Significance
Aluminum is an earth-abundant metal used in a great many structural and technological applications. All aluminum is terminated with a surface oxide; it is the properties of this alumina layer that govern the interactions of aluminum with molecules and materials. Here, we show that the alumina layer surrounding aluminum nanocrystals can be altered in multiple ways by annealing the nanocrystals in various chemical ambients. The crystalline phase, type of defect, and defect density can all be modified by this straightforward approach. Changing the alumina layer in this manner affects its catalytic properties, particularly for light-driven chemical reactions where the aluminum nanocrystal core acts as an optical frequency antenna and the defects induced in the aluminum surface oxide play the role of reactive sites.
Keywords: aluminum, alumina, surface oxide
Abstract
Aluminum nanocrystals (AlNCs) are of increasing interest as sustainable, earth-abundant nanoparticles for visible wavelength plasmonics and as versatile nanoantennas for energy-efficient plasmonic photocatalysis. Here, we show that annealing AlNCs under various gases and thermal conditions induces substantial, systematic changes in their surface oxide, modifying crystalline phase, surface morphology, density, and defect type and concentration. Tailoring the surface oxide properties enables AlNCs to function as all-aluminum-based antenna-reactor plasmonic photocatalysts, with the modified surface oxides providing varying reactivities and selectivities for several chemical reactions.
Alumina is a highly versatile material, with applications as diverse as catalyst supports, cutting tools, and surgical implants (1–5). In addition to its stable α-Al2O3 phase, alumina can exist in a range of metastable “transition alumina” phases, each with unique properties, allowing specific alumina phases to be selected for a given application (6, 7). For example, η- and γ-aluminas are used as catalyst supports for alcohol dehydration and hydrogen sulfide decomposition (the Claus process) due to their hydrothermal stability, controllable surface area, rich defect chemistry, and the presence of both acidic and basic surface sites (8–10). However, the wide bandgap of the various alumina polymorphs, nominally 7 eV, has precluded their use in photocatalysis.
Leveraging the versatile surface chemistry of the aluminas for visible photocatalysis requires a new approach. One particularly attractive strategy would be to use an alumina polymorph with catalytically active surface sites as a reactor, in combination with the light-harvesting properties and nonequilibrium processes of a plasmonic antenna (11). Plasmonic antenna-reactor photocatalysis is of rapidly growing interest and importance because it can dramatically lower the effective activation energy of multiple chemical reactions, enabling highly endothermic reactions to proceed without an external heat source (12–14). Coinage metals such as Au, Ag, or Cu that sustain localized surface plasmons make outstanding optical antennas for photocatalysis. Aluminum, the most abundant metal in the Earth’s crust, supports a size- and morphology-tunable plasmon resonance, making it a promising and sustainable optical antenna material (15, 16). Aluminum nanocrystals (AlNCs) have recently been demonstrated as plasmonic photocatalysts and as antennas in antenna-reactor photocatalytic complexes for reactions such as methanol decomposition and acetylene hydrogenation (17, 18). A thin (~4 nm), self-limiting native oxide grows on the AlNC surface, protecting it from further uncontrolled oxidation and degradation under ambient conditions (19).
Here, we demonstrate all-aluminum-based plasmonic antenna-reactor photocatalysts, nanoparticle complexes composed of an Al core and different transition alumina polymorphs with their corresponding active sites. Thermal annealing of colloidally synthesized AlNCs under various conditions controllably modifies the surface alumina, transforming several properties of the native oxide. We examine how this approach modulates oxide porosity and coordination environment, altering surface chemistry, strain, and catalytic properties. By comparing plasmonic photocatalytic activity of AlNCs under three different annealing conditions, we observe that photocatalytic activity is dependent upon defect density and type. The modified oxide can provide electron traps for reactions favoring basic surfaces or, by enhancing the crystallinity of the surface oxide, can reduce the density of chemically reactive trap states compared to trap-rich surface oxides. The ability to modify AlNC surface oxide properties by such a straightforward annealing step enables a surprisingly wide range of chemistries that can be facilitated by this all-Al antenna-reactor plasmonic photocatalyst complex.
Results
AlNCs were synthesized in an Ar-filled glovebox following a previously described procedure (20), purified by centrifugation, and then dried in a vacuum desiccator. Upon exposure to ambient postsynthesis, the pristine AlNCs react with ambient atmosphere to grow an ultrathin, self-limiting native oxide, which can be observed using energy-dispersive X-ray (EDX) imaging (Fig. 1A). The amorphous nature of the native oxide can be confirmed by an apparent lack of sharp oxide peaks in its X-ray diffraction spectrum (XRD, SI Appendix, Fig. S1). The AlNCs were annealed at 500 °C for 60 min under three different ambient conditions: A) low vacuum (~10−2 torr), B) ultrahigh purity helium, or C) 10% oxygen in helium atmosphere. Following these annealing protocols, EDX measurements showed that all samples retained their essential morphology (Fig. 1 B–D). The He-annealed AlNCs retained a similar oxide percentage relative to the unannealed AlNCs, the O2-annealed AlNCs exhibited a significantly higher percentage of oxide, strongest at the AlNC edges, and the vacuum-annealed samples also appeared to have a greater oxygen content, indicating a thicker oxide shell. This was confirmed by EDX analysis which also showed an increased atomic fraction of oxygen for O2− and vacuum-annealed AlNCs (Fig. 1E and SI Appendix, Fig. S2).
Fig. 1.
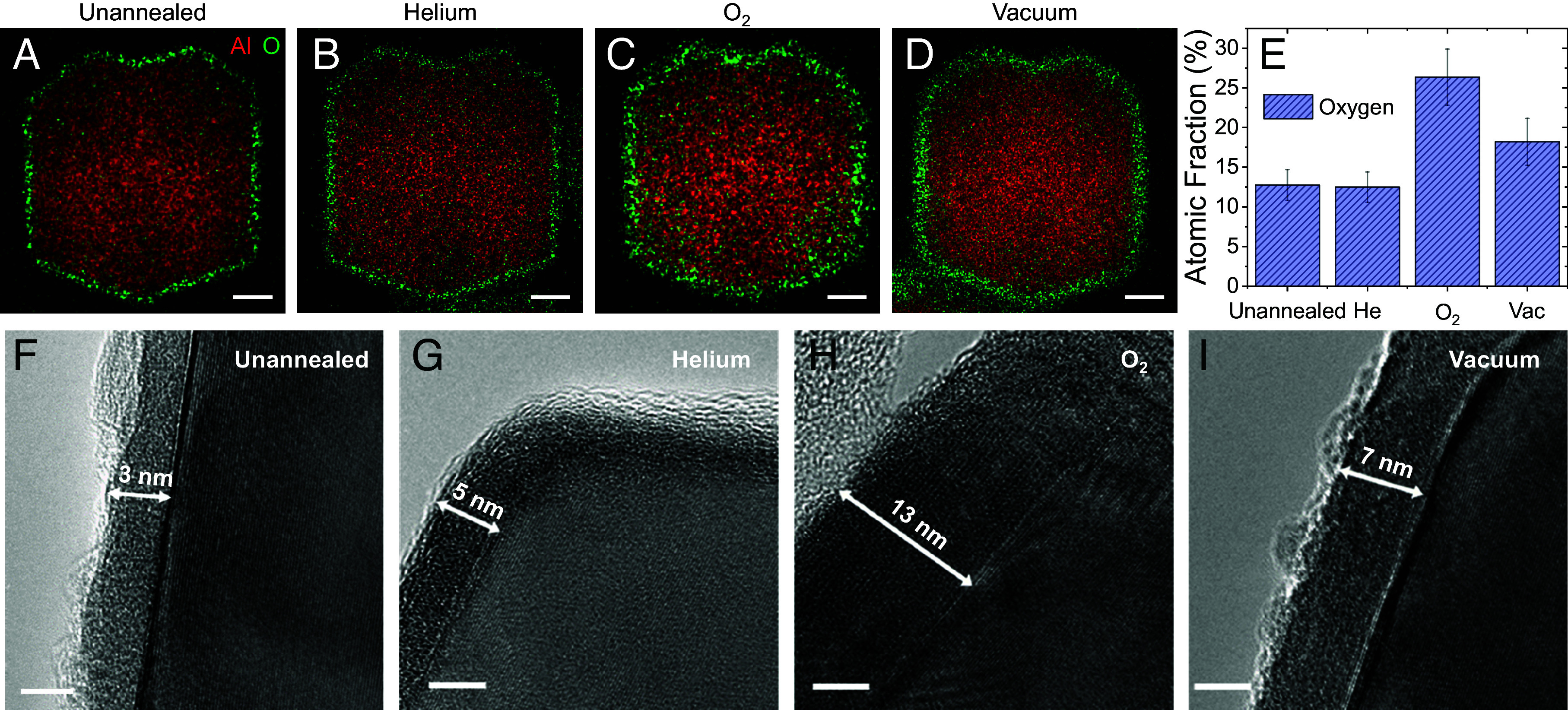
Morphologies of AlNC surface oxides that have undergone different annealing protocols. EDX elemental mapping of (A) unannealed, (B) He-annealed, (C) O2-annealed, and (D) vacuum-annealed single AlNCs. Al is red, O is green, and scale bars are 50 nm. (E) EDX atomic fraction of oxygen for each sample. (F−I) HRTEM of AlNC oxide layers. The measured oxide thickness is marked. (Scale bars are 5 nm.)
Transmission Electron Microscopy Imaging.
To examine the morphology of the various oxide layers with greater resolution, high-resolution transmission electron microscopy (HRTEM) imaging was performed. Several significant changes in oxide thickness and properties upon application of various annealing conditions were observed. He-annealed particles possess a characteristic ~4-nm alumina shell, while O2-annealed and vacuum-annealed AlNCs possess oxide layers of 11 ± 2.2 nm and 7.6 ± 1.2 nm thickness, respectively (Fig. 1 F−I and SI Appendix, Fig. S3). While the native oxide thickness was unchanged by He annealing relative to the unannealed case, the substantially increased thickness of the O2-annealed AlNCs is likely due to oxidation of the AlNC core during the annealing process. The increased thickness of the vacuum-annealed AlNCs likely originates from a weaker oxidation of the AlNC core due to the lower oxygen content of its annealing ambient, followed by subsequent air-ambient exposure. For the vacuum-annealed case, there is a distinct surface roughness observable at the oxide–air interface not present for AlNCs that have undergone other annealing treatments (Fig. 1I).
All AlNCs possess a crystalline metallic core with a 4.04 Å lattice spacing. The alumina layer of the O2-annealed AlNCs also exhibits a crystalline lattice, with a 3.90 Å lattice constant (SI Appendix, Fig. S4), while the other annealing conditions yielded amorphous oxide shells. This is confirmed by XRD measurements, where the well-defined Al oxide peaks at 46° and 67° are only observed for O2-annealed AlNCs (SI Appendix, Fig. S5) (21). By lengthening the O2 annealing time, the intensity of the oxide XRD peaks increased at the expense of the metal peaks (Fig. 2A), confirming that the crystalline oxide shell grows and the oxide mass ratio (ratio of oxide to metal) increases as the Al core is oxidized (Fig. 2 A, Inset). The metal peaks progressively shift to lower angles, indicating increasing Al core strain (Fig. 2 A, Inset); HRTEM reveals Moiré fringe patterns radiating from the oxide–metal interface due to strain induced in the Al core by surface oxide crystallization and the resulting lattice mismatch (Fig. 2B).
Fig. 2.
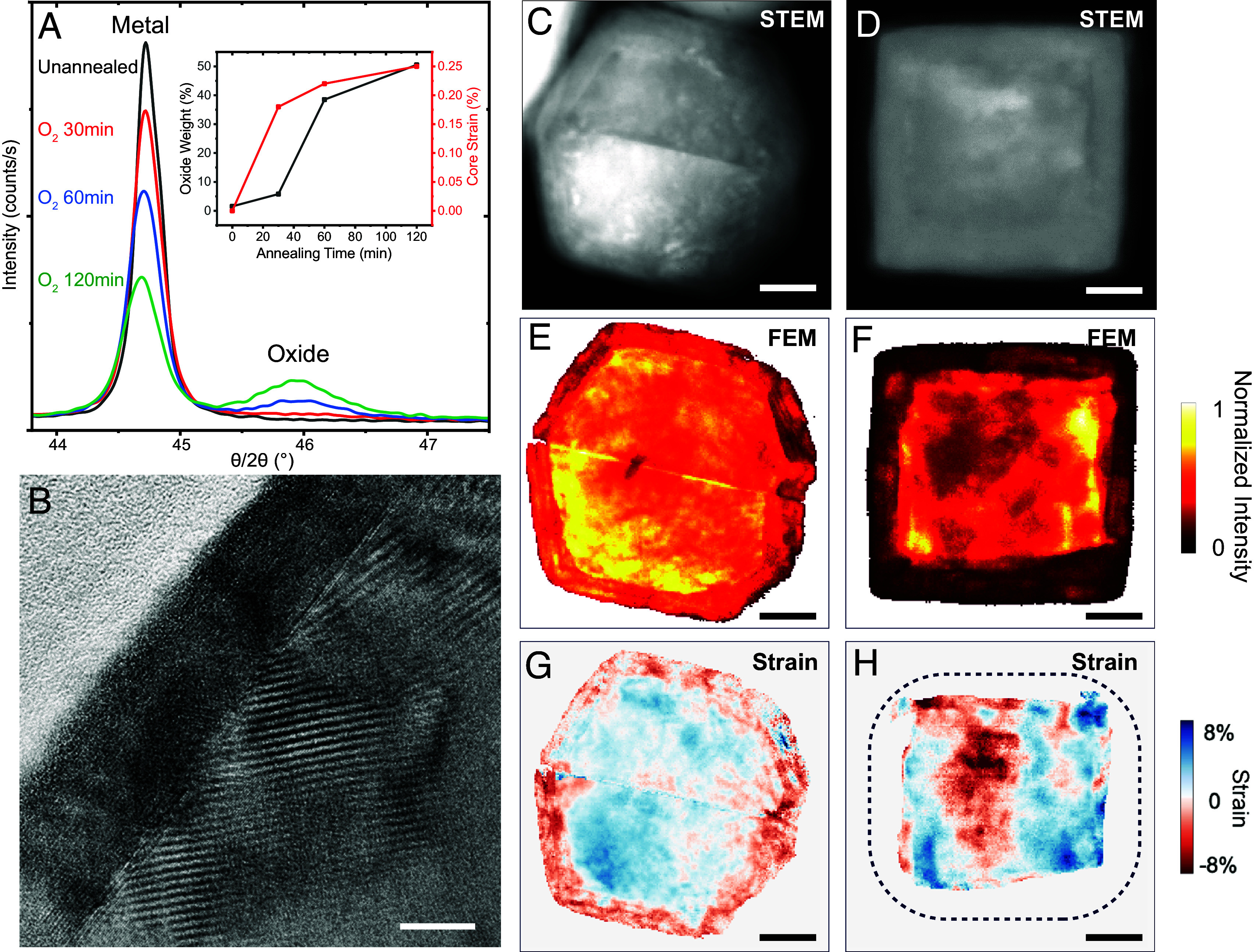
Structural and strain mapping analysis of O2-annealed AlNCs. (A) XRD of AlNCs unannealed and annealed in O2 for different times. (Inset) Oxide weight ratio and Al core strain calculated from the XRD peak analysis. (B) HRTEM of the edge of a cuboctahedron particle following O2 annealing, demonstrating additional strain-induced fringe patterns radiating from metal-oxide interface. The scale bar is 10 nm. HAADF-STEM image of an O2-annealed (C) twinned AlNC and (D) Al nanocube. (E and F) Corresponding FEM maps for the twinned particle particles. (G and H) Corresponding strain maps for the twinned and cube particles. Dashed outline of cube represents the approximate location of the surface oxide-vacuum interface. (Scale bars are 20 nm.)
The degree of crystallinity and intraparticle strain induced by O2 annealing was investigated in further detail using 4D-Scanning TEM (4D-STEM) (22, 23). Al nanocubes were synthesized following a previously described procedure (24), annealed in O2, and compared to O2-annealed twinned particles. High-angle annular dark field (HAADF) STEM images revealed particles of both morphologies to have a thicker oxide shell while overall particle shape was conserved. (Fig. 2 C and D). Fluctuation Electron Microscopy (FEM) allows for crystallinity information to be extracted from a 4D-STEM dataset, in which higher intensity correlates to higher crystallinity (25). Interestingly, while both twinned and nanocube particles demonstrate a highly crystalline core, the twinned particle surface oxide is primarily crystalline while the cube surface oxide appears substantially more amorphous (Fig. 2 E and F). This is attributed to the possibility that the {100} facets of the cube may potentially frustrate oxide crystallization, an observation that merits further study. The nanocube core appears distorted after annealing when compared to an unannealed nanocube (SI Appendix, Fig. S6) due to preferential oxidation at its vertices. Strain mapping reveals the presence of strain variation across the oxide and core of the twinned particle, which arises from lattice distortion near the metal-oxide interface (Fig. 2G). The Al nanocube images suggest that the core also undergoes residual strain, although the amorphous character of the oxide precludes the ability to extract strain information from that region (Fig. 2H). Images of the He-, and Vac-annealed AlNCs reveal low core strain (SI Appendix, Fig. S7).
Plasmonic Properties.
Individual nanoparticle dark-field scattering spectra provide information regarding how annealing affects the plasmonic properties of the AlNCs (ensemble extinction spectra shown in SI Appendix, Fig. S8). The annealing process induces two primary trends that can modify the plasmon resonance frequency in different ways. Growth of a thicker oxide dielectric layer surrounding the AlNC metallic core, observed for O2 and vacuum annealing (Fig. 1E), should redshift the plasmon resonance. But since the oxide layer grows by oxidization of the metallic core (Fig. 2A), and the plasmon resonance frequency of AlNCs depends upon Al core size, reduction of the core diameter should blueshift the plasmon resonance. Consequently, individual dark-field spectra of pre- and postannealed AlNCs should reflect these competing effects.
A characteristic single-particle dark field scattering spectrum, obtained for an individual AlNC before and after O2 annealing, is shown (Fig. 3A). The O2 annealing process blueshifts the dipolar plasmon resonance relative to the unannealed resonance at ~3.3 eV. O2 annealing also decreases the magnitude of the quadrupolar plasmon feature at nominally 4.7 to 4.8 eV, consistent with a decrease in Al core size. Thus, the decrease of the Al core size has a greater effect on the plasmonic properties than the oxide growth. To examine the subtleties of these two opposing effects, we obtained dark-field spectra of multiple AlNCs, before and after O2 annealing (Fig. 3B). We observe a blueshifted plasmon for this cohort of annealed AlNCs relative to the unannealed case, with statistical variations. The theoretically calculated dipolar plasmon peak maximum for this range of AlNC sizes, for two different oxide thicknesses, is overlaid on the experimental spectral maxima, highlighting the statistical variation observed.
Fig. 3.
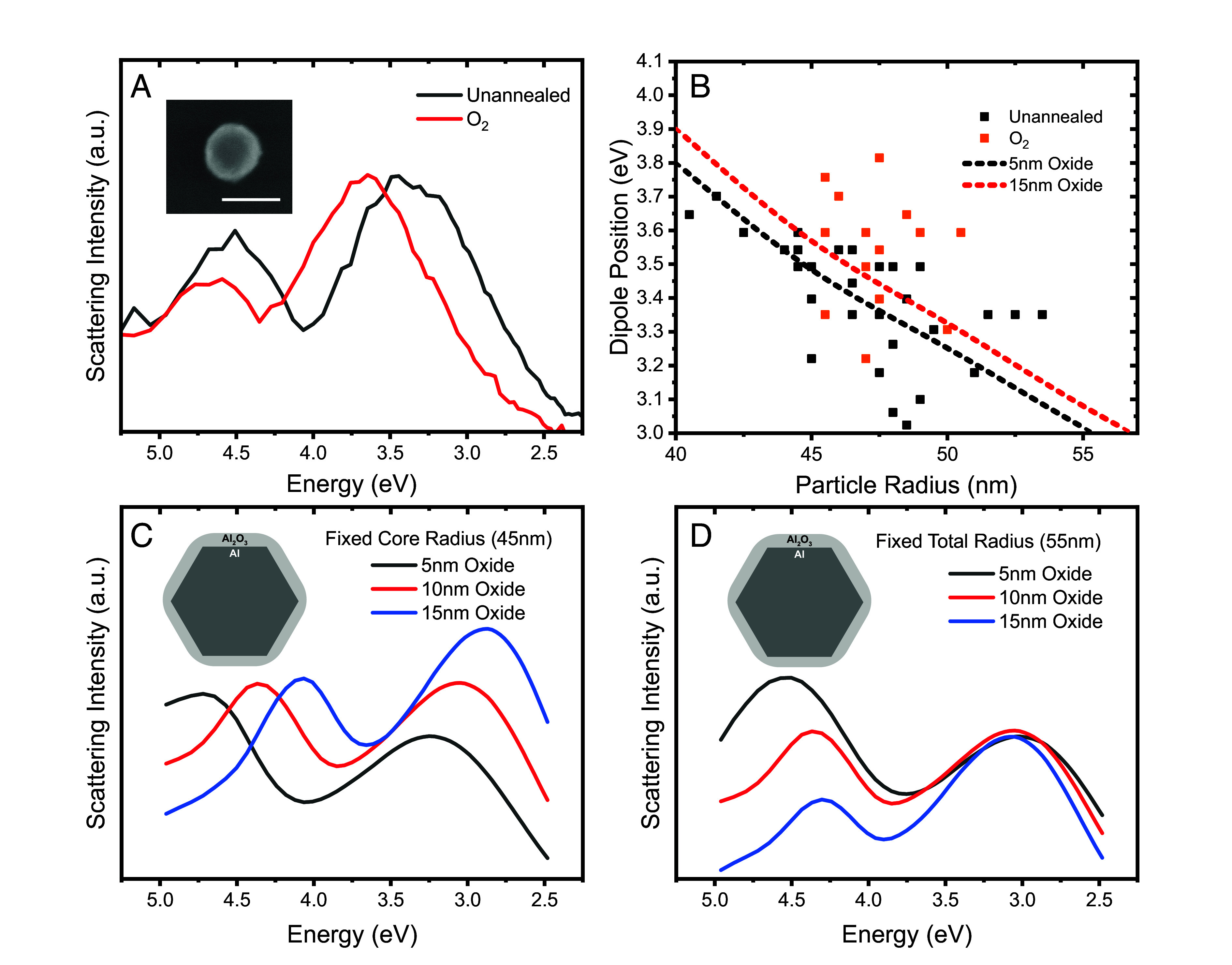
Plasmonic properties of individual AlNCs before and after O2 annealing. (A) Experimental single-particle scattering spectra of an AlNC before and after O2-annealing. (Inset) Scanning electron microscope image of the AlNC after annealing. The scale bar is 100 nm. (B) Plot of measured AlNC diameters and corresponding dipole peak positions for a cohort of annealed and unannealed AlNCs. Dashed lines represent the calculated dipolar plasmon energies for AlNCs with a fixed 5-nm (black) or 15-nm (red) surface oxide layer. (C) Calculated dark-field spectra of a 45-nm fixed radius AlNC core with changing shell thicknesses. (D) Calculated dark-field spectra of a 55-nm total radius AlNC with changing core radii and oxide layer thicknesses. (C and D, Inset) Schematic of theoretical model for AlNC.
Theoretical spectra allow us to examine the effect of oxide growth distinct from the experimental case where the core size is also simultaneously reduced, showing that this effect alone would produce an easily detectable redshifted plasmon resonance (Fig. 3C). A combination of both oxide layer growth and core size reduction can be modeled by keeping the total size of the Al NC constant, reducing the Al core while increasing the oxide layer thickness (Fig. 3D). This is consistent with our images of AlNCs before and after annealing, where changes in total particle diameter are not typically observed. In this case, the primary modification to the plasmonic response is the decreased amplitude of the quadrupolar plasmon, consistent with our single-particle spectra (Fig. 3A).
NMR Spectroscopy.
The types of defects present in the oxide layer are strongly dependent upon the annealing process. Solid-state NMR (ssNMR) spectroscopy was performed to investigate the relative concentrations of the primary alumina coordination states: AlO4 (tetrahedral), AlO5 (pentavalent), and AlO6 (octahedral) (Fig. 4A) (26, 27). Both 4- and 6-coordinate sites are characteristic of crystalline morphologies; the 5-coordinate sites are defect sites with an unsaturated Al valence and a square pyramidal morphology (28, 29). 27Al ssNMR spectra reveal how the three different annealing conditions affect oxide coordination ratios (Fig. 4 B–E), quantified by integrating the corresponding 27Al NMR spectral peaks. The unannealed AlNCs had weaker NMR signal intensities than the annealed samples, likely due to relative oxide thickness, NMR parameters, or rotor packing (Fig. 4B and SI Appendix, Fig. S9).
Fig. 4.
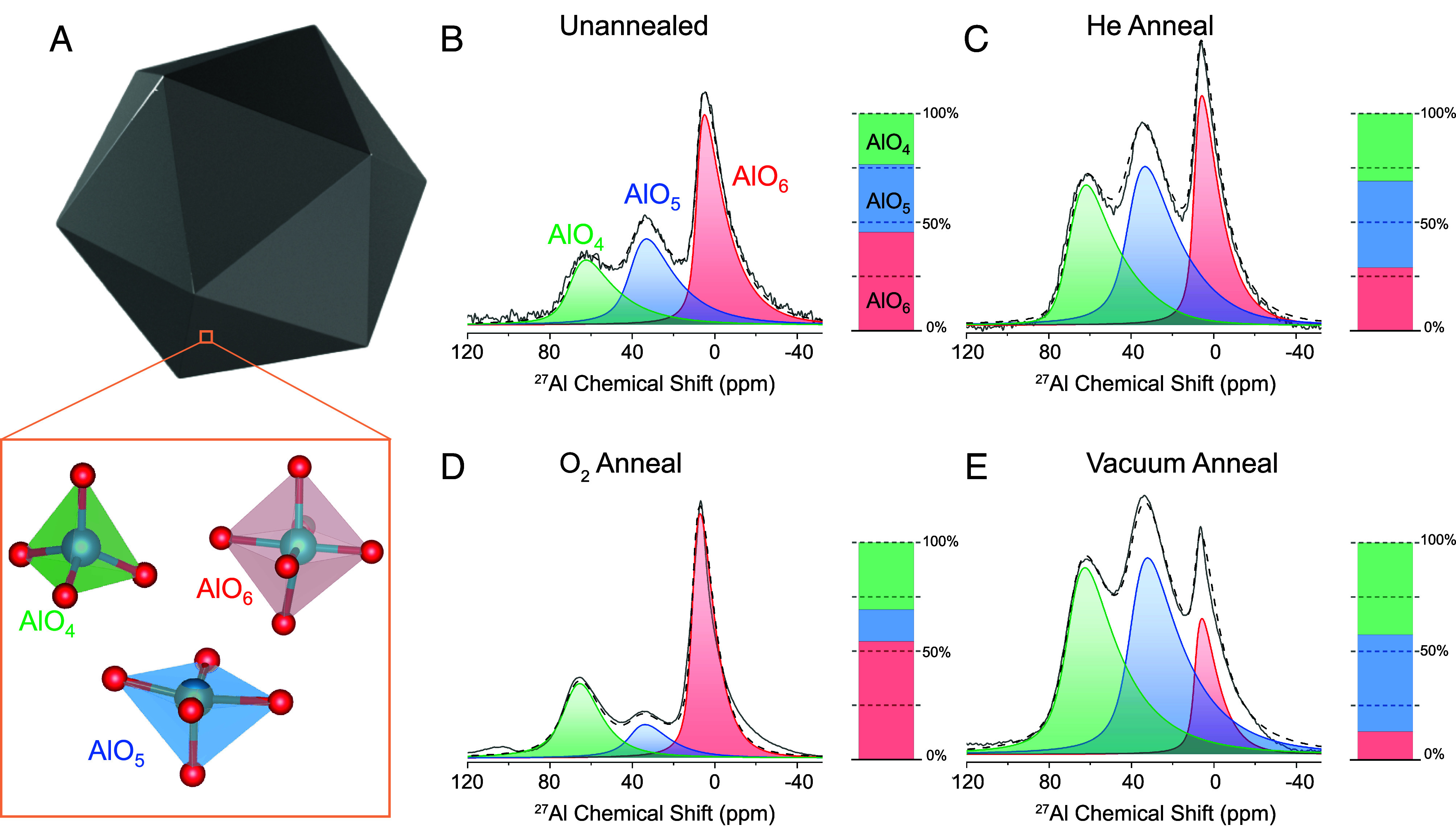
27Al ssNMR spectra of AlNC oxide following various annealing protocols. (A) AlNC illustration showing the most common Al-O coordination states present in the oxide shell. 27Al ssNMR spectra of the oxide region of (B) unannealed AlNCs, (C) He-annealed AlNCs, (D) O2-annealed AlNCs, and (E) vacuum-annealed AlNCs, with accompanying bar plot showing relative quantities of 4-coordinate (green), 5-coordinate (blue), and 6-coordinate (red) alumina sites. The solid gray line represents experimental data, and the dashed black line represents the numerical fit to the data.
The relative concentrations of tetra-, penta-, and hexavalent sites were significantly modified by the various annealing protocols (SI Appendix, Fig. S10). For annealing under a He ambient (Fig. 4C), the relative concentrations of each type of coordination site were nominally equivalent, each at ~30 to 40% of the oxide. This is similar to the unannealed AlNCs, except that He-annealed AlNCs exhibited a lower relative percentage of 6-coordinate sites. In contrast, following O2 annealing, the relative contribution of 5-coordinate sites was markedly decreased (Fig. 4D), while after annealing in vacuum, the AlNCs exhibited the largest relative fraction of 4- and 5-coordinate sites (Fig. 4E) obtained for all treatments. This was confirmed by cryogenic O2 pulse chemisorption (SI Appendix, Fig. S11), which allows for quantification of surface defect sites.
Comparison of these spectra to reported 27Al ssNMR spectra of Al polymorphs indicates that the unannealed and He-annealed AlNC oxide appear most similar to ρ-alumina, also known as “hydratable alumina,” due to its strong interaction with H2O to form surface hydroxyl groups (30). The oxide of the O2-annealed AlNCs has a spectrum most similar to γ-alumina, one of the most studied alumina polymorphs due to its widespread use in thermocatalysis (31). However, the oxide of the vacuum-annealed AlNCs presents a defect-rich spectrum that cannot be attributed to any single naturally occurring alumina phase, to our knowledge. AlO5-rich 2D materials have been produced by chemical vapor deposition (32) and solvothermal methods (33), but this work represents evidence of predominantly 5-coordinate alumina in colloidal nanocrystals.
Comparing the relative intensities of the metal and oxide peaks in 27Al ssNMR allows for the calculation of total oxide mass within a given AlNC powder sample. With this information, we may estimate oxide density (SI Appendix, Note S1). From our analysis, the oxide layer of both the He- and vacuum-annealed AlNCs are ~30% less dense than the γ-alumina of the O2-annealed AlNCs, and the unannealed particles are ~72% less dense than the γ-alumina of the O2-annealed AlNCs (SI Appendix, Fig. S12). BET measurements support this large variation in oxide densities, with unannealed AlNCs being the most porous, followed by He-, vacuum-, and then O2-annealed AlNCs, in order of decreasing porosity (SI Appendix, Fig. S13).
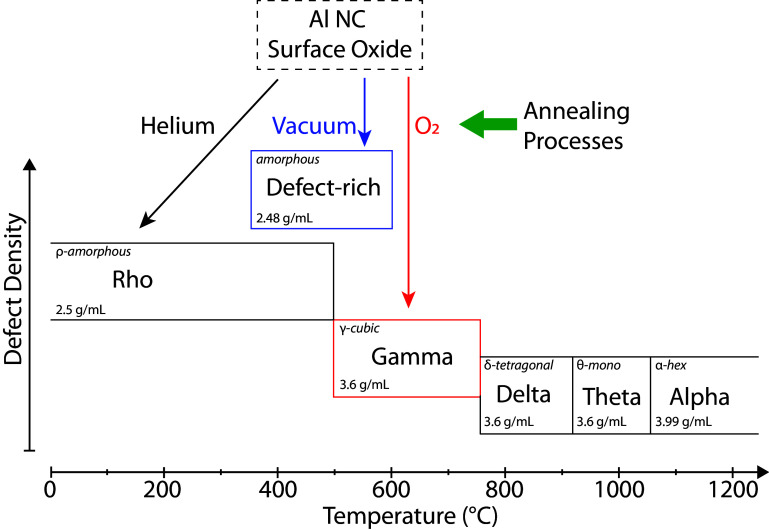
The relationship between density, phase transition temperature, and defect density of amorphous oxide, the meta-stable polymorphs, and the most thermodynamically stable phase of alumina is summarized in Fig. 5. Literature information regarding the δ-, θ-, and α-phases is shown to provide context, but these polymorphs are not experimentally observed in this study due to the relatively low annealing temperatures used (7, 27).
Fig. 5.
Schematic representation of the effect of annealing on alumina phase, density, and defect density. The experimentally observed phases in this study (ρ, γ, and defect-rich) are plotted as a function of their transition temperature (x axis) and defect density (y axis) with arrows showing the corresponding annealing ambient. Density and defect information for the high-temperature phases was adapted from refs. 7 and 27, respectively.
Types of Defects induced by Annealing.
The concentration and types of active sites present and available are critical for both thermocatalysis and plasmonic photocatalysis, lowering activation barriers for key reactions (34). Acidic sites accept electrons from adsorbates, while basic sites are electron donors (21). Because pyridine selectively binds to acidic sites and CO2 binds to basic sites (35), those molecules were used to study chemisorption on the annealed AlNCs (Fig. 6A). The He-annealed AlNCs exhibit almost exclusively basic sites, while the vacuum-annealed AlNCs possess more acidic sites than basic sites. O2 annealing appears to passivate the surface, exhibiting the fewest active sites resulting from the three types of annealing conditions. Acidic sites on alumina surfaces are attributed to exposed lower-coordination Al centers (tetrahedral and defect sites) (36). Basic sites are typically attributed to hydroxyl groups (−OH) formed concomitant with initial oxide growth upon exposure to ambient atmospheric conditions (37). We hypothesize that -OH groups are unaffected by He annealing, effectively creating a protective layer around the AlNC that shields the acidic sites from adsorbates. However, O2 annealing oxidizes the −OH groups, generating H2O and leaving a surface primarily composed of neutral AlO6. This is confirmed by mass spectrometry (MS) measurements, which allow for the tracking of desorbed species during He and O2 annealing (SI Appendix, Fig. S14). At lower annealing temperatures there is substantial desorption of physisorbed H2O and carbon species for both samples. This is followed by the emergence of an H2O peak above 480 °C during O2 annealing which gradually returns to baseline levels, indicating oxidation of the hydroxyl sites. No H2O was observed at elevated temperatures for the He-annealed AlNCs during the annealing process. Some exposed tetrahedral sites and remaining hydroxyl groups account for the low but measurable active site populations on O2-annealed AlNCs. Vacuum annealing appears to remove the O atoms from the oxide lattice through dehydroxylation and dehydration, which simultaneously generate lower coordination sites and remove many surface −OH groups, allowing adsorbates to interact with the now-exposed acidic sites (38). The effect of annealing for each ambient condition is summarized in SI Appendix, Fig. S15.
Fig. 6.
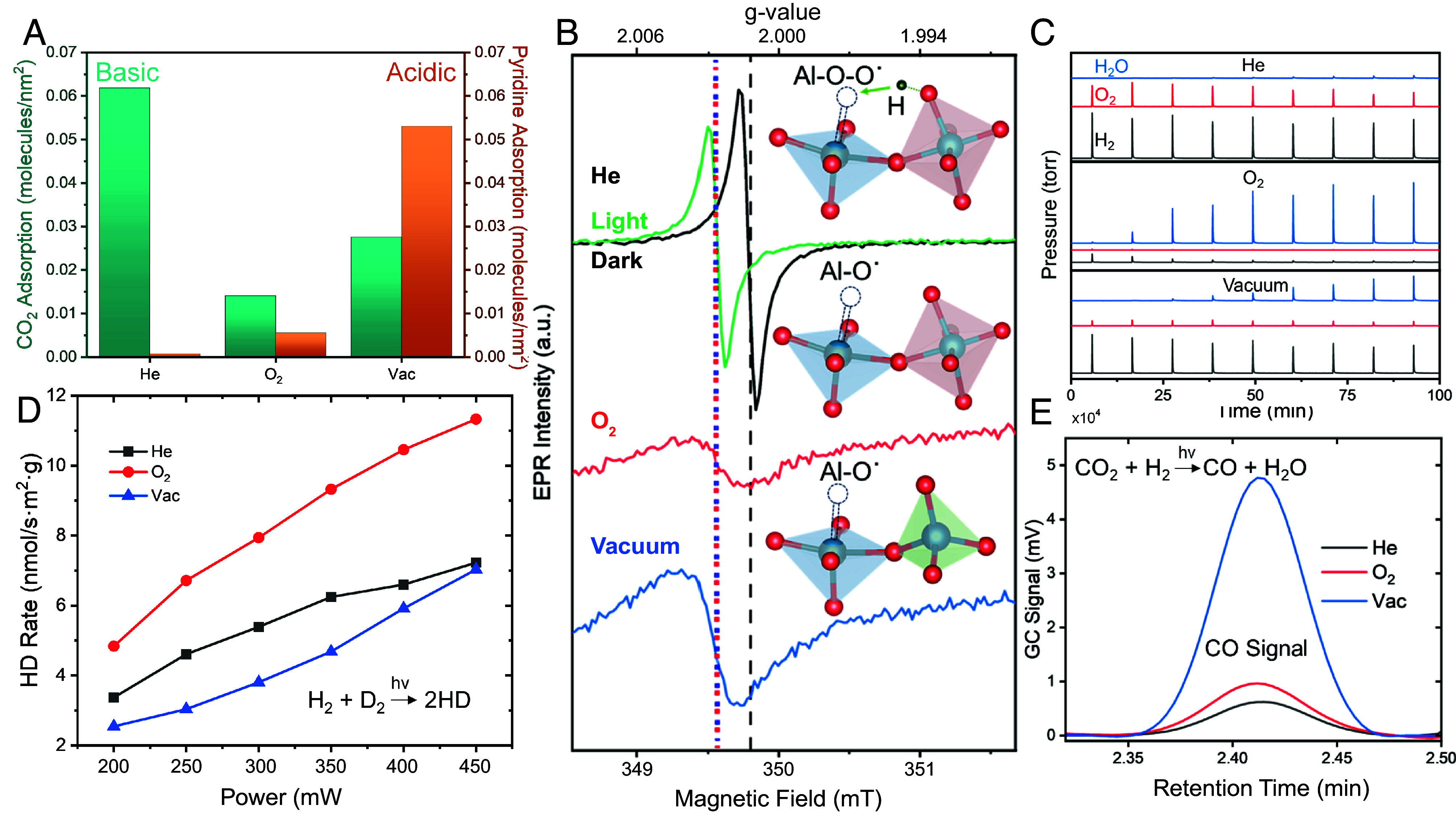
Variations in defect sites and photocatalytic activity depending upon annealing of AlNCs. (A) Measurement of CO2 and pyridine chemisorption to basic and acidic sites, respectively, on AlNCs. (B) X-Band room temperature ssEPR spectra of annealed samples. Above each curve is the proposed structure for surface defect sites in that sample. The green curve represents ssEPR of He-annealed AlNCs under illumination, while the blue-red dotted and black dashed vertical lines denote Al-O˙ and Al-O-O˙ defect signals, respectively. (C) Mass spectrometer reading of gas flow over annealed AlNCs heated to 500 °C and exposed to pulses of H2O vapor. (D) Power dependence of photocatalytic H2 dissociation under broadband illumination. (E) Measurement of GC CO signal during laser illumination.
Solid-state electron paramagnetic resonance (ssEPR) was used to probe the interaction of electrons with active sites on the AlNC surface. The measured ssEPR spectrum of He-annealed AlNCs indicates the presence of electrons occupying pentavalent defect sites stabilized by adjacent -OH groups (Al-O-O˙ sites) at the surface (Fig. 6 B, Top) (39). In contrast, the spectra of both O2− and vacuum-annealed AlNCs indicate electrons localized at pentavalent defects (Al-O˙ sites) with no neighboring hydroxyl groups (Fig. 6 B, Middle and Bottom) (39). When O2− and vacuum-annealed samples are illuminated with a broadband visible light source, they exhibit no light-induced changes (SI Appendix, Fig. S16). However, when He-annealed AlNCs are illuminated, the spectrum shifts and decreases to become more similar to the spectra of the O2− and vacuum-annealed AlNCs. This light-induced shift, which possesses an extremely long relaxation time (hours), is quite possibly related to a spin-forbidden relaxation from an excited state of the pentavalent site to its ground state, and merits further study.
Catalytic Properties.
To probe how changes to the AlNC surface oxide affect catalytic behavior, several chemical reactions were investigated (Fig. 6 C–E). Vapor-phase thermocatalytic water splitting was chosen due to its reliance on different active sites for both oxygen and hydrogen evolution (40). AlNCs were heated to 500 °C under He gas flow followed by exposure to controlled pulses of H2O vapor in the He carrier gas: desorbed H2O and the O2 and H2 produced were measured by MS (Fig. 6C). For the He-annealed AlNCs, high levels of both O2 and H2 and the absence of H2O in the product stream during the first five pulses indicate water splitting facilitated by the surface oxide layer. After that, the O2 decreases and H2O increases with each pulse, most likely indicating a subsequent slow oxidation of the Al core. The vacuum-annealed sample initially produced H2, but with little O2 generated and a delayed, slow rise in H2O. This indicates that H2O molecules quickly oxidized the surface defects, then likely started oxidizing the Al core. For the O2-annealed AlNCs, the H2O signal rises rapidly, but there is no O2 observed, and the weak H2 signal disappears after the first few H2O pulses. It is likely that the crystalline oxide protects the core from oxidation but, perhaps due to its strongly reduced density of defect sites, also does not contribute to catalysis. We conclude that the basic sites present on the He-annealed AlNC surface show a capacity for thermocatalytic splitting of water, while the crystalline oxide generated by O2 annealing enhances AlNC stability.
Next, we explored the influence of surface oxide annealing on the plasmonic H2 dissociation through the probe reaction of HD exchange (H2 + D2 ⟶ 2HD) (Fig. 6D). Our previous study of this light-induced reaction on AlNCs suggested that injection of a nonequilibrium hot electron into the antibonding orbital of H2 could facilitate its dissociation on AlNCs, in direct analogy to HD exchange on plasmonic Au nanoparticles (41). For AlNCs, the surface oxide layer is sufficiently porous to allow H2 (and D2) diffusion to the Al metal core surface, allowing hot electron transfer from metal to adsorbate molecule to occur (41). Here, we observed an increase in the HD production rate for all three catalysts with increasing light intensity from a pulsed broadband laser source (Fianium). The O2-annealed AlNCs showed slightly better photocatalytic activity toward H2 dissociation than the vacuum- and He-annealed AlNCs. Since this reaction occurs at the Al interface, where a hot electron can transfer into the H2 LUMO orbital, this variation in reactivity probes differences in diffusion of H2 through the different surface oxides. The differences in reactivity observed likely indicate that H2 diffusion through the vacuum-annealed and He-annealed alumina polymorphs is slightly reduced by the presence of disorder in those oxides relative to the substantially more crystalline γ-alumina (42).
While stoichiometric oxides generally exhibit low H2 diffusion and uptake capacity, the presence of defects in the oxide layer (i.e., the amorphous alumina layer) was previously demonstrated to substantially enhance H2 uptake and transportation through alumina (43). In light of those previous results, the observed HD exchange reactivity trend in the annealed AlNCs appears quite surprising, since the vacuum-annealed catalyst with the highest defect density from unsaturated Al(V) sites (Fig. 4) would be expected to show a higher reactivity compared to the O2-annealed catalyst with fewer oxide defects. However, the observed photocatalytic reactivity trend implies that hot-electron transfer and injection into the H2 LUMO orbital at the Al interface could play a key role on determining the outcome of photocatalysis, largely independent of oxide defect density.
The reverse water–gas shift reaction (rWGS) was also tested due to its importance for generating fuel from greenhouse gases and its known preference for basic catalytic sites under thermocatalytic conditions (44). Under white light illumination with no external heating, vacuum-annealed AlNCs exhibited a substantially higher photocatalytic CO production rate compared to the other catalysts with almost similar reactivity, as measured by gas chromatography (GC) (Fig. 6E). To probe this behavior, we performed CO2 physisorption experiments (SI Appendix, Fig. S17), which showed the highest CO2 uptake for He-annealed AlNCs, followed by vacuum- and then O2-annealed AlNCs, in accordance with the relative abundance of basic sites in Fig. 6A. In contrast, the photocatalytic rWGS rates better correlate with the concentration of acidic sites, not with the concentration of basic sites in Fig. 6A (vacuum > O2 > He). This is quite possible, since CO2 can also adsorb at surface defect sites formed by dehydroxylation (Lewis acid sites) (45). Consequently, it appears that the trend in photocatalytic activity is not determined solely by differences in CO2 adsorption: the photoexcitation of AlNCs also affects the observed relative catalytic activities. We hypothesize that while basic sites provide adsorption sites for CO2, plasmonic hot electron generation drives H2 dissociation at the Al core surface, followed by the possibility of H2- or H atoms diffusing to the CO2 binding sites to generate CO and H2O. It is possible that the combination of high defect density and the presence of hot electron-generated reactive H atoms or H2- intermediates may make the vacuum-annealed AlNCs the most effective of these photocatalysts for the rWGS reaction. Although the high degree of oxide crystallinity of O2-annealed AlNCs eliminated most defects, its moderate activity is caused by its retention of some basic sites and its ability to facilitate hot electron-driven H2 dissociation. Although He-annealed AlNCs have many basic sites, it is possible that the high concentration of surface hydroxyl groups may screen the interaction of adsorbates with those defect sites, leading to less photocatalytic activity. While the precise reaction mechanism would require extensive further study, this reaction illustrates how tailoring defects in surface oxides provides different reaction pathways in plasmonic photocatalysis relative to thermocatalytic approaches.
Discussion and Conclusions
We have shown that the native surface oxide of colloidally synthesized AlNCs can be altered by annealing under various conditions, modifying many of its properties. Our work demonstrates that thermal annealing under various ambient gases can dramatically modify the type and concentration of defects in and on the surface oxide, which in turn modifies their behavior as reactive sites under thermocatalytic and plasmonic photocatalytic conditions. This simple approach can be expanded to tailor the properties of a much wider range of metal oxides, grown onto plasmonic nanoparticle surfaces, to facilitate plasmonic photocatalysts for a wider range of chemical reactions yet to be studied.
Materials and Methods
AlNC Preparation.
All chemicals were purchased from Sigma-Aldrich unless otherwise noted. Dimethylethylamine alane (DMEAA) was obtained as 0.5 M solution in toluene. 1,4-dioxane and tetrahydrofuran (THF) and toluene were dried and sparged using a solvent system. High purity titanium (IV) isopropoxide (99.99%) was used. All above reagents and solvents for AlNC synthesis were handled under an inert atmosphere using a glovebox. ACS Grade isopropanol (IPA) was used. All glassware was dried in an oven at 120 °C before use. In an argon-filled glovebox, 80 mL 1,4-dioxane in a stoppered 250 mL Erlenmeyer flask was stirred vigorously for at least an hour with the hot plate set to 45 °C to ensure temperature uniformity. Twenty mL of DMEAA was added and stirred for 30 min. Two mL of titanium (IV) isopropoxide (50 mM in toluene) was added, turning the solution from clear to brown immediately and then slowly proceeding to black and gray. The reaction proceeded for 24 h and was quenched by the addition of 100 mL toluene. Products were removed from the glovebox and sonicated in loosely capped centrifuge tubes. AlNCs were isolated by multiple centrifugation cycles in IPA and then dried under house vacuum until solvent completely evaporated. At this scale, synthesis yields 200 to 240 mg of AlNC powder. Larger particles can be obtained by using a mixture of 1,4-dioxane and THF in place of pure 1,4-dioxane.
AlNC Treatment.
The dried AlNC powder was ground in a mortar and pestle to break up aggregates and then divided into separate vials. He and O2 annealing was performed in an AutoChem II 2920 using UHP He and 10% O2 in He from Airgas. For He annealing, samples were ramped in 50 sccm He flow at 30 °C/min, and then held at 500 °C for 1 h before being cooled via liquid nitrogen pump at 30 °C/min to ambient temperature. For O2 annealing, samples were ramped in He at 30 °C/min to 500 °C. Once maximum temperature was achieved, O2 flow was introduced into the system for 1 h. Then, the O2 flow was replaced by He before the 30 °C/min cooling ramp began. Vacuum annealing was performed in an MTI 53L vacuum oven with attached mechanical pump, generating 10-2 torr vacuum. The sample was heated to 500 °C at 1 °C/min, and then held at 500 °C for 1 h before being cooled back to room temperature.
Material Characterization.
Solid-state NMR measurements were performed on a 500-MHz Bruker AVIII HD wide-bore spectrometer with a zirconia 4 mm rotor at a spin rate of 12 KHz at a 10-degree pulse. Fitting was completed using Czjzek distribution on the ssNake software, a cross-platform open-source NMR data processing and fitting application (46). EPR measurements at X-Band ~9.8 GHz were performed on a Bruker ELEXSYS E500 spectrometer fitted with an ER4102ST resonator at room temperature using a 4 mm quartz sample holder. Illuminated EPR experiments were performed using a 150W Xe arc lamp coupled to a fiber that aligned into the optical port of the ER4102ST resonator at room temperature using a 4-mm quartz sample holder. TEM imaging was performed on a JEOL 2100F transmission electron microscope operating at 200 kV. HAADF-STEM, EDX, and 4D-STEM measurements were performed at 300 kV on an FEI Titan Themis3 (scanning) transmission electron microscope (S/TEM) equipped with EMPAD detector. XRD measurements were performed on a Rigaku Smartlab II with zero-background sample holder. Extinction spectra were measured on a Cary 5000 UV/Vis/NIR spectrometer.
Chemisorption and Physisorption.
All chemisorption experiments were performed on an AutoChem II 2920 equipped with thermal conductivity detector and online mass spectrometer (MKS Cirrus II). Annealed samples (50 to 80 mg) were introduced into the U-shaped sample tube and treated in 50 sccm He for one hour at 500 °C to remove any adsorbed water and atmospheric contaminants. For basic site measurements, the sample was ramped to 45 °C under 40 sccm He, and then exposed to 0.5 mL pulses of 10% CO2 in He (Airgas) until saturation. For acidic site measurements, the sample was ramped to 120 °C, and a vapor generator set to 90 °C was used to introduce pulses of anhydrous pyridine (Sigma Aldrich) until saturation. For O2 chemisorption, the sample was cooled to −90 °C and then exposed to pulses of 10% O2 in He. For H2O vapor splitting, the sample was ramped to 500 °C and a vapor generator set to 85 °C was used to introduce 0.5 mL pulses of MilliQ H2O. Pore volume and CO2 uptake were measured using a Quantachrome Autosorb-iQ3-MP/Kr BET Surface Analyzer at 273 K up to 1 bar. Samples were degassed for 4 h under vacuum at 150 °C prior to measurement.
Catalysis.
The nanoparticles were mixed with Aerosil SiO2 support particles at 10% loading, and the mixture was ground thoroughly to homogenize the catalyst. All catalytic activities were measured at a total pressure of 1 atm using about 10 mg of the catalyst loaded into a customized stainless-steel chamber flow fixed-bed reactor (Harrick Scientific Products Inc.). The photocatalytic experiments were performed at room temperature without external heating. The catalyst was illuminated using a supercontinuum fiber laser (6 ps, 80 MHz; Fianium) with 750 nm shortpass and no focusing lens. For the rWGS reaction, a 1:1 ratio of CO2/H2 (research purity, Airgas) was continuously flowed into the reaction chamber at a total flow rate of 20 sccm. For hydrogen dissociation, a 1:1 ratio of H2/D2 was flowed instead. The effluent composition was measured using a customized gas chromatograph (Shimadzu) equipped with a pulsed discharge helium ionization detector (for rWGS) and Hiden online mass spectrometer (for H2 dissociation).
4D-STEM Data Acquisition.
The 4D-STEM datasets were taken on an aberration-corrected FEI Titan Themis with an Electron Microscope Pixel Array Detector (EMPAD). As the focused electron beam scans over an AlNC sample, a diffraction pattern is recorded at each scan position by the EMPAD. Due to its high dynamic range (1,000,000:1), lattice strain can be measured with subpicometer precision through the diffraction. The 4D datasets of the AlNCs were acquired at 300 kV. A 1.76-mrad convergence angle was used, leading to a ~0.69-nm probe size. For a 300 kV electron beam, 579 ADUs represent one electron per pixel. For all the datasets, an exposure time of 1.86 ms (1 ms acquisition time along with 0.86 ms readout time) was employed when acquiring the EMPAD 4D datasets. The scan size in real space (the number of pixels the beam scans across) can be set from 64 × 64 to 512 × 512. The scan size of the data used in this work was 256 × 256.
4D-STEM Data Processing.
Domains with different lattice orientations in the 4D-STEM datasets are first segmented through unsupervised hierarchical clustering and we map the strain of each domain individually (SI Appendix, Fig. S18) (47). To avoid the dynamical multiscattering and the lattice mistilt, we utilized the exit-wave power-cepstrum (EWPC) method to measure the lattice strain (48). The raw diffraction patterns are transformed as logarithmic intensity scale first and then the power cepstrum image will be calculated as the fast Fourier transform (FFT) of the log scale diffraction (SI Appendix, Fig. S19). Since the diffraction pattern is in reciprocal space, the power cepstrum, which is FFT of diffraction, is transformed back to real space. Therefore, the real space lattice vector can be directly measured in each cepstrum image. Subsequently, the transformation matrix can be calculated through . Through polar decomposition, the transformation matrix can be separated into a rotation matrix and a strain matrix . The in-plane uniaxial strain can be computed by , , and the shear strain and rotation can be calculated by and . The biaxial strain shown in Fig. 2 can be calculated by , which represent the Al lattice size changes compared with the unstrained reference.
Fluctuation electron microscopy (FEM) images are measured by the normalized variance of the cepstrum intensity in the medium-range order (SI Appendix, Fig. S20) (25, 49). The anisotropy crystalline regions with strong peaks give higher FEM score while the isotropy disordered regions with uniform amorphous rings give lower FEM score.
Dark-Field Scattering.
Fused silica substrates from Silicon Valley Microelectronics were cleaned by sonication in acetone followed by a rinse with isopropanol (IPA). A correlation grid was formed by deposition of a 50-nm Au layer with a 2-nm Ti adhesion layer through a TEM grid with no carbon film (Ted Pella Micron Index 1). Particle samples were diluted in IPA to an appropriate degree and then ∼10 μL was drop-cast onto a prepared substrate. Reflection-mode measurements were carried out using a custom-built instrument capable of measuring from 225 to 700 nm. Measurements were taken with a 5-nm step size and an integration time of 15 to 20 s. Correlation was done with SEM on an FEI Quanta 650.
Optical Simulations.
Dark-field scattering simulations were performed with the finite-difference time-domain (FDTD) method (Lumerical Solutions 2023 R1.3). Aluminum cuboctahedrons were modeled with rounded edges of 10-nm radius of curvature. The aluminum cuboctahedrons were surrounded with a 5-, 10-, or 15-nm-thick oxide layer with rounded edges of the same radius of curvature. The particles are on top of quartz substrate. A single frequency total-field scattered-field approach was used to obtain dark-field scattering spectra at a 60° angle from substrate normal. The cuboctahedron is aligned such that light is incident on a face of the cuboctahedron. Scattering cross-sections in simulations were calculated by integration of corresponding far-field differential scattering cross-sections in a hollow cone 8-16° from substrate normal. The energy range was swept over from 2.5 to 5 eV, and the permittivity of each material at the simulated energies was interpolated from corresponding dielectric functions. For the aluminum core, alumina shell, and silica substrate, Palik’s optical constant data were used. The external medium on the incident side was assumed to be vacuum. A 1.5-nm mesh size was used for scattering cross-section simulations. Far-field scattering patterns were calculated with a custom program, based on the Schelkunoff equivalence theorem and the Lorentz reciprocity principle, that performs the near-to-far field transformation of equivalent electric and magnetic currents on a closed surface enclosing the nanoparticle. Equivalent currents were derived from fields calculated using Lumerical FDTD.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We gratefully acknowledge the support of our research from the Air Force Office of Scientific Research under Grant no. FA9550-15-1-0022, the Defense Threat Reduction Agency under Grant no. HDTRA1-16-1-0042, NSF NEWT (EEC-1449500), NSF under Grant no. DMR-1905757 (G.F.S.), and the Robert A. Welch Foundation under Grant Nos. C-1220 (N.J.H.), C-1222 (P.N.), and C-2065 (Y.H.). C.J.F. acknowledges support from the Department of Defense SMART Scholarship under OUSD/R&E, NDEP/BA-1, and Y.M.C. acknowledges support from Fulbright Colombia-Pasaporte a la Ciencia. C.S. and Y. H. also acknowledge support from NSF (CMMI–2239545). We further acknowledge the use of the Rice University Shared Equipment Authority facilities, most notably the Electron Microscopy Center, the use of the Materials Characterization Laboratory (FSU075000MAC) and the SSNMR laboratory (NSF-CHE1126587) at the Florida State University Department of Chemistry and Biochemistry.
Author contributions
H.R., Y.H., P.N., H.O.E., and N.J.H. designed research; A.B., C.J.F., C.S., L.Y., Y.Y., N.C., C.R.J., P.D., A.O., Y.M.C., and B.C. performed research; A.B., C.J.F., H.R., Y.H., and H.O.E. analyzed data; and A.B., C.J.F., C.S., H.R., Y.H., G.F.S., P.N., H.O.E., and N.J.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: A.B., Purdue University West Lafayette; and M.P., Texas Tech University.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Liu X. L., et al. , Deactivation and regeneration of Claus catalyst particles unraveled by pore network model. Chem. Engineering Sci. 211, 115305 (2020), 10.1016/j.ces.2019.115305. [DOI] [Google Scholar]
- 2.Aghaeinejad-Meybodi A., Ebadi A., Shafiei S., Khataee A., Kiadehi A. D., Degradation of Fluoxetine using catalytic ozonation in aqueous media in the presence of nano-gamma-alumina catalyst: Experimental, modeling and optimization study. Sep. Purif. Technol. 211, 551–563 (2019), 10.1016/j.seppur.2018.10.020. [DOI] [Google Scholar]
- 3.Lee W., Ji R., Gosele U., Nielsch K., Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 5, 741–747 (2006), 10.1038/nmat1717. [DOI] [PubMed] [Google Scholar]
- 4.Iwai M., Kikuchi T., Suzuki R. O., Self-ordered nanospike porous alumina fabricated under a new regime by an anodizing process in alkaline media. Sci. Rep. 11, 7240 (2021), 10.1038/s41598-021-86696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besisa N. H. A., Besisa D. H. A., Ewais E. M. M., Processing of high temperature alumina/aluminum titanate ceramic composites from clean sources. Sci. Rep. 12, 5957 (2022), 10.1038/s41598-022-09670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busca G., Advances in Catalysis, Jentoft F. C., Ed. (2014), vol. 57, pp. 319–404.
- 7.Levin I., Brandon D., Metastable alumina polymorphs: Crystal structures and transition sequences. J. Am. Ceramic Soc. 81, 1995–2012 (1998). [Google Scholar]
- 8.Lodziana Z., Topsoe N. Y., Norskov J. K., A negative surface energy for alumina. Nat. Mater. 3, 289–293 (2004), 10.1038/nmat1106. [DOI] [PubMed] [Google Scholar]
- 9.Leach B. E., Applied Industrial Catalysis, Leach B. E., Ed. (Academic Press, 1983), pp. 1–30. [Google Scholar]
- 10.Knozinger H., Ratnasamy P., Catalytic aluminas–Surface models and characterization of surface sites. Catalysis Rev. Sci. Engineering 17, 31–70 (1978), 10.1080/03602457808080878. [DOI] [Google Scholar]
- 11.Brongersma M. L., Halas N. J., Nordlander P., Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 10, 25–34 (2015), 10.1038/nnano.2014.311. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C., et al. , Al-Pd nanodisk heterodimers as antenna-reactor photocatalysts. Nano Lett. 16, 6677–6682 (2016), 10.1021/acs.nanolett.6b03582. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L. A., et al. , Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69 (2018), 10.1126/science.aat6967. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L. A., et al. , Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 5, 61–70 (2020), 10.1038/s41560-019-0517-9. [DOI] [Google Scholar]
- 15.Knight M. W., et al. , Aluminum for plasmonics. ACS Nano 8, 834–840 (2014), 10.1021/nn405495q. [DOI] [PubMed] [Google Scholar]
- 16.Sundararaman R., Narang P., Jermyn A. S., Goddard W. A., Atwater H. A., Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 5, 5788 (2014), 10.1038/ncomms6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swearer D. F., et al. , Heterometallic antenna-reactor complexes for photocatalysis. Proc. Natl. Acad. Sci. U.S.A. 113, 8916–8920 (2016), 10.1073/pnas.1609769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayles A., et al. , Al@TiO2 core-shell nanoparticles for plasmonic photocatalysis. Acs Nano 16, 5839–5850 (2022), 10.1021/acsnano.1c10995. [DOI] [PubMed] [Google Scholar]
- 19.McClain M. J., et al. , Aluminum nanocrystals. Nano Lett. 15, 2751–2755 (2015), 10.1021/acs.nanolett.5b00614. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson C. R., et al. , Shining light on aluminum nanoparticle synthesis. Acc. Chem. Res. 53, 2020–2030 (2020), 10.1021/acs.accounts.0c00419. [DOI] [PubMed] [Google Scholar]
- 21.Campelo J. M., Garcia A., Gutierrez J. M., Luna D., Marinas J. M., Alkali-promoted AlPO4 catalysis. 1. Acid-base and oxidizing-reducing properties. J. Colloid Interf. Sci. 95, 544–550 (1983), 10.1016/0021-9797(83)90213-8. [DOI] [Google Scholar]
- 22.Han Y. M., et al. , Strain mapping of two-dimensional heterostructures with subpicometer precision. Nano Lett. 18, 3746–3751 (2018), 10.1021/acs.nanolett.8b00952. [DOI] [PubMed] [Google Scholar]
- 23.Tate M. W., et al. , High dynamic range pixel array detector for scanning transmission electron microscopy. Microsc. Microanal. 22, 237–249 (2016), 10.1017/s1431927615015664. [DOI] [PubMed] [Google Scholar]
- 24.Clark B. D., et al. , Aluminum nanocubes have sharp corners. ACS Nano 13, 9682–9691 (2019), 10.1021/acsnano.9b05277. [DOI] [PubMed] [Google Scholar]
- 25.Voyles P. M., Muller D. A., Fluctuation microscopy in the STEM. Ultramicroscopy 93, 147–159 (2002), 10.1016/S0304-3991(02)00155-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee S. K., Lee S. B., Park S. Y., Yi Y. S., Ahn C. W., Structure of amorphous aluminum oxide. Phys. Rev. Lett. 103, 095501 (2009), 10.1103/PhysRevLett.103.095501. [DOI] [PubMed] [Google Scholar]
- 27.Chandran C. V., et al. , Alumina: Discriminative analysis using 3D correlation of solid-state NMR parameters. Chem. Soc. Rev. 48, 134–156 (2019), 10.1039/c8cs00321a. [DOI] [PubMed] [Google Scholar]
- 28.Kwak J. H., et al. , Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on gamma-Al2O3. Science 325, 1670–1673 (2009), 10.1126/science.1176745. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z. C., et al. , Nature of five-coordinated AI in gamma-Al2O3 revealed by ultra-high-field solid-state NMR. Acs Central Sci. 8, 795–803 (2022), 10.1021/acscentsci.1c01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinhold R. H., Slade R. C. T., Newman R. H., High-field MAS NMR, with simulations of the effects of disorder on lineshape, applied to thermal transformations of alumina hydrates. Appl. Magn. Reson. 4, 121–140 (1993), 10.1007/bf03162559. [DOI] [Google Scholar]
- 31.Waterhouse G. I. N., et al. , Structural, optical, and catalytic support properties of gamma-Al2O3 inverse opals. J. Phys. Chem. C 119, 6647–6659 (2015), 10.1021/acs.jpcc.5b00437. [DOI] [Google Scholar]
- 32.Baggetto L., et al. , Atomic scale structure of amorphous aluminum oxyhydroxide, oxide and oxycarbide films probed by very high field Al-27 nuclear magnetic resonance. Phys. Chem. Chem. Phys. 19, 8101–8110 (2017), 10.1039/c6cp07937g. [DOI] [PubMed] [Google Scholar]
- 33.Shi L., et al. , Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation. Angew. Chem. Int. Ed. Engl. 54, 13994–13998 (2015), 10.1002/anie.201507119. [DOI] [PubMed] [Google Scholar]
- 34.Corma A., Garcia H., Lewis acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Rev. 103, 4307–4365 (2003), 10.1021/cr030680z. [DOI] [PubMed] [Google Scholar]
- 35.Fierro J. L. G., Tejuca L. G., Surface interactions of carbon-dioxide and pyridine with LaCRO3 perovskite-type oxide. J. Chem. Technol. Biotechnol. Chem. Technol. 34, 29–37 (1984). [Google Scholar]
- 36.Coster D., Blumenfeld A. L., Fripiat J. J., Lewis-acid sites and surface aluminum in aluminas and zeolites–A high-resolution NMR-study. J. Phys. Chem. 98, 6201–6211 (1994), 10.1021/j100075a024. [DOI] [Google Scholar]
- 37.Hoggan P. E., Aboulayt A., Pieplu A., Nortier P., Lavalley J. C., Mechanism of COS hydrolysis on alumina. J. Catalysis 149, 300–306 (1994), 10.1006/jcat.1994.1298. [DOI] [Google Scholar]
- 38.Gasenkova I. V., Mukhurov N. I., Zhvavyi S. P., Kolesnik E. E., Stupak A. P., Effect of heat treatment in vacuum on photoluminescence of anodic alumina. Luminescence 34, 520–525 (2019), 10.1002/bio.3670. [DOI] [PubMed] [Google Scholar]
- 39.Ishizaka T., Tero-Kubota S., Kurokawa Y., Ikoma T., EPR studies on defects in sol-gel derived alumina films. J. Phys. Chem. Solids 64, 801–806 (2003), 10.1016/s0022-3697(02)00377-3. [DOI] [Google Scholar]
- 40.Seal N., Karmakar A., Kundu S., Neogi S., Undulated Ni(II)-framework with in situ-grafted open-metal and basic sites for high-performance electrochemical water oxidation and flexible composite-driven size-exclusive autotandem catalysis. Acs Sustain. Chem. Engg. 11, 979–993 (2023), 10.1021/acssuschemeng.2c05673. [DOI] [Google Scholar]
- 41.Zhou L., et al. , Aluminum nanocrystals as a plasmonic photocatalyst for hydrogen dissociation. Nano Lett. 16, 1478–1484 (2016), 10.1021/acs.nanolett.5b05149. [DOI] [PubMed] [Google Scholar]
- 42.Salomone L. S., Campabadal F., Faigon A., Electron trapping in amorphous Al2O3. J. Appl. Phys. 123, 085304 (2018), 10.1063/1.5005546. [DOI] [Google Scholar]
- 43.Wang Y., Palsson G. K., Raanaei H., Hjorvarsson B., The influence of Al2O3 coating on hydrogen uptake of materials. J. Alloys Comp. 464, L13–L16 (2008). [Google Scholar]
- 44.Gonzalez-Castano M., Dorneanu B., Arellano-Garcia H., The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 6, 954–976 (2021), 10.1039/d0re00478b. [DOI] [Google Scholar]
- 45.Szanyi J., Kwak J. H., Dissecting the steps of CO2 reduction: 1. The interaction of CO and CO2 with c-Al2O3: An in situ FTIR study. Phys. Chem. Chem. Phys. 16, 15117 (2014). [DOI] [PubMed] [Google Scholar]
- 46.van Meerten S. G. J., Franssen W. M. J., Kentgens A. P. M., ssNake: A cross-platform open-source NMR data processing and fitting application. J. Magn. Reson. 301, 56–66 (2019), 10.1016/j.jmr.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Shi C., et al. , Uncovering material deformations via machine learning combined with four-dimensional scanning transmission electron microscopy. npj Comput. Mater. 8, 114 (2022), 10.1038/s41524-022-00793-9. [DOI] [Google Scholar]
- 48.Padgett E., et al. , The exit -wave power-cepstrum transform for scanning nanobeam electron diffraction: Robust strain mapping at subnanometer resolution and subpicometer precision. Ultramicroscopy 214, 112994 (2020), 10.1016/j.ultramic.2020.112994. [DOI] [PubMed] [Google Scholar]
- 49.Pidaparthy S., Ni H., Hou H., Abraham D. P., Zuo J.-M., Fluctuation cepstral scanning transmission electron microscopy of mixed-phase amorphous materials. Ultramicroscopy 248, 113718 (2023), 10.1016/j.ultramic.2023.113718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.