno.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Niu G.; Wu H.; Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin αvβ3. Theranostics 2016, 6, 78–92. 10.7150/thno.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Xie J.; Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem. Rev. 2010, 110, 3087–3111. 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. A.; Lee Y. J.; Lee J. W.; Lee K. C.; An G. I.; Kim K. M.; Kim B. I.; Kim T. J.; Kim J. Y. Cyclic RGD peptides incorporating cycloalkanes: synthesis and evaluation as PET radiotracers for tumor imaging. ACS Med. Chem. Lett. 2014, 5, 979–982. 10.1021/ml500135t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J. J.; Kelly T. S.; Andrews R.; Rogers B. E. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin (7–14) analogues containing different amino acid linker moieties. Bioconjugate Chem. 2007, 18, 1110–1117. 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- Wong P.; Li L.; Chea J.; Hu W.; Poku E.; Ebner T.; Bowles N.; Wong J. Y.; Yazaki P. J.; Sligar S.; Shively J. E. Antibody targeted PET imaging of 64Cu-DOTA-anti-CEA PEGylated lipid nanodiscs in CEA positive tumors. Bioconjugate Chem. 2020, 31, 743–753. 10.1021/acs.bioconjchem.9b00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K.; Yu J.; Ishizaki A.; Yokokawa M.; Kitamura M.; Kitamura Y.; Shiba K.; Odani A. Radiogallium complex-conjugated bifunctional peptides for detecting primary cancer and bone metastases simultaneously. Bioconjugate Chem. 2015, 26, 1561–1570. 10.1021/acs.bioconjchem.5b00186. [DOI] [PubMed] [Google Scholar]
- Li J.; Shi Y.; Zhang Z.; Liu H.; Lang L.; Liu T.; Chen X.; Liu Z. A metabolically stable boron-derived tyrosine serves as a theranostic agent for positron emission tomography guided boron neutron capture therapy. Bioconjugate Chem. 2019, 30, 2870–2878. 10.1021/acs.bioconjchem.9b00578. [DOI] [PubMed] [Google Scholar]
- Boswell C. A.; Sun X.; Niu W.; Weisman G. R.; Wong E. H.; Rheingold A. L.; Anderson C. J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004, 47, 1465–1474. 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- Wadas T. J.; Wong E. H.; Weisman G. R.; Anderson C. J. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des 2007, 13, 3–16. 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Li Z. B.; Cao Q.; Liu S.; Wang F.; Chen X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J. Nucl. Med. 2009, 50, 1168–1177. 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- Selvaraj R.; Liu S.; Hassink M.; Huang C. W.; Yap L. P.; Park R.; Fox J. M.; Li Z.; Conti P. S. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg. Med. Chem. Lett. 2011, 21, 5011–5014. 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S.; Bhatt N.; Ha Y. S.; Huynh P. T.; Soni N.; Lee W.; Lee Y. J.; Kim J. Y.; Pandya D. N.; An G. I.; Lee K. C.; Chang Y.; Yoo J. High in vivo stability of 64Cu-labeled cross-bridged chelators is a crucial factor in improved tumor imaging of RGD peptide conjugates. J. Med. Chem. 2018, 61, 385–395. 10.1021/acs.jmedchem.7b01671. [DOI] [PubMed] [Google Scholar]
- Evangelista L.; Jori G.; Martini D.; Sotti G. Boron neutron capture therapy and 18F-labelled borophenylalanine positron emission tomography: A critical and clinical overview of theliterature. Appl. Radiat. Isot. 2013, 74, 91–101. 10.1016/j.apradiso.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Fu Q.; Li J.; Liu H.; Zhang Z.; Liu T.; Liu Z. 2020. Covalent organic polymer as a carborane carrier for imaging-facilitated boron neutron capture therapy. ACS Appl. Mater. Interfaces 2020, 12, 55564–55573. 10.1021/acsami.0c15251. [DOI] [PubMed] [Google Scholar]
- Nomoto T.; Yao Y.; Inoue Y.; Suzuki M.; Kanamori K.; Takemoto H.; Matsui M.; Tomoda K.; Nishiyama N. Fructose-functionalized polymers to enhance therapeutic potential of p-boronophenylalanine for neutron capture therapy. J. Controlled Release 2021, 332, 184–193. 10.1016/j.jconrel.2021.02.021. [DOI] [PubMed] [Google Scholar]
- Yanagie H.; Higashi S.; Seguchi K.; Ikushima I.; Fujihara M.; Nonaka Y.; Oyama K.; Maruyama S.; Hatae R.; Suzuki M.; Masunaga S. I.; et al. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer involving the intra-arterial injection of a 10BSH-containing WOW emulsion. Appl. Radiat. Isot. 2014, 88, 32–37. 10.1016/j.apradiso.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Iguchi Y.; Michiue H.; Kitamatsu M.; Hayashi Y.; Takenaka F.; Nishiki T. I.; Matsui H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. 10.1016/j.biomaterials.2015.03.061. [DOI] [PubMed] [Google Scholar]
- Henriksson R.; Capala J.; Michanek A.; Lindahl S.Å.; Salford L. G.; Franzén L.; Blomquist E.; Westlin J. E.; Bergenheim A. T. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA). Radiother. Oncol 2008, 88, 183–191. 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


