Abstract
Spontaneous rupture of the patellar (PTR) and quadriceps (QTR) tendon is infrequent. Systemic diseases such as diabetes mellitus, CKD, and secondary hyperparathyroidism (SHPT) are risk factors. The present cohort study aimed to evaluate risk factors associated with tendon rupture in hemodialysis (HD) patients with SHPT, as well as outcomes including surgical complications, re-ruptures, and fracture. Baseline clinical, laboratorial data, and radiographs were analyzed. Patients were followed up from March 2012 to March 2020. One-hundred thirty-one patients (≥18 yr of age, on HD ≥ 6 mo, with SHPT) were included. Incidence rates of PTR and QTR were 2.3 and 1.7/10000 HD patients/yr, respectively. The mean age of patients with tendon rupture was 44.0 ± 11.2 yr. These patients exhibited higher serum levels of phosphorus (6.3 ± 1.5 mg/dL vs 5.6 ± 1.1 mg/dL; P = .005), PTH (2025.7 ± 667.6 pg/mL vs 1728.4 ± 684.8 pg/mL; P = .035), and C-reactive-protein (35.4 ± 32.9 mg/dL vs 17 ± 24.5 mg/dL; P = .002) compared to the group without tendon rupture. The mean follow-up was 56.7 ± 27.1 mo. No patient required a new surgical approach or experienced re-rupture. Of all patients, 31% experienced hip fracture: 50% in the group with rupture (29.5 ± 17.4 mo after the tendon rupture) vs 26% without tendon rupture (P = .015). After adjustment, the hazard ratio for hip fracture was 2.87 (95% CI, 1.27–6.49; P = .012). Patients with SHPT and high levels of phosphorus, PTH, and inflammatory markers were at greater risk for tendon rupture. Surgical complication rates were low. However, results suggest that tendon rupture of knee extensor mechanism in HD patient with SHPT should be regarded as a “red flag” for future hip fracture.
Keywords: hemodialysis, hip fracture, tendon rupture, secondary hyperparathyroidism
Introduction
The quadriceps (QTR) tendon, patella, and patellar (PTR) tendon are part of the knee extensor mechanism and are essential for ambulation. Spontaneous rupture of the PTR and QTR tendon appears to be infrequent in the general population, although the exact incidence among hemodialysis (HD) patients remains unknown. Bilateral rupture of these tendons is even rarer; however, in recent years, some case reports in the literature have been described.1–12 Reported risk factors for tendon rupture include the following: use of specific drugs, such as quinolones and corticoids; autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis; and systemic diseases, such diabetes mellitus, obesity, CKD, and secondary hyperparathyroidism (SHPT).13–18
The prevalence of CKD is increasing worldwide, with an estimated 850 million affected individuals in 2021.19 The number of complications related to this disease is also increasing, including bone alterations and SHPT. With a reduction in renal function, there is a change in calcium, phosphorus, and vitamin D levels, which, if not corrected promotes a progressive increase in PTH levels. These alterations, known as mineral and bone disorder related to CKD (CKD-MBD), are associated with extra skeletal calcifications (including vascular and tendon), fractures, and histopathological bone alterations (renal osteodystrophy).20 The fracture incidence is higher in HD patients and it occurs earlier in these patients than in the general population.21,22 Many factors are related to fracture,22–24 and it is crucial to recognize new risk factors, because fracture increases hospitalization, morbidity, mortality, and costs.
In 1962, Preston was the first to associate tendon ruptures with hyperparathyroidism.25 Since then, several case reports describing QTR or PTR in patients on HD have been published.26–40 Two studies evaluated HD patients with hyperparathyroidism versus HD patients without SHPT (controls).41,42 The authors observed that patients who experienced tendon rupture were younger and exhibited higher levels of serum calcium, phosphorus, alkaline phosphatase, and PTH. However, not all patients with severe SHPT experience tendon rupture. As such, this study aimed to evaluate the incidence and risk factors associated with tendon tears of the knee extensor mechanism in HD patients with severe hyperparathyroidism, as well as perioperative complications, re-ruptures, and associated fracture during follow-up.
Patients and Methods
The present study was an observational, prospective, longitudinal study, involving a cohort of patients followed at the Renal Osteodystrophy outpatient clinic of the National Institute of Traumatology and Orthopedics Jamil Haddad (INTO) and University Hospital Clementino Fraga Filho (UFRJ). The former is an orthopedic referral hospital, which offers specialized care in highly complex orthopedic procedures, while the latter is a tertiary hospital that offers specialized care, such as an outpatient clinic to evaluate bone and mineral disease related to CKD, bone biopsy, and parathyroidectomy. This study was approved by the local ethics committee, and was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria were as follows: age > 18 yr, undergoing HD for at least 6 mo, and severe SHPT (PTH > 1000 pg/mL), and in outpatient follow-up for >12 mo. Patients with known bone disease (Paget, myeloma) or who used medications that affect bone metabolism such as anticonvulsants, corticoids, antiretrovirals, and anti-osteoporotic drugs were excluded.
Clinical, laboratory, and radiographic data were analyzed at baseline. Clinical (age, gender, time on dialysis, medication use, smoking, underlying disease for CKD, and comorbidities) and anthropometric (weight, height, BMI) data were collected.
Laboratory investigations included: serum total calcium (8.5–10.5 mg/dL), phosphorus (2.5–4.5 mg/dL), alkaline phosphatase (65–300 U/L), iPTH (second generation chemiluminescentimmunometric assay, 12–65 pg/mL), 25(OH) vitamin D (30 ng/mL), albumin (3.6–4.8 g/dL), C-reactive protein (0–5 mg/dL), ferritin (28–365 ng/mL), transferrin saturation index (20%–50%), hemoglobin (12–16 g/dL), and bicarbonate (22–26 mmol/L).
Patients underwent X-ray examinations of the hands, thoracolumbar spine, pelvis, femur, and knees to assess fracture, tendon calcification and the presence of bone cysts.
β2-microglobulin amyloidosis was assessed according to the presence of amyloid deposits detected by Congo red staining in the histopathological examination of the tendon or by the presence of bone cysts in the femoral head, humerus, and hands, in addition to the presence of carpal tunnel syndrome.
The site of tendon rupture, type of treatment (conservative or surgical), surgical method, and time between rupture and surgery (early, if surgery occurred within 30 d or late, if longer than 30 d) were noted.
This cohort study was divided in 2 phases. The first step was to analyze the incidence and risk factors for tendon rupture in HD patients with severe SHPT referred to our institutions. Thereafter, these patients were prospectively followed from March 2012 to March 2020 to assess surgical complications, the need for a new surgical approach, and the occurrence of skeletal events, such as new tendon ruptures and fractures.
After Kolmogorov–Smirnov normality test, Student’s t-test (parametric continuous variables), Mann–Whitney (non-parametric continuous variable) and Chi-squared test (categorical variables) were used for comparison between 2 independent groups (with or without tendon rupture). We calculated incidence rate of tendon rupture per 10 000 HD patients per year. The patients were distributed according to the type of tendon rupture (PTR, QTR, unilateral, or bilateral). Kaplan–Meier survival analysis with Log rank test was used to evaluate the probability of fracture over time. We performed the univariable analysis to identify which variables were associated with fracture. Those variables with a P value of .25 or lower on univariable analysis were included in multivariate analysis. Cox regression model was performed to estimate the hazard ratio for fracture adjusted for risk factors. The hazard ratios were plotted in Forest plot to better visualize these data. Continuous variables are expressed as mean ± SD or median and interquartile. Categorical variables are presented as number and percentage. All tests were 2-sided, and the significance level was fixed at 0.05. Statistical analysis was performed using SPSS version 28 (IBM Corporation).
Results
In total, 131 patients with severe SHPT were evaluated. An incidence of 49 tendon ruptures (28 PTR and 21 QTR) in 31 patients (24%) was found. The incidence rate was 2.3 and 1.7 per 10 000 HD patients/yr for PTR and QTR, respectively. Of the 31 patients, 58% were male. There were no cases of rupture of other tendons.
Differences between groups with and without tendon rupture were analyzed in clinical characteristics and risk factors for rupture (Table 1). None of the 6 tendons subjected to histopathological evaluation tested positive for amyloid deposit by Congo red staining. Practically, all patients with tendon rupture exhibited tendon calcification on knee radiography.
Table 1.
Demographic, laboratory, and radiographic data of patients with and without tendon rupture.
| Without tendon rupture (n = 100) | Tendon rupture (n = 31) | P value | |
|---|---|---|---|
| Gender (Male) | 45 (45%) | 18 (58%) | .203 |
| Age | 47.4 ± 10.8 | 44.0 ± 11.2 | .134 |
| HD vintage | 115.3 ± 55.5 | 117.4 ± 32.2 | .838 |
| BMI | 24.0 ± 4.6 | 24.7 ± 4.3 | .493 |
| BMI > 30 | 6 (6%) | 2 (6.5%) | .927 |
| Smoking | 8 (8%) | 2 (7%) | .776 |
| Cause of CKD | |||
| Unknown | 55 (55%) | 11 (35%) | .058 |
| Hypertension | 30 (30%) | 14 (45%) | .118 |
| Diabetes mellitus | 0 (0%) | 1 (3%) | |
| Others | 15 (15%) | 5 (17%) | .878 |
| Ca | 9.6 ± 0.9 | 9.4 ± 0.8 | .449 |
| P | 5.6 ± 1.1 | 6.3 ± 1.5 | .005a |
| ALP | 1377.7 ± 1133.1 | 1634.6 ± 1343.5 | .293 |
| iPTH | 1728.4 ± 684.8 | 2025.7 ± 667.6 | .035a |
| 25OHD | 27.2 ± 11.4 | 29.1 ± 10.5 | .442 |
| Bicarbonate | 22.4 ± 4.8 | 21.3 ± 7 | .301 |
| Albumin | 3.9 ± 0.5 | 3.7 ± 0.5 | .047a |
| CRP | 17 ± 24.5 | 35.4 ± 32.9 | .002a |
| Ferritin | 821.4 ± 720.2 | 1144.8 ± 975.1 | .054 |
| IST | 28.9 ± 13.6 | 28.8 ± 14.8 | .982 |
| Hemoglobin | 11.2 ± 2.5 | 10.7 ± 1.8 | .344 |
| Colesterol | 160.2 ± 37.8 | 181.5 ± 50.2 | .069 |
| Triglycerides | 140.3 ± 61.2 | 165.9 ± 60.3 | .140 |
| Bone cyst+CTS | 16 (16%) | 5 (16%) | .986 |
| Corticoid use | 10 (10%) | 4 (12%) | .647 |
| Tendon calcification | 32 (32%) | 30 (97%) | <.001a |
| Previous hip fracture | 13 (13%) | 5 (16%) | .658 |
To compare groups, we used the t-Student test for continuous variable and Chi-Square for categorical variables
a:p<0.05
Abbreviations: ALP, alkaline phosphatase (U/L); Ca, calcium (mg/dL); CRP, C-reactive protein (mg/dL); CTS, Carpal Tunnel Syndrome; HD, hemodialysis; iPTH, intact parathormone (pg/mL); P, phosphorus (mg/dL). Values expressed by mean ± SD, or absolute number (%).
Same clinical and laboratory parameters were studied in patients with unilateral rupture (n = 13) versus those patients with bilateral rupture (n = 18). There was no significant difference in any of these parameters, with the exception of albumin level, which was lower in patients with bilateral rupture (3.9 ± 0.2 g/dL vs 3.5 ± 0.6 g/dL; P = .015).
Sudden pain and inability to sustain leg extension were the main symptoms in complete extensor mechanism rupture. Patella displacement, with patella alta (infrapatellar gap) in PTR and patella baja (gap suprapatellar) in QTR, was evidenced in the physical exam and X-ray. Seventy percent underwent early surgical treatment (10.7 ± 8 d from the occurrence of the rupture until surgery), while 26% received late treatment (88.6 ± 55.2 d).
The number of early complications was low, with the occurrence of 1 case (3%) of dehiscence and 1 case (3%) of superficial surgical infection, both of which were treated with antibiotics. Patients returned to their daily activities in approximately 6 mo after surgery. There were no cases of deep venous thrombosis. Patients were prospectively followed up for 56.7 ± 27.1 mo. No patient required a new surgical approach, and there were no reports of re-rupture or rupture of other tendons.
All patients with severe SHPT were in the queue for parathyroidectomy, and while waiting for the procedure, they underwent drug therapy with phosphorus binders, calcium carbonate, and calcimimetic, and vitamin D analogs. Fifty-six patients (43%) underwent parathyroidectomy, 55% of whom after tendon rupture (mean 39.6 ± 20.4 mo).
Despite clinical or surgical treatment of SHPT, 31% of all patients experienced hip fracture: 50% in the group with tendon rupture versus 26% in the group without (P = .015). Among patients with tendon rupture who later experienced hip fracture, 15% did not undergo parathyroidectomy, whereas 80% did (P = .009). There was one vertebral fracture in each group (with and without rupture), with no significant difference. There was no correlation between rupture site (ie, PTR or QTR) and the occurrence of fracture. Hip fracture occurred approximately 29.5 ± 17.4 mo after tendon rupture. Figure 1 shows Kaplan–Meier curve to fracture event. After adjustments for age, calcium, phosphorus, alkaline phosphatase, PTH, CRP, and albumin levels, and parathyroidectomy, tendon rupture was an independent risk factor, as shown in Figure 2. The hazard ratio for fracture was 2.87 (95% CI, 1.27–6.49, P = .012).
Figure 1.
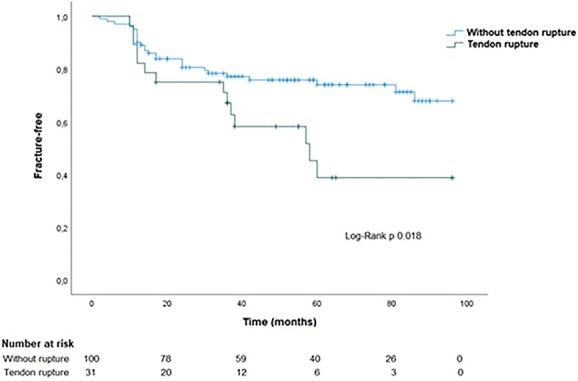
Kaplan–Meier curve to fracture event.
Figure 2.
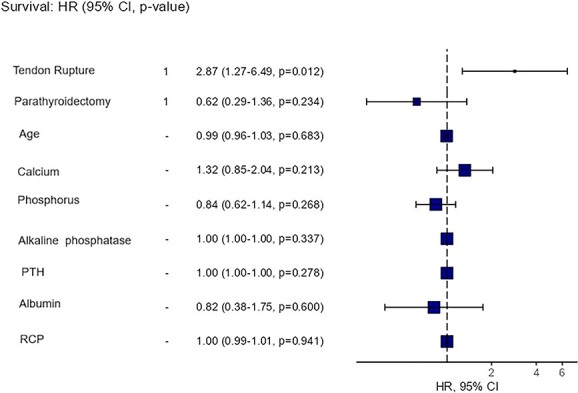
Forest plot of hazard ratio for hip fracture.
Discussion
It has been established that CKD and SHPT are risk factors for spontaneous PTR and/or QTR. However, not every patient undergoing HD and with SHPT experience tendon rupture. To our knowledge, this was the first study to assess risk factors for tendon rupture and outcomes in this specific population. We observed that higher serum levels of phosphorus and PTH were more strongly associated with spontaneous tendon rupture, as well as patients exhibiting inflammation (elevated CRP and ferritin levels). In the longitudinal evaluation, these patients experienced few surgical complications and no re-ruptures; however, they were twice as likely to experience hip fracture.
Spontaneous PTR and QTR appear to be infrequent in the general population, although the exact incidence is unknown in HD patients. A previous study reported incidences of 1.37/100 000 for QTR and 0.68/100 000 for PTR in the general population of Edinburgh (Scotland) between 1996 and 2000.43 However, a Finnish population-based study observed that annual incidence of QTR increased by over 400% between 1997 and 2014.44 In our study, involving patients with risk factors for tendon rupture, such as CKD and SHPT, the incidence rate was 2.3 and 1.7 per 10 000 HD patients per year for PTR and QTR, respectively. Koh and Arimuthu45 reported a case series in patients undergoing HD and with PTH levels >600 pg/mL, in whom the incidence rate of QTR was 7.27 per 10 000 person-years between 2012 and 2021, illustrating the high incidence of tendon rupture in this population.
Currently, there are pharmacological treatments for SHPT, such as calcimimetics and vitamin D analogs. However, adverse events associated with drugs (nausea, vomiting, gastrointestinal intolerance, hypo- or hypercalcemia) and high pill burden limit medication adherence.46 When patients do not respond adequately to pharmacological treatment, parathyroidectomy is the treatment of choice.47 However, these medications and surgery are not widely accessible and are associated with high costs and socioeconomic impact.48–50 Then, the prevalence of SHPT (PTH > 600 pg/mL) is high, mainly in developing countries, varying between 18% and 28.3% and between 10.7% and 13.3% for severe SHPT (PTH > 1000 pg/mL).51–55 Of note, the incidence of patients undergoing HD increases each year, as well as patients with SHPT and consequent skeletal complications, such as tendon rupture.
In CKD, uremic toxins, acidosis and changes in mineral, hormone and bone metabolism (CKD-MBD) may be involved in the pathophysiology of tendon rupture. A progressive increase in PTH levels results in an increase in bone turnover. At very high PTH levels (>1000 pg/mL), characterizing severe hyperparathyroidism, there is a greater predominance of bone resorption, cortical thinning, and, consequently, weakening of the tendon insertion site in the bone.56,57 The majority of QTR and PTR occur at the osseous insertion site.17,18,57–59 Furthermore, a direct effect of PTH on the tendon matrix by depolymerization of glycoproteins is possible.60 Hyperparathyroidism promotes greater extra-skeletal calcification, such as in vessels, soft tissues, and tendons, rendering these structures less flexible and with a weakened less response to microtrauma correction. In our population, virtually all patients exhibited tendon calcification, similar to Tsourvakas et al.59 and Wu et al.,61 who found ectopic calcification and there were calcifications in the ruptured end of the tendon in all patients. Extra-skeletal calcification can also affect vessels, leading to hypovascularization and ischemia.
The loss of tendon elasticity can also occur in patients with β2-microglobulin accumulation. This is a medium-size molecule removed by high-flux dialysis capillaries, but its use has only recently expanded. Therefore, patients in whom low-flux or bioincompatible dialysis membranes are used are at a higher risk of accumulating this molecule, which has a tendency to deposit in the synovium, bones, ligaments, and tendons. This accumulation is usually proportional to HD vintage.62,63 Our patients were on HD for long time and, despite the possibility of β2-microglobulin amyloidosis, there was no difference between the groups with and without tendon rupture. Among samples analyzed in our study, none exhibited amyloid deposits, similar to that found in other study.59 Consistent with Jones et al.41 and De Franco et al.,64 we believe that amyloid and tendon ruptures are not necessarily correlated, although both occur in patients who have been undergoing dialysis for many years.
Systemic inflammation is prevalent among HD patients and is correlated with high CRP and low albumin levels.65,66 Patients who experienced tendon rupture in our study exhibited higher CRP and ferritin and lower albumin levels, mainly in those with bilateral rupture. Therefore, they are more susceptible to direct tendon injuries from inflammatory cytokines and decreased healing potential after repeated microtrauma.
Regarding surgical complications, the main prognostic factors influencing outcomes are delayed diagnosis and timing of surgery.67 In our study, most patients underwent surgery early. Complications as re-rupture or inability to fully extend the knee were not observed in our patients and only one patient exhibited wound infection, fewer than that reported by Ciriello et al.17
Severe hyperparathyroidism and HD are established risk factors for hip fracture. Persistently high PTH levels have catabolic effects and are associated with thinning cortical, increased cortical porosity, and even endocortical “trabecularization,”68–71 resulting in increased bone fragility. However, in our study, high levels of PTH and inflammatory markers, which lead to an increased risk for tendon rupture, did not exhibit a significant association with hip fracture after adjustment. Parathyroidectomy remains the definitive therapy for refractory SHPT; however, it was also not associated as a significant protective factor against hip fracture, probably due to late execution of this treatment in our study. In a previous case series, fractures were not reported as a complication among HD patients with severe SHPT and QTR, who underwent tendon repair followed by parathyroidectomy (follow-up of 3.46 ± 1.37 yr).61 However, there was a limitation in this outcome due to the absence of controls (patients without surgical treatment).
Tendon rupture was an independent risk for fracture. Of note, patients lose their ambulatory capacity after tendon rupture and require prolonged immobilization after surgery until total recovery (4–6 wk). It has been established that mechanical stimuli and motion with load are essential to bone metabolism.72 Immobilization leads an imbalance between bone formation and resorption, resulting in structural deterioration and low bone density. Both the cortical and trabecular compartments are affected; however, the decrease of cortical thickness appears to be more important in loss of bone strength after nonuse. One study suggested that the subendocortical layer can be transformed into trabecular bone in response to immobilization and it would be associated with risk for fracture,73 similar to severe SHPT.
The present study had some limitations. First, it was performed in a referral hospital for orthopedic surgery and parathyroidectomy, which would explain the high incidence of tendon rupture. In addition, we evaluated a population undergoing HD and with severe SHPT, known risk factors for tendon rupture and hip fracture, so our results should not be extrapolated to other populations. Furthermore, this study was unable to determine whether parathyroidectomy could prevent hip fracture, as patients underwent this surgery late.
To our knowledge, no study has addressed the occurrence of fracture as an outcome of tendon rupture. Because the probable cause of tendon rupture among our patients was the increase in bone turnover due to hyperparathyroidism, we assessed whether they would also have a higher risk for developing fractures. We found that the incidence of hip fracture was significantly higher among patients with tendon rupture than in those without, despite the fact that all patients had severe hyperparathyroidism, with a 2-fold increased risk. It is noteworthy that immobilization, rehabilitation, and non-deambulatory status after knee surgery could contribute to decreased bone mass in a bone already affected by SHPT.
Conclusion
HD patients with hyperparathyroidism exhibiting high serum levels of phosphorus, PTH, and inflammatory markers (CRP and ferritin) or low levels of albumin were at greater risk for tendon rupture. If diagnosed and treated early, complication rates were low. Our results suggest that extensor mechanism rupture in HD patient with SHPT should be considered as a “red flag” for future hip fracture.
Contributor Information
Alinie Pichone, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil; Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
Elicivaldo Lima Juvencio, Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
Bernardo Crespo, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil; Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
Carlos Perez Gomes, Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
Renata de Souza Mendes, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil; Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
Marise Rocha Godinho, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil.
Aline Cordeiro Fernandes Ladeira, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil.
Maurilo Leite, Jr, Department of Nephrology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-617, Brazil.
João Antônio Matheus Guimarães, Department of Nephrology, Knee Surgery and Research, National Institute of Traumatology and Orthopedics Jamil Haddad, Rio de Janeiro, RJ 20940-070, Brazil.
Author contributions
Alinie Pichone (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—original draft, Writing—review & editing), Elicivaldo Lima Juvencio (Data curation, Investigation, Writing—original draft, Writing—review & editing), Bernardo Crespo (Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing), Carlos Perez Gomes (Conceptualization, Formal analysis, Methodology, Software, Supervision, Writing—original draft, Writing—review & editing), Renata de Souza Mendes (Data curation, Formal analysis, Methodology, Validation, Writing—review & editing), Marise Rocha Godinho (Data curation, Formal analysis, Visualization, Writing—review & editing), Aline Cordeiro Fernandes Ladeira (Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Writing—review & editing), Maurilo Leite Jr (Formal analysis, Methodology, Project administration, Supervision, Writing—review & editing), and João Antônio Matheus Guimarães (Formal analysis, Methodology, Project administration, Supervision, Writing—review & editing)
Funding
This research received no external funding.
Conflicts of interest
All authors declare no conflict of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval
This study was approved by the ethic committee of National Institute of Traumatology and Orthopedics Jamil Haddad (60486222.8.0000.5273) and University Hospital Clementino Fraga Filho (62657316.8.0000.5257), in accordance with the Declaration of Helsinki.
References
- 1. Doehring MC, Propst SL. Simultaneous bilateral quadriceps tendon rupture in an adult man. Clin Pract Cases Emerg Med. 2022;6(2):192–193. 10.5811/cpcem.2022.3.55675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Govindu R, Ammar H, George V. Bilateral quadriceps tendon rupture. J Clin Rheumatol. 2019;25(5):e63–e66. 10.1097/RHU.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 3. Onuoha KM, Ajiboye OK, Kumar R. Spontaneous bilateral quadriceps tendon rupture: a case report. Pan Afr Med J. 2020;23(37):84. 10.11604/pamj.2020.37.84.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Ouali Z, Nassar K, Bassa E, et al. Bilateral patellar tendon rupture on lupus undergoing corticosteroids: a case report. BMC Musculoskelet Disord. 2020;21(1):477. 10.1186/s12891-020-03513-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foley J, Elhelali R, Moiloa D. Spontaneous simultaneous bilateral patellar tendon rupture. BMJ Case Rep. 2019;12(2):e227931. 10.1136/bcr-2018-227931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sasaki R, Nagashima M, Aibara N, et al. Simultaneous bilateral quadriceps tendon rupture in a healthy young male: a case report. J Med Case Rep. 2023;17(1):85. 10.1186/s13256-023-03802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Umbel B, Triplet JJ, Johnson DB, Taylor BC. Atraumatic bilateral quadriceps tendon rupture in a patient with hypothyroidism. J Long-Term Eff Med Implants. 2018;28(4):271–275. 10.1615/JLongTermEffMedImplants.2019029541. [DOI] [PubMed] [Google Scholar]
- 8. Lin C, Shen J, Jiang Z, et al. Spontaneous tendon rupture in a patient with systemic sclerosis: a case report. BMC Musculoskelet Disord. 2022;23(1):1001. 10.1186/s12891-022-05967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juliato RH, Boschi LH, Juliato RF, Freitas AP, França AF, Bottura LB. Bilateral atraumatic patellar ligament rupture-case report. Rev Bras Ortop. 2019;54(02):223–227. 10.1016/j.rbo.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasen SK, Foster AJ, Thakrar RR. Non-identical bilateral rupture of the extensor mechanism of the knee in a patient with hyperlipidemia: a case study. J Orthop Case Rep. 2019;9(4):88–91. 10.13107/jocr.2250-0685.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barner KL, Gluck C, Witte KA. Simultaneous bilateral rupture of patellar tendons in a young man without predisposing factors or systemic disease. Cureus. 2019;11(8):e5469. 10.7759/cureus.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moura DL, Marques JP, Pinheiro JP, Fonseca F. Total bilateral ruptures of the knee extensor apparatus. Rev Bras Ortop. 2017;52(6):663–669. 10.1016/j.rbo.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garner MR, Gausden E, Berkes MB, Nguyen JT, Lorich DG. Extensor mechanism injuries of the knee: demographic characteristics and comorbidities from a review of 726 patient records. J Bone Joint Surg Am. 2015;97(19):1592–1596. 10.2106/JBJS.O.00113. [DOI] [PubMed] [Google Scholar]
- 14. Malta LMA, Dos Santos A, Malta MC, Machado LM, Lugon JR. Treatment of quadriceps tendon rupture in hemodialysis patients: a 2020 update. Acta Ortop Bras. 2022;30(spe1):e245692. 10.1590/1413-785220223001e245692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibounig T, Simons TA. Etiology, diagnosis and treatment of tendinous knee extensor mechanism injuries. Scand J Surg. 2016;105(2):67–72. 10.1177/1457496915598761. [DOI] [PubMed] [Google Scholar]
- 16. Tandogan RN, Terzi E, Gomez-Barrena E, Violante B, Kayaalp A. Extensor mechanism ruptures. EFORT Open Rev. 2022;7(6):384–395. 10.1530/EOR-22-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931–1938. 10.1016/j.injury.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 18. Camarda L, D'Arienzo A, Morello S, Guarneri M, Balistreri F, D'Arienzo M. Bilateral ruptures of the extensor mechanism of the knee: A systematic review. J Orthop. 2017;14(4):445–453. 10.1016/j.jor.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sozio SM, Pivert KA, Caskey FJ, Levin A. The state of the global nephrology workforce: a joint ASN-ERA-EDTA-ISN investigation. Kidney Int. 2021;100(5):995–1000. 10.1016/j.kint.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1-130. 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 21. McNerny EMB, Nickolas TL. Bone quality in chronic kidney disease: definitions and diagnostics. Curr Osteoporos Rep. 2017;15(3):207–213. 10.1007/s11914-017-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358–1366. 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 23. Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis. 2013;62(4):747–754. 10.1053/j.ajkd.2013.02.368. [DOI] [PubMed] [Google Scholar]
- 24. Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47(1):149–156. 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 25. Preston FS, Adicoff A. Hyperparathyroidism with avulsion of three major tendons. Report of a case. N Engl J Med. 1962;266(19):968–971. 10.1056/NEJM196205102661903. [DOI] [PubMed] [Google Scholar]
- 26. Wu W, Wang C, Ruan J, et al. Simultaneous spontaneous bilateral quadriceps tendon rupture with secondary hyperparathyroidism in a patient receiving hemodialysis: A case report. Medicine. 2019;98(10):e14809. 10.1097/MD.0000000000014809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allata Y, Chouhani BA, El Bardai G, Kabbali N, Sqalli HT. A spontaneous bilateral quadriceps tendon rupture in a patient undergoing long-term hemodialysis. Cureus. 2023;15(3):e36059. 10.7759/cureus.36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartono F, Besinga KE, Tjie H, Marpaung D, Ananditya T, Gabriel HRN. Considerations in spontaneous quadriceps tendon rupture repair in end-stage renal disease patients: a case report. Int J Surg Case Rep. 2021;86:106298. 10.1016/j.ijscr.2021.106298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vemuri VN, Venkatesh M, Kada V, Chakkalakkoombil SV. Spontaneous bilateral quadriceps tendon rupture in a patient with renal failure. BMJ Case Rep. 2018;2018:bcr2017223191. 10.1136/bcr-2017-223191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torkaman A, Yousof Gomrokchi A, Elahifar O, Barmayoon P, Shojaei SF. Simultaneous bilateral rupture of patellar tendons in diabetic hemodialysis patient: a case report. Caspian J Intern Med. 2018;9(3):306–311. 10.22088/cjim.9.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zribi W, Zribi M, Guidara AR, et al. Spontaneous and simultaneous complete bilateral rupture of the quadriceps tendon in a patient receiving hemodialysis: a case report and literature review. World J Orthop. 2018;9(9):180–184. 10.5312/wjo.v9.i9.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radaideh AM, Audat ZA, Sunallah AW, Bani-Younes HM, Obeidat O. A simultaneous rupture of the patellar tendon and the con-tralateral quadriceps tendon in a patient with chronic renal failure undergoing long term hemodialysis. Med Arch. 2021;75(4):317–320. 10.5455/medarh.2021.75.317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tao Z, Liu W, Ma W, Luo P, Zhi S, Zhou R. A simultaneous bilateral quadriceps and patellar tendons rupture in patients with chronic kidney disease undergoing long-term hemodialysis: a case report. BMC Musculoskelet Disord. 2020;21(1):179. 10.1186/s12891-020-03204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cherrad T, Louaste J, Kasmaoui el H, Bousbaä H, Rachid K. Neglected bilateral rupture of the patellar tendon: acase report. J Clin Orthop Trauma. 2015;6(4):296–299. 10.1016/j.jcot.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allon M. Acute bilateral knee pain in a dialysis patient with severe secondary hyperparathyroidism. Kidney360. 2020;1(2):151. 10.34067/KID.0000122019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taşoğlu Ö, Ekiz T, Yenigün D, Akyüz M, Özgirgin N. Bilateral quadriceps and triceps tendon rupture in a hemodialysis patient. Hemodial Int. 2016;20(1):E19–E21. 10.1111/hdi.12319. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Lin Z, Zhong J, Nie D, Gao S, Zhang J. Spontaneous rupture of the right quadriceps tendon in a patient undergoing long-term hemodialysis: a case report. J Int Med Res. 2020;48(10):300060520959221. 10.1177/0300060520959221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moerenhout K, Gkagkalis G, Benoit B, Laflamme GY. Simultaneous ipsilateral quadriceps and triceps tendon rupture in a patient with end-stage renal failure. Case Rep Orthop. 2018;2018:7602096. 10.1155/2018/7602096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basic-Jukic N, Juric I, Racki S, Kes P. Spontaneous tendon ruptures in patients with end-stage renal disease. Kidney Blood Press Res. 2009;32(1):32–36. 10.1159/000201792. [DOI] [PubMed] [Google Scholar]
- 40. Thaunat M, Gaudin P, Naret C, Beaufils P, Thaunat O. Role of secondary hyperparathyroidism in spontaneous rupture of the quadriceps tendon complicating chronic renal failure. Rheumatology. 2006;45(2):234–235. 10.1093/rheumatology/kei022. [DOI] [PubMed] [Google Scholar]
- 41. Jones N, Kjellstrand CM. Spontaneous tendon ruptures in patients on chronic dialysis. Am J Kidney Dis. 1996;28(6):861–866. 10.1016/S0272-6386(96)90386-8. [DOI] [PubMed] [Google Scholar]
- 42. Malta LM, Gameiro VS, Sampaio EA, Gouveia ME, Lugon JR. Quadriceps tendon rupture in maintenance haemodialysis patients: results of surgical treatment and analysis of risk factors. Injury. 2014;45(12):1970–1973. 10.1016/j.injury.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 43. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338–1344. 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 44. Reito A, Paloneva J, Mattila VM, Launonen AP. The increasing incidence of surgically treated quadriceps tendon ruptures. Knee Surg Sports Traumatol Arthrosc. 2019;27(11):3644–3649. 10.1007/s00167-019-05453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koh KH, Arimuthu DA. Association of incidence rate of spontaneous tendon rupture and renal bone disease in end-stage renal disease patients' cohort. Semin Dial. 2023;36(6):462–467. 10.1111/sdi.13145. [DOI] [PubMed] [Google Scholar]
- 46. Fuller DS, Hallett D, Dluzniewski PJ, et al. Predictors of cinacalcet discontinuation and reinitiation in hemodialysis patients: results from 7 European countries. BMC Nephrol. 2019;20(1):169. 10.1186/s12882-019-1355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steinl GK, Kuo JH. Surgical Management of Secondary Hyperparathyroidism. Kidney Int Rep. 2021;6(2):254–264. 10.1016/j.ekir.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ponce D, Cardoso MMA, Rúgolo JRM, Molina SA, Andrade LGM, Curado DDSP. Cost-effectiveness analysis of cinacalcet vs. paricalcitol in the treatment of hyperparathyroidism secondary to chronic kidney disease. J Bras Nefrol. 2023;45(3):365–372. 10.1590/2175-8239-JBN-2022-0126en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Z, Cai L, Wu H, et al. Paricalcitol versus calcitriol + cinacalcet for the treatment of secondary hyperparathyroidism in chronic kidney disease in China: a cost-effectiveness analysis. Front Public Health. 2021;9:712027. 10.3389/fpubh.2021.712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma A, Marshall TS, Khan SS, Johns B. Cost effectiveness of paricalcitol versus cinacalcet with low-dose vitamin D for management of secondary hyperparathyroidism in haemodialysis patients in the USA. Clin Drug Investig. 2014;34(2):107–115. 10.1007/s40261-013-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Douthat WG, Castellano M, Berenguer L, et al. High prevalence of secondary hyperparathyroidism in chronic kidney disease patients on dialysis in Argentina. Nefrologia. 2013;33(5):657–666. 10.3265/Nefrologia.pre2013.May.12009. [DOI] [PubMed] [Google Scholar]
- 52. Barretti P. The new Brazilian Dialysis Census. J Bras Nephrol. 2022;44(3):308–309. 10.1590/2175-8239-jbn-2022-e006en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oliveira RB, Silva EN, Charpinel DM, et al. Secondary hyperparathyroidism status in Brazil: Brazilian census of parathyroidectomy. J Bras Nefrol. 2011;33(4):457–462. 10.1590/S0101-28002011000400011. [DOI] [PubMed] [Google Scholar]
- 54. Al Salmi I, Bieber B, Al Rukhaimi M, et al. Parathyroid hormone serum levels and mortality among hemodialysis patients in the Gulf Cooperation Council Countries: results from the DOPPS (2012-2018). Kidney360. 2020;1(10):1083–1090. 10.34067/KID.0000772020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J, Bieber BA, Hou FF, Port FK, Anand S. Mineral and bone disorder and management in the China Dialysis Outcomes and Practice Patterns Study. Chin Med J. 2019;132(23):2775–2782. 10.1097/CM9.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ryuzaki M, Konishi K, Kasuga A, et al. Spontaneous rupture of the quadriceps tendon in patients on maintenance hemodialysis--report of three cases with clinicopathological observations. Clin Nephrol. 1989;32(3):144–148. [PubMed] [Google Scholar]
- 57. Meneghello A, Bertoli M. Tendon disease and adjacent bone erosion in dialysis patients. Br J Radiol. 1983; 56(672):915–920. 10.1259/0007-1285-56-672-915. [DOI] [PubMed] [Google Scholar]
- 58. McKinney B, Cherney S, Penna J. Intra-articular knee injuries in patients with knee extensor mechanism ruptures. Knee Surg Sports Traumatol Arthrosc. 2008;16(7):633–638. 10.1007/s00167-008-0516-z. [DOI] [PubMed] [Google Scholar]
- 59. Tsourvakas S, Alexandropoulos C, Gouvalas K, Gimtsas C, Tsianas N, Founta P. Spontaneous major tendon ruptures in patients on chronic hemodialysis. Eur J Orthop Surg Traumatol. 2005;15(2):109–112. 10.1007/s00590-004-0213-7. [DOI] [Google Scholar]
- 60. Preston ET. Avulsion of both quadriceps tendons in hyperparathyroidism. JAMA. 1972;221(4):406–407. 10.1001/jama.1972.03200170050017. [DOI] [PubMed] [Google Scholar]
- 61. Wu S, Wang H, Zhu Y, Fu W. A retrospective case series of the treatment of spontaneous quadriceps tendon rupture in patients with uremia and secondary hyperparathyroidism. Front Surg. 2023;10:961188. 10.3389/fsurg.2023.961188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Portales-Castillo I, Yee J, Tanaka H, Fenves AZ. Beta-2 microglobulin amyloidosis: past, present, and future. Kidney360. 2020;1(12):1447–1455. 10.34067/KID.0004922020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jadoul M, Drüeke TB. β2 microglobulin amyloidosis: an update 30 years later. Nephrol Dial Transplant. 2016;31(4):507–509. 10.1093/ndt/gfv318. [DOI] [PubMed] [Google Scholar]
- 64. De Franco P, Varghese J, Brown WW, Bastani B. Secondary hyperparathyroidism, and not beta 2-microglobulin amyloid, as a cause of spontaneous tendon rupture in patients on chronic hemodialysis. Am J Kidney Dis. 1994;24(6):951–955. 10.1016/S0272-6386(12)81067-5. [DOI] [PubMed] [Google Scholar]
- 65. Valga F, Monzón T, Vega-Diaz N, Rodriguez-Perez JC, Ruiz-Santana S. Inflammation and hemodialysis adequacy: are C-reactive protein levels influenced by the dose of dialysis? Nefrologia. 2021;42(2):163–170. 10.1016/j.nefroe.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 66. Nowak KL, Chonchol M. Does inflammation affect outcomes in dialysis patients? Semin Dial. 2018;31(4):388–397. 10.1111/sdi.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures-is there a complete functional restitution? Injury. 2004;35(9):922–926. 10.1016/S0020-1383(03)00261-4. [DOI] [PubMed] [Google Scholar]
- 68. Hong AR, Lee JH, Kim JH, Kim SW, Shin CS. Effect of endogenous parathyroid hormone on bone geometry and skeletal microarchitecture. Calcif Tissue Int. 2019;104(4):382–389. 10.1007/s00223-019-00517-0. [DOI] [PubMed] [Google Scholar]
- 69. Rejnmark L, Ejlsmark-Svensson H. Effects of PTH and PTH hypersecretion on bone: a clinical perspective. Curr Osteoporos Rep. 2020;18(3):103–114. 10.1007/s11914-020-00574-7. [DOI] [PubMed] [Google Scholar]
- 70. Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(8):1811–1820. 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Negri AL, Del Valle EE, Zanchetta MB, et al. Evaluation of bone microarchitecture by high-resolution peripheral quantitative computed tomography (HR-pQCT) in hemodialysis patients. Osteoporos Int. 2012;23(10):2543–2550. 10.1007/s00198-011-1890-9. [DOI] [PubMed] [Google Scholar]
- 72. Wang L, You X, Zhang L, Zhang C, Zou W. Mechanical regulation of bone remodeling. Bone Res. 2022;10(1):16. 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rittweger J, Simunic B, Bilancio G, et al. Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone. 2009;44(4):612–618. 10.1016/j.bone.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


