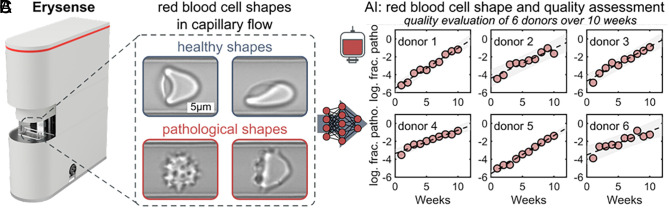
Recently, Isiksacan et al. introduced in their perspective article the assessment of stored red blood cells (RBCs) through lab-on-a-chip (LOC) technologies for precision transfusion medicine (1). The article presents a timely vision and we share and support the central message; however, a broader perspective is warranted in light of the European experience. Europe accounts for more than 35% of the annual 56 million blood transfusions worldwide registered by the World Health Organization (2). It is thus worthy to emphasize that the European Commission will impose quality control requirements for stored RBC through the regulation of quality and safety standards for substances of human origin (SoHO) intended for human application (3) repealing previous regulations (Directive 2002/98EC, 2004/23EC). The proposal of Isiksacan et al. (1) is thus not simply visionary but a compulsory requirement and calls for the timely implementation of novel technologies. One such LOC device is already commercially available on the European market specifically designed for RBC quality assessment (Erysense, Cysmic GmbH, Germany) (4) and meets most of the requirements defined by Isiksacan et al. (1). In contrast to other more academic approaches, chip geometry is kept simple by design (4): Capillary flow is mimicked with no constrictions and thus avoids the risk of channel clogging (Fig. 1A). Furthermore, several relevant cellular properties govern RBC morphology in flow, including cytosolic viscosity (and thus hydration status), cytoskeleton integrity, and also plasma membrane properties. This means shape changes during flow (Fig. 1B) reflect the biophysical and biochemical status of RBC and thus reveal signs of storage lesions. It was shown that the use of Erysense in combination with AI–based prediction models (5) can monitor RBC quality during standardized storage conditions, revealing i) a constant decline in RBC quality and ii) a donor-related variability (6) (Fig. 1C). If incorporated into routine blood banking, regular testing of blood units may not only impact clinical outcomes from a personalized medicine perspective (1) but would also reduce the number of blood units discarded owing to the current use of one-size-fits-all expiration dates [approximately 1.4 million units annually worldwide (2)]. To fully realize the potential of such LOC devices, we urge that the use of AI in “In vitro diagnostic” devices needs simplifying; current approval procedures by regulatory bodies (e.g., Food and Drug Administration; Medical Drug Administration) do not yet facilitate the advantages of the “self-improving” properties of AI (6, 7).
Fig. 1.
Erysense device, principle of measurement, and AI–based analysis of single-cell microfluidic flow behavior of stored RBCs. (A) Image of the Erysense device. A micro-structured chip without the need to connect any tubes (i.e., no cleaning requirements, no risk for contamination) requires very small volumes of packed RBCs (1 µL). It is an all-integrated “point-of-care” device with a footprint of an Espresso machine. (B) Representative images of healthy croissant-shaped (Top Left) and slipper-shaped RBCs (Top Right) at low (1 mm/s) and high (10 mm/s) velocities, respectively. Representative images of pathological RBC shapes during capillary flow (Lower images). Flow is from Left to Right in all images. (C) AI-based evaluation of donor-dependent (donors 1 to 6) changes in the RBC microcapillary flow behavior. Dashed black lines correspond to linear fits. Gray areas indicate an estimate of a 95% prediction interval. The y-axis shows the logarithm of the fraction of pathological RBC shapes based on the shape phase diagrams. Panels A and B are reproduced with permission from Recktenwald et al. 2022 (4) and panel C with permission from Lopes et al. 2023 (6).
Last but not least, an additional aspect should be considered as part of the vision of precision transfusion medicine extending the perspective of Isiksacan et al. (1): A number of European research institutions and companies focus on the in vitro production of RBCs at a scale relevant for transfusions (8–10). These cultured RBCs can be “designed” in terms of optimizing allosteric properties. Table 1 summarizes the conceptual outline and provides the state-of-the-art. In future, the transfusion of tailored in vitro-produced RBCs may represent the most sophisticated approach for personalized transfusions.
Table 1.
Conceptual use of in vitro produced RBCs in personalized transfusion medicine
| Order | Requirement/procedure | Example | State-of-the-art |
|---|---|---|---|
| 1 | Patient (group)-specific allosteric requirement(s) | Rare blood group types and allo-immunized patients | Undertreated patients due to lack of matching blood; need for cultured RBCs |
| 2 | Source of precursor cells | Inducible pluripotent stem cells or embryonic stem cells | Isolation protocols are well established; use of embryonic stem cells ethically banned in some countries |
| 3 | Genetic manipulation | Knock-out or replacement of particular proteins | CRISPR/Cas systems allow for straightforward manipulations |
| 4 | Expansion and differentiation | Upscaling in bioreactors, culture medium composition, postprocess purification of enucleated cells | Stirred bioreactors and perfusion, free of animal/human components, leukocyte depletion filters |
| 5 | Quality control | Investigation of capillary flow properties | Under development; clinical trials with the transfusion of cultured RBCs are ongoing |
| 6 | RBC conservation | Storage conditions, freezing protocols | Available protocols for erythrocytes may not suit cultured reticulocytes |
| 7 | Quality control | Capillary flow properties | So far only based on hemolysis, but potential for LOC devices like Erysense (Fig. 1) |
| 8 | Personalized transfusion upon request | For planned surgeries McLeod patients can get autologous blood transfusions | Although a “misuse “, there are established protocols for autologous blood doping based on frozen blood samples (can still be refined) |
Acknowledgments
Author contributions
L.K. developed the idea; and L.K., P.S., M.v.L., and W.E.N. wrote the paper.
Competing interests
L.K. is a shareholder of Cysmic GmbH. The other authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Isiksacan Z., et al. , Assessment of stored red blood cells through lab-on-a-chip technologies for precision transfusion medicine. Proc. Natl. Acad. Sci. U.S.A. 120, e2115616120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, “Global status report on blood safety and availability 2021” (World Health Organisation, Geneva, Switzerland, 2022). [Google Scholar]
- 3.European Commission Safety Directorate-General for Health and Food, “Proposal for a Regulation of the European Parliament and of the council on standards of quality and safety for substances of human origin intended for human application and repealing directives 2002/98/EC and 2004/23/EC” (Document 52022PC0338, European Commission, Brussels, Belgium, 2022). [Google Scholar]
- 4.Recktenwald S. M., et al. , Erysense, a lab-on-a-chip-based point-of-care device to evaluate red blood cell flow properties with multiple clinical applications. Front. Physiol. 13, 884690 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kihm A., Kaestner L., Wagner C., Quint S., Classification of red blood cell shapes in flow using outlier tolerant machine learning. PLoS Comput. Biol. 14, e1006278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes M. G. M., et al. , Big data in transfusion medicine and artificial intelligence analysis for red blood cell quality control. Transf. Med. Hemother. 50, 163–173 (2023), 10.1159/000530458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaestner L., Artificial intelligence: Training the trainer. Br. J. Haematol. 198, 805–806 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Gallego-Murillo J. S., et al. , Expansion and differentiation of ex vivo cultured erythroblasts in scalable stirred bioreactors. Biotechnol. Bioeng. 119, 3096–3116 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernecker C., et al. , Enhanced ex vivo generation of erythroid cells from human induced pluripotent stem cells in a simplified cell culture system with low cytokine support. Stem Cells Dev. 28, 1540–1551 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrin S., Severn C. E., Toye A. M., Towards manufactured red blood cells for the treatment of inherited anemia. Haematologica 106, 2304–2311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]