Abstract
Adolescent idiopathic scoliosis (AIS) is a common and progressive spinal deformity in children that exhibits striking sexual dimorphism, with girls at more than fivefold greater risk of severe disease compared to boys. Despite its medical impact, the molecular mechanisms that drive AIS are largely unknown. We previously defined a female-specific AIS genetic risk locus in an enhancer near the PAX1 gene. Here, we sought to define the roles of PAX1 and newly identified AIS-associated genes in the developmental mechanism of AIS. In a genetic study of 10,519 individuals with AIS and 93,238 unaffected controls, significant association was identified with a variant in COL11A1 encoding collagen (α1) XI (rs3753841; NM_080629.2_c.4004C>T; p.(Pro1335Leu); p=7.07E–11, OR = 1.118). Using CRISPR mutagenesis we generated Pax1 knockout mice (Pax1-/-). In postnatal spines we found that PAX1 and collagen (α1) XI protein both localize within the intervertebral disc-vertebral junction region encompassing the growth plate, with less collagen (α1) XI detected in Pax1-/- spines compared to wild-type. By genetic targeting we found that wild-type Col11a1 expression in costal chondrocytes suppresses expression of Pax1 and of Mmp3, encoding the matrix metalloproteinase 3 enzyme implicated in matrix remodeling. However, the latter suppression was abrogated in the presence of the AIS-associated COL11A1P1335L mutant. Further, we found that either knockdown of the estrogen receptor gene Esr2 or tamoxifen treatment significantly altered Col11a1 and Mmp3 expression in chondrocytes. We propose a new molecular model of AIS pathogenesis wherein genetic variation and estrogen signaling increase disease susceptibility by altering a PAX1-COL11a1-MMP3 signaling axis in spinal chondrocytes.
Research organism: Human, Mouse
eLife digest
Adolescent idiopathic scoliosis (AIS) is a twisting deformity of the spine that occurs during periods of rapid growth in children worldwide. Children with severe cases of AIS require surgery to stop it from getting worse, presenting a significant financial burden to health systems and families.
Although AIS is known to cluster in families, its genetic causes and its inheritance pattern have remained elusive. Additionally, AIS is known to be more prevalent in females, a bias that has not been explained. Advances in techniques to study the genetics underlying diseases have revealed that certain variations that increase the risk of AIS affect cartilage and connective tissue. In humans, one such variation is near a gene called Pax1, and it is female-specific.
The extracellular matrix is a network of proteins and other molecules in the space between cells that help connect tissues together, and it is particularly important in cartilage and other connective tissues. One of the main components of the extracellular matrix is collagen. Yu, Kanshour, Ushiki et al. hypothesized that changes in the extracellular matrix could affect the cartilage and connective tissues of the spine, leading to AIS.
To show this, the scientists screened over 100,000 individuals and found that AIS is associated with variants in two genes coding for extracellular matrix proteins. One of these variants was found in a gene called Col11a1, which codes for one of the proteins that makes up collagen.
To understand the relationship between Pax1 and Col11a1, Yu, Kanshour, Ushiki et al. genetically modified mice so that they would lack the Pax1 gene. In these mice, the activation of Col11a1 was reduced in the mouse spine. They also found that the form of Col11a1 associated with AIS could not suppress the activation of a gene called Mmp3 in mouse cartilage cells as effectively as unmutated Col11a1. Going one step further, the researchers found that lowering the levels of an estrogen receptor altered the activation patterns of Pax1, Col11a1, and Mmp3 in mouse cartilage cells. These findings suggest a possible mechanism for AIS, particularly in females.
The findings of Yu, Kanshour, Ushiki et al. highlight that cartilage cells in the spine are particularly relevant in AIS. The results also point to specific molecules within the extracellular matrix as important for maintaining proper alignment in the spine when children are growing rapidly. This information may guide future therapies aimed at maintaining healthy spinal cells in adolescent children, particularly girls.
Introduction
The human spinal column is a dynamic, segmented, bony, and cartilaginous structure that is essential for integrating the brain and nervous system with the axial skeleton while simultaneously providing flexibility in three dimensions (Richards et al., 2020). Idiopathic scoliosis is the most common developmental disorder of the spine, typically appearing during the adolescent growth spurt. Adolescent idiopathic scoliosis (AIS) is reported in all major ancestral groups, with a population prevalence of 1.5–3% (Wise, 2014; Hresko, 2013). Children with AIS usually present with a characteristic right-thoracic major curve pattern and a compensatory lumbar curve. Major thoracolumbar and lumbar curves are less frequent (Richards et al., 2020). The three-dimensional nature of the deformity results in torsion in the spine that is most significant at the apex of the major curve, and changes in the structures of the vertebrae and ribs may develop as the curve worsens or progresses (Richards et al., 2020). Children with thoracic curves, with larger curves at first presentation, and/or with greater remaining growth potential are at increased risk of progression, but this risk decreases sharply after skeletal maturity (Richards et al., 2020). Sex is a recognized risk factor for AIS, with girls having at least a fivefold greater risk of progressive deformity requiring treatment compared to boys (Karol et al., 1993). This well-documented sexual dimorphism has prompted speculation that levels of circulating endocrine hormones, particularly estrogen, are important exposures in AIS susceptibility (Liang et al., 2021).
The genetic architecture of human AIS is complex, and underlying disease mechanisms remain uncertain. Heritability studies of Northern European (Wynne-Davies, 1968; Grauers et al., 2012), North American (Riseborough and Wynne-Davies, 1973; Kruse et al., 2012), and South Asian (Tang et al., 2012) ancestral groups suggest that disease risk is multifactorial, caused by genetic and environmental contributions (Wise, 2014; Wise et al., 2020). Accordingly, population-based genome-wide association studies (GWAS) in multiple ancestral groups have identified several AIS-associated susceptibility loci, mostly within non-coding genomic regions (Wise et al., 2020). In particular, multiple GWAS have implicated non-coding regions near the LBX1 (Takahashi et al., 2011), ADGRG6 (also known as GRP126) (Kou et al., 2013), and BNC2 (Ogura et al., 2015) genes. An association with alleles in an enhancer distal to PAX1, encoding the transcription factor paired box 1, was primarily driven by females, suggesting that it contributes to the sexual dimorphism observed in AIS (Sharma et al., 2015). Subsequent meta-analysis of combined AIS GWAS identified additional susceptibility loci. These included variants in an intron of SOX6, a transcription factor, that along with PAX1, is important in early spinal column formation (Smits and Lefebvre, 2003). Furthermore, gene enrichment analyses found significant correlation of AIS-associated loci with biological pathways involving cartilage and connective tissue development (Khanshour et al., 2018). A more recent GWAS in a Japanese population identified 14 additional AIS loci that are candidates for further evaluation (Kou et al., 2019). In separate studies, genome sequencing in AIS cases and families identified enrichment of rare variants in the COL11A2 (Haller et al., 2016) and HSPG2 (Baschal et al., 2014) genes, encoding components of the cartilage extracellular matrix (ECM). Hence, variation affecting cartilage and connective tissue ECM is an emerging theme in the heterogeneous genetic architecture of AIS.
Pre-clinical animal models are essential tools for accelerating mechanistic understanding of AIS and for therapeutic testing (Wise et al., 2020). In zebrafish, several genetic mutants with larval or later-onset spinal deformity have been described, including ptk7 (Hayes et al., 2014; Van Gennip et al., 2018), c21orf59 (Jaffe et al., 2016), ccdc40 (Becker-Heck et al., 2011), ccdc151 (Bachmann-Gagescu et al., 2011), dyx1c1, and kif6 (Konjikusic et al., 2018). In rescue experiments, Rebello et al. recently showed that missense variants in COL11A2 associated with human congenital scoliosis fail to rescue a vertebral malformation phenotype in a zebrafish col11a2 knockout line (Rebello et al., 2023). In mouse, conditional deletion of Adgrg6 in skeletal cartilage (using Col2a1-Cre) produces a progressive scoliosis of the thoracic spine during postnatal development that is marked by herniations within the cartilaginous endplates of involved vertebrae. Progressive scoliosis, albeit to a lesser extent, was also observed when Adgrg6 was deleted from committed chondrocytes (using ATC:Cre) (Long et al., 2001; Liu et al., 2019; Liu et al., 2021). These studies demonstrate that cartilage and possibly other osteochondroprogenitor cells contribute to the scoliosis phenotype in these models (Liu et al., 2019). Taken together, genetic and functional studies in mouse, although limited, support the hypothesis that deficiencies in biogenesis and/or homeostasis of cartilage, intervertebral disc (IVD), and dense connective tissues undermine the maintenance of proper spinal alignment during the adolescent growth spurt (Wise et al., 2020).
The combined contribution of reported AIS-associated variants is broadly estimated to account for less than 10% of the overall genetic risk of the disease (Kou et al., 2019). To address this knowledge gap, we sought to define novel loci associated with AIS susceptibility in genes encoding proteins of the ECM (i.e. the ‘matrisome’; Naba et al., 2012b; Naba et al., 2012a). Here, we identify new genetic associations with AIS. Further, our functional assessments support a new disease model wherein AIS-associated genetic variation and estrogen signaling perturb a PAX1-COL11a1-MMP3 axis in chondrocytes.
Results
Nonsynonymous variants in matrisome genes are associated with increased risk of AIS
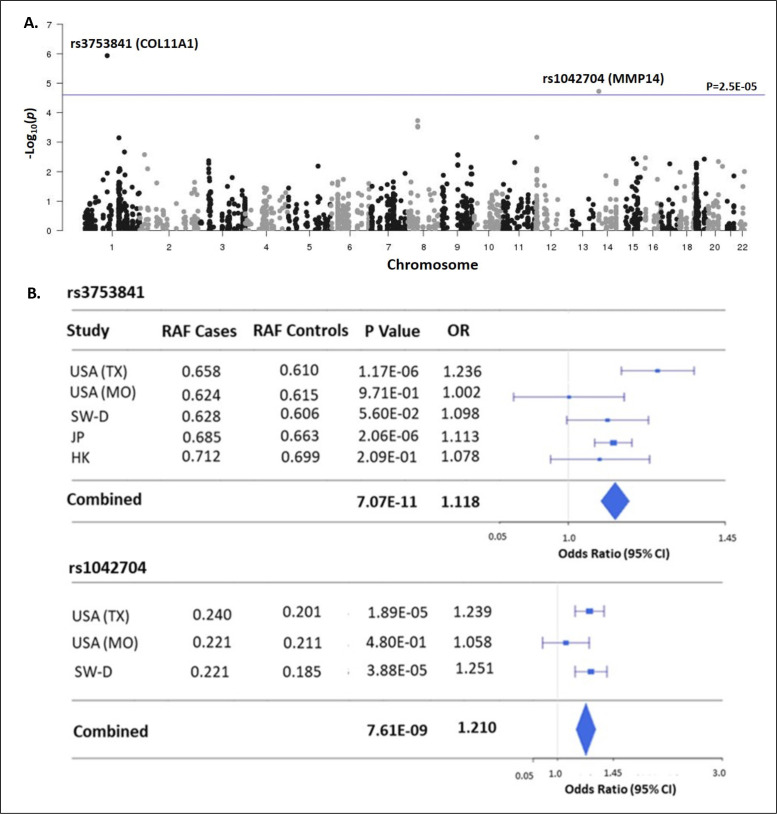
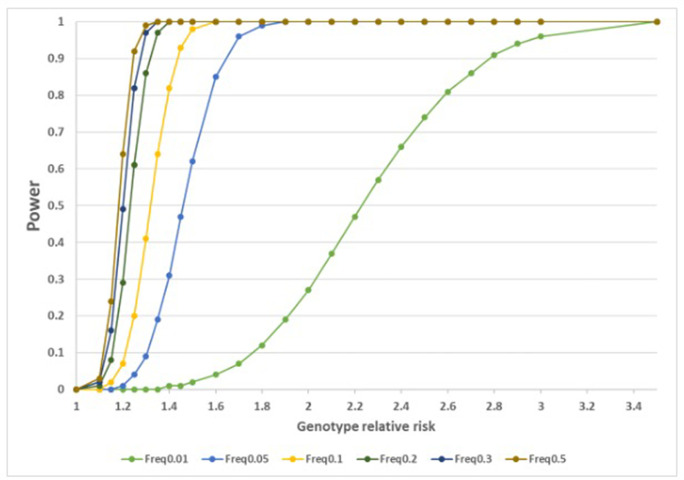
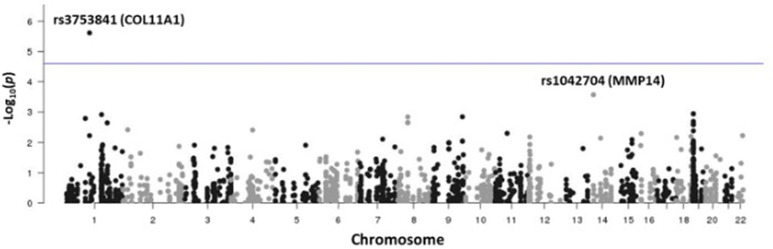
The ‘matrisome’ has been defined as ‘all genes encoding structural ECM components and those encoding proteins that may interact with or remodel the ECM’ (Hynes and Naba, 2012). Proteins comprising the global ECM as currently defined have been identified by both experimental and bioinformatic methods (Naba et al., 2012b). We assembled 1027 matrisome genes as previously identified (Naba et al., 2016), including 274 core-matrisome and 753 matrisome-associated genes (N=1027 total). For the genes encoding these 1027 proteins, we identified all nonsynonymous common variants (MAF>0.01) queried by the Illumina HumanCoreExome-24v1.0 beadchip and determined their genotypes in a discovery cohort of 1358 cases and 12,507 controls, each of European ancestry (Table 1). After applying multiple quality control measures (see Methods section), we retained 2008 variants in 597 matrisome genes for association testing (Supplementary file 1). This sample size was estimated to provide at least 80% power to detect significant associations at the matrisome-wide level (α≤2.5E–05), for alleles with population frequency ≥0.05 and OR ≥1.5 (Figure 1—figure supplement 1). Two nonsynonymous variants, in COL11A1 (rs3753841; NM_080629.2_c.4004C>T; p.(Pro1335Leu); odds ratio (OR)=1.236 [95% CI = 1.134–1.347], p=1.17E–06) and MMP14 (rs1042704; NM_004995.4_c.817G>A; p.(Asp273Asn); OR = 1.239 [95% CI = 1.125–1.363], p=1.89E–05) were significantly associated with AIS (Figure 1A). Given the sexual dimorphism in AIS and our prior observation of a female-predominant disease locus (Sharma et al., 2015), we tested the 2008 variants separately in females (N=1157 cases and 7138 controls). In females, the association with rs3753841 remained statistically significant, whereas rs1042704, near MMP14, was not associated with AIS in females (Figure 1—figure supplement 2). Our study was not sufficiently powered to test males separately.
Table 1. Study cohorts.
| Cohort | Ethnicity | Stage | Subjects | Cases | Controls | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| USA (TX) | NHW | Discovery | 13,865 | 201 | 1157 | 5369 | 7138 |
| USA (MO) | NHW | Replication | 2951 | 201 | 1102 | 1049 | 689 |
| SW-D | NHW | Replication | 4627 | 222 | 1409 | 505 | 2491 |
| JP | EAS (Japanese) | Replication | 79,211 | 323 | 5004 | 40,205 | 33,679 |
| HK | EAS (HAN Chinese) | Replication | 3103 | 178 | 812 | 858 | 1255 |
| Total | 103,757 | 10,519 | 93,238 | ||||
USA (TX): Texas cohort; USA (MO): Missouri cohort; SW-D: Danish cohort; JP: Japanese cohort; HK: Hong Kong cohort; NHW: Non-Hispanic White; EAS: East Asian.
Figure 1. Matrisome-wide association study.
(A) Manhattan plot showing –log10 p-values (y-axis) versus chromosomal position (x-axis) for the 2008 common coding variants tested in the discovery study USA (TX). The horizontal line represents the threshold for significance level (p-value <2.5 × 10–5) after Bonferroni multiple testing correction. (B) Tests of association for SNPs rs3753841 and rs1042704 in discovery and independent replication cohorts. RAF – reference allele frequency; OR – odds ratio; CI –confidence interval.
Figure 1—figure supplement 1. Statistical power as a function of the genotype relative risk (OR) to detect significant association at α=2.5E–05 for different disease allele frequencies, using 1358 cases and 12,507 controls in the discovery study.
Figure 1—figure supplement 2. Manhattan plot showing –log10 p-values (y-axis) plotted versus chromosomal position (x-axis) for the 2009 common coding variants tested for females in the discovery study USA (TX).
Figure 1—figure supplement 3. Tests of association of SNP rs1042704 with adolescent idiopathic scoliosis (AIS) in East Asian cohorts.
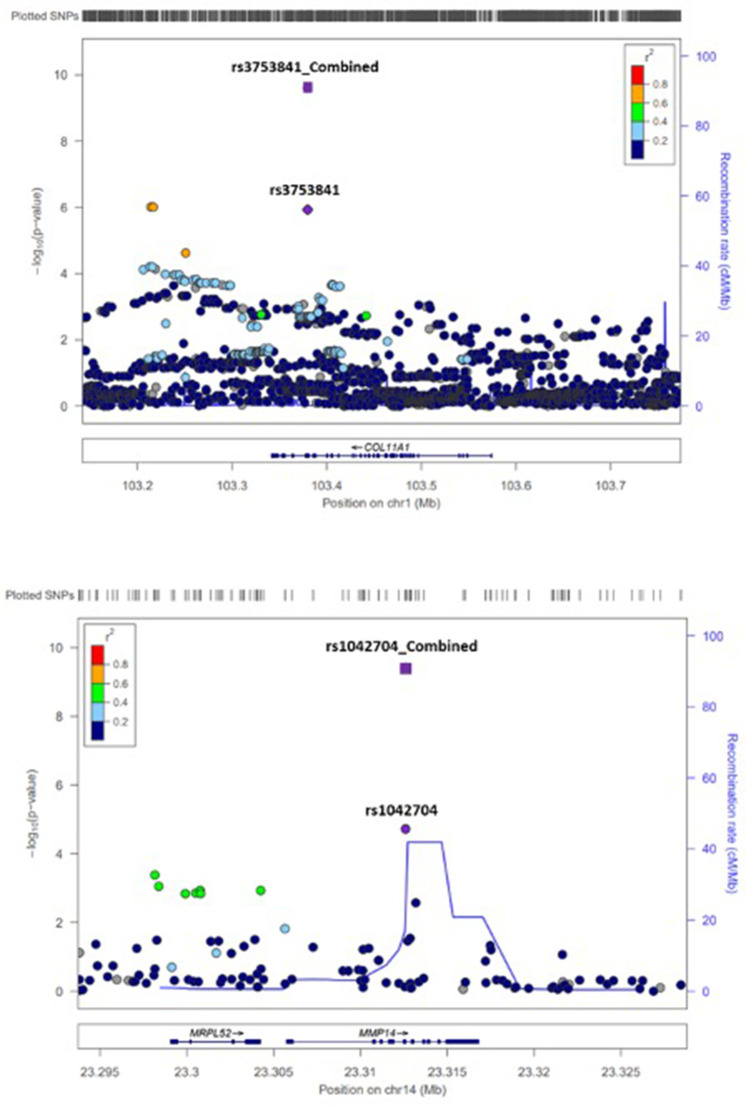
Figure 1—figure supplement 4. LocusZoom plots of SNPs in genomic regions of SNPs rs3753841 (top) and rs1042704 (bottom).
To validate these results, we sought to replicate the associations of rs3753841 and rs1042704 in four independent AIS case-control cohorts, from North America, Europe, and eastern Asia, representing multiple ethnicities (total N=9161 AIS cases, 80,731 healthy controls, Table 1). Genotypes for both variants were extracted from these datasets and tested for association by meta-analysis together with the discovery cohort (see Methods). Meta-analysis of all cohorts together increased the evidence for association of both variants with AIS risk (Figure 1B). While a similar effect size was noted for rs1042704 in Japanese and Han Chinese cohorts, the results were less significant, likely due to lower minor allele frequencies (East Asian MAF = 0.02 compared to total non-Asian cohort MAF = 0.20) in these populations (Figure 1—figure supplement 3). Plotting recombination across both regions suggested that these signals were likely confined to blocks of linkage disequilibrium within the COL11A1 and MMP14 genes, respectively (Figure 1—figure supplement 4).
Rare dominant mutations in COL11A1, often disrupting a Gly-X-Y sequence, can cause Marshall (MRSHS) (OMIM #154780) or Stickler syndromes (STL2) (OMIM #604841) marked variously by facial anomalies, sensineural hearing loss, short stature, spondyloepiphyseal dysplasia, eye anomalies, ectodermal features, and scoliosis. Notably, our AIS cohort and particularly individuals carrying the rs3753841 risk allele were negative for co-morbidities or obvious features of Marshall or Stickler syndromes. Thus, variation in COL11A1 is independently associated with AIS. Notably, we did not detect common variants in linkage disequilibrium (R2>0.6) with the top SNP rs3753841 (Figure 1—figure supplement 4). Further, analysis of 625 exomes from the discovery cohort (46%) identified only three rare COL11A1 variants in five individuals (Supplementary file 2), and rare variant burden testing for COL11A1 was not significant as expected (data not shown). These observations suggested that rs3753841 itself could confer disease risk, although our methods would not detect deep intronic variants that could contribute to the overall association signal.
COL11A1 is expressed in adolescent spinal tissues
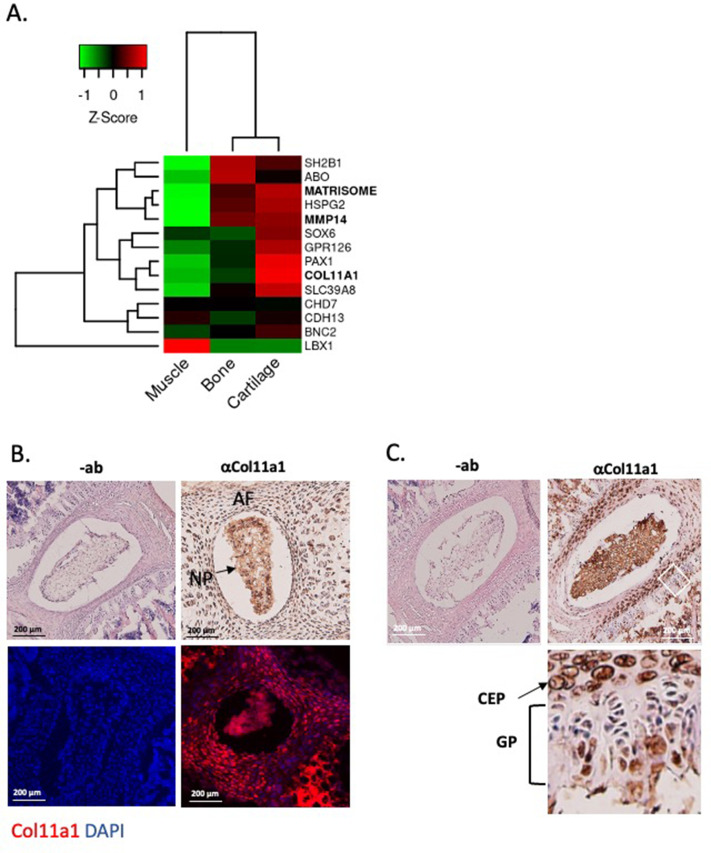
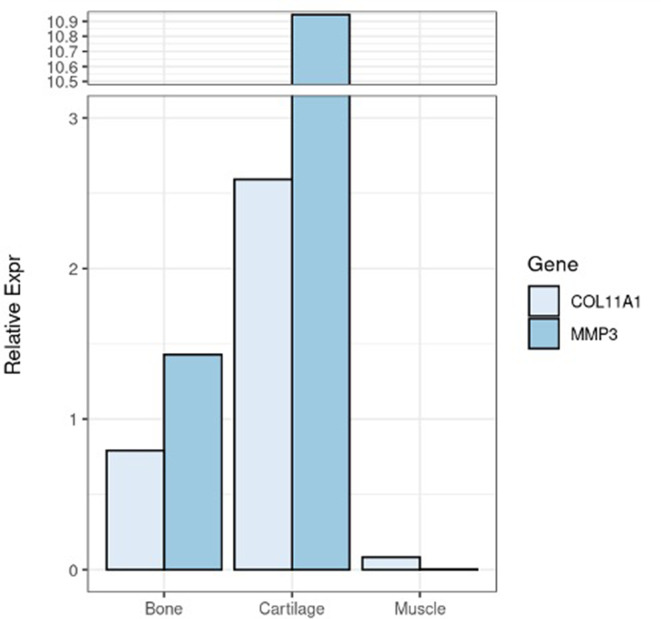
We next characterized COL11A1 in postnatal spine development. COL11A1 encodes one of three alpha chains of type XI collagen, a member of the fibrillar collagen subgroup and regulator of nucleation and initial fibril assembly, particularly in cartilage (Fernandes et al., 2007). Spinal deformity is well described in Col11a1-deficient (cho/cho) embryos (Hafez et al., 2015; Seegmiller et al., 1971). In mouse tendon, Col11a1 mRNA is abundant during development but barely detectable at 3 months of age (Wenstrup et al., 2011). We analyzed RNAseq datasets derived from adolescent human spinal tissues (Makki et al., 2020), finding that COL11A1 was upregulated in cartilage relative to bone and muscle. In cartilage, PAX1 and COL11A2 showed the strongest expression levels relative to other published human AIS-associated genes (Kou et al., 2013; Ogura et al., 2015; Sharma et al., 2015; Khanshour et al., 2018; Haller et al., 2016; Baschal et al., 2014; Gao et al., 2007; Figure 2A). In all, most AIS-associated genes showed the strongest expression levels in cartilage relative to other adolescent spinal tissues.
Figure 2. Col11a1 and Mmp14 expression in spine.
(A) A heatmap of transcript per million (TPM) values of COL11A1, MMP14, and other published genes associated with adolescent idiopathic scoliosis (AIS). The average TPM value of matrisome genes is represented as MATRISOME. (B) Detection of collagen a1(XI) in P0.5 mouse spine. Immunohistochemistry (IHC) shown at top, with immunofluorescence (IF) staining below. ‘-ab’ refers to negative controls lacking primary antibody (shown at left). Results are representative of N≥3 technical replicates in whole spines. (C) Detection of collagen a1(XI) in P28 mouse spine. Negative antibody IHC control shown at left; antibody-positive IHC shown at right. Enlarged, rotated view of white boxed area shows a biphasic staining pattern. CEP – cartilage endplate; GP – growth plate. Results are representative of N≥3 technical replicates in whole spines.
Figure 2—figure supplement 1. Immunofluorescence (IF) staining using collagen a1(XI) antibody in P28 ribs (top).
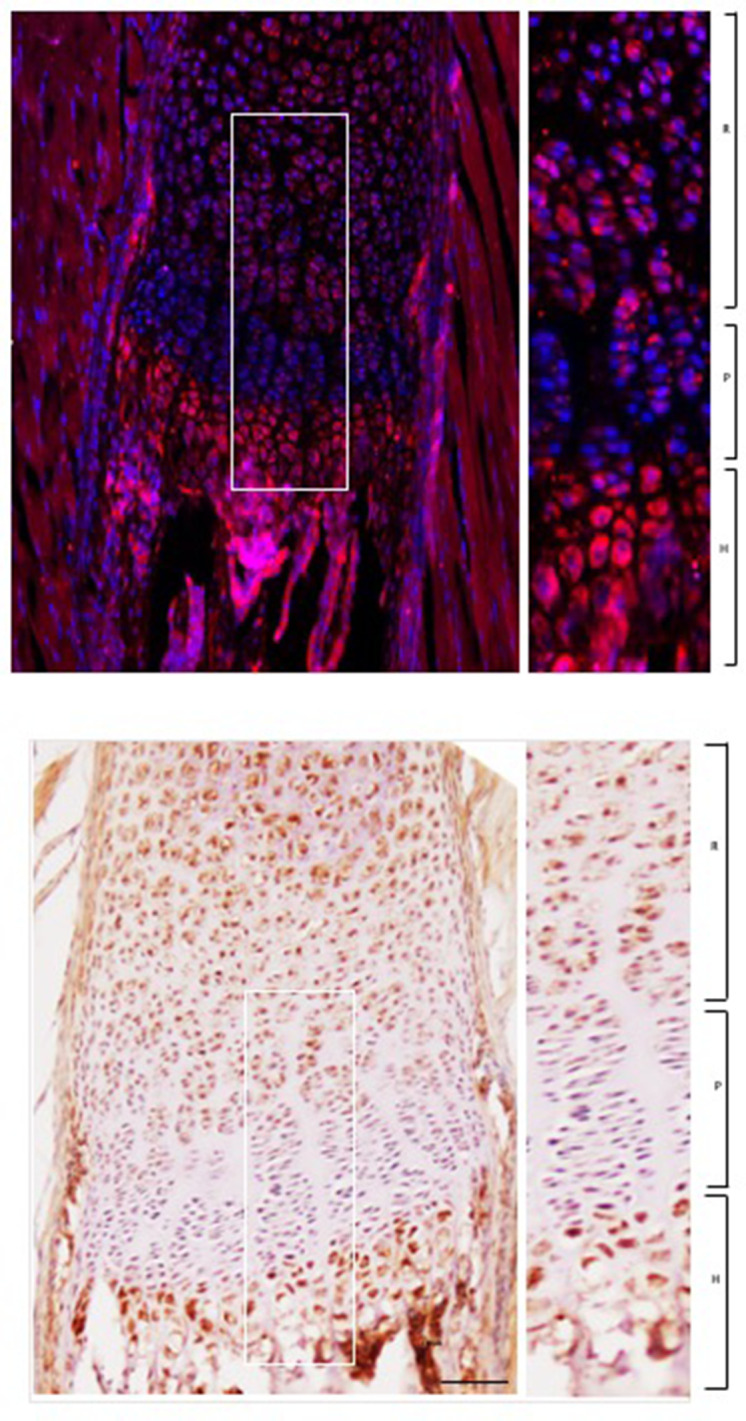
We next sought to characterize Col11a1 expression in spines of postnatal mice. To detect COL11A1 protein (collagen α1(XI)), we performed immunohistochemistry (IHC) and immunofluorescence (IF) microscopy using a collagen α1(XI) reactive antibody (Sun et al., 2020) in newborn (P0.5) and adolescent (P28) mice. In spines of P0.5 mice, strong staining was observed in the nucleus pulposus (NP) and in surrounding annulus fibrosus (AF) (Figure 2B). In thoracic spines of P28 mice, the compartments of the IVD were more distinct, and strong collagen α1(XI) staining was observed in each (Figure 2C). In regions of the cartilage endplate (CEP)-vertebral bone transition, collagen α1(XI) was detected in columnar chondrocytes, particularly in the hypertrophic zone adjacent to condensing bone (Figure 2C). We also examined collagen α1(XI) expression in ribs, as these structures are also involved in the scoliotic deformity (Richards et al., 2020). In P28 rib growth plates, as in spine, a biphasic pattern was observed in which collagen α1(XI) reactivity was most pronounced around cells of the presumed resting and pre-hypertrophic/hypertrophic zones (Figure 2—figure supplement 1). These data show that in mouse, collagen α1(XI) is detectable in all compartments of young postnatal IVD and, at the thoracic level, is particularly abundant in the chondro-osseous junction region of IVD and vertebral growth plate.
Col11a1 is downregulated in the absence of Pax1 in mouse spine and tail
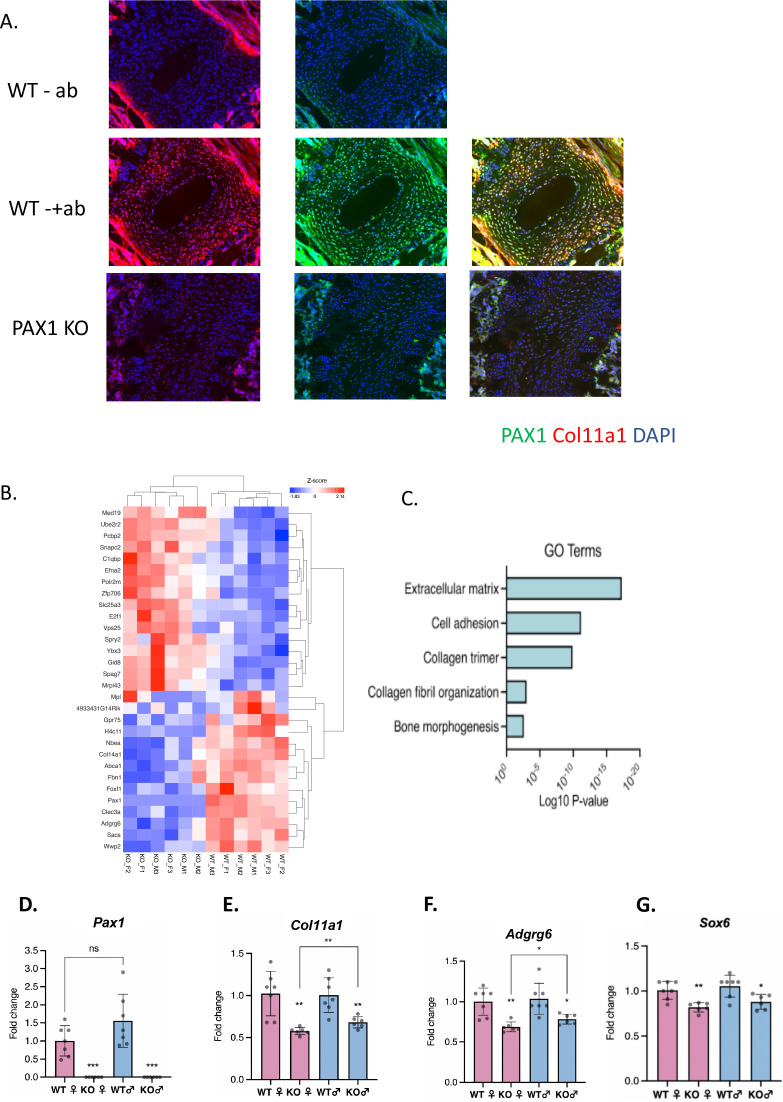
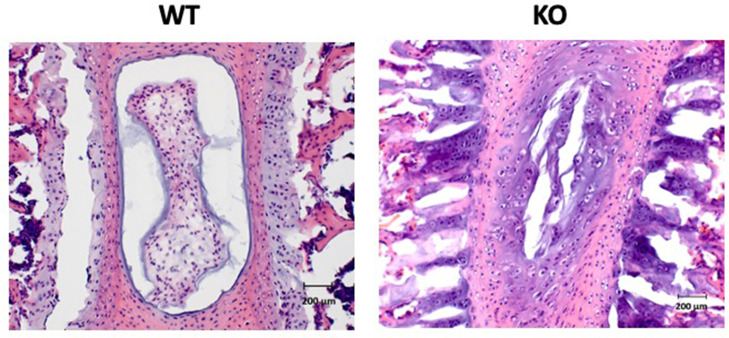
We previously identified AIS-associated variants within a putative enhancer of PAX1 encoding the transcription factor Paired Box 1 (Sharma et al., 2015; Khanshour et al., 2018). Pax1 is a well-described marker of condensing sclerotomal cells as they form segments that will eventually become the IVD and vertebrae of the spine (Chan et al., 2014; Aszódi et al., 1998; Smith et al., 2011). We generated Pax1 knockout mice (Pax1-/-) using CRISPR-Cas9 mutagenesis and validated them using sequencing and southern blot (Figure 3—figure supplement 1). Homozygous Pax1-/- mice were viable and developed spine deformity and kinks in the tail, as observed in other Pax1-deficient mice (Wilm et al., 1998). We next compared the expression of collagen α1(XI) protein in IVD and condensing bone of wild-type and Pax1-/- mice by performing IF staining in P28 spines (Figure 3A). In wild-type IVD, strong overlapping expression of collagen α1(XI) and PAX1 cells was observed, mostly within the CEP and chondro-osseous interface (Figure 3A). PAX1 staining was negative in Pax1-/- mice as expected, and collagen α1(XI) staining was dramatically diminished in CEP and the chondro-osseous vertebral borders. Moreover, the IVD in Pax1-/- mice was highly disorganized, without discernable NP, AF, and CEP structures as has been reported (Figure 3—figure supplement 2; Wallin et al., 1994). To test the effect of Pax1 on expression of Col11a1 and other AIS-associated genes during embryonic development, RNA was isolated from vertebral tissue dissected from the tails of embryonic stage 12.5 (E12.5) wild-type and Pax1-/- mice and subjected to bulk RNAseq and quantitative real-time PCR (qRT-PCR) (Figure 3B). Gene-set enrichment analysis of RNAseq was most significant for the gene ontology term ‘extracellular matrix’ (Figure 3C). By qRT-PCR analysis, expression of Col11a1, Adgrg6, and Sox6 was significantly reduced in female and male Pax1-/- mice compared to wild-type mice (Figure 3D–G). These data show that loss of Pax1 leads to reduced expression of Col11a1 and the AIS-associated genes Adgrg6 and Sox6 in affected tissue of the developing tail.
Figure 3. Assessing Pax1 regulation of Col11a1 expression.
(A) Immunofluorescence (IF) staining of P28 intervertebral disc (IVD) from thoracic regions of Pax1-/- (bottom) and wild-type (WT) littermate (middle, top) mice using PAX1- (green) and collagen a1(XI)-specific (red) antibodies and DAPI nuclear counterstain. Antibody-negative controls are shown at top as (-ab). Results are representative of N≥3 technical replicates in whole spines. (B) Heatmap of differentially expressed genes (p-value <0.0001) in embryonic stage 12.5 (E12.5) tails of WT and Pax1-/- mice. (C) Gene ontology (GO) analysis of differentially expressed genes in E12.5 tail WT and Pax1-/- mice. (D–G) Gene expression levels dissected from E12.5 mouse tail from WT and Pax1-/- (knockout [KO]) mice as determined by quantitative real-time PCR (qRT-PCR). Each value represents the ratio of each gene expression to that of β-actin, and values are mean ± standard deviation. The expression value of WT female group was arbitrarily set at 1.0. Each dot represents one embryo and statistical differences were determined using a two-sided unpaired t-test (*p<0.05, **p<0.01, ***p<0.001).
Figure 3—figure supplement 1. Design and validation of Pax1 knockout in mouse using CRISPR-mediated gene targeting.
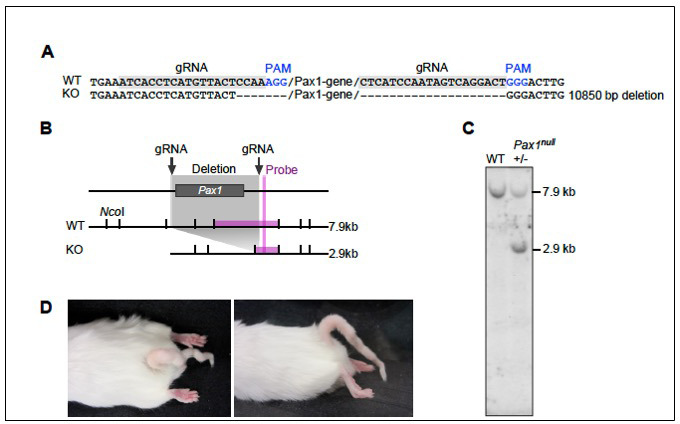
Figure 3—figure supplement 2. HE staining of sectioned lumbar spines from wild-type (left) and Pax1-/- (right) mice.
Col11a1 regulates Mmp3 expression in chondrocytes
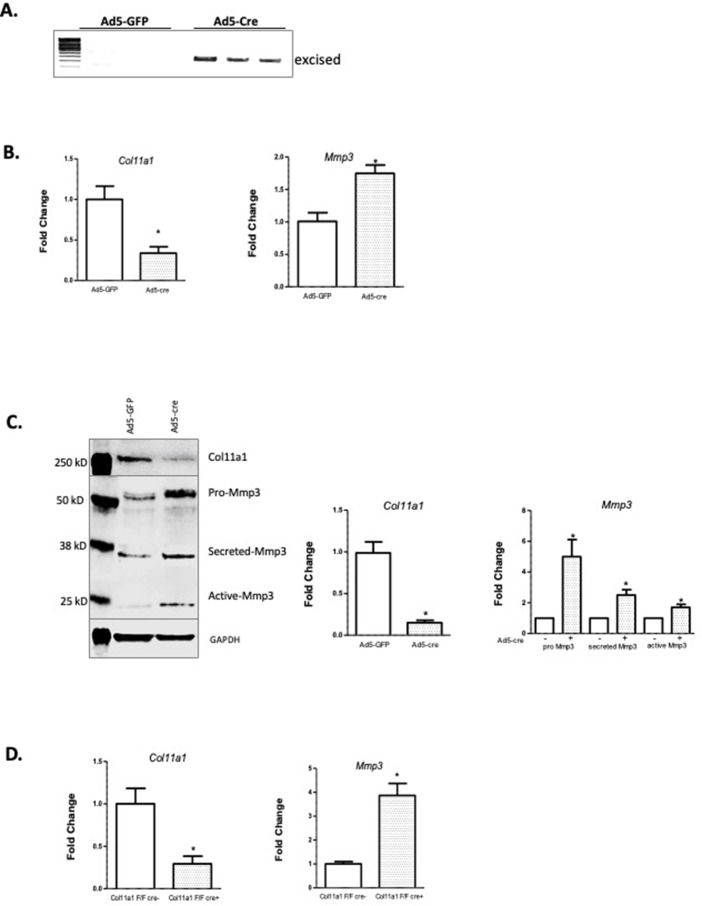
COL11A1 has been linked with ECM remodeling and invasiveness in some cancers (Wu et al., 2014). In solid tumors, COL11A1 has been shown to alter ECM remodeling by enhancing MMP3 expression in response to TGFΒ1 (Wu et al., 2014). MMP3 encodes matrix metalloproteinase 3, also known as stromolysin, an enzyme implicated in matrix degradation and remodeling in connective tissues (Mudgett et al., 1998). We confirmed strong MMP3 mRNA expression, relative to COL11A1, in human spinal cartilage and bone, but minimal expression in spinal muscle (Figure 4—figure supplement 1). We next cultured costal chondrocytes from P0.5 Col11a1fl/fl mice (Sun et al., 2020) and subsequently removed Col11a1 by treating with Cre-expressing adenoviruses. After confirming Col11a1 excision (Figure 4A), we compared Mmp3 expression in these cells to cells treated with GFP-expressing adenoviruses lacking Cre activity. We found that Mmp3 expression was significantly increased in cells where Col11a1 mRNA expression was downregulated by about 70% compared to untreated cells (Figure 4B). Furthermore, western blotting in these cells demonstrated an ~2- to 5-fold increase in pro-, secreted, and active forms of Mmp3 protein when collagen α1(XI) was reduced. The proteolytic processing per se of precursor MMP3 into active forms (Sun et al., 2014) did not appear to be affected by Col11a1 expression (Figure 4C). These results suggest that Mmp3 expression is negatively regulated by Col11a1 in mouse costal chondrocytes.
Figure 4. Col11a1 regulation of Mmp3 expression in cartilage.
(A) PCR assay of Col11a1 excision in Col11a1fl/fl cultured costal chondrocytes. (B) Gene expression levels from Col11a1fl/fl cultured costal chondrocytes transduced with green fluorescent protein (GFP) (Ad5-GFP, left) or Cre-expressing adenovirus (Ad5-cre, right) as determined by quantitative real-time PCR (qRT-PCR). Values represent the ratio of each gene expression to that of GAPDH, and values are mean ± standard deviation. The expression value of control Ad5-GFP results was arbitrarily set at 1.0. Statistical differences were determined using a two-sided paired t-test (*p<0.05). Results shown for N≥3 biologic replicates, each including three technical replicates. (C) Western blot detection of collagen a1(XI), MMP3, and GAPDH loading control in cultured costal chondrocytes after Ad5-GFP or Ad5-cre transduction. Results are representative of N=4 biologic replicates. Protein size ladder is shown in lane 1. Quantification of bands detected by western blotting, where Ad5-GFP was set to 1.0, is shown at right. Statistical differences were determined using a two-sided paired t-test (*p<0.05). (D) Gene expression levels from dissected Col11a1fl/fl:ATC costal cartilage, analyzed as described in (A). Results shown for N=3 biologic replicates, each including three technical replicates.
Figure 4—figure supplement 1. Relative expression of MMP3 compared to COL11A1 in human spinal tissues.
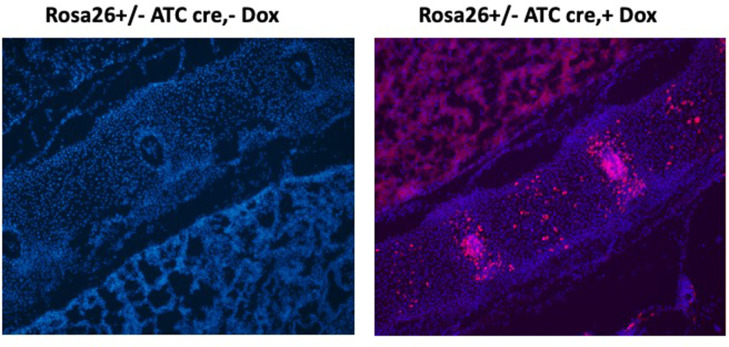
Figure 4—figure supplement 2. Immunofluorescence microscopy of Rosa26+/-:ATC P0 spines, without doxycycline treatment (left) and after doxycycline treatment starting at embryonic stage 15.5 (E15.5) (right).
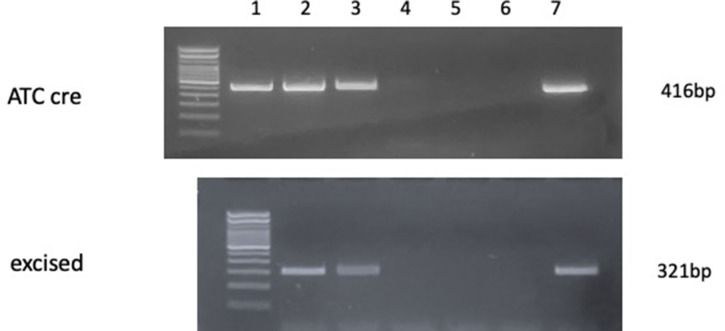
Figure 4—figure supplement 3. PCR assays in DNA from costal cartilage.
To test whether Col11a1 affects Mmp3 expression in vivo, we bred Col11a1fl/fl female mice with Col11a1fl/fl:ATC males carrying the Acan enhancer-driven, doxycycline-inducible Cre (ATC) transgene (Dy et al., 2012). ATC has been shown to harbor Cre-mediated recombination activity in most differentiated chondrocytes and in NP within 2 days of treating pregnant mothers with doxycycline starting at E15.5 (Dy et al., 2012). ATC activity was confirmed by crossing this line to the R26td[Tomato] reporter that ubiquitously expresses the fluorescent gene Tomato after Cre recombination. Strong Cre activity was seen in P0 pups of mothers treated with doxycycline at E15.5 in the NP, CEP, and AF of the IVD and in chondrocytes of the growth plates (Figure 4—figure supplement 2). Pregnant Col11a1fl/fl females were treated with doxycycline water from E15.5 to induce Cre expression in differentiated chondrocytes. Excision of Col11a1 was confirmed in DNA from costal cartilage of Col11a1fl/fl:ATC-positive offspring (Figure 4—figure supplement 3). Consistent with results obtained by in vitro excision of Col11a1, cartilage from mice deficient in Col11a1 showed ~4-fold upregulation of Mmp3 mRNA expression relative to Col11a1fl/fl mice (Figure 4D).
AIS-associated variant in COL11A1 perturbs its regulation of MMP3
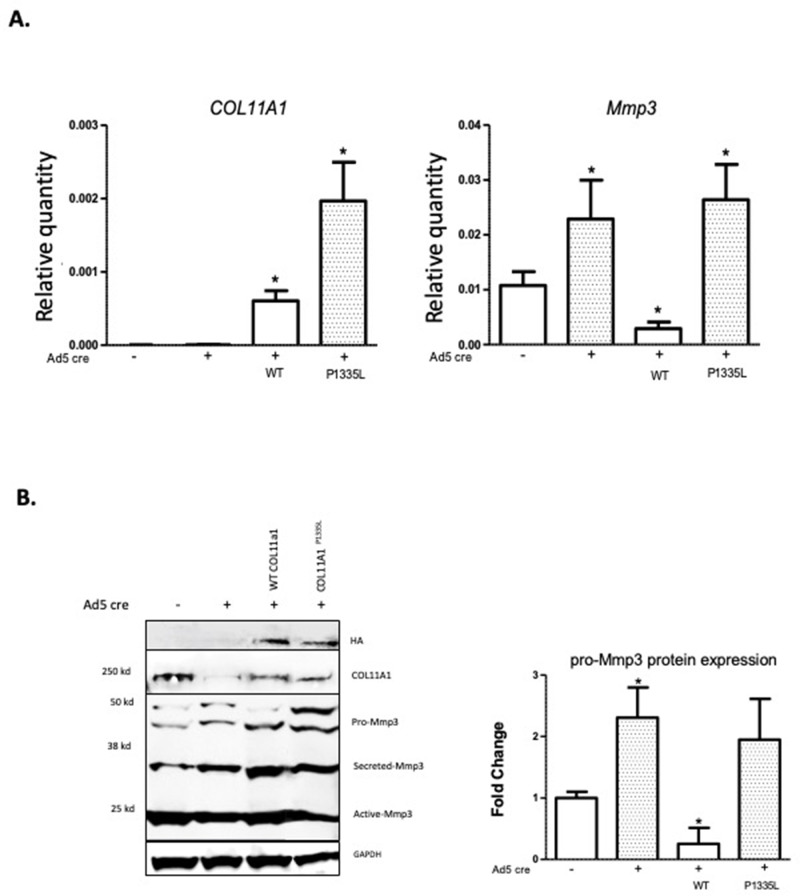
Although low-resolution structures currently available for collagen triple helices are not useful for modeling the effects of individual variants on protein stability, we noted that the AIS-associated variant P1335L occurs at the third position of a Gly-X-Y repeat and consequently could be structurally important in promoting stability of the triple helix, particularly if it is hydroxylated. We also noted that this variant is predicted to be deleterious by Combined Annotation Dependent Depletion (CADD) (Rentzsch et al., 2021) and Genomic Evolutionary Rate Profiling (GERP) (Cooper et al., 2005) analysis (CADD = 25.7; GERP = 5.75). Further, COL11A1 missense variants have been shown to evoke transcriptional changes in ECM genes in cancer cells (Lee et al., 2021). We therefore tested whether the COL11A1P1335L sequence variant alters its regulation of Mmp3 in chondrocytes. For this, SV40-immortalized cell lines were established from Col11a1fl/fl mouse costal chondrocytes and transduced with lentiviral vectors expressing green fluorescent protein (GFP) and COL11A1wt, COL11A1P1335L, or vector alone. After transduction, GFP-positive cells were grown to 50% confluence and treated with Cre-expressing adenovirus (ad5-Cre) to remove endogenous mouse Col11a1 (Figure 5A). Using a human-specific COL11A1 qRT-PCR assay, we detected overexpression of COL11A1wt and COL11A1P1335L compared to untransduced cells regardless of Cre expression (Figure 5A). Western blotting with an antibody directed against the HA epitope tag confirmed overexpression of human collagen α1(XI) protein (Figure 5B). Endogenous Mmp3 mRNA and protein upregulation was evident by qRT-PCR and western blotting, respectively, in untransduced cells treated with Ad5-Cre, as expected. Overexpressing human wild-type COL11A1 suppressed Mmp3 expression, consistent with the negative regulation we previously observed (Figure 5A and B). However, the COL11A1P1335L mutant failed to downregulate Mmp3 expression despite being overexpressed (Figure 5A and B). Thus, regulation of Mmp3 appeared to be perturbed in the presence of the COL11A1P1335L variant in these cells.
Figure 5. Col11a1P1335L regulation of Mmp3 expression in lentiviral transduced mouse GPCs.
(A) Quantitative real-time PCR (qRT-PCR) of human COL11A1 and endogenous mouse Mmp3 in SV40-immortalized mouse costal chondrocytes transduced with the lentiviral vector only (lanes 1,2), human wild-type (WT) COL11A1 (lane 3), or COL11A1P1335L. Values represent the ratio of each gene expression to that of GAPDH, and values are mean ± standard deviation. Significant quantitative changes (p≤0.05) relative to vector-only transfected cells as measured by unpaired t-tests are shown by *. Results shown for N=4 biologic replicates, each including three technical replicates. (B) Western blot corresponding to experiments shown in (A) using HA antibody to detect epitope-tagged human collagen a1(XI), COL11A1 antibody to detect mouse and human collagen a1(XI), MMP3 antibody to detect endogenous mouse MMP3, and GAPDH. Values are mean after normalization to GAPDH, ± standard deviation. Significant differences (p≤0.05) relative to vector-only, Ad5-negative transfected cells as measured by unpaired t-tests are shown by *.
Col11a1 and Mmp3 are responsive to estrogen receptor signaling in chondrocytes
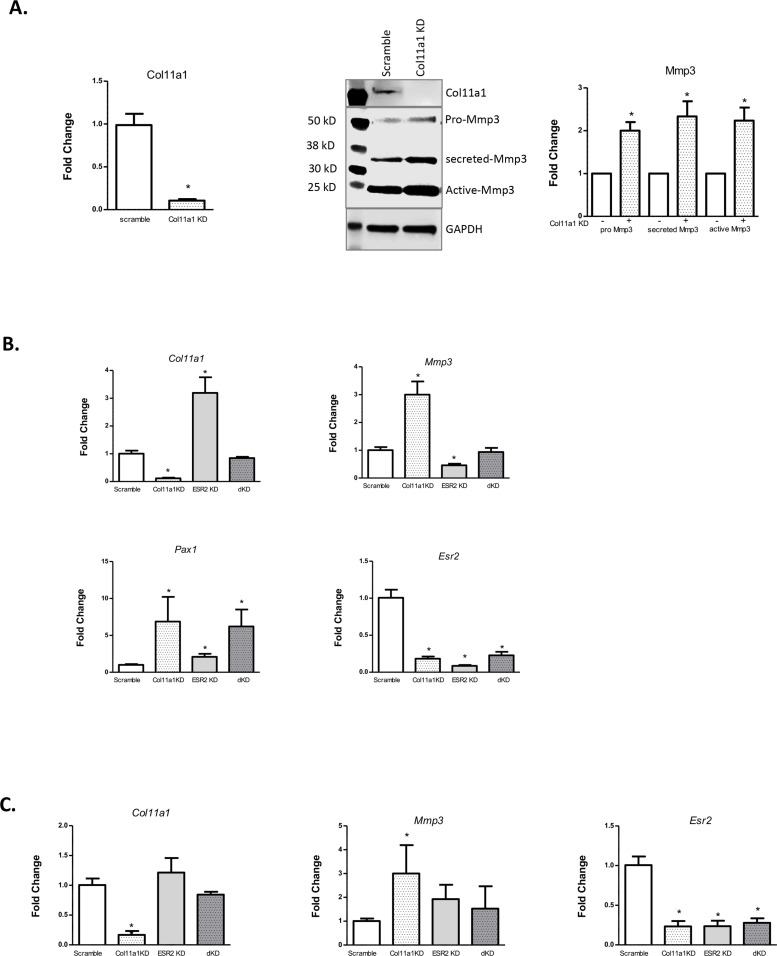
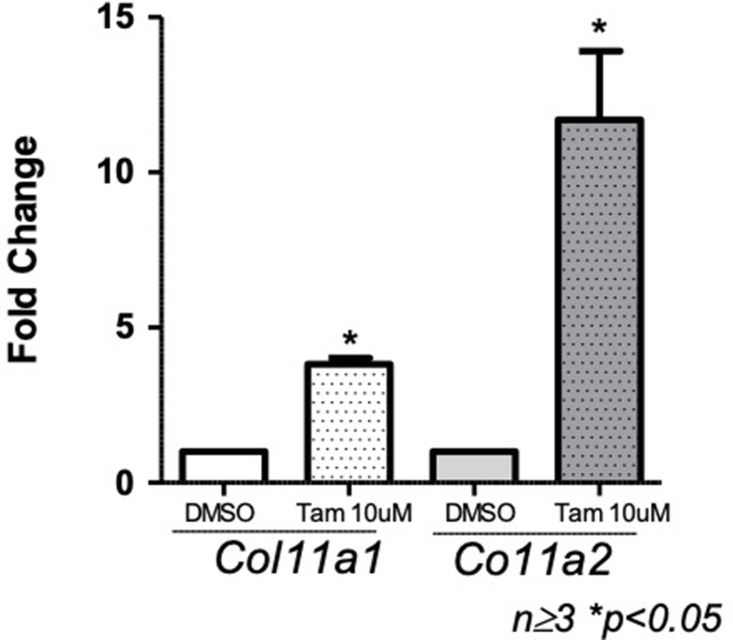
The expression of Col11a1, and of other ECM genes, is known to be estrogen- responsive in certain tissues, such as ovarian follicular cells (Zalewski et al., 2012). Because of the suspected role of endocrine hormones in AIS, we investigated whether Col11a1 expression was responsive to estrogen receptor siRNA-mediated knockdown in cultured chondrocytes. We first validated that Mmp3 mRNA and protein levels were significantly increased after Col11a1 knockdown in wild-type chondrocytes, as observed by Cre-mediated deletion in Col11a1fl/fl chondrocytes (Figure 6A). Estrogen receptor 2 (Esr2), but not estrogen receptor alpha (Esr1), was detected in mouse chondrocytes by qRT-PCR (data not shown). We therefore tested the consequences of Esr2 siRNA-mediated knockdown on gene expression in chondrocytes. After Esr2 knockdown, Col11a1 as well as Pax1 was significantly upregulated compared to scramble control, while Mmp3 expression was significantly downregulated (Figure 6B). We also performed Col11a1 knockdowns in these cells and noted upregulation of Pax1 expression, suggesting a negative feedback loop between Pax1 and Col11a1 in these cells (Figure 6B). Simultaneous knockdown of Col11a1 and Esr2 expression reduced Mmp3 expression to normal levels, supporting a possible interaction between Col11a1 and Esr2 in regulating Mmp3. Treating chondrocytes with tamoxifen, an estrogen receptor modulator, also upregulated Col11a1 expression to similar levels as observed after Esr2 knockdown, compared to cells treated with DMSO carrier (Figure 6—figure supplement 1). These results suggest that estrogen signaling suppresses Col11a1 expression. In cultured rat CEP cells, Esr2 mRNA was downregulated, and Mmp3 mRNA was upregulated after Col11a1 knockdown, as observed in mouse chondrocytes (Figure 6C, Figure 6—figure supplement 2). However, Esr2 knockdown did not significantly impact Col11a1 or Mmp3 expression in these cells (Figure 6C). Hence, we conclude that in cultured mouse chondrocytes, ESR2 signaling disrupts the suppression of Mmp3 by Col11a1.
Figure 6. Effects of estrogen receptor beta on Col11a1-Mmp3 signaling axis.
(A) RT-qPCR (left) of Col11a1 expression after siRNA-mediated knockdown as shown at left. Representative western blot (of N=4 biologic replicates) of cultured costal chondrocytes after scramble or Col11a1-specific siRNA knockdown is shown in middle. Protein size ladder is shown in lane 1. Quantification of bands detected by western blotting is shown at right, where scramble results were set to 1.0. Values are mean after normalization to GAPDH, ± standard deviation. (B) Gene expression levels of Col11a1, Mmp3, Pax1, and Esr2 mRNA in cultured costal chondrocytes showing fold change relative to the scramble control. dKD = double Col11a1-Esr2-specific siRNA knockdowns. Each value represents the ratio of each gene expression to that of GAPDH, and values are mean ± standard deviation. Results are representative of N≥3 biologic replicates, each including three technical replicates. (C) Gene expression levels from rat cartilage endplate (CEP) cells, as described in (B).
Figure 6—figure supplement 1. Quantitative real-time PCR (qRT-PCR) of Col11a1 and Col11a2 mRNA in cultured costal chondrocytes treated with DMSO carrier or tamoxifen (N≥3 independent experiments).
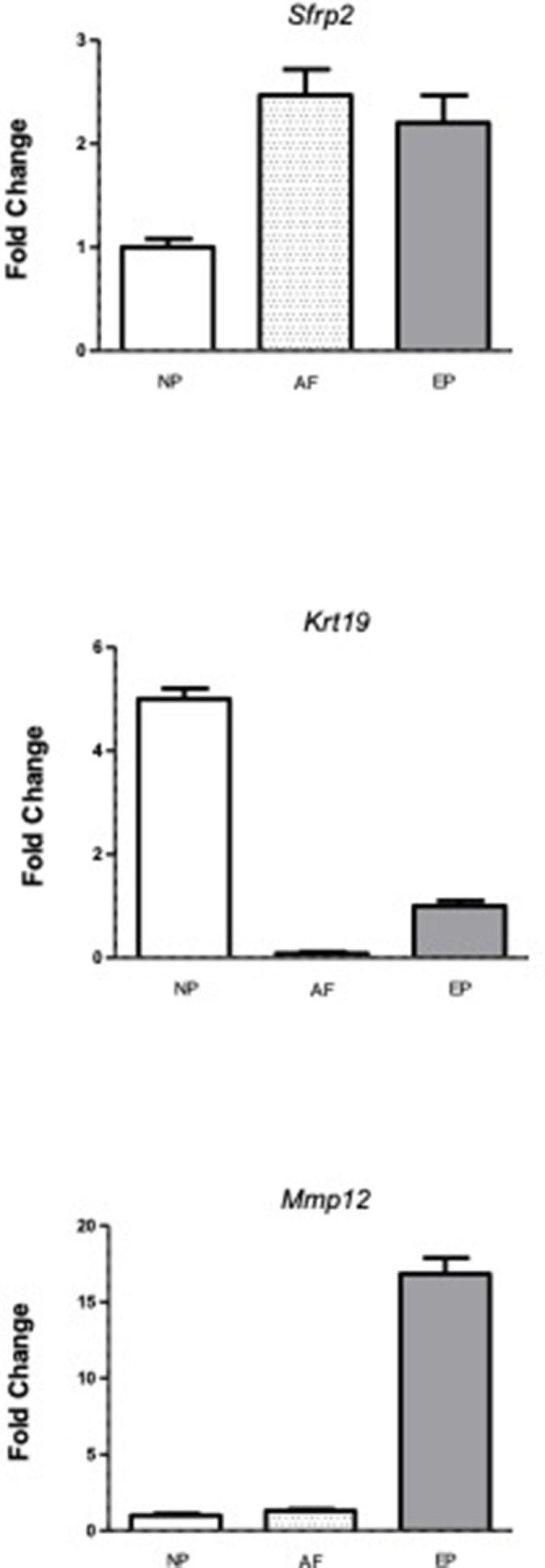
Figure 6—figure supplement 2. Quantitative real-time PCR (qRT-PCR) of Sfrp2, Krt19, and Mmp12 mRNA to validate expression of these marker genes in cultured rat nucleus pulposus (NP), annulus fibrosus (AF), and cartilage endplate (CEP) cells.
Discussion
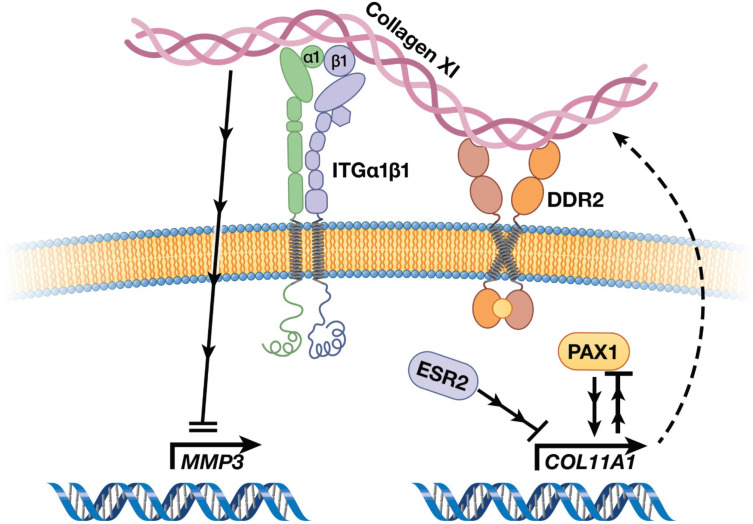
AIS has been described in the medical literature for centuries, yet its underlying etiology has remained enigmatic (Wise and Sharma, 2010). Given that AIS originates in children who appear to be otherwise healthy, even its tissue of origin has been difficult to discern, and long debated (Wise et al., 2020). The advent of powerful genotyping and sequencing methods in the last two decades has led to breakthrough discoveries of genetic loci associated with AIS, most in non-coding regions of the genome that are difficult to interpret biologically (Wise et al., 2020). Aggregating these results, however, provided supportive evidence that pathways of cartilage and connective tissue ECM development are relevant in AIS etiology (Wise et al., 2020; Khanshour et al., 2018). Here, in the largest multi-ethnic human cohort studied to date, we elected to test the hypothesis that alterations in ECM proteins themselves contribute to AIS susceptibility. This approach yielded most significant evidence for a common protein-altering variant in the COL11A1 gene encoding collagen α1(XI), a minor yet critical component of cartilaginous ECM. Moreover, our studies define a COL11A1-mediated disease pathway (Figure 7) and point to the chondro-osseous junction of IVD and vertebrae spine as a relevant cellular compartment in AIS etiology.
Figure 7. Cartoon depiction of a collagen XI-mediated signaling axis in chondrocytes.
Collagen XI is held in the pericellular space by integrins and DDR2. COL11A1, under the regulation of ESR2 and PAX1, signals through unknown mechanisms and inhibits MMP3 transcription.
The results of this study together with the previous observation of COL11A2 rare variant enrichment in AIS support a role for the collagen α1(XI) heterotrimer itself in its pathogenesis (Haller et al., 2016). Collagen type XI, composed of three chains encoded by the COL11A1, COL11A2, and COL2A1 genes (OMIM #s 120280,120290, 120140, respectively), is a minor component of collagen type II fibrils that are abundant in cartilage. Collagen type XI is also broadly expressed in testis, trachea, tendons, trabecular bone, skeletal muscle, placenta, lung, brain neuroepithelium, the vitreous of the eye, and IVDs (Yoshioka et al., 1995). In the pericellular space, collagen α1(XI) initiates fibrillogenesis with collagen type II fibrils, maintaining regular spacing and diameter of the collagen fibrils, while organizing the pericellular surface by interaction with cartilage proteoglycans (Smith et al., 1989; Luo and Karsdal, 2019). Purified human collagen type XI, when added back to chondrocytes in in vitro culture, stimulates chondrogenesis while inhibiting hypertrophy, as measured by histological staining, proliferation assays, and relative expression of chondrogenic early marker genes (Li et al., 2018). In newborn and 1-month-old mice, we found that collagen α1(XI) was abundant in IVD and at the chondro-osseous junction of IVD and vertebrae, particularly concentrated in pre-hypertrophic/hypertrophic chondrocytic cells. In long bone growth plates, Long et al., 2022, recently identified eight distinct cell clusters after unsupervised analysis of single cell (scRNAseq) of flow-sorted hypertrophic chondrocytes from Col10a1Cre;Rosa26fs-tdTomato mice. At E16.5, Col11a1 expression was highest in cells with signatures of pre-hypertrophic to hypertrophic transition, and lowest in cells with osteogenic signatures (M Hilton, personal communication) (Long et al., 2022). Taken together, these results suggest that collagen α1(XI) normally participates in maintaining growth plate cells in a hypertrophic, pre-osteogenic state, although little is known about its precise molecular function in that compartment, or in the IVD, during spinal development. Spines of Col11a1-deficient mice (cho/cho) show incompletely formed vertebral bodies, spinal curvatures, and decreased separation between vertebrae, which are themselves less mineralized than in wild-type mice (Hafez et al., 2015). Notably, common COL11A1 variants also have been associated with adult lumbar disc herniation and lumbar disc degeneration, as well as DXA-measured bone size, spinal stenosis, and spondylolisthesis (Jiang et al., 2017; Mio et al., 2007; Styrkarsdottir et al., 2019). Although gain-of-function or dominant-negative effects of the rs3753841 variant would not have been revealed in our assays, the spinal deformity noted in the cho/cho loss-of-function model, and failure of missense variants in Col11a2 to rescue congenital scoliosis (Rebello et al., 2023), leads us to surmise that reduction in the components of collagen type XI disrupts spinal development.
Pax1 is a well-described marker of early spine development, where it activates a gene expression cascade starting at E12.5–13.5 in mouse development (Wilm et al., 1998; Rodrigo et al., 2003; Sivakamasundari et al., 2017). Our data showed that loss of Pax1 leads to decreased expression of Col11a1, Sox6, and Adgrg6 in E12.5 tails of both male and female mice. The downregulation of Col11a1 is consistent with a prior study of gene expression in flow-sorted GFP-labeled Pax1-/- embryonic IVD cells (Sivakamasundari et al., 2017). However, from these experiments we cannot discern if Pax1 directly regulates Col11a1 in cis, or by an indirect effect. It is likely, however, that Col11a1 expression in developing tail is directly activated by binding SOX transcription factors, as a prior genomic study using chromatin immunoprecipitation and sequencing in rat chondrosarcoma cells identified super enhancers near the Col11a1 gene that were bound multiple times by SOX9 and SOX6 (Liu and Lefebvre, 2015). The SOX5/6/9 trio is known to regulate many of the same genes as PAX1 (Sivakamasundari et al., 2017), but whether this includes Col11a1 is unknown.
In mouse postnatal spines, we observed co-localization of collagen α1(XI) and PAX1 proteins specifically within the cartilaginous endplate-vertebral junction region that includes the vertebral growth plate. The endplate, which is important as the site allowing diffusion of nutrients from the circulation into the avascular inner IVD, harbors subpopulations of cells expressing type II collagen presumably organized by collagen type XI (Chan et al., 2014; Smith et al., 2011). While the endplate is continuous with the vertebral growth plate in mice, it is important to note that in humans the endplate and vertebrae become distinctly separate structures with closure of the growth plates at puberty (Chan et al., 2014). This is also the site of the ring apophyses that form the insertion of the IVD into vertebrae (Costa et al., 2021). Lagging maturity of the ring apophysis, combined with mechanical forces across the IVD in the horizontal plane, has been proposed as an initiating factor leading to rotatory decompensation in the adolescent spine in AIS (Costa et al., 2021; Castelein et al., 2020). Recently, Sun et al. reported the discovery of a vertebral skeletal stem cell (vSSC) residing in the endplate and marked by expression of the genes Zic1 and Pax1, along with other cell surface markers (Sun et al., 2023). These vSSCs also express high levels of Col11a1 (M Greenblatt, personal communication). It is interesting to consider that AIS-associated variation in collagenα1(XI), perhaps together with mechanical forces, could alter the differentiation trajectory of this cell niche. Altogether, extant data and our results strongly suggest that cell populations at the IVD-vertebral junction region are relevant in AIS pathogenesis. Further investigation is warranted to understand the developmental programs of cells in this region of the spine.
Matrix metalloproteinase 3, also known as stromolysin, is a secreted enzyme expressed in connective tissues and in regions of endochondral ossification (Ortega et al., 2004). MMP3 has degradative activity toward a variety of ECM components, including proteoglycans, fibronectin, laminin, but notably not type I collagen (Sellers and Murphy, 1981). Additionally, in chondrocytes MMP3 also has been shown to translocate to the nucleus, where it activates transcription of connective tissue growth factor (CTGF/CCN2) by binding to an element known as transcription enhancer dominant in chondrocytes (TRENDIC) (Eguchi et al., 2008; Cui et al., 2017). Our observations of a Col11a1-Mmp3 signaling axis in chondrocytes and CEP cells raise the possibility that Col11a1 variation may have consequences for both MMP3 enzymatic activity levels and MMP3-mediated transcriptional programming in these cells. COL11A1 missense variants, usually altering glycine or proline in Gly-X-Y repeats in the collagen α1(XI) helical domain as with COL11A1P1335L, are reported to be frequent in cutaneous squamous cell carcinomas and have been linked to transcriptional changes and tumor invasiveness (Lee et al., 2021). The mechanisms by which chondrocytes or other cells sense such single amino acid changes in collagen α1(XI) and induce subsequent transcriptional responses are unknown but may involve direct interaction with integrins in the pericellular space (Lee et al., 2021).
We found that Col11a1 expression is sensitive to estrogen receptor blockade or knockdown in chondrocytes. Type XI collagen is also a key player in organizing the pericellular space, which is critical for transmitting mechanical forces from the ECM to the cell (Xu et al., 2016). Thus, it is interesting to consider that type XI collagen may effectively act as a receptor for environmental cues, i.e., mechanical forces and estrogen signaling, in the adolescent spine. Our study provides new insights into the regulation and signaling role of Col11a1 in chondrocytes, and it suggests potential mechanisms by which its genetic variation contributes to AIS susceptibility.
Methods
Discovery study
The cases in the discovery stage (USA TX: n=1358) were recruited at Scottish Rite for Children. Informed consent to participate in this research was obtained as approved by the Institutional Review Board of the University Texas Southwestern Medical Center, protocol STU 112010-150. Subjects were genotyped on the Illumina HumanCoreExome BeadChip (Illumina, San Diego, CA, USA). For controls, we utilized 12,507 non-AMD GRU (non-age-related macular degeneration general research use) subjects of the European ancestry downloaded from dbGaP website (https://www.ncbi.nlm.nih.gov/gap/) from the International Age-Related Macular Degeneration Genomics Consortium study (IAMDGC: phs001039.v1.p1.c1). The subjects from the IAMDGC study were also genotyped on the Illumina HumanCoreExome Beadchip-24v1.0 platform (Fritsche et al., 2016). We merged cases and controls and applied quality controls to the genotypes for 468,801 overlapping SNPs using PLINK.1.9 (Chang et al., 2015) as described in Khanshour et al., 2018. In summary, samples with sex inconsistencies or from duplicated or related individuals or ancestral outliers as identified by principal component analysis (PCA) were removed, leaving 13,865 samples in the analysis. Genotypes were corrected for strand direction, and SNPs with call-rate per marker <95%, deviating from Hardy-Weinberg equilibrium (cutoff p-value = 10–4), or with significant missingness rate between cases and controls (cutoff p-value = 10–4) were removed, leaving 341,759 SNPs in the analysis. Genotypes for SNPs across autosomal chromosomes were imputed using Minimac3 with the 1000G-Phase3.V.5 reference panel as described in the instructions available from the software website (Das et al., 2016). Protein-coding changes were annotated with ANNOVAR using RefSeq-based transcripts (Wang et al., 2010). External databases included allele frequencies from gnomAD (Karczewski et al., 2020) variant pathogenicity in Clinvar (Landrum et al., 2018); CADD scores (Rentzsch et al., 2019) GERP scores (Davydov et al., 2010), and protein domains in IntroPro (Blum et al., 2021). Only bi-allelic common (MAF > 0.01) protein-altering SNPs with imputation quality Rsq ≥ 0.3 within matrisome genes (Naba et al., 2016) were included for further analysis. Matrisome genes used can be found in the Molecular Signature Database (MsigDB) (Subramanian et al., 2005; Liberzon et al., 2015; https://www.gsea-msigdb.org/gsea/msigdb/cards/NABA_MATRISOME). Genetic association for the imputed allele dosages in the discovery cohort (USA TX) was performed in Mach2dat (Li et al., 2009) using logistic regression with gender and 10 principal components (PCs) as covariates. The genomic regions of the associated loci were visualized with LocusZoom software (Pruim et al., 2010) utilizing linkage disequilibrium information from 1000 Genomes EUR populations.
Meta-analysis study
For the meta-analysis stage we utilized four cohorts – USA MO: n=2951 (1213 cases and 1738 controls), Swedish-Danish populations (SW-D: n=4627 [1631 cases and 2996 controls]), Japan (JP: n=79,211 [5327 cases and 73,884 controls]), and Hong Kong (HK: n=3103 [990 cases and 2113 controls]) – to check significant candidates from the discovery study. Summary statistics across the discovery study and the four replication cohorts (total N=103,757 [10,519 cases and 93,238 controls]) were combined as previously described (Khanshour et al., 2018) using METAL (Willer et al., 2010).
SW-D cohort
All patients provided written informed consent. Patients were recruited according to protocols approved by the institutional review boards in Stockholm (protocol #290/202906, #2009/1124-31/2, #2012/1595-31/2), Lund (protocol #LU 200-95, #LU 280-99, #LU 363-02, #567/2008, #2014/804), and Southern Denmark (protocol #S-2011002). Genotyping and analyses were performed as described in Khanshour et al., 2018; Ameur et al., 2017.
USA MO cohort
Whole exome sequencing data from 1213 unrelated idiopathic scoliosis cases of European ancestry with spinal curvature greater than 10-degree Cobb angle were derived from the Adolescent Idiopathic Scoliosis 1000 Exomes Study (dbGAP accession number: phs001677), and patients recruited from St. Louis Children’s Hospital, and St. Louis Shriners Hospital for Children. Patients and/or parents provided consent to participate in the study, and IRB approval was obtained from Washington University (protocol #201102118). For controls, exome data from 1738 unrelated samples of European ancestry were provided by investigators at Washington University School of Medicine in St. Louis, MO (dbGAP accession numbers: phs000572.v8.p4 and phs000101.v5.p1), and Oregon Health & Science University in Portland, OR (https://gemini.conradlab.org/). Exome data were aligned to the human genome reference (GRCh37) using BWA-MEM (v0.7.15). Variant calling of single nucleotide variants and insertion and deletion variants were generated first for each single sample in cases and controls and then combining all samples with joint genotyping method, described in GATK Best-Practices (Genome Analysis Toolkit [GATK v3.5] https://gatk.broadinstitute.org/hc/en-us/sections/360007226651-Best-Practices-Workflows). All cases and controls samples were classified as unrelated and of European ancestry using relationship inference (Manichaikul et al., 2010) and PCA (Chang et al., 2015). Association analysis of variants rs3753841 and rs1042704 were performed using logistic regression adjusting for sex and PCs in PLINK (Chang et al., 2015).
JP cohort
Informed consents were obtained from all the subjects or their parents, and the ethics committee of the Keio University Hospital, Tokyo, approved the study protocol (approved protocol #20080129). 5327 case subjects were recruited from collaborating hospitals (Japanese Scoliosis Clinical Research Group) as previously described (Kou et al., 2019). For controls, 73,884 subjects were randomly selected from the BioBank Japan Project, and subjects were genotyped on Illumina Human BeadChips as previously described (Ogura et al., 2015, Kou et al., 2013). Imputation and association analyses in JP were performed as previously described (de Klerk et al., 1988).
HK cohort
3103 subjects were recruited at The Duchess of Kent Children’s Hospital. Informed consent to participate in research was obtained as approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB approval number: UW 08-158). All 990 cases were characterized by Cobb angles greater than 40 degrees with onset age between 10 and 18 years. Congenital, neuromuscular, and syndromic scoliosis samples were excluded. We used 2113 controls from the Chinese population with no spinal deformities on MRI scans (Song et al., 2013). Cases and controls were genotyped using the Illumina Infinium OmniZhongHua-8 BeadChip and analyzed with GenomeStudio 2.0 software. The quality control approach adopted the GWA tutorial developed by Marees et al., 2018. The filtered genotyping data of cases and controls was phased and imputed using SHAPEIT (Delaneau et al., 2011) and IMPUTE2 (Howie et al., 2009), respectively. Logistic model association analysis was performed using PLINK 1.9 (Chang et al., 2015).
Stratification-by-sex test
To investigate sex specificity in the COL11A1 and MMP14 loci, we performed stratification-by-sex analysis in the discovery study (USA_TX). Association for the imputed allele dosages in rs3753841 and rs1042704 was computed separately for females (1157 cases and 7138 controls) using logistic regression with 10 PCs as covariates in Mach2dat (Li et al., 2009).
RNAseq of human tissues
RNAseq was performed as previously described (Makki et al., 2021). Read counting and transcript quantification were performed using HTSeq (Anders et al., 2015). Finally, reads were normalized using DESeq2 tools (Love et al., 2014) and TPM values were generated using the Kalisto pipeline (Bray et al., 2016).
Animal studies
Mouse and rat work was conducted per IACUC approved protocols at University of Texas Southwestern Medical Center (approved protocol #2016-101455) and University of California San Francisco (approved protocol #AN181381) and was in accordance with AALAC and NIH guidelines.
Generation of Pax1 knockout mice
Two gRNAs were designed to target the 5′ and 3′ ends of Pax1 gene (gRNA sequence shown in Figure 3—figure supplement 1) using the gRNA design tool on the Integrated DNA Technologies (IDT, Newark, NJ, USA) website and selected based on low off-target and high on-target scores. The knockout allele was generated using i-GONAD (Gurumurthy et al., 2019) as previously described (Ushiki et al., 2021).
To validate proper generation of the knockout, mice were analyzed by genotyping (with primers shown in Appendix 1), Sanger sequencing of PCR-amplified DNA, and southern blot (Figure 3—figure supplement 1). For southern blot analyses, genomic DNA were treated with NcoI (Cat #R0193, New England Biolabs, MA, USA) and fractionated by agarose gel electrophoreses. Following capillary transfer onto nylon membranes, blots were hybridized with digoxigenin (DIG)-labeled DNA probes (corresponding to chr2:147,202,083–147,202,444; mm9) amplified by the PCR DIG Probe Synthesis Kit (Cat #11636090910, Sigma-Aldrich, MO, USA). The hybridized probe was immunodetected with antidigoxigenin Fab fragments conjugated to alkaline phosphatase (Cat #11093274910, Sigma-Aldrich, MO, USA) and visualized with a CDP star (Cat #11685627001, Sigma-Aldrich, MO, USA) according to the manufacturer’s protocol. Chemiluminescence was detected using the FluorChem E (Cat #92-14860-00, ProteinSimple, CA, USA).
Col11a1fl/fl and ATC mice
The Col11a1fl/fl mouse line (Sun et al., 2020) was kindly provided by Dr. Lou Soslowsky with permission from Dr. David Birk. ATC mice (Dy et al., 2012) were kindly provided by Dr. Ryan Gray, with permission from Dr. Veronique Lefebvre.
Other mice
Cartilage was harvested from C57B/6 wild-type mice for siRNA-mediated knockdown experiments.
Histological methods
For thin cryostat sections, P0.5 mouse whole body was fixed in 4% paraformaldehyde (PFA) for 6 hr followed by 10% sucrose for 12 hr, then transferred to 18% sucrose for 24 hr. Tissues were then embedded in optimal cutting temperature compound (OCT) and sectioned using low-profile blades on a Thermo Shandon Cryostar NX70 cryostat and all sections were lifted on APES clean microscope slides. For whole mount images, samples were treated similarly with the addition of 2% polyvinylpyrrolidone (PVP) during the cryoprotection step and frozen in 8% gelatin (porcine) in the presence of 20% sucrose and 2% PVP. Samples were sectioned at a thickness of 10 μm. Slides were stored at –80°C until ready for use. For P28 and older mice, spines were removed then fixed, decalcified, and embedded in OCT. Spines were processed by making 7 µm thick lateral cuts the length of the spine.
Collagen α1(XI) was detected by IHC staining using affinity-purified antisera against peptide (C) YGTMEPYQTETPRR-amide (Genescript, NJ, USA) as described (Sun et al., 2020), and secondary horseradish peroxidase (HRP)-conjugated affinity-purified secondary antibody (Cat #AP187P, MilliporeSigma Aldrich, MO, USA). Briefly, frozen sections were equilibrated to room temperature for 1 hr, then fixed with 4% PFA in PBS at 4°C for 20 min. Slides were washed, treated with 3% H2O2 in methanol for 10 min to block endogenous peroxidase, washed, and transferred to PBS with 0.05% Tween 20 (Cat #P3563-10PAK, Sigma-Aldrich, MO, USA) pH 7.4. Slides were blocked with 0.5% goat serum in PBS mix with 0.2% Triton 100 (Cat #T8787, Sigma-Aldrich, MO, USA) at room temperature for 1.5 hr. The primary collagen α1(XI) affinity-purified antibody was applied at 0.40 mg/ml and slides were incubated overnight at 4°C. Afterward slides were washed in PBS Tween 20 for three times and treated with goat anti-rabbit-HRP for 1.5 hr, then washed three times in PBS Tween 20. After applying 3,3’-diaminobenzidine solution, slides were washed and counterstained with Mayer’s hematoxylin (Cat #MHS80, Sigma-Aldrich, MO, USA), washed, dehydrated, and mounted.
For collagen α1(XI) and PAX1 IF studies, P0.5 mice, P28 spine, and ribs sections were fixed in 4% PFA for 20 min then washed with PBS + 0.1% Triton three times, before incubation with 10% normal goat serum in PBS + 0.1% Triton for 30 min to block the background. Slides were incubated with goat anti-mouse collagen α1(XI) antibody at 1:500 dilution and mouse anti-rat PAX1(Cat #MABE1115M, Sigma-Aldrich, MO, USA), in PBS + 0.1% Triton + 1% normal goat serum at 4°C overnight. Secondary antibodies used were 1:5000 anti-rat Alexa488 and anti-mouse Alexa594-conjugated antibodies (Cat #A32740 Invitrogen, CA, USA). The sections were mounted using ProLong Gold with DAPI (Cat #S36964 Invitrogen, CA, USA) for imaging as described (Yu et al., 2018). All images were taken with Carl Zeiss Axio Imager.M2 fluorescence microscope (Zeiss, Oberkochen, DE).
Rib cartilage and IVD cell culture
All cell culture experiments utilized primary cells. Cell cultures were negative for mycoplasma contamination as determined by random, monthly testing. Mouse costal chondrocytes were isolated from the rib cage and sternum of P0.5 mice. Rat IVD was removed intact from 1-month female rats and immediately separated into NP, AF, and CEP isolates. Subsequently, tissues were incubated and shaken with 2 mg/ml Pronase solution (Cat #10165921001 Sigma-Aldrich, Inc, St. Louis, MO, USA) for 1 hr, followed by 1.5 hr digestion with 3 mg/ml Collagenase D solution (Cat #11088882001 Sigma-Aldrich, Inc, St. Louis, MO, USA), then 5 hr digestion with 0.5 mg/ml Collagenase D solution before three times PBS wash. Filtered, dissociated cells were seeded in Dulbecco’s modified Eagle’s medium (DMEM; Cat #MT15017CV Thermo Fisher Scientific, MA, USA) containing 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100 IU/ml penicillin. Remaining cartilage tissues underwent further digestion in 86 U/ml type 1 collagenase (Cat #SCR103 Sigma-Aldrich, Inc, St. Louis, MO, USA) overnight. Cells were collected and cultured in DMEM with 10% FBS plus 100 μg/ml streptomycin and 100 IU/ml penicillin.
SV40 immortalization and transfection of primary chondrocytes
Col11a1fl/fl mouse costal chondrocytes were isolated from the rib cage and sternum of P0.5 mice. The cells were transduced with pRRLsin-sv40 T antigen-IRES-mCherry lentivirus (Jha et al., 1998) for 48 hr, then sorted for mCherry-positive cells by flow cytometry. mCherry-positive cells were then infected with plv-eGFP, plv-eGFP-COL11A1-HA, plveGFP-COL11A1P1335L-HA constructs. After expansion, GFP-positive cells were sorted by flow cytometry and seeded in 24-well plates.
Adenovirus treatment
SV40-induced Col11a1fl/fl mouse costal chondrocytes were grown to 50% confluency. Afterward, cells were treated with 2 µl Ad5-CMV-cre adenovirus (titer 1.8×1011 pfu/ml) and Ad5-CMV-eGFP (titer 1.65×1010 pfu/ml) as control. Both virus strains were from the Gene Vector Core facility, Baylor College of Medicine. After 48 hr the cells were harvested for mRNA and protein lysate.
RNAseq and qRT-PCR
For Pax1 knockout studies, total RNA was collected from E12.5 tails using TRIzol (Cat #15596026, Thermo Fisher Scientific, MA, USA) and converted to cDNA using ReverTra Ace qPCR-RT master mix with genomic DNA remover (Cat #FSQ-301, Toyobo, Osaka, Japan). Sequencing was done using an Illumina Novaseq platform and the data were analyzed using Partek Flow (version 10.0) and gene ontology (Ashburner et al., 2000). qPCR was performed using SsoFast EvaGreen supermix (Cat #1725205, Bio-Rad, CA, USA). Primer sequences used for qPCR are shown in Appendix 1.
To quantify the expression level of Col11a1, Mmp3, and marker genes in IVD compartments and rib cartilage, cultured cells were collected in RNeasy (QIAGEN, Inc) for RNA purification. Taqman Reverse Transcription Kit (Cat #4387406 Thermo Fisher Scientific, MA, USA) was used to reverse-transcribe mRNA into cDNA. Following this, RT-qPCR was performed using a Power SYBR Green PCR Master Mix Kit (Cat #1725271, Bio-Rad, CA, USA). The primer sequences for the genes used in this study are listed in Appendix 1. Gene expression was calculated using the ΔΔCT method after normalizing to GAPDH.
siRNA knockdown
Mouse rib cartilage cells seeded in six-well plates were 60–80% confluent at transfection. Lipofectamine RNAiMAX reagent (Cat #13778030 Thermo Fisher, Waltham, MA, USA) was diluted (9 µl in 500 µl) in Opti-MEM Medium (Cat #31985070 Thermo Fisher, MA, USA). 30 pmol siRNA was diluted in 500 µl Opti-MEM medium, then added to diluted Lipofectamine RNAiMAX Reagent. siRNA-lipid complex was added to cells after 5 min incubation at room temperature. Cells were harvested after 72 hr.
Western blotting
For MMP3 western blotting, a total of 30 µg protein mixed with SDS-PAGE buffer was loaded on 12% SDS-polyacrylamide gel for electrophoresis. For collagen α1(XI) western blotting, 50 µg protein mixed with SDS-PAGE buffer was loaded on 4–20% SDS-polyacrylamide gel. The separated proteins were then transferred to nitrocellulose membranes (Cat #77010 Thermo Fisher Waltham, MA, USA) at 100 V for 2–3 hr. The membrane was first incubated with blocking buffer containing 5% defatted milk powder, and then exposed to 0.1 mg/ml mouse anti-rabbit Mmp3 (Cat #ab214794 Abcam, Cambridge, MA, USA) or anti-rabbit Col11a1 (Cat #PA5-38888 Thermo Fisher, Waltham, MA, USA) overnight. The samples were then washed thoroughly with TBS buffer, followed by incubation with HRP-labeled anti-rabbit IgG secondary antibodies 1:5000 (Cat #32460 Thermo Fisher, Waltham, MA, USA) overnight. The membranes were then washed with TBS buffer. GAPDH was detected by a rabbit anti-mouse antibody (Cat #14C10 Cell Signaling, MA, USA) and used as the internal loading control.
Acknowledgements
We thank the patients and their families who participated in these studies. We are grateful to the clinical investigators who referred patients into the study from the Japan Scoliosis Clinical Research Group (JSCRG), and the Scottish Rite for Children Clinical Group (SRCCG). We are grateful for the Scoliosis and Genetics in Scandinavia (ScoliGeneS) study group for help with patient recruitment and analyses of the Swedish-Danish cohort. The names and affiliations for JSCRG, Scottish Rite for Children Clinical Group (SRCCG), ScoliGeneS are included in Appendix 2. We also thank Drs. Carlos Cruchaga, Sanjay Jain, Matthew Harms, and Don Conrad for allowing us to use exome data from their cohort studies. We are grateful to Dr. James Lupski and the Baylor-Hopkins Center for Mendelian Genomics for providing exome sequencing of AIS cases. The University of Pennsylvania is acknowledged for providing the Col11a1fl/fl mouse line. We thank Drs. D Burns for help in interpreting histological studies. We thank Dr. M Hilton for sharing scRNAseq data as described in reference (51). This study was funded by JSPS KAKENHI Grants (22H03207 to SI), the Swedish Research Council (number K-2013–52X-22198-01-3 and 2017-01639 to PG and EE), the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (to PG), Center for Innovative Medicine (CIMED), Karolinska Institutet (to PG), the Department of Research and Development of Vasternorrland County Council (to PG), and Karolinska Institutet research funds (to PG) and Research Grants Council of Hong Kong (No: 17114519 to YQS), The National Institutes of Health GM127390 (to NG) and the Eunice Kennedy Shriver National Institute of Child Health & Development of the National Institutes of Health (P01HD084387 to CAW and NA). Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The data used for the analyses described in the USA MO cohort were obtained from the database of Genotypes and Phenotypes (dbGaP) Adolescent Idiopathic Scoliosis 1000 Exomes Study (phs001677.v1.p1) which included investigators at Washington University in St. Louis (M Dobbs), Shriners Hospital for Children, St. Louis (M Dobbs), University of Colorado (N Miller), University of Iowa (S Weinstein and J Morcuende), University of Wisconsin (P Giampietro), and Hospital for Special Surgery (C Raggio). Sequencing of these samples was funded by NIH NIAMS 1R01AR067715 (M Dobbs and CG). Use of the dbGAP dataset phs001039.v1.p1 is gratefully acknowledged. All contributing sites and additional funding information for dbGAP dataset phs001039.v1.p1 are acknowledged in this publication: Fritsche et al. (2016) Nature Genetics 48 134–143, (doi:10.1038 /ng.3448); The International AMD Genomics consortium’s web page is: http://eaglep.case.edu/iamdgc_web/, and additional information is available on: http://csg.sph.umich.edu/abecasis/public/amd015/. The authors acknowledge the Texas Advanced Computing Center (TACC) at The University of Texas at Austin for providing computing resources that have contributed to the research results reported within this paper. URL: http://www.tacc.utexas.edu.
Appendix 1
siRNA and primer sequences
Appendix 1—table 1. RNA and DNA oligonucleotide primers used for siRNA knockdown, RT-qPCR, and genotyping experiments.
| Mouse Esr2 siRNA | CAAGUGUUACGAAGUAGGAdT |
| Mouse Col11a1 siRNA | GAAAGAAGGUGCAAAGGGUdT |
| Mouse Mmp3 F | CTCTGGAACCTGAGACATCACC |
| Mouse Mmp3 R | AGGAGTCCTGAGAGATTTGCGC |
| Mouse Col11a1 F | AGGAGAGTTGAGAATTGGGAATC |
| Mouse Col11a1 R | TGGTGATCAGAATCAGAAGTT |
| Mouse Col11a2 F | CTCATCTTCCTGCATCAGAC |
| Mouse Col11a2 R | ACTTGGAAAGCGAGGTCCT |
| Mouse Adgrg6 F | AGAGGATGGACTGAGGCTGTGT |
| Mouse Adgrg6 R | CCAGGCTTGTTTGGACATGGTTG |
| Mouse Sox6 F | GCATAAGTGACCGTTTTGGCAGG |
| Mouse Sox6 R | GGCATCTTTGCTCCAGGTGACA |
| Mouse Mmp14 F | GCCTTCTGTTCCTGATAA |
| Mouse Mmp14 R | CCATCCTTCCTCTCGTAG |
| Mouse Pax1 F | AACCAGCACGGAGTATACAGC |
| Mouse Pax1 R | TGTAAGCTACCGAGTGCATCC |
| Mouse Esr2 F | GGTCCTGTGAAGGATGTAAGGC |
| Mouse Esr2 R | TAACACTTGCGAAGTCGGCAGG |
| Mouse Gapdh F | CATCACTGCCACCCAGAAGACTG |
| Mouse Gapdh R | ATGCCAGTGAGCTTCCCGTTCAG |
| Rat Sfrp2 F | CGTGAAACGGTGGCAGAAG |
| Rat Sfrp2 R | CGGATGCTGCGGGAGAT |
| Rat Krt19 F | AAGACACACTGGCAGAAACG |
| Rat Krt19 R | GATTCTGCCGCTCACTATCA |
| Rat Mmp12 F | TTGGCCATTCCTTGGGGCTGC |
| Rat Mmp12 R | TGTTGGTGGCTGGACTCCCAGG |
| Mouse Pax1 F (Figure 5) | CCGCACATTCAGTCAGCAAC |
| Mouse Pax1 R (Figure 5) | CATCTTGGGGGAGTAGGCAG |
| Mouse Col11a1 F (Figure 5) | CACAAAACCCCTCGATAGAAGTG |
| Mouse Col11a1 R (Figure 5) | CCTGTGATCAGGAACTGCTGAA |
| Mouse Adgrg6 F (Figure 5) | TCCTGTCCATCTCTGGCTCA |
| Mouse Adgrg6 R (Figure 5) | CACAAGACAGAGCTGCTCCA |
| Mouse Sox6 F (Figure 5) | TGCGACAGTTCTTCACTGTGG |
| Mouse Sox6 R (Figure 5) | CGTCCATCTTCATACCATACG |
| Mouse β-Actin F (Figure 5) | GGCACCACACCTTCTACAATG |
| Mouse β-Actin R (Figure 5) | GGGGTGTTGAAGGTCTCAAAC |
| Pax1-genotyping F | CAGAACCTGGAATGCTGTGCTC |
| Pax1-genotyping R | AAAGGGTTGCAGTGCCTTCAC |
Appendix 2
Clinical groups
Scottish Rite for Children Clinical Group
Lori A Karol1, Karl E Rathjen1, Daniel J Sucato1, John G Birch1, Charles E Johnston III1, Benjamin S Richards1, Brandon Ramo1, Amy L McIntosh1, John A Herring1, Todd A Milbrandt2, Vishwas R Talwakar3, Henry J Iwinski3, Ryan D Muchow3, J Channing Tassone4, X-C Liu4, Richard Shindell5, William Schrader6, Craig Eberson7, Anthony Lapinsky8, Randall Loder9, and Joseph Davey10
Department of Orthopaedic Surgery, Texas Scottish Rite Hospital for Children, Dallas, Texas, USA
Department of Orthopaedic Surgery, Mayo Clinic, Rochester, Minnesota, USA
Department of Orthopaedic Surgery, Shriners Hospitals for Children, Lexington, Kentucky, USA
Department of Orthopaedic Surgery, Children's Hospital of Wisconsin, Milwaukee, Wisconsin, USA
OrthoArizona, Phoenix, Arizona, USA
Departments of Orthopedics, Sports Medicine, and Surgical Services, Akron Children’s Hospital, Akron, Ohio, USA
Pediatric Orthopaedics and Scoliosis, Hasbro Children’s Hospital, Providence, Rhode Island, USA
University of Massachusetts Memorial Medical Center, Worcester, Massachusetts, USA
Indiana University-Purdue University Indianapolis, Indianapolis, Indiana, USA
University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA
Japan Scoliosis Clinical Research Group
Kota Watanabe1, Nao Otomo1,2, Kazuki Takeda1,2, Yoshiro Yonezawa1,2, Yoji Ogura1,2, Yohei Takahashi1,2, Noriaki Kawakami3, Taichi Tsuji4, Koki Uno5, Teppei Suzuki5, Manabu Ito6, Shohei Minami7, Toshiaki Kotani7, Tsuyoshi Sakuma7, Haruhisa Yanagida8, Hiroshi Taneichi9, Satoshi Inami9, Ikuho Yonezawa10, Hideki Sudo11, Kazuhiro Chiba12, Naobumi Hosogane12, Kotaro Nishida13, Kenichiro Kakutani13, Tsutomu Akazawa14, Takashi Kaito15, Kei Watanabe16, Katsumi Harimaya17, Yuki Taniguchi18, Hideki Shigemats19, Satoru Demura20, Takahiro Iida21, Ryo Sugawara22, Katsuki Kono23, Masahiko Takahata24, Norimasa Iwasaki24, Eijiro Okada1, Nobuyuki Fujita1, Mitsuru Yagi1, Masaya Nakamura1, Morio Matsumoto1
Department of Orthopaedic Surgery, Keio University School of Medicine, Tokyo, Japan
Laboratory of Bone and Joint Diseases, Center for Integrative Medical Sciences, RIKEN, Tokyo, Japan
Department of Orthopaedic Surgery, Meijo Hospital, Nagoya, Japan
Department of Orthopaedic Surgery, Toyota Kosei Hospital, Nagoya, Japan
Department of Orthopaedic Surgery, National Hospital Organization, Kobe Medical Center, Kobe, Japan
Department of Orthopaedic Surgery, National Hospital Organization, Hokkaido Medical Center, Sapporo, Japan
Department of Orthopaedic Surgery, Seirei Sakura Citizen Hospital, Sakura, Japan
Department of Orthopaedic Surgery, Fukuoka Children’s Hospital, Fukuoka, Japan
Department of Orthopaedic Surgery, Dokkyo Medical University School of Medicine, Mibu, Japan
Department of Orthopaedic Surgery, Juntendo University School of Medicine, Tokyo, Japan
Department of Advanced Medicine for Spine and Spinal Cord Disorders, Hokkaido University Graduate School of Medicine, Sapporo, Japan
Department of Orthopaedic Surgery, National Defense Medical College, Tokorozawa, Japan
Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine, Kobe, Japan
Department of Orthopaedic Surgery, St. Marianna University School of Medicine, Kawasaki, Japan
Department of Orthopaedic Surgery, Osaka University Graduate School of Medicine, Suita, Japan
Department of Orthopaedic Surgery, Niigata University Hospital, Niigata, Japan
Department of Orthopaedic Surgery, Graduate School of Medical Sciences, Kyushu University Beppu Hospital, Fukuoka, Japan
Department of Orthopaedic Surgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
Department of Orthopaedic Surgery, Nara Medical University, Nara, Japan
Department of Orthopaedic Surgery, Kanazawa University School of Medicine, Kanazawa, Japan
Department of Orthopaedic Surgery, Dokkyo Medical University Koshigaya Hospital, Koshigaya, Japan
Department of Orthopaedic Surgery, Jichi Medical University, Simotsuke, Japan
Department of Orthopaedic Surgery, Kono Othopaedic Clinic, Tokyo, Japan
Department of Orthopaedic Surgery, Hokkaido University, Sapporo, Japan
Scoliosis and Genetics in Scandinavia study group
Tian Cheng1, Juha Kere2, Aina Danielsson3, Kristina Åkesson4, Ane Simony5, Mikkel Andersen5, Steen Bach Christensen5, Maria Wikzén6, Luigi Belcastro6
Department of Clinical Sciences and Technology, Karolinska Institutet, Huddinge, Sweden
Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden
Department of Orthopedics, Sahlgrenska University Hospital, Gothenburg, Sweden
Department of Orthopedics and Clinical Sciences, Lund University, Skane University Hospital, Malmö, Sweden
Sector for Spine Surgery and Research, Middelfart Hospital, Middelfart, Denmark
Department of Reconstructive Orthopaedics, Karolinska University Hospital, Stockholm, Sweden
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Carol A Wise, Email: Carol.Wise@tsrh.org.
Michel Bagnat, Duke University, United States.
Sofia J Araújo, University of Barcelona, Spain.
Funding Information
This paper was supported by the following grants:
Japan Society for the Promotion of Science 22H03207 to Shiro Ikegawa.
Swedish Research Council K-2013-52X-22198-01-3 to Elisabet Einarsdottir.
Swedish Research Council 2017-01639 to Elisabet Einarsdottir.
Regional Agreement on Medical Training and Clinical Research to Paul Gerdhem.
Center for Innovative Medicine to Paul Gerdhem.
Department of Research and Development of Vasternorrland County Council to Paul Gerdhem.
Research Grants Council, University Grants Committee 17114519 to You-qiang Song.
National Institutes of Health GM127390 to Nick V Grishin.
National Institutes of Health P01HD084387 to Carol A Wise.
National Institutes of Health 1R01AR067715 to Christina A Gurnett.
Additional information
Competing interests
No competing interests declared.
Author contributions
Investigation, Methodology, Writing – original draft.
Data curation, Formal analysis, Writing – original draft.
Investigation, Writing – original draft.
Formal analysis, Writing – review and editing.
Formal analysis.
Formal analysis, Writing – review and editing.
Formal analysis, Writing – review and editing.
Formal analysis, Writing – review and editing.
Formal analysis, Writing – review and editing.
Methodology, Writing – review and editing.
Investigation.
Investigation.
Formal analysis.
Formal analysis.
Methodology, Writing – review and editing.
Validation, Writing – review and editing.
Investigation, Writing – review and editing.
Supervision, Writing – review and editing.
Validation, Writing – review and editing.
Validation, Writing – review and editing.
Validation, Writing – review and editing.
Supervision, Writing – review and editing.
Supervision, Writing – review and editing.
Supervision, Writing – original draft.
Conceptualization, Supervision, Writing – original draft, Writing – review and editing.
Ethics
The cases in the discovery stage (USA TX: n=1,358) were recruited at Scottish Rite for Children. Informed consent to participate in this research was obtained as approved by the Institutional Review Board of the University Texas Southwestern Medical Center, protocol STU 112010-150. Cases in the replication cohort SW-D were recruited according to protocols approved by the institutional review boards in Stockholm (protocol #290/202906, #2009/1124-31/2, #2012/1595-31/2), Lund (protocol #LU 200-95, #LU 280-99, #LU 363-02, #567/2008, #2014/804), and Southern Denmark (protocol #S-2011002). Cases in the replication cohort USA-MO cohort were recruited from St. Louis Children's Hospital, and St. Louis Shriners Hospital for Children. Informed consent to participate in the study was obtained from each case as approved by the Washington University Institutional Review Board (protocol #201102118). Cases in the replication cohort JP provided informed consents as approved by the ethics committee of the Keio University Hospital, Tokyo (approved protocol #20080129). For the replication cohort HK, subjects were recruited at The Duchess of Kent Children's Hospital and provided informed consent to participate in research as approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Institutional Review Board approval number: UW 08-158).
Mouse and rat work was conducted per IACUC approved protocols at University of Texas Southwestern Medical Center (approved protocol #2016-101455) and University of California San Francisco (approved protocol #AN181381) and was in accordance with AALAC and NIH guidelines.
Additional files
Data availability
Summary data for GWAS3 is deposited in the NHGRI-EBI GWAS catalog (accession #GCST006902).
The following previously published datasets were used:
Khanshour AM, Kou I, Fan Y, Einarsdottir E, Makki N, Kidane YH, Kere J, Grauers A, Johnson TA, Paria N, Patel C, Singhania R, Kamiya N, Takeda K, Otomo N, Watanabe K, Luk KDK, Cheung KMC, Herring JA, Rios JJ, Ahituv N, Gerdhem P, Gurnett CA, Song YQ, Ikegawa S, Wise CA. 2018. Genome-wide meta-analysis and replication studies in multiple ethnicities identify novel adolescent idiopathic scoliosis susceptibility loci. GWAS catalog. GCST006902
Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Saïd S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanché H, Deleuze JF, Igo RP, Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA, Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NT, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CC, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JR, Allikmets R, Wang JJ, Schaumberg DA, Klein BE, Hagstrom SA, Chowers I, Lotery AJ, Léveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BH, Abecasis GR, Heid IM. 2016. International Age-Related Macular Degeneration Genomics Consortium - Exome Chip Experiment. NCBI. phs001039.v1.p1
Gurnett CA, Dobbs MB, Miller N, Morcuende J, Giampietro P, Raggio C, Wise C. 2019. Adolescent Idiopathic Scoliosis (AIS) 1000 Exomes Study. NCBI. phs001677.v1.p1
References
- Ameur A, Dahlberg J, Olason P, Vezzi F, Karlsson R, Martin M, Viklund J, Kähäri AK, Lundin P, Che H, Thutkawkorapin J, Eisfeldt J, Lampa S, Dahlberg M, Hagberg J, Jareborg N, Liljedahl U, Jonasson I, Johansson Å, Feuk L, Lundeberg J, Syvänen A-C, Lundin S, Nilsson D, Nystedt B, Magnusson PK, Gyllensten U. SweGen: a whole-genome data resource of genetic variability in a cross-section of the Swedish population. European Journal of Human Genetics. 2017;25:1253–1260. doi: 10.1038/ejhg.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq—a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A, Chan D, Hunziker E, Bateman JF, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. The Journal of Cell Biology. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Human Molecular Genetics. 2011;20:4041–4055. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschal EE, Wethey CI, Swindle K, Baschal RM, Gowan K, Tang NLS, Alvarado DM, Haller GE, Dobbs MB, Taylor MRG, Gurnett CA, Jones KL, Miller NH. Exome sequencing identifies a rare HSPG2 variant associated with familial idiopathic scoliosis. G3: Genes, Genomes, Genetics. 2014;5:167–174. doi: 10.1534/g3.114.015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, McSheene J, Loges NT, Olbrich H, Haeffner K, Fliegauf M, Horvath J, Reinhardt R, Nielsen KG, Marthin JK, Baktai G, Anderson KV, Geisler R, Niswander L, Omran H, Burdine RD. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nature Genetics. 2011;43:79–84. doi: 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Chang H-Y, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, Richardson L, Salazar GA, Williams L, Bork P, Bridge A, Gough J, Haft DH, Letunic I, Marchler-Bauer A, Mi H, Natale DA, Necci M, Orengo CA, Pandurangan AP, Rivoire C, Sigrist CJA, Sillitoe I, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Bateman A, Finn RD. The interpro protein families and domains database: 20 years on. Nucleic Acids Research. 2021;49:D344–D354. doi: 10.1093/nar/gkaa977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nature Biotechnology. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Castelein RM, Pasha S, Cheng JC, Dubousset J. Idiopathic scoliosis as a rotatory decompensation of the spine. Journal of Bone and Mineral Research. 2020;35:1850–1857. doi: 10.1002/jbmr.4137. [DOI] [PubMed] [Google Scholar]
- Chan WCW, Au TYK, Tam V, Cheah KSE, Chan D. Coming together is a beginning: the making of an intervertebral disc. Birth Defects Research. Part C, Embryo Today. 2014;102:83–100. doi: 10.1002/bdrc.21061. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Stone EA, Asimenos G, NISC Comparative Sequencing Program. Green ED, Batzoglou S, Sidow A. Distribution and intensity of constraint in mammalian genomic sequence. Genome Research. 2005;15:901–913. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L, de Reuver S, Kan L, Seevinck P, Kruyt MC, Schlosser TPC, Castelein RM. Ossification and fusion of the vertebral ring apophysis as an important part of spinal maturation. Journal of Clinical Medicine. 2021;10:3217. doi: 10.3390/jcm10153217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Progress in Molecular Biology and Translational Science. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh P-R, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C. Next-generation genotype imputation service and methods. Nature Genetics. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++ PLOS Computational Biology. 2010;6:e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk JB, Duran M, Dorland L, Brouwers HA, Bruinvis L, Ketting D. A patient with mevalonic aciduria presenting with hepatosplenomegaly, congenital anaemia, thrombocytopenia and leukocytosis. Journal of Inherited Metabolic Disease. 1988;11 Suppl 2:233–236. doi: 10.1007/BF01804244. [DOI] [PubMed] [Google Scholar]
- Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature Methods. 2011;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, Lefebvre V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Developmental Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T, Ibaragi S, Sasaki A, Kuboki T, Takigawa M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Molecular and Cellular Biology. 2008;28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes RJ, Weis M, Scott MA, Seegmiller RE, Eyre DR. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biology. 2007;26:597–603. doi: 10.1016/j.matbio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JNC, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HPN, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YTE, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GHS, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Saïd S, Sahel J-A, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanché H, Deleuze J-F, Igo RP, Jr, Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA, Jr, Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NTM, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CCW, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JRW, Allikmets R, Wang JJ, Schaumberg DA, Klein BEK, Hagstrom SA, Chowers I, Lotery AJ, Léveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BHF, Abecasis GR, Heid IM. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature Genetics. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Gordon D, Zhang D, Browne R, Helms C, Gillum J, Weber S, Devroy S, Swaney S, Dobbs M, Morcuende J, Sheffield V, Lovett M, Bowcock A, Herring J, Wise C. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. American Journal of Human Genetics. 2007;80:957–965. doi: 10.1086/513571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauers A, Rahman I, Gerdhem P. Heritability of scoliosis. European Spine Journal. 2012;21:1069–1074. doi: 10.1007/s00586-011-2074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy CB, Sato M, Nakamura A, Inui M, Kawano N, Islam MA, Ogiwara S, Takabayashi S, Matsuyama M, Nakagawa S, Miura H, Ohtsuka M. Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Nature Protocols. 2019;14:2452–2482. doi: 10.1038/s41596-019-0187-x. [DOI] [PubMed] [Google Scholar]
- Hafez A, Squires R, Pedracini A, Joshi A, Seegmiller RE, Oxford JT. Col11a1 regulates bone microarchitecture during embryonic development. Journal of Developmental Biology. 2015;3:158–176. doi: 10.3390/jdb3040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller G, Alvarado D, Mccall K, Yang P, Cruchaga C, Harms M, Goate A, Willing M, Morcuende JA, Baschal E, Miller NH, Wise C, Dobbs MB, Gurnett CA. A polygenic burden of rare variants across extracellular matrix genes among individuals with adolescent idiopathic scoliosis. Human Molecular Genetics. 2016;25:202–209. doi: 10.1093/hmg/ddv463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M, Gao X, Yu LX, Paria N, Henkelman RM, Wise CA, Ciruna B. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nature Communications. 2014;5:4777. doi: 10.1038/ncomms5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLOS Genetics. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko MT. Clinical practice: idiopathic scoliosis in adolescents. The New England Journal of Medicine. 2013;368:834–841. doi: 10.1056/NEJMcp1209063. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspectives in Biology. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe KM, Grimes DT, Schottenfeld-Roames J, Werner ME, Ku T-SJ, Kim SK, Pelliccia JL, Morante NFC, Mitchell BJ, Burdine RD. c21orf59/kurly controls both cilia motility and polarization. Cell Reports. 2016;14:1841–1849. doi: 10.1016/j.celrep.2016.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha KK, Banga S, Palejwala V, Ozer HL. SV40-Mediated immortalization. Experimental Cell Research. 1998;245:1–7. doi: 10.1006/excr.1998.4272. [DOI] [PubMed] [Google Scholar]
- Jiang H, Yang Q, Jiang J, Zhan X, Xiao Z. Association between COL11A1 (rs1337185) and ADAMTS5 (rs162509) gene polymorphisms and lumbar spine pathologies in Chinese Han population: an observational study. BMJ Open. 2017;7:e015644. doi: 10.1136/bmjopen-2016-015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD, Brand H, Solomonson M, Watts NA, Rhodes D, Singer-Berk M, England EM, Seaby EG, Kosmicki JA, Walters RK, Tashman K, Farjoun Y, Banks E, Poterba T, Wang A, Seed C, Whiffin N, Chong JX, Samocha KE, Pierce-Hoffman E, Zappala Z, O’Donnell-Luria AH, Minikel EV, Weisburd B, Lek M, Ware JS, Vittal C, Armean IM, Bergelson L, Cibulskis K, Connolly KM, Covarrubias M, Donnelly S, Ferriera S, Gabriel S, Gentry J, Gupta N, Jeandet T, Kaplan D, Llanwarne C, Munshi R, Novod S, Petrillo N, Roazen D, Ruano-Rubio V, Saltzman A, Schleicher M, Soto J, Tibbetts K, Tolonen C, Wade G, Talkowski ME, Genome Aggregation Database Consortium. Neale BM, Daly MJ, MacArthur DG. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karol LA, Johnston CE, Browne RH, Madison M. Progression of the curve in boys who have idiopathic scoliosis. The Journal of Bone & Joint Surgery. 1993;75:1804–1810. doi: 10.2106/00004623-199312000-00010. [DOI] [PubMed] [Google Scholar]
- Khanshour AM, Kou I, Fan Y, Einarsdottir E, Makki N, Kidane YH, Kere J, Grauers A, Johnson TA, Paria N, Patel C, Singhania R, Kamiya N, Takeda K, Otomo N, Watanabe K, Luk KDK, Cheung KMC, Herring JA, Rios JJ, Ahituv N, Gerdhem P, Gurnett CA, Song Y-Q, Ikegawa S, Wise CA. Genome-wide meta-analysis and replication studies in multiple ethnicities identify novel adolescent idiopathic scoliosis susceptibility loci. Human Molecular Genetics. 2018;27:3986–3998. doi: 10.1093/hmg/ddy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjikusic MJ, Yeetong P, Boswell CW, Lee C, Roberson EC, Ittiwut R, Suphapeetiporn K, Ciruna B, Gurnett CA, Wallingford JB, Shotelersuk V, Gray RS. Mutations in kinesin family member 6 reveal specific role in ependymal cell ciliogenesis and human neurological development. PLOS Genetics. 2018;14:e1007817. doi: 10.1371/journal.pgen.1007817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nature Genetics. 2013;45:676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- Kou I, Otomo N, Takeda K, Momozawa Y, Lu H-F, Kubo M, Kamatani Y, Ogura Y, Takahashi Y, Nakajima M, Minami S, Uno K, Kawakami N, Ito M, Yonezawa I, Watanabe K, Kaito T, Yanagida H, Taneichi H, Harimaya K, Taniguchi Y, Shigematsu H, Iida T, Demura S, Sugawara R, Fujita N, Yagi M, Okada E, Hosogane N, Kono K, Nakamura M, Chiba K, Kotani T, Sakuma T, Akazawa T, Suzuki T, Nishida K, Kakutani K, Tsuji T, Sudo H, Iwata A, Sato T, Inami S, Matsumoto M, Terao C, Watanabe K, Ikegawa S. Genome-wide association study identifies 14 previously unreported susceptibility loci for adolescent idiopathic scoliosis in Japanese. Nature Communications. 2019;10:3685. doi: 10.1038/s41467-019-11596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse LM, Buchan JG, Gurnett CA, Dobbs MB. Polygenic threshold model with sex dimorphism in adolescent idiopathic scoliosis: the carter effect. Journal of Bone and Joint Surgery. 2012;94:1485–1491. doi: 10.2106/JBJS.K.01450. [DOI] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W, Karapetyan K, Katz K, Liu C, Maddipatla Z, Malheiro A, McDaniel K, Ovetsky M, Riley G, Zhou G, Holmes JB, Kattman BL, Maglott DR. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Research. 2018;46:D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Siprashvili Z, Mah A, Bencomo T, Elcavage LE, Che Y, Shenoy RM, Aasi SZ, Khavari PA. Mutant collagen COL11A1 enhances cancerous invasion. Oncogene. 2021;40:6299–6307. doi: 10.1038/s41388-021-02013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annual Review of Genomics and Human Genetics. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Wei Y, Hung C, Vunjak-Novakovic G. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials. 2018;173:47–57. doi: 10.1016/j.biomaterials.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Liang ZT, Guo CF, Li J, Zhang HQ. The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB Journal. 2021;35:e21839. doi: 10.1096/fj.202100759R. [DOI] [PubMed] [Google Scholar]
- Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular signatures database (MSigDB) hallmark gene set collection. Cell Systems. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Lefebvre V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Research. 2015;43:8183–8203. doi: 10.1093/nar/gkv688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Easson GWD, Zhao J, Makki N, Ahituv N, Hilton MJ, Tang SY, Gray RS. Dysregulation of STAT3 signaling is associated with endplate-oriented herniations of the intervertebral disc in Adgrg6 mutant mice. PLOS Genetics. 2019;15:e1008096. doi: 10.1371/journal.pgen.1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hussien AA, Wang Y, Heckmann T, Gonzalez R, Karner CM, Snedeker JG, Gray RS. An adhesion G protein-coupled receptor is required in cartilaginous and dense connective tissues to maintain spine alignment. eLife. 2021;10:e67781. doi: 10.7554/eLife.67781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Long JT, Leinroth A, Liao Y, Ren Y, Mirando AJ, Nguyen T, Guo W, Sharma D, Rouse D, Wu C, Cheah KSE, Karner CM, Hilton MJ. Hypertrophic chondrocytes serve as a reservoir for marrow-associated skeletal stem and progenitor cells, osteoblasts, and adipocytes during skeletal development. eLife. 2022;11:e76932. doi: 10.7554/eLife.76932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YY, Karsdal MA. In: Biochemistry of Collagens, Laminins and Elastin. Morten A K, editor. London: Academic Press; 2019. Type XI collagen; pp. 99–106. [Google Scholar]
- Makki N, Zhao J, Liu Z, Eckalbar WL, Ushiki A, Khanshour AM, Wu Z, Rios J, Gray RS, Wise CA, Ahituv N. Genomic Characterization of the Adolescent Idiopathic Scoliosis Associated Transcriptome and Regulome. bioRxiv. 2020 doi: 10.1101/2020.03.02.973735. [DOI] [PMC free article] [PubMed]
- Makki N, Zhao J, Liu Z, Eckalbar WL, Ushiki A, Khanshour AM, Wu J, Rios J, Gray RS, Wise CA, Ahituv N. Genomic characterization of the adolescent idiopathic scoliosis-associated transcriptome and regulome. Human Molecular Genetics. 2021;29:3606–3615. doi: 10.1093/hmg/ddaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie‐Claire C, Derks EM. A tutorial on conducting genome‐wide association studies: quality control and statistical analysis. International Journal of Methods in Psychiatric Research. 2018;27:e1608. doi: 10.1002/mpr.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio F, Chiba K, Hirose Y, Kawaguchi Y, Mikami Y, Oya T, Mori M, Kamata M, Matsumoto M, Ozaki K, Tanaka T, Takahashi A, Kubo T, Kimura T, Toyama Y, Ikegawa S. A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. American Journal of Human Genetics. 2007;81:1271–1277. doi: 10.1086/522377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, Stevens K, Chen H, Trumbauer M, Visco DM. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis & Rheumatism. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Naba A, Hoersch S, Hynes RO. Towards definition of an ECM parts list: an advance on GO categories. Matrix Biology. 2012a;31:371–372. doi: 10.1016/j.matbio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Molecular & Cellular Proteomics. 2012b;11:M111. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “omics” era. Matrix Biology. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Kou I, Miura S, Takahashi A, Xu L, Takeda K, Takahashi Y, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yonezawa I, Yanagida H, Taneichi H, Zhu Z, Tsuji T, Suzuki T, Sudo H, Kotani T, Watanabe K, Hosogane N, Okada E, Iida A, Nakajima M, Sudo A, Chiba K, Hiraki Y, Toyama Y, Qiu Y, Shukunami C, Kamatani Y, Kubo M, Matsumoto M, Ikegawa S. A functional SNP in BNC2 is associated with adolescent idiopathic scoliosis. American Journal of Human Genetics. 2015;97:337–342. doi: 10.1016/j.ajhg.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends in Cell Biology. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello D, Wohler E, Erfani V, Li G, Aguilera AN, Santiago-Cornier A, Zhao S, Hwang SW, Steiner RD, Zhang TJ, Gurnett CA, Raggio C, Wu N, Sobreira N, Giampietro PF, Ciruna B. COL11A2 as a candidate gene for vertebral malformations and congenital scoliosis. Human Molecular Genetics. 2023;32:2913–2928. doi: 10.1093/hmg/ddad117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Research. 2019;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch P, Schubach M, Shendure J, Kircher M. CADD-Splice-improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Medicine. 2021;13:31. doi: 10.1186/s13073-021-00835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards BS, Sucato DJ, Johnston CE. In: Tachdjian’s Pediatric Orthopaedics. Herring JA, editor. Amsterdam: Elsevier; 2020. Scoliosis. [Google Scholar]
- Riseborough EJ, Wynne-Davies R. A genetic survey of idiopathic scoliosis in Boston, Massachusetts. The Journal of Bone and Joint Surgery. American Volume. 1973;55:974–982. [PubMed] [Google Scholar]
- Rodrigo I, Hill RE, Balling R, Münsterberg A, Imai K. Pax1 and Pax9 activate Bapx1 to induce chondrogenic differentiation in the sclerotome. Development. 2003;130:473–482. doi: 10.1242/dev.00240. [DOI] [PubMed] [Google Scholar]
- Seegmiller R, Fraser FC, Sheldon H. A new chondrodystrophic mutant in mice: electron microscopy of normal and abnormal chondrogenesis. The Journal of Cell Biology. 1971;48:580–593. doi: 10.1083/jcb.48.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A, Murphy G. Collagenolytic enzymes and their naturally occurring inhibitors. International Review of Connective Tissue Research. 1981;9:151–190. doi: 10.1016/b978-0-12-363709-3.50010-3. [DOI] [PubMed] [Google Scholar]
- Sharma S, Londono D, Eckalbar WL, Gao X, Zhang D, Mauldin K, Kou I, Takahashi A, Matsumoto M, Kamiya N, Murphy KK, Cornelia R, TSRHC Scoliosis Clinical Group. Japan Scoliosis Clinical Research Group. Herring JA, Burns D, Ahituv N, Ikegawa S, Gordon D, Wise CA. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nature Communications. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakamasundari V, Kraus P, Sun W, Hu X, Lim SL, Prabhakar S, Lufkin T. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biology Open. 2017;6:187–199. doi: 10.1242/bio.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GN, Hasty KA, Brandt KD. Type XI collagen is associated with the chondrocyte surface in suspension culture. Matrix. 1989;9:186–192. doi: 10.1016/s0934-8832(89)80049-6. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Disease Models & Mechanisms. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- Song Y-Q, Karasugi T, Cheung KMC, Chiba K, Ho DWH, Miyake A, Kao PYP, Sze KL, Yee A, Takahashi A, Kawaguchi Y, Mikami Y, Matsumoto M, Togawa D, Kanayama M, Shi D, Dai J, Jiang Q, Wu C, Tian W, Wang N, Leong JCY, Luk KDK, Yip S, Cherny SS, Wang J, Mundlos S, Kelempisioti A, Eskola PJ, Männikkö M, Mäkelä P, Karppinen J, Järvelin M-R, O’Reilly PF, Kubo M, Kimura T, Kubo T, Toyama Y, Mizuta H, Cheah KSE, Tsunoda T, Sham P-C, Ikegawa S, Chan D. Lumbar disc degeneration is linked to a carbohydrate sulfotransferase 3 variant. The Journal of Clinical Investigation. 2013;123:4909–4917. doi: 10.1172/JCI69277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, Stefansson OA, Gunnarsdottir K, Thorleifsson G, Lund SH, Stefansdottir L, Juliusson K, Agustsdottir AB, Zink F, Halldorsson GH, Ivarsdottir EV, Benonisdottir S, Jonsson H, Gylfason A, Norland K, Trajanoska K, Boer CG, Southam L, Leung JCS, Tang NLS, Kwok TCY, Lee JSW, Ho SC, Byrjalsen I, Center JR, Lee SH, Koh J-M, Lohmander LS, Ho-Pham LT, Nguyen TV, Eisman JA, Woo J, Leung P-C, Loughlin J, Zeggini E, Christiansen C, Rivadeneira F, van Meurs J, Uitterlinden AG, Mogensen B, Jonsson H, Ingvarsson T, Sigurdsson G, Benediktsson R, Sulem P, Jonsdottir I, Masson G, Holm H, Norddahl GL, Thorsteinsdottir U, Gudbjartsson DF, Stefansson K. GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nature Communications. 2019;10:2054. doi: 10.1038/s41467-019-09860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. PNAS. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Bay-Jensen A-C, Karsdal MA, Siebuhr AS, Zheng Q, Maksymowych WP, Christiansen TG, Henriksen K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskeletal Disorders. 2014;15:93. doi: 10.1186/1471-2474-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Luo EY, Adams SM, Adams T, Ye Y, Shetye SS, Soslowsky LJ, Birk DE. Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon. Matrix Biology. 2020;94:77–94. doi: 10.1016/j.matbio.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hu L, Bok S, Yallowitz AR, Cung M, McCormick J, Zheng LJ, Debnath S, Niu Y, Tan AY, Lalani S, Morse KW, Shinn D, Pajak A, Hammad M, Suhardi VJ, Li Z, Li N, Wang L, Zou W, Mittal V, Bostrom MPG, Xu R, Iyer S, Greenblatt MB. A vertebral skeletal stem cell lineage driving metastasis. Nature. 2023;621:602–609. doi: 10.1038/s41586-023-06519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kou I, Takahashi A, Johnson TA, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Tsuji T, Suzuki T, Sudo H, Kotani T, Watanabe K, Chiba K, Hosono N, Kamatani N, Tsunoda T, Toyama Y, Kubo M, Matsumoto M, Ikegawa S. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nature Genetics. 2011;43:1237–1240. doi: 10.1038/ng.974. [DOI] [PubMed] [Google Scholar]
- Tang NLS, Yeung H-Y, Hung VWY, Di Liao C, Lam T-P, Yeung H-M, Lee K-M, Ng BK-W, Cheng JC-Y. Genetic epidemiology and heritability of AIS: a study of 415 Chinese female patients. Journal of Orthopaedic Research. 2012;30:1464–1469. doi: 10.1002/jor.22090. [DOI] [PubMed] [Google Scholar]
- Ushiki A, Zhang Y, Xiong C, Zhao J, Georgakopoulos-Soares I, Kane L, Jamieson K, Bamshad MJ, Nickerson DA, University of Washington Center for Mendelian Genomics. Shen Y, Lettice LA, Silveira-Lucas EL, Petit F, Ahituv N. Deletion of CTCF sites in the SHH locus alters enhancer-promoter interactions and leads to acheiropodia. Nature Communications. 2021;12:2282. doi: 10.1038/s41467-021-22470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gennip JLM, Boswell CW, Ciruna B. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Science Advances. 2018;4:eaav1781. doi: 10.1126/sciadv.aav1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. The Journal of Biological Chemistry. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Dahl E, Peters H, Balling R, Imai K. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. PNAS. 1998;95:8692–8697. doi: 10.1073/pnas.95.15.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise CA, Sharma S. In: The Genetics and Development of Scoliosis. Kusumi K, Dunwoodie S, editors. New York: Springer; 2010. Current understanding of genetic factors in idiopathic Scoliosis; pp. 167–190. [Google Scholar]
- Wise CA. In: Molecular Genetics of Pediatric Orthopaedic Disorders. Carol A, Wise JJR, editors. New York: Springer; 2014. The genetic architecture of idiopathic Scoliosis; pp. 71–90. [DOI] [Google Scholar]
- Wise CA, Sepich D, Ushiki A, Khanshour AM, Kidane YH, Makki N, Gurnett CA, Gray RS, Rios JJ, Ahituv N, Solnica-Krezel L. The cartilage matrisome in adolescent idiopathic scoliosis. Bone Research. 2020;8:13. doi: 10.1038/s41413-020-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33:3432–3440. doi: 10.1038/onc.2013.307. [DOI] [PubMed] [Google Scholar]
- Wynne-Davies R. Familial (idiopathic) scoliosis: a family survey. The Journal of Bone and Joint Surgery. British Volume. 1968;50:24–30. [PubMed] [Google Scholar]
- Xu X, Li Z, Leng Y, Neu CP, Calve S. Knockdown of the pericellular matrix molecule perlecan lowers in situ cell and matrix stiffness in developing cartilage. Developmental Biology. 2016;418:242–247. doi: 10.1016/j.ydbio.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Iyama K, Inoguchi K, Khaleduzzaman M, Ninomiya Y, Ramirez F. Developmental pattern of expression of the mouse alpha 1 (XI) collagen gene (Col11a1) Developmental Dynamics. 1995;204:41–47. doi: 10.1002/aja.1002040106. [DOI] [PubMed] [Google Scholar]
- Yu H, Harrison FE, Xia F. Altered DNA repair; an early pathogenic pathway in Alzheimer’s disease and obesity. Scientific Reports. 2018;8:5600. doi: 10.1038/s41598-018-23644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski A, Cecchini EL, Deroo BJ. Expression of extracellular matrix components is disrupted in the immature and adult estrogen receptor β-null mouse ovary. PLOS ONE. 2012;7:e29937. doi: 10.1371/journal.pone.0029937. [DOI] [PMC free article] [PubMed] [Google Scholar]