Abstract
In 2020 the World Health organization announced a pandemic due to the outbreak of the Coronavirus disease 19. Pneumonia was the most common manifestation of the Sars-Cov-2 infection, however, clinical papers describe Sars-Cov-2 associated cardiovascular pathologies, such as ACS, myopericarditis, cardiomyopathies, dysrhythmias, as leading causes of increased morbidity and mortality. The short and long term prognosis of Sars-Cov-2-related cardiovascular diseases was defined not only by the disease severity itself but also by associated conditions and complications, among which mental health issues (stress, depression and anxiety) have a negative impact. The interplay between Sars-Cov-2 infection, cardiovascular disease and depression may be explained by hyperinflammation, unhealthy lifestyle and inter-organ communication, mediated by extracellular vesicles (EV) and non-coding MicroRNA (miRNA). The long Covid syndrome is characterized with orthostatic hypotension, impaired cardiac and cerebral perfusion, postural orthostatic tachycardia syndrome (POTS), syncope, chest pain, dyspnea, palpitation, chronic fatigue syndrome, ‘brain fog’, memory, cognitive and sleep difficulties, depression and anxiety. From a clinical point of view these symptoms may be considered as common symptoms representing not only a cardiac but also a neurological/psychiatric problem. Consequently assessment of these symptoms are of paramount importance. Due to their complexity, management of these patients requires multidisciplinary care.
Keywords: ACS, Covid 19, Depression, Interplay, Brain-heart crosstalk
In 2020 the World Health Organization announced a pandemic due to the outbreak of the Coronavirus disease 19 (Sars-Cov-2) [1,2], Pneumonia was the most common manifestation of the Sars-Cov-2 infection, however, clinical data from multiple reports suggest cardiovascular manifestations, thromboembolism and multiorgan failure as Sars-Cov-2 infection related complications [[3], [4], [5]].
1. Mechanisms
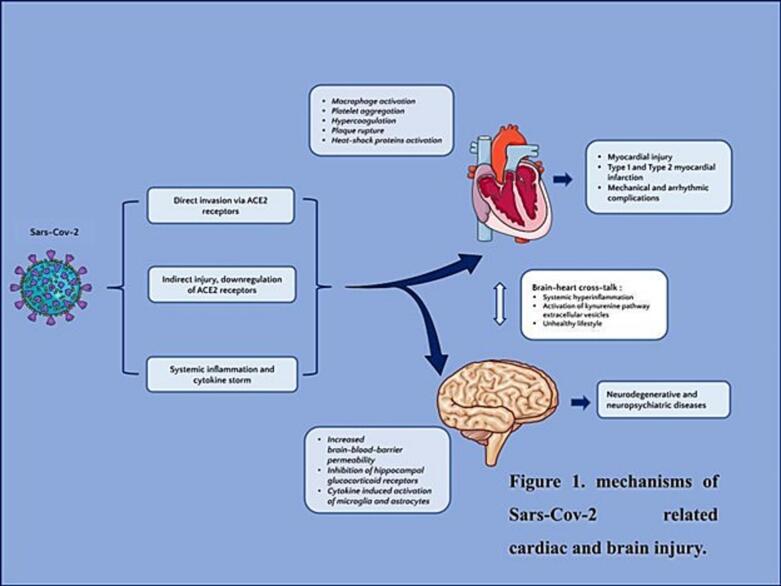
Three following mechanisms were suggested as mediators of Sars-Cov-2-related inflammation:
-
1.
Direct invasion - the virus binds to ACE 2 receptors and is then cleaved by type 2 transmembrane serine protease (TMPRSS2), which facilitates the fusion of viral and cellular membranes [6].
-
2.
An Indirect injury - viral replication induces the downregulation of ACE 2 receptors and activation of Angiotensin II type 1 receptors (Ang II/AT1) leading to vasoconstrictive, proinflammatory, prooxidant, and procoagulant effects [7].
-
3.
Systemic inflammatory response - activation of T and B lymphocytes (B/T cells) and inflammatory cytokines (interleukines, tumor necrosis factor-a (TNF-a)) promotes oxidative stress and production of free radicals resulting in endothelial dysfunction [7,8].
2. Sars-Cov-2 and cardiovascular inflammation
Immune-mediated cytokine storm promotes activation of platelets, toll-like receptors (TLRs), and macrophages leading to platelet aggregation, hypercoagulation, micro and macroangiopathy, and consequent vascular thrombosis [7,9]. Macrophage-induced disruption of atherosclerotic plaques results in type 1 myocardial infarction [7,10,11]. Hyperinflammation also may induce a mismatch in myocardial supply-demand and type 2 myocardial infarction [7].
Data from the largest cohort study (36 309 patients) show that 565 patients with out-of-hospital ST-segment elevation myocardial infarction (STEMI) and 359 patients with in-hospital STEMI had a concomitant Sars-Cov-2 infection [12]. Studies report that there was no evidence of obstructive coronary lesions in most patients (around 40%) with STEMI and Sars-Cov-2 infection [13,14]. An observational study of 115 patients demonstrated an increased thrombotic burden and poor prognosis in patients with confirmed STEMI and concomitant Sars-Cov-2 [15].
Apart from myocardial infarction cardiomyocyte damage may be clinically manifested as myocarditis, cardiomyopathy, and even cardiogenic shock [16]. Cytokine-induced myocardial cell injury contributes to the activation of heat-shock proteins, leading to further inflammation, mechanical and arrhythmic complications, and cardiomyopathy [16]. Symptoms related to mechanical and arrhythmic complications include chest pain, dyspnea, and palpitation [10]. A multicenter study across six acute hospitals used cardiac magnetic resonance imaging (MRI) for the assessment of Sars-Cov-2 related myocardial injury [17]. Non-ischemic type of late gadolinium enhancement (LGE) was demonstrated in 26 % of patients, ischemic type LGE was seen in 22 % of patients, and dual pathology was seen in 6 % of patients [17]. It should be emphasized that 66 % of patients did not have a known history of ischemic heart disease or pre-existing heart disease [17,18]. According to the German cohort study of 100 Sars-Cov-2 patients myocardial inflammation was the most prevalent (60 % of cases) abnormality, detected by cardiac MRI [19]. Other abnormalities included late gadolinium enhancement (LGE) typical for myocardial ischemia and pericardial enhancement [19].
Clinical papers report increased morbidity and mortality in patients with Sars-Cov-2 infection and concomitant cardiovascular pathologies, such as acute coronary syndrome (ACS), myopericarditis, cardiomyopathies (including takotsubo cardiomyopathy) and cardiac dysrhythmias [20] [21]. According to a Chinese study predictors of adverse clinical outcomes were severe cardiac injury (OR 2.4, 95%CI 1.8–20.1), hypotension during treatment (OR 3.4, 95%CI 2.1–17.1), and pericardial effusion (OR = 3.5, 95%CI 1.8–15.1) [22]. The short-term mortality was 20 % and major adverse events occurred in 35 % of patients with Sars-Cov-2 and myocardial injury [23]. Both cardiac MRI and high-sensitive cardiac troponin T (hs-cTnT) above sex-specific 99th percentile URLs were considered as independent predictors of prognosis in patients with Sars-Cov-2 infection [23,24]. A mortality of 22 % was seen in patients with cTn above URL, versus 61.5 % of those with cTn levels >10 times the URL [25]. Findings from a multicenter cohort study of patients with Sars-Cov-2 infection suggest that hs-cTnT <6 ng/l was related to better outcomes, with a negative predictive value of 94.9 % (87.5–98.6 95 % CI), [23]. However, according to the COVID-HEART prospective, longitudinal, multicenter, observational cohort study only late gadolinium enhancement (LGE) but not troponin was an independent predictor of major adverse cardiac events (MACE) - odds ratio, 2.25 (1.12–4.57 95%CI) [24].
The short and long-term prognosis of Covid-19-related cardiovascular diseases was defined not only by the disease severity itself but also by associated conditions such as stress, depression, and anxiety [26]. 20 % of patients with acute coronary disease have major depression, and depressive symptoms are even more frequent [27]. According to the National Adult of Cardiac Rehabilitation (NACR) registry, patients with coronary artery bypass grafting (CABG) and heart failure had an increased risk of newly developed depressive symptoms (odds ratio 1.47 (1.25–1.73 95%CI) and 1.33 (1.19–1.48 95%CI) respectively) [28]. See Table 1.
Table 1.
Depressive symptoms in patients with Sars-Cov-2 infection and long Covid syndrome.
| Paper | Study sample | Results |
|---|---|---|
| Serdar Sever [28] | The National Audit of Cardiac Rehabilitation (NACR) registry | Receiving CABG or other treatments were associated with an increase in the odds of having new-onset depressive symptoms at the start of cardiac rehabilitation with 47 % and 24 % respectively (OR: 1.47, 95%CI: 1.25, 1.73; OR: 1.24, 95%CI: 1.08, 1.43). Patients who had heart failure were 33 % more likely to have new onset depressive symptoms (OR: 1.33, 95%CI: 1.19, 1.48). |
| Bai [70] | A single-center prospective cohort study was conducted at San Paolo Hospital in Milan, Italy. Study population: adult patients who were evaluated at the post-COVID outpatient service. Participants were individuals who had clinically recovered from COVID-19 and in whom virological clearance had occurred. A total of 377 patients were enrolled in the study. |
A diagnosis of long COVID syndrome was made in 260/377 (69 %) patients. The most common reported symptoms were fatigue (149/377, 39.5 %), exertional dyspnoea (109/377, 28.9 %), musculoskeletal pain (80/377, 21.2 %), and “brain fog” (76/377, 20.2 %). Anxiety symptoms were ascertained in 71/377 (18.8 %) individuals, whereas 40/377 (10.6 %) patients presented symptoms of depression. Post-traumatic stress disorder (defined by a pathological IES-R score) was diagnosed in one-third of patients (85/275, 31 %). |
3. Sars-Cov-2 neuroinvasion and neuroinflammation and brain-heart cross-talk
Neuroinvasion of the virus occurs either via hematogenous or neuronal routes [6].
The virus binds to ACE2 in vascular endothelium or infects leukocytes (macrophages) that cross the blood-cerebrospinal fluid barrier (BCSFB) or brain-blood barrier (BBB), known as the Trojan horse mechanism [6,29,30].
Neuronal invasion of the virus occurs via olfactory, trigeminal, and vagus nerves through the, nasal cavity, rhinopharynx and lower respiratory tract [6].
Viral-induced cytokine storm increases the permeability of BBB [31,32], leading to inflammation in neuronal tissue and activation of microglia and astrocytes and subsequent neurodegenerative and neuropsychiatric diseases (stroke, mental health-related, conditions such as depression, anxiety, insomnia, dementia etc.) [31,32]. Activated cytokines inhibit hippocampal glucocorticoid receptors and contribute to the generation of reactive oxygen species [31,33]. High levels of pro-inflammatory cytokines (IL-6 and C-reactive protein) and glial activation enhance the conversion of tryptophan into kynurenine and hyperactivation of the kynurenine pathway and it's toxic metabolites (quinolinic acid, 30-hydroxykynurenine, and 3-hydroxy-anthranilic acid) leading to decrease serotonin synthesis and therefore depressive disorder [34,35]. In addition, the virus can reach brain micro vessels leading to endothelial dysfunction and microthrombotic events [36].
Some studies found higher levels of C-reactive protein (CRP) in Sars-Cov 2 patients with self-reported depression [37]. A Chinese cross-sectional study of Sars-Cov 2 patients showed a positive correlation between depression severity and CRP, while the decreased level of CRP from the baseline was related to a decreased depressive score [38].
A case-control observational study findings demonstrated that decreased tryptophan and increased kynurenine levels were correlated with increased levels of Il-6 and therefore with disease severity in patients with Sars-Cov-2 [34]. Results from an Italian cohort study indicate that Sars-Cov-2 positive patients with lymphopenia had an increased kynurenine/tryptophan ratio that reflects an increased inflammatory burden [39]. Findings from the UPBEAT UK study indicate that CRP levels, IL-6 expression, and kynurenine/tryptophan (KYN/TRP) ratio were significantly higher in depressed patients with coronary heart disease compared with non-depressed ones [40].
Overactivation of the kynurenine pathway is associated with a high risk of acute myocardial infarction in patients with stable angina [41]. In mice models, acute myocarditis was linked with decreased tryptophan and increased kynurenine levels [42].
Taken together, these findings indicate that the activation of kynurenine pathway in Sars-Cov-2 could be responsible for both cardiovascular and psychiatric (depression) complications and may play an important role in brain-heart crosstalk [34,40,73] (Fig. 1).
Fig. 1.
Mechanisms of Sars-cov-2 related cardiac and brain injury.
Along with systemic inflammation, brain-heart crosstalk is based on extracellular vesicles (EV), which carry biological information and can mediate inter-organ communication between the heart and brain in patients with Sars-Cov-2 infection [43]. These extracellular vesicles are secreted by cells, containing miRNAs and mRNA proteins [43]. Brain-derived extracellular vesicles may promote the release of inflammatory proteins, leading to systemic inflammation and involvement of the cardiovascular system [43]. In patients with Sars-Cov-2 infection, high levels of EV were related to increased coagulation and thrombotic events [44]. Owing to their mediating role in interorgan communication, non-coding miRNAs could be used as biomarkers of Sars-Cov-2-related inflammation as well as for prognostic purposes [44].
Apart from systemic inflammation, social context should be considered as an important contributor to depressive symptoms. The covid-19 pandemic was related to isolation, financial issues, stress, and depressive mood, which itself led to unhealthy behaviors, such as increased smoking, limited physical activity, consumption of cholesterol-rich food, carbohydrates, and weight gain, which are well-known traditional risk factors for cardiovascular diseases [6,8]. Increased levels of stress and anxiety could trigger cardiac arrhythmias, acute myocardial infarction and takotsubo cardiomyopathy [36]. During the Covid-19 pandemic patients with cardiovascular diseases experienced even more worsening of their mental health status (mainly due to restrictions and isolation), affecting the quality of their life [8]. HeartSleep study findings indicate that insomnia, anxiety, and depression have a negative impact on the coping process in patients with heart failure, especially during a stressful time such as a COVID-19 pandemic [45].
In summary, the interplay between Sars-Cov-2 infection, cardiovascular diseases, and depression can be attributed to multiple mechanisms, involving hyperinflammation, non-coding miRNA, unhealthy lifestyle and social restrictions [16].
4. Gender difference in Sars-Cov-2 related inflammation
Studies report sex-related differences in ACE 2 expression. In particular, western analyses of human airway smooth muscle cell lysates demonstrated lower expression of ACE 2 in females vs males, which was explained by possible protective effects of estrogens [46]. Males are more susceptible to the Sars-Cov-2 infection due to the following factors contributing to virus entry: androgen-induced expression of transmembrane serine protease 2 (TMPRSS2) [47] and testosterone-related overexpression of ACE 2 receptors [46,48].
The evidence from multiple studies highlighted that sex hormones influence different innate and adaptive immune responses [49,50]. Testosterone is recognized as immunosuppressive, while estrogens are promoting the immune response [50]. Due to the anti-inflammatory properties of estrogens, women have a more benign time course of the infection and a better prognosis compared to men [47]. The same trend was observed in animal studies - mice males were more susceptible to Sars-Cov infection and were characterized by higher inflammatory response and more severe lung injury [51].
Along with sex hormones, increased immune response and consequent adverse outcomes in men could be attributed to increased levels of proinflammatory cytokines, ferritin, and CRP, especially in elderly age [52,53].
However, the protective effect of the female sex is mitigated by the presence of classic cardiovascular risk factors, and coronary calcifications in women as demonstrated by the sCORE COVID-19 (calcium score for COVID-19 Risk Evaluation) study [54].
A particular attention should be paid to Sars-Cov-2 related takotsubo syndrome, which was more common in Sars-Cov-2 positive elderly females compared to males [55]. Age-related estrogen deprivation, increased cortisol and catecholamine release, and renin-angiotensin-aldosterone system (RAAs) system dysfunction may facilitate a high strain to cardiac muscle, leading to takotsubo syndrome [36].
5. Long Covid syndrome, neuro-cardiologic complications and prognosis
The long Covid syndrome (post-Covid syndrome) is defined as: “the condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of Sars-Cov-2, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis” [56]. Long Covid syndrome is a complex condition that involves multiple organ systems [57]. It has two phases: post-acute (from 3 weeks to 3 months from the onset of symptoms) and chronic (>3 months) [58].
A long Covid syndrome may be clinically manifested with orthostatic hypotension, postural orthostatic tachycardia syndrome (POTS), syncope, chest pain, dyspnea, palpitation, chronic fatigue syndrome, ‘brain fog’, memory, cognitive and sleep difficulties, anosmia, headache, signs of impaired cardiac and cerebral perfusion, depression and anxiety [57,59,60]. A higher prevalence of psychiatric syndromes - post-traumatic stress disorder (PTSD) (28 %), depression (31 %), anxiety (42 %), insomnia (40 %), and obsessive-compulsive (OS) symptoms (20 %) was also reported [61]. Long-Covid has a negative impact on patient's quality of life. A German cross-sectional study of 1027 patients demonstrated that 49 % of patients with post-covid syndrome reported activity limitations and participation restrictions [62].
Main mechanisms of long-Covid syndrome involve autonomic dysfunction (imbalance of parasympathetic and sympathetic systems) due to cytokine-induced hyperinflammation, oxidative stress, and mitochondrial damage [57,59]. The imbalance of parasympathetic and sympathetic systems leads to impaired function of cardiovascular and neurological systems [59]. A small cross-sectional study of long Covid patients demonstrated that patients had a higher mean heart rate and higher low frequency (LF) indices, indicating vagal dysfunction, dysautonomia, and increased pro-inflammatory state [63]. Similar findings were seen in a prospective study of 103 patients who had disturbed diurnal heart rate variability, and an impaired sympathovagal balance 252 days after infection [64].
Prolonged autoimmune neuroinflammation and persistent viral load in the central nervous system can contribute to late psychiatric syndromes, such as depression, anxiety, and PTSD [29]. Sars-Cov-2-related, prolonged immune response affects brain vessels leading to destruction of BBB, brain cell infiltration and astroglial inflammation, which can result in depression, anxiety, and insomnia [65].
According to an Italian study, the baseline systemic immune inflammation index (SH) was positively associated with anxiety and depression [61]. However, severity of anxiety, insomnia, and PTSD decreased within a 3 months period [66]. More stronger correlation was observed between depression and SH in the follow-up period [66]. Depression symptoms severity was decreased in patients, who showed a marked decrease of SH at 3 months follow-up period, while in those with minor changes in SH depressive symptoms persisted or even worsened [66]. Severe depressive symptoms also predicted poor performance in information processing in the follow-up period [66]. Findings from a Chinese cross-sectional study suggest that the 10-year coronary heart disease risk was higher in post-covid 19 patients with major depressive disorder and higher SH index [67].
An Italian prospective cohort study of Sars-Cov-2 patients showed that depression and PTSD were related to severe inflammation and decreased gray matter volumes in the anterior cingulate gyrus or insular cortex [68]. Authors suggest that gray matter and white matter microstructure and function may mediate a relationship between systemic illness (Sars-Cov-2) and psychiatric outcomes [68].
Along with biological mechanisms, other factors such as social isolation, confinement, as well as acute infection-related trauma, and persistive fatigue could be considered as possible contributors to neuropsychiatric manifestations of long-Covid syndrome [69].
Evidence from clinical studies demonstrated that the risk of long-Covid syndrome is 3 times higher in women and characterized by both physical and psychological burdens [70]. Women with long Covid syndrome reported persistive and residual symptoms of fatigue, dyspnea, increased levels of breathlessness, musculoskeletal pain, and disabilities (visual, memory, walking) compared to males [70,71]. The high prevalence of long Covid syndrome in female patients was explained by a high level of IgG antibodies [72].
From a clinical point of view symptoms of dyspnea, palpitation, and chest pain can be considered as shared symptoms representing not only a cardiac but also a neurological/psychiatric problem. Consequently, assessment of these symptoms is of paramount importance, and due to their complexity, management of these patients requires multidisciplinary care.
In summary, Sars-Cov-2-related cardiovascular and neuropsychiatric manifestations are based on complex mechanisms that can share common pathways and symptoms and therefore their management requires the use of an integrated approach.
Financial disclosure
No funding.
CRediT authorship contribution statement
Ana Gonjilashvili: Formal analysis, Resources, Visualization. Sophio Tatishvili: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
We have no conflicts of interest to disclose.
Contributor Information
Ana Gonjilashvili, Email: agonjilashvili@newvision.ge.
Sophio Tatishvili, Email: statishvili@newvision.ge.
References
- 1.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. Jun 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 — navigating the uncharted. N. Engl. J. Med. Mar 26 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese L., Ruiz D., Tehrani B., Sinha S. Acute coronary thrombosis as a complication of COVID-19. BMJ Case Reports. Mar 2021;14(3) doi: 10.1136/bcr-2020-238218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduction and Targeted Therapy. Jul 25 2020;5(1) doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. May 14 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Generoso J.S., Barichello de Quevedo J.L., Cattani M., et al. Neurobiology of COVID-19: how can the virus affect the brain?. Braz. J. Psychiatry. 2021;43(6):650–664. doi: 10.1590/1516-4446-2020-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Prete A., Conway F., Della Rocca D.G., Biondi-Zoccai G., De Felice F., Musto C., et al. COVID-19, acute myocardial injury, and infarction. Cardiac Electrophysiology Clinics. Mar 2022;14(1):29–39. doi: 10.1016/j.ccep.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucciarelli V., Nasi M., Bianco F., Seferovic J., Ivkovic V., Gallina S., et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc. Med. Jan 2022;32(1):12–17. doi: 10.1016/j.tcm.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramadan M.S., Bertolino L., Marrazzo T., Florio M.T., Durante-Mangoni E., The Monaldi Hospital Cardiovascular Infection Study Group Cardiac complications during the active phase of COVID-19: review of the current evidence. Intern. Emerg. Med. 2021;16(8):2051–2061. doi: 10.1007/s11739-021-02763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. Jul 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fard M.B., Fard S.B., Ramazi S., Atashi A., Eslamifar Z. Thrombosis in COVID-19 infection: role of platelet activation-mediated immunity. Thromb. J. Aug 23 2021;19(1) doi: 10.1186/s12959-021-00311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanaroff A.C., Garcia S., Giri J. Myocardial infarction during the COVID-19 pandemic. JAMA. Nov 16 2021;326(19):1916. doi: 10.1001/jama.2021.19608. [DOI] [PubMed] [Google Scholar]
- 13.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., et al. ST-elevation myocardial infarction in patients with COVID-19. Circulation. Jun 23 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., et al. ST-segment elevation in patients with Covid-19 — a case series. N. Engl. J. Med. Jun 18 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudry F.A., Hamshere S.M., Rathod K.S., Akhtar M.M., Archbold R.A., Guttmann O.P., Woldman S., Jain A.K., Knight C.J., Baumbach A., Mathur A., Jones D.A. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasab E.M., Aghajani H., Makoei R.H., Athari S.S. COVID-19’s immuno-pathology and cardiovascular diseases. J. Investig. Med. Jan 16 2023;71(2):71–80. doi: 10.1177/10815589221141841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. Mar 26 2020;368. [DOI] [PMC free article] [PubMed]
- 19.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) [published correction appears in JAMA Cardiol. 2020 Nov 1;5(11):1308] JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minhas A.S., Scheel P., Garibaldi B., Liu G., Horton M., Jennings M., et al. Takotsubo syndrome in the setting of COVID-19. JACC: Case Reports. Jul 2020;2(9):1321–1325. doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasudeva R., Challa A., Al Rifai M., Polana T., Duran B., Vindhyal M., et al. Prevalence of cardiovascular diseases in COVID-19 related mortality in the United States. Prog. Cardiovasc. Dis. Sep 2022;74:122–126. doi: 10.1016/j.pcad.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q., Xu L., Dai Y., et al. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin. Cardiol. 2020;43(7):796–802. doi: 10.1002/clc.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Michieli Laura, Ola Olatunde, Knott Jonathan D., Akula Ashok, Mehta Ramila A., Hodge David O., Dworak Marshall, Yang Eric H., Gharacholou Michael, Singh Gurpreet, Singh Ripudamanjit, Gulati Rajiv, Jaffe Allan S., Sandoval Yader. High-sensitivity cardiac troponin T for the detection of myocardial injury and risk stratification in COVID-19. Clin. Chem. August 2021;67(8):1080–1089. doi: 10.1093/clinchem/hvab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artico J., Shiwani H., Moon J.C., et al. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter. observational study. Circulation. 2023;147(5):364–374. doi: 10.1161/CIRCULATIONAHA.122.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cenko E., Badimon L., Bugiardini R., Claeys M.J., De Luca G., de Wit C., et al. Cardiovascular disease and COVID-19: a consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA) Cardiovasc. Res. Sep 16 2021;117(14):2705–2729. doi: 10.1093/cvr/cvab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueno-Notivol J., Gracia-García P., Olaya B., Lasheras I., López-Antón R., Santabárbara J. Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int. J. Clin. Health Psychol. Jan 2021;21(1) doi: 10.1016/j.ijchp.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtman J.H., Bigger J.T., Jr., Blumenthal J.A., Frasure-Smith N., Kaufmann P.G., Lespérance F., et al. Depression and coronary heart disease. Circulation. Oct 21 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 28.Sever S., Harrison A., Doherty P. Factors associated with new-onset depressive symptoms in patients starting cardiac rehabilitation: pre-COVID-19 and COVID-19 period comparison. J. Psychosom. Res. 2023;170 doi: 10.1016/j.jpsychores.2023.111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postolache T.T., Benros M.E., Brenner L.A. Targetable biological mechanisms implicated in emergent psychiatric conditions associated with SARS-CoV-2 infection. JAMA Psychiatry. July 31, 2020 doi: 10.1001/jamapsychiatry.2020.2795. (Published online) [DOI] [PubMed] [Google Scholar]
- 30.Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. Dec 20 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingoti M.E.D., Bertollo A.G., Simões J.L.B., Francisco G.R., Bagatini M.D., Ignácio Z.M. COVID-19, oxidative stress, and neuroinflammation in the depression route. J. Mol. Neurosci. Mar 23 2022;72(6):1166–1181. doi: 10.1007/s12031-022-02004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilotto A., Padovani A. Reply to the letter “COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation”. Ann. Neurol. 2020;88:861–862. doi: 10.1002/ana.25856. ([PMC free article] [PubMed] [CrossRef] [Google Scholar] [Ref list]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.-K., Na K.-S., Myint A.-M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Thomas T., Stefanoni D., Reisz J.A., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. Jul 23 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouças A.P., Rheinheimer J., Lagopoulos J. Why severe COVID-19 patients are at greater risk of developing depression: a molecular perspective. Neurosci. 2020 doi: 10.1177/1073858420967892. ([PubMed] [Ref list]) [DOI] [PubMed] [Google Scholar]
- 36.Lionetti V., Bollini S., Coppini R., et al. Understanding the heart-brain axis response in COVID-19 patients: a suggestive perspective for therapeutic development. Pharmacol. Res. 2021;168 doi: 10.1016/j.phrs.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan B., Li W., Liu H., et al. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Q., Zheng Y., Shi J., et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav. Immun. 2020;88:17–27. doi: 10.1016/j.bbi.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lionetto L., Ulivieri M., Capi M., et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: an observational cohort study. Biochim. Biophys. Acta Mol. basis Dis. 2021;1867(3) doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikkheslat N., Zunszain P.A., Horowitz M.A., et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav. Immun. 2015;48:8–18. doi: 10.1016/j.bbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen E.R., Tuseth N., Eussen S.J., et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler. Thromb. Vasc. Biol. 2015;35(2):455–462. doi: 10.1161/ATVBAHA.114.304674. [DOI] [PubMed] [Google Scholar]
- 42.Hoshi M., Matsumoto K., Ito H., et al. L-tryptophan-kynurenine pathway metabolites regulate type I IFNs of acute viral myocarditis in mice. J. Immunol. 2012;188(8):3980–3987. doi: 10.4049/jimmunol.1100997. [DOI] [PubMed] [Google Scholar]
- 43.Jusic A., Stellos K., Ferreira L., Baker A.H., Devaux Y. (Epi)transcriptomics in cardiovascular and neurological complications of COVID-19. Journal of Molecular and Cellular Cardiology Plus. Sep 2022;1 doi: 10.1016/j.jmccpl.2022.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosell A., Havervall S., von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality—brief report. Arterioscler. Thromb. Vasc. Biol. Feb 2021;41(2):878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connell M., Jeon S., Conley S., Linsky S., Redeker N.S. Coping, symptoms, and insomnia among people with heart failure during the COVID-19 pandemic. Eur. J. Cardiovasc. Nurs. 2023;22(3):291–298. doi: 10.1093/eurjcn/zvac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalidhindi R.S.R., Borkar N.A., Ambhore N.S., Pabelick C.M., Prakash Y.S., Sathish V. Sex steroids skew ACE2 expression in human airway: a contributing factor to sex differences in COVID-19? Am. J. Phys. Lung Cell. Mol. Phys. 2020;319(5):L843–L847. doi: 10.1152/ajplung.00391.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marik PE, DePerrior SE, Ahmad Q, Dodani S. Gender-based disparities in COVID-19 patient outcomes. J. Investig. Med. March 2021;12 doi: 10.1136/jim-2020-001641. Published online. [DOI] [PubMed] [Google Scholar]
- 48.Tukiainen T., Villani A.C., Yen A., et al. Landscape of X chromosome inactivation across human tissues [published correction appears in Nature. 2018 Mar 7;555(7695):274] Nature. 2017;550(7675):244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. Aug 22 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 50.Roved J., Westerdahl H., Hasselquist D. Sex differences in immune responses: hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. Feb 2017;88:95–105. doi: 10.1016/j.yhbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. May 15 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin L., Li X., Shi J., Yu M., Wang K., Tao Y., et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J. Med. Virol. Jun 19 2020;92(11):2684–2692. doi: 10.1002/jmv.26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Márquez E.J., Chung C. han, Marches R., Rossi R.J., Nehar-Belaid D., Eroglu A., et al. Sexual-dimorphism in human immune system aging. Nat. Commun. Feb 6 2020;11(1) doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cereda A., Toselli M., Palmisano A., Vignale D., Leone R., Nicoletti V., et al. The hidden interplay between sex and COVID-19 mortality: the role of cardiovascular calcification. GeroScience. Jul 14 2021;43(5):2215–2229. doi: 10.1007/s11357-021-00409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghasemi H., Kazemian S., Nejadghaderi S.A., Shafie M. Takotsubo syndrome and COVID-19: a systematic review. Health Sci Rep. Dec 2 2022;6(1):e972. doi: 10.1002/hsr2.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Decline in Trust in the Centers for Disease Control and Prevention During the COVID-19 Pandemic. RAND Corporation; 2021. (cited 2023 Aug 24, Internet) [DOI] [Google Scholar]
- 57.DePace N.L., Colombo J. Long-COVID syndrome and the cardiovascular system: a review of neurocardiologic effects on multiple systems. Curr. Cardiol. Rep. Sep 30 2022;24(11):1711–1726. doi: 10.1007/s11886-022-01786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Titze-de-Almeida R., da Cunha T.R., dos Santos Silva L.D., Ferreira C.S., Silva C.P., Ribeiro A.P., et al. Persistent, new-onset symptoms and mental health complaints in Long COVID in a Brazilian cohort of non-hospitalized patients. BMC Infect. Dis. Feb 8 2022;22(1) doi: 10.1186/s12879-022-07065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombo J., Weintraub M.I., Munoz R., Verma A., Ahmad G., Kaczmarski K., et al. Long COVID and the autonomic nervous system: the journey from dysautonomia to therapeutic neuro-modulation through the retrospective analysis of 152 patients. NeuroSci. May 23 2022;3(2):300–310. doi: 10.3390/neurosci3020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poenaru S., Abdallah S.J., Corrales-Medina V., Cowan J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infect. Dis. Apr 20 2021;8 doi: 10.1177/20499361211009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemhöfer C., Sturm C., Loudovici-Krug D., Best N., Gutenbrunner C. The impact of Post-COVID-Syndrome on functioning - results from a community survey in patients after mild and moderate SARS-CoV-2-infections in Germany. J Occup Med Toxicol. Oct 7 2021;16(1):45. doi: 10.1186/s12995-021-00337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menezes Junior A.D.S., Schröder A.A., Botelho S.M., Resende A.L. Cardiac autonomic function in long COVID-19 using heart rate variability: an observational cross-sectional study. J. Clin. Med. Dec 22 2022;12(1):100. doi: 10.3390/jcm12010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mooren F.C., Böckelmann I., Waranski M., et al. Autonomic dysregulation in long-term patients suffering from Post-COVID-19 Syndrome assessed by heart rate variability. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-42615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubota T., Kuroda N., Sone D. Neuropsychiatric aspects of long COVID: a comprehensive review. Psychiatry Clin. Neurosci. 2023;77(2):84–93. doi: 10.1111/pcn.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazza M.G., Palladini M., De Lorenzo R., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L., Ma X., Wang W. Inflammation and coronary heart disease risk in patients with depression in China mainland: a cross-sectional study. Neuropsychiatr. Dis. Treat. Jan 9 2020;16:81–86. doi: 10.2147/NDT.S216389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benedetti F., Palladini M., Paolini M., et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav Immun Health. 2021;18 doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Premraj L., Kannapadi N.V., Briggs J., et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai F., Tomasoni D., Falcinella C., Barbanotti D., Castoldi R., Mulè G., et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin. Microbiol. Infect. Apr 2022;28(4):611.e9–611.e16. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sigfrid L., Drake T.M., Pauley E., et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J. Med. Virol. May 22 2020;92(10):2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.https://smart.servier.com/?fbclid=IwAR3_1q-dCnQRB72bEY6VLCPp00C8Kc66wUOz4gMXap3Xebcu9Z-ICYT-LSI