Abstract
Study objective
This study investigated whether schizophrenia and the duration of schizophrenia were associated with cardiovascular autonomic neuropathy (CAN) by using heart rate variability (HRV) as a marker.
Design
Cross-sectional study.
Setting
The examinations were conducted at the Centre for Psychosis Research and at the Department of Cardiology, Aalborg University Hospital, Aalborg, Denmark.
Participants
240 patients with first-episode and chronic schizophrenia and 180 controls.
Interventions
CAN was assessed by the cardiovascular reflex tests (CARTs): HR, RS ratio, E:I ratio, and VM using a handheld device.
Main outcome measures
One abnormal CART was interpreted as borderline CAN and ≥2 abnormal CARTs established definitive CAN. Borderline CAN and definitive CAN together was categorized as overall CAN. Analyses were adjusted for age, sex, smoking, overweight, and hypercholesterolemia.
Results
A total of 240 patients with schizophrenia (median age 42.5 [28.8, 52.3], 42.9 % women) and 180 controls (median age 45.8 [24.0, 60.1], 47.8 % women) were included, with 50.8 % of patients with schizophrenia having overall CAN compared to 27.2 % among controls. Dividing patients into patients with first-episode and chronic schizophrenia, 32.9 % vs 10 % (p < 0.001) and 59.1 % vs 41 % (p < 0.001) had overall CAN compared with controls, respectively. Schizophrenia was significantly associated with overall CAN (OR, 2.80; 95%CI, 1.75–4.50), with an OR of 2.31 (95%CI, 1.14–4.68) for first-episode schizophrenia and an OR of 2.97 (95%CI, 1.81–4.87) for chronic schizophrenia.
Conclusion
It was demonstrated that a diagnosis of schizophrenia was associated with CAN. Patients with chronic schizophrenia had a significantly higher prevalence of CAN compared to patients with first-episode schizophrenia, suggesting an association between the duration of schizophrenia and CAN.
Keywords: Cardiovascular autonomic neuropathy, Cardiovascular autonomic reflex tests, Patients with schizophrenia, Schizophrenia
1. Introduction
Patients with schizophrenia have a two- to three-fold increased risk of mortality [[1], [2], [3], [4], [5], [6]], with a reduced life expectancy of 15–20 years compared to the general population [7,8], in part due to a higher prevalence of cardiovascular diseases. Other underlying mechanisms for reduced life expectancy in this patient group due to cardiovascular diseases have been suggested to be lifestyle factors, use of psychotropics, and comorbidities such as hypercholesterolaemia, hypertension, obesity, metabolic syndrome, and diabetes [7,9,10].
An important factor affecting the high cardiovascular disease burden and increased risk of cardiovascular mortality in this population is dysfunction of the autonomic nervous system, as indicated by cardiovascular autonomic neuropathy (CAN) [11]. CAN is a condition that leads to sympathetic predominance due to reduced vagus nerve activity, inducing an increased heart rate (HR), stroke volume, systemic vascular resistance, and renin-angiotensin-aldosterone system overactivity, leading to left ventricular dysfunction and potential heart failure [12]. Heart rate variability (HRV) is a reliable marker of CAN, and studies have suggested a correlation between a reduced HRV and increased CAN in patients with schizophrenia [4,13]. This is a complex association, with reduced treatment of cardiovascular disease and an increased presence of cardiac risk factors making patients with schizophrenia a vulnerable group regarding cardiovascular disease. The gold standard clinical test of CAN in patients with diabetes, according to the American Diabetes Association, is based on controlled active tests [11,14], referred to as Cardiovascular Autonomic Reflex Tests (CARTs) [11]. This approach could be applied to patients with schizophrenia considering their higher risk of cardiovascular diseases. However, CAN assessment using recommended CARTs has previously required advanced and time-consuming techniques [15]. Yet, modern techniques have made detection of CAN in patients with schizophrenia feasible as shown in a previous Danish study using a hand-held device [16].
The aim of this cross-sectional study was to investigate whether patients with schizophrenia have a higher prevalence of CAN compared to the general population and to investigate whether the severity of CAN is associated with a longer duration of the schizophrenia diagnosis based on patients with first-episode and chronic schizophrenia.
2. Methods and materials
2.1. Study design and population
This cross-sectional study analysed data from the CardioSchizoStudyGroup, based on data from a large prospective clinical cohort study in patients diagnosed with schizophrenia [17], of which a subpopulation has already been used in another study [18].
In total, 300 patients with schizophrenia >18 years of age were planned to be enrolled in this study. Patients were diagnosed according to the International Classification of Disease (ICD-10) using the codes F20 (Schizophrenia) and F25 (Schizo-affective disorder) [17]. Other inclusion criteria were residency in the North Denmark Region and being able to give an informed statement of consent. Patients were categorized based on the duration of the mental illness into first-episode (n = 100) and chronic schizophrenia (n = 200). Criteria for patients with first-episode schizophrenia were receiving the diagnosis within the last 2 years from study inclusion, while criteria for patients with chronic schizophrenia were having the diagnosis for >10 years from study inclusion.
For the patients with first-episode schizophrenia, a control group of 100 participants with no history of mental illness and no history of congenital heart disease were planned to be recruited for the study.
Due to lack of controls for patients with chronic schizophrenia from the larger prospective clinical cohort study [17], 100 healthy controls from another independent cross-sectional study of patients with diabetes were used. Only controls with no diabetes or impaired glucose tolerance by oral glucose tolerance test were included due to the nature of the independent diabetes study [19].
Exclusion criteria for all participants were the lack of ability to provide informed consent or to cooperate with the planned study programme, pregnant or lactating women, and severe claustrophobia [19].
2.2. Data collection and management
For the screening of CAN, a handheld instrument Vagus ™ (Medicus Engineering, Aarhus, Denmark) [20] was used. The device recorded and calculated RS ratio, E:I ratio, and VM based on a standard lead I ECG recording. CARTs, as recommended by the American Diabetic Association, were evaluated according to published age-related reference intervals (Table 1) [15,[20], [21], [22]]. This study utilized the definition of CAN according to the American Diabetic Association [22] of no, borderline, and definitive CAN. Borderline CAN and definitive CAN together were categorized as overall CAN.
Table 1.
Presentation of the different CARTs, how they are performed, and the limit for an abnormal test result.
| CARTs | Examination activity | Abnormal test result |
|---|---|---|
| HR | The participant rested for 4 min in a lying position during ECG-recording. | >100 bpm |
| RS | The participant stood up quickly for 1 min after lying down. As soon as the participant was standing, the ECG-recording started. The ratio was calculated as the difference between the minimum and maximum HR. | <1.03 |
| E:I | The participant breathed deeply, 6 breaths per min, for a minute, following the pace of a bar on the display of the device. The participant was sitting down during the examination, and the ECG-recording was performed during the deep breathing. The ratio was calculated as the mean maximum of HR. | <1.17 |
| VM | The participant performed a forced exhale through a mouthpiece for 15 s against a pressure of 40 mmHg. The ECG-recording started at the beginning of the exhale and lasted for 45 s after the exhale with normal breathing. The ratio was calculated as the difference between the minimum and maximum HR during forced expiration and normal breathing. | <1.20 |
Abbreviations: HR = heart rate. RS = response to standing. E:I = expiration:inspiration. VM = valsalva maneuver.
Adapted from (Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes [17]).
2.3. Covariates
Data on smoking habits, body mass index (BMI), diabetes, hypercholesterolemia, and the use of antipsychotics were included. Patients gave information regarding smoking habits, height, and weight through a questionnaire. BMI was then calculated from height and weight and categorized as normal when BMI < 25 and overweight when BMI > 25 based on WHO criteria [23]. The definition of diabetes and hypercholesterolaemia was based on ICD diagnosis codes or use of antidiabetics or lipid-lowering drugs through Anatomical Therapeutic Chemical Classification System (ATC). The use of antipsychotics was classified according to ATC.
2.4. Statistical analysis
For descriptive statistics, categorical variables were presented as number of cases and percentages, while continuous variables were presented as medians with first and third quartiles. Chi-square tests were performed for comparison of categorical data, and unpaired Wilcoxon tests were performed for comparison of continuous data.
Multivariable logistic regression analysis was used to investigate the association between schizophrenia and the presence/absence of CAN, adjusted for age, sex, smoking, overweight, and hypercholesterolemia. In case of missing data for CART results, the CART variable was interpreted as normal. This was done to ensure complete-case analysis.
Due to a low occurrence of diabetes, adjustments were not made in the general model, but a sensitivity analysis excluding all patients with diabetes was performed due to an overweight of diabetes among patients with schizophrenia compared with controls. Moreover, a subgroup analysis was performed on the following: patients with first-episode vs. chronic schizophrenia, patients with first-episode schizophrenia vs. controls, and patients with chronic schizophrenia vs. controls.
A two-sided p-value <0.05 was considered statistically significant. All data management and analysis were performed using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
2.5. Ethics
The above-mentioned studies obtained ethical approval by the research ethics committee (N-20140047 and M-20080059) and were conducted in accordance with the Declaration of Helsinki. All study subjects gave written informed consent prior to enrolment.
3. Results
3.1. Study population
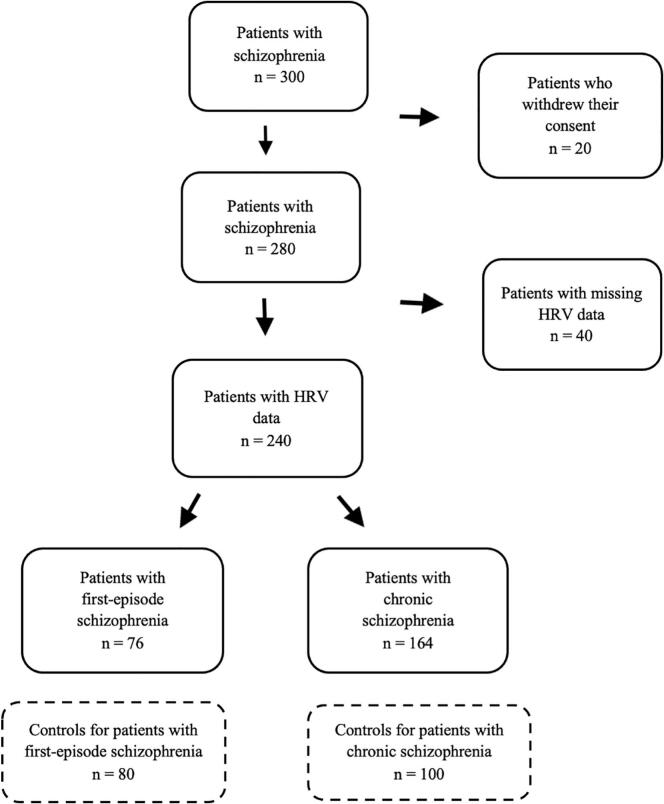
A total of 300 patients, subdivided in 100 patients with first-episode schizophrenia and 200 patients with chronic schizophrenia, were enrolled in this study. A total of 76 patients with first-episode schizophrenia and 164 patients with chronic schizophrenia were eligible in the present study, with 80 and 100 controls, respectively (Fig. 1).
Fig. 1.
Flowchart of the study population.
3.2. Characteristics of patients with schizophrenia and controls
The median age of patients with schizophrenia was 42.5 years compared to 45.8 years for controls. 42.9 % of patients with schizophrenia were female compared to 47.8 % of controls. Patients with schizophrenia had a significantly higher prevalence of smoking and overweight compared to controls, whereas the prevalence of hypercholesterolemia did not differ significantly between the two groups. 90 % of patients with schizophrenia were using antipsychotics and had a significantly higher median HR of 75 compared to a median HR of 62 for controls (Table 2.1). Table 2.2, Table 2.3 show characteristics for each schizophrenia group compared to their controls.
Table 2.1.
Demographics for patients with schizophrenia and controls.
| Control (N = 180) | Schizophrenia (N = 240) | P-value | |
|---|---|---|---|
| Gender | 0.372 | ||
| Female | 86 (47.8 %) | 103 (42.9 %) | |
| Male | 94 (52.2 %) | 137 (57.1 %) | |
| Age (years) | 0.285 | ||
| Median [q25, q75] | 45.8 [24.0, 60.1] | 42.5 [28.8, 52.3] | |
| Smoking | <0.001 | ||
| Smoker | 54 (30.0 %) | 115 (47.9 %) | |
| Former smoker | 37 (20.6 %) | 63 (26.2 %) | |
| Non-smoker | 89 (49.4 %) | 62 (25.8 %) | |
| Overweight | 84 (46.7 %) | 164 (68.3 %) | <0.001 |
| DM | 1 (0.6 %) | 35 (14.6 %) | <0.001 |
| Hypercholesterolaemia | 19 (10.6 %) | 33 (13.8 %) | 0.404 |
| Antipsychotics | 0 (0 %) | 216 (90.0 %) | <0.001 |
Table 2.2.
Demographics for patients with first-episode schizophrenia and controls.
| First-episode schizophrenia (N = 76) | Control (N = 80) | P-value | |
|---|---|---|---|
| Gender | |||
| Female | 34 (44.7 %) | 38 (47.5 %) | 0.853 |
| Male | 42 (55.3 %) | 42 (52.5 %) | |
| Age (years) | |||
| Median [q25, q75] | 24.0 [21.0, 28.0] | 23.0 [20.0, 27.0] | 0.341 |
| Smoking | |||
| Smoker | 45 (59.2 %) | 33 (41.2 %) | 0.00633 |
| Former smoker | 8 (10.5 %) | 3 (3.8 %) | |
| Non-smoker | 23 (30.3 %) | 43 (53.8 %) | |
| Missing | 0 (0 %) | 1 (1.2 %) | |
| Overweight | 49 (64.5 %) | 25 (31.2 %) | <0.001 |
| DM | 4 (5.3 %) | 1 (1.2 %) | 0.317 |
| Hypercholesterolaemia | |||
| Yes | 3 (3.9 %) | 1 (1.2 %) | 0.576 |
| Antipsychotics | |||
| Yes | 66 (86.8 %) | 0 (0 %) | <0.001 |
Abbreviations: DM = diabetes mellitus.
Table 2.3.
Demographics for patients with chronic schizophrenia and controls.
| Chronic schizophrenia (N = 164) | Control (N = 100) | P-value | |
|---|---|---|---|
| Gender | |||
| Female | 69 (42.1 %) | 48 (48.0 %) | 0.416 |
| Male | 95 (57.9 %) | 52 (52.0 %) | |
| Age (years) | |||
| Median [q25, q75] | 49.0 [41.8, 56.0] | 59.0 [52.2, 64.6] | <0.001 |
| Smoking | |||
| Smoker | 70 (42.7 %) | 21 (21.0 %) | <0.001 |
| Former smoker | 53 (32.3 %) | 33 (33.0 %) | |
| Non-smoker | 39 (23.8 %) | 46 (46.0 %) | |
| Missing | 2 (1.2 %) | 0 (0 %) | |
| Overweight | 115 (70.1 %) | 59 (59 %) | 0.0863 |
| DM | 31 (18.9 %) | 0 (0 %) | <0.001 |
| Hypercholesterolaemia | |||
| Yes | 30 (18.3 %) | 18 (18.0 %) | 0.999 |
| Antipsychotics | |||
| Yes | 150 (91.5 %) | 0 (0 %) | <0.001 |
Abbreviations: DM = diabetes mellitus.
3.3. Association between schizophrenia and CAN
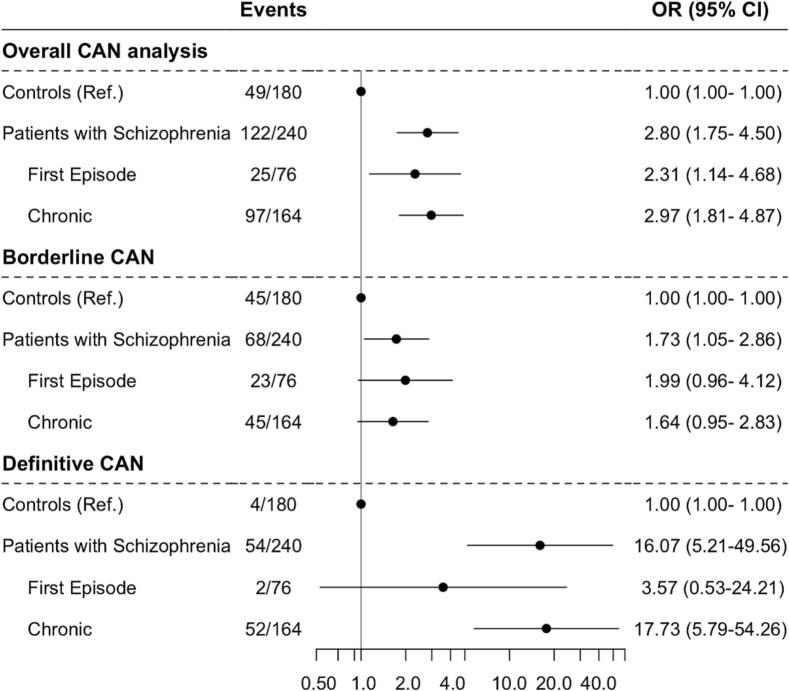
A total of n = 122 (50.8 %) of patients with schizophrenia had overall CAN compared to n = 49 (27.2 %) of controls. Overall CAN was detected in n = 25 (32.9 %) in patients with first-episode schizophrenia and n = 97 (59.1 %) in patients with chronic schizophrenia (Fig. 2).
Fig. 2.
CAN analysis in patients with schizophrenia and controls stratified by type of schizophrenia as well as the degree of CAN.
Schizophrenia was associated with a significantly increased risk of overall CAN (OR, 2.80; 95%CI, 1.75–4.50) (Fig. 2), both in patients with first-episode (OR 2.31; 95%CI, 1.14–4.68) and patients with chronic schizophrenia (OR, 2.97; 95%CI, 1.81–4.87), adjusted for age, sex, smoking, overweight, and hypercholesterolemia. Borderline CAN was associated with schizophrenia (OR 1.73, 95%CI, 1.05–2.86), albeit not significantly when stratified by first-episode and chronic schizophrenia. For definitive CAN, patients with schizophrenia had an increased OR of 16.07 (95 % 95%CI, 5.21–49.56), solely driven by the findings in the group of patients with chronic schizophrenia (OR; 17.73; 5.79–54.26), whereas no association was shown with first-episode schizophrenia (Fig. 2).
3.4. Association between schizophrenia and individual abnormal CARTs
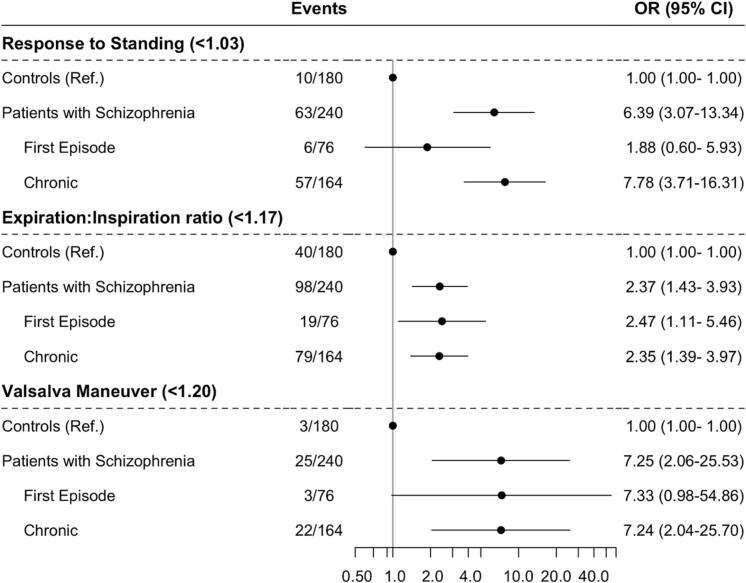
Schizophrenia was significantly associated with all of the individual abnormal CART parameters including RS (OR, 6.39; 95%CI, 3.07–13.34), E:I (OR, 2.37; 95%CI, 1.43–3.93), and VM (OR, 7.25; 95%CI, 2.06–25.53), with analyses divided by first-episode and chronic groups shown in Fig. 3. HR was not included in this analysis, as it cannot be relied upon as an individual marker of CAN due to its susceptibility to other factors such as stress [12].
Fig. 3.
The association between schizophrenia and individual abnormal HRV measurements. The threshold of an abnormal test is shown for each individual abnormal HRV parameter.
3.5. Supplementary analyses
Comparing demographics of patients with schizophrenia and their controls, patients with first-episode schizophrenia were significantly more overweight than controls (Table 2.2). No statistical significance was observed regarding overweight in patients with chronic schizophrenia vs controls (Table 2.3). In the same comparisons, it was also observed that the patients with schizophrenia had a significantly higher prevalence of smokers than their controls.
Significantly lower individual CART parameters and significantly higher HR measurements were found in patients with schizophrenia compared to controls, also when divided into first-episode and chronic groups (Tables S1, S2 and S3).
A significantly lower E:I was observed in patients with first-episode schizophrenia vs controls (Table S2). All three variables RS, E:I, and VM were significantly lower in patients with chronic schizophrenia compared to controls (Table S3).
The analysis of the association between schizophrenia and CAN, where all patients with prior diabetes were excluded (Fig. S1), showed that patients with schizophrenia were significantly associated with overall CAN (OR, 2.90; 95%CI, 1.75–4.78). When assessing the degree of CAN, schizophrenia was associated with borderline CAN (OR, 1.87; 95%CI, 1.09–3.19), both in patients with first-episode schizophrenia (OR, 2.26; 95%CI, 1.04–4.91) and in patients with chronic schizophrenia (OR, 1.73; 95%CI, 0.96–3.10). Schizophrenia was also associated with definitive CAN (OR, 16.52; 95%CI, 5.14–53.09), both in patients with first-episode (OR, 2.47; 95%CI, 0.22–27.33) and in patients with chronic schizophrenia (OR, 18.33; 95%CI, 5.76–58.30).
4. Discussion
In this cross-sectional study, it was observed that schizophrenia was associated with a significantly increased prevalence of overall CAN. More than a 2-fold increased likelihood for overall CAN in patients with schizophrenia was observed compared to controls. The highest probability for overall CAN was observed among patients with chronic schizophrenia, showing a 17-fold increased likelihood of developing definitive CAN compared to controls, while patients with first-episode schizophrenia had a 3.6-fold increased likelihood in developing definitive CAN. Looking at borderline CAN, patients with schizophrenia had almost a 2-fold increased likelihood of developing borderline CAN compared to controls, but results were non-significant when divided by first-episode and chronic groups. In general, patients with schizophrenia had a 2.37–7.25-fold increased likelihood of expressing an abnormal individual CART parameter compared to controls (Fig. 3).
The pathophysiological mechanisms explaining the association between schizophrenia and the development of CAN remain multifactorial, but a well-known factor to influence CAN is age [11,24,25]. However, in the analysis of this study, when adjusting for several confounding factors including age, schizophrenia remained significantly associated with CAN. This suggests that schizophrenia is a risk factor for developing CAN, and clinicians need to be aware of this potential risk factor for the development of CAN.
As mentioned previously, patients with chronic schizophrenia had a higher prevalence of CAN than patients with first-episode schizophrenia, which could be due to the difference in time since diagnosis. This difference in CAN could possibly be explained by a longer exposure to unhealthy lifestyle habits, cardiac risk factors, comorbidities, and antipsychotics [26].
The existence of comorbidities and altered metabolic profiles has been shown to be a risk factor for CAN [18,24]. A study by Smith et al. found a significantly higher prevalence of comorbidities in patients with schizophrenia compared to controls [27], while a reduced HRV in another study was associated to an increase in BMI, diastolic pressure, triglycerides, and high- and low-density lipoproteins in patients with schizophrenia [28]. In the present study, patients with schizophrenia likewise demonstrated a higher prevalence of smoking and overweight compared to controls which might in part explain the association between schizophrenia and CAN. However, a contradictory cross-sectional study found an under-recording of cardiovascular diseases in patients with schizophrenia compared to controls [27]. This unexpected finding might be explained by a systematic under-recognition and resulting undertreatment of cardiovascular disease in patients with schizophrenia [27]. This is supported by Attar et al., who found a significant difference in the offer and acceptance of cardiac treatment following myocardial infarction in patients with schizophrenia, where patients with schizophrenia were more prone to decline examination and treatment [29].
Regarding the use of antipsychotics, a meta-analysis highlights the importance of using a medication-free group as an ideal approach in order to analyse the effects of schizophrenia [24], and it has been highlighted that antipsychotics leads to a significantly more severe reduction in HRV compared to unmedicated patients with schizophrenia [30]. This effect may be due to antipsychotics' effect on the development of cardiometabolic disorders both directly by affecting the lipid metabolism and indirectly by increasing appetite, food intake, and weight gain [7,26]. In this study, after consultation with resident psychiatrists, it was chosen not to include information regarding type of antipsychotics (first and second generation) or e.g. chlorpromazine equivalents for the patients with schizophrenia, as it was considered only to be a “snapshot in time” and not taking into consideration what might have gone before regarding type and dose of antipsychotics, thus possibly providing an unclear association in the analysis.
Another explanation for the association between schizophrenia and a higher prevalence of cardiovascular disease is demonstrated by a study that investigates advanced glycation end products (AGEs) and schizophrenia [31]. AGEs accumulate during the human lifespan, thus a biomarker for ageing, and are strongly linked to cardiovascular mortality [31]. The study found a 15.1 % elevation of AGEs in recent-onset psychosis compared to healthy controls which equals an accumulation that normally occurs in approximately 10 years. They also found that duration of illness and antipsychotic treatment correlated with AGEs [31].
As for clinical implications of the study findings, it has already been suggested to implement screening for CAN among patients with diabetes [15,22]. A similar approach to patients with schizophrenia could be advisable, as early recognition and treatment could possibly reduce the future prevalence of CAN and development of cardiovascular disease [15,22], as suggested with other cardiovascular risk stratification tools such as the ECG [18,32,33]. In diabetes patients, it has been observed that CAN may be reduced through exercise, lifestyle interventions, ACE inhibitors, and beta-blockers [12,15,22] which could again be considered in the treatment of patients with schizophrenia. Also, the use of metformin to reduce and prevent antipsychotic weight gain in addition to regulating the glucose metabolism would be an option to consider for this patient group [34,35].
5. Limitations
Due to problems with completing the CARTs, many patients with schizophrenia had missing data in individual HRV parameters which was instead interpreted as normal to ensure complete-case analysis. However, this approach may underestimate the true association between schizophrenia and CAN. Exclusion of these participants from the analysis was not possible due to resulting small study populations, as the percentage of missing data in one single CART measurement ranged from 13.2 % to 37.2 %. Therefore, missing data was interpreted as normal.
Statistically, a larger sample size could have been beneficial, e.g. it would have been possible to only include participants with full CAN assessment and match patients with controls 1:1. Especially the VM test had a high number of missing data and might need some modification for future application.
Additionally, patients could have been grouped regarding the use of antipsychotics and other medications such as ACE-inhibitors and beta-blockers, and analyses could have been adjusted for more factors to eliminate possible confounders. Regarding the use of antipsychotics, it would likewise be of interest to transform antipsychotics into e.g. haloperidol equivalent doses in a way to see whether the dose influences HRV.
6. Conclusion
This cross-sectional study demonstrated that schizophrenia was significantly associated with CAN. A correlation between the duration of schizophrenia and definitive CAN was also observed, meaning that patients with chronic schizophrenia had a significantly higher rate of CAN relative to controls as well as patients with first-episode schizophrenia. Early recognition and screening of CAN among patients with schizophrenia may help in detecting otherwise unidentified patients at high cardiovascular risk.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jesper Fleischer reports a relationship with Medicus Engineering that includes: equity or stocks.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2023.100252.
Appendix A. Supplementary data
Supplementary material
References
- 1.Fan Z., Wu Y., Shen J., Ji T., Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J. Psychiatr. Res. 2013;47(11):1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Koponen H., Alaräisänen A., Saari K., et al. Schizophrenia and sudden cardiac death: a review. Nord. J. Psychiatry. 2008;62(5):342–345. doi: 10.1080/08039480801959323. [DOI] [PubMed] [Google Scholar]
- 3.Fujibayashi M., Matsumoto T., Kishida I., et al. Autonomic nervous system activity and psychiatric severity in schizophrenia: regular article. Psychiatry Clin. Neurosci. 2009;63(4):538–545. doi: 10.1111/j.1440-1819.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- 4.Jindal R., MacKenzie E.M., Baker G.B., Yeragani V.K. Cardiac risk and schizophrenia. J. Psychiatry Neurosci. 2005;30(6):393–395. [PMC free article] [PubMed] [Google Scholar]
- 5.Lomholt L.H., Andersen D.V., Sejrsgaard-Jacobsen C., et al. Mortality rate trends in patients diagnosed with schizophrenia or bipolar disorder: a nationwide study with 20 years of follow-up. Int. J. Bipolar Disord. 2019;7(1):6. doi: 10.1186/s40345-018-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen R.E., Uggerby A.S., Jensen S.O.W., McGrath J.J. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades—a Danish nationwide study from 1980 to 2010. Schizophr. Res. 2013;146(1–3):22–27. doi: 10.1016/j.schres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Henderson D.C., Vincenzi B., Andrea N.V., Ulloa M., Copeland P.M. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2(5):452–464. doi: 10.1016/S2215-0366(15)00115-7. [DOI] [PubMed] [Google Scholar]
- 8.Laursen T.M., Nordentoft M., Mortensen P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 9.Emul M., Kalelioglu T. Etiology of cardiovascular disease in patients with schizophrenia: current perspectives. Neuropsychiatr. Dis. Treat. 2015;11:2493–2503. doi: 10.2147/NDT.S50006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hingorani P., Karnad D.R., Natekar M., Kothari S., Narula D., Lokhandwala Y. QTc interval and its variability in patients with schizophrenia and healthy subjects: implications for a thorough QT study. Int. J. Neuropsychopharmacol. 2012;15(10):1535–1540. doi: 10.1017/S1461145712000077. [DOI] [PubMed] [Google Scholar]
- 11.Bissinger A. Cardiac autonomic neuropathy: why should cardiologists care about that? J. Diabetes Res. 2017;2017 doi: 10.1155/2017/5374176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinik A.I., Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 13.Bär K.J., Letzsch A., Jochum T., Wagner G., Greiner W., Sauer H. Loss of efferent vagal activity in acute schizophrenia. J. Psychiatr. Res. 2005;39(5):519–527. doi: 10.1016/j.jpsychires.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Didangelos T., Moralidis E., Karlafti E., et al. A comparative assessment of cardiovascular autonomic reflex testing and cardiac 123I-metaiodobenzylguanidine imaging in patients with type 1 diabetes mellitus without complications or cardiovascular risk factors. Int. J. Endocrinol. 2018;2018 doi: 10.1155/2018/5607208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulichsen E., Fleischer J., Ejskjaer N., Eldrup E., Tarnow L. Screening for diabetic cardiac autonomic neuropathy using a new handheld device. J. Diabetes Sci. Technol. 2012;6(4):965–972. doi: 10.1177/193229681200600430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobbs W.C., Fedewa M.V., MacDonald H.V., et al. The accuracy of acquiring heart rate variability from portable devices: a systematic review and meta-analysis. Sports Med. 2019;49(3):417–435. doi: 10.1007/s40279-019-01061-5. [DOI] [PubMed] [Google Scholar]
- 17.Aagaard J., Kugathasan P., Jensen S. Coronary artery disease as a cause of morbidity and mortality in patients suffering from schizophrenia: protocol for a prospective cohort study with long-term follow-up. Clin. Trials Degener. Dis. 2016;1(4):141. [Google Scholar]
- 18.Omar M., Wieben E.S., Polcwiartek C., et al. Cardiovascular autonomic neuropathy in patients with schizophrenia. Nord. J. Psychiatry. 2021;0(0):1–6. doi: 10.1080/08039488.2021.1902566. [DOI] [PubMed] [Google Scholar]
- 19.Høyem P., Hansen T.K., Kim W.Y.C.J. 2008. Immune Profile and Complication Risk in Type 2 Diabetes. [Google Scholar]
- 20.Andersen S.T., Witte D.R., Fleischer J., et al. Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes: addition-Denmark. Diabetes Care. 2018;41(12):2586–2594. doi: 10.2337/dc18-1411. [DOI] [PubMed] [Google Scholar]
- 21.Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab. J. 2019;43(1):3–30. doi: 10.4093/dmj.2018.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulton Andrew J.M., Vinik A.I.A.J., et al. Diabetic neuropathies - a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Body Mass Index.
- 24.Clamor A., Lincoln T.M., Thayer J.F., Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br. J. Psychiatry. 2016;208(1):9–16. doi: 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- 25.Fleischer J., Nielsen R., Laugesen E., Nygaard H., Poulsen P.L., Ejskjaer N. Self-monitoring of cardiac autonomic function at home is feasible. J. Diabetes Sci. Technol. 2011;5(1):107–112. doi: 10.1177/193229681100500115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen R.E., Banner J., Jensen S.E. Cardiovascular disease in patients with severe mental illness. Nat. Rev. Cardiol. 2020;18:136–145. doi: 10.1038/s41569-020-00463-7. [DOI] [PubMed] [Google Scholar]
- 27.Smith D.J., Langan J., McLean G., Guthrie B., Mercer S.W. Schizophrenia is associated with excess multiple physical-health comorbidities but low levels of recorded cardiovascular disease in primary care: cross-sectional study. BMJ Open. 2013;3(4):1–9. doi: 10.1136/bmjopen-2013-002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung M.-S., Yang A.C., Lin Y.-C., et al. Association of altered cardiac autonomic function with psychopathology and metabolic profiles in schizophrenia. Psychiatry Res. 2013;210(3):710–715. doi: 10.1016/j.psychres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Attar R., Johansen M.B., Valentin J.B., Aagaard J., Jensen S.E. Treatment following myocardial infarction in patients with schizophrenia. PLoS One. 2017;12(12):1–9. doi: 10.1371/journal.pone.0189289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung W., Jang K.-I., Lee S.-H. Heart and brain interaction of psychiatric illness: a review focused on heart rate variability, cognitive function, and quantitative electroencephalography. Clin. Psychopharmacol. Neurosci. 2019;17(4):459–474. doi: 10.9758/cpn.2019.17.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagen J.M., Sutterland A.L., Koeter M.W., Lutter R., Cohen D., de Haan L. Advanced glycation end products in recent-onset psychosis indicate early onset of cardiovascular risk. J. Clin. Psychiatry. 2017;78(9):1395–1401. doi: 10.4088/JCP.16m10972. [DOI] [PubMed] [Google Scholar]
- 32.Polcwiartek C., Kragholm K., Hansen S.M., et al. Electrocardiogram characteristics and their association with psychotropic drugs among patients with schizophrenia. Schizophr. Bull. 2020;46(2):354–362. doi: 10.1093/schbul/sbz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polcwiartek C., Atwater B.D., Kragholm K., et al. Association between ECG abnormalities and fatal cardiovascular disease among patients with and without severe mental illness. J. Am. Heart Assoc. 2021;10(2) doi: 10.1161/JAHA.120.019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yerevanian A., Soukas A.A. Metformin: mechanisms in human obesity and weight loss. Curr. Obes. Rep. 2019;8(2):156–164. doi: 10.1007/s13679-019-00335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seifarth C., Schehler B., Schneider H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes Off J. Ger. Soc. Endocrinol. [and] Ger Diabetes Assoc. 2013;121(1):27–31. doi: 10.1055/s-0032-1327734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material