Abstract
The translation elongation factor 1δ (EF-1δ) consists of two forms, a hypophosphorylated form (apparent Mr, 38,000) and a hyperphosphorylated form (apparent Mr, 40,000). Earlier Y. Kawaguchi, R. Bruni, and B. Roizman (J. Virol. 71:1019–1024, 1997) reported that whereas mock-infected cells accumulate the hypophosphorylated form, the hyperphosphorylated form of EF-1δ accumulates in cells infected with herpes simplex virus 1. We now report that the accumulation of the hyperphosphorylated EF-1δ is due to phosphorylation by UL13 protein kinase based on the following observations. (i) The relative amounts of hypo- and hyperphosphorylated EF-1δ in Vero cells infected with mutant virus lacking the UL13 gene could not be differentiated from those of mock-infected cells. In contrast, the hyperphosphorylated EF-1δ was the predominant form in Vero cells infected with wild-type viruses, a recombinant virus in which the deleted UL13 sequences were restored, or with a virus lacking the US3 gene, which also encodes a protein kinase. (ii) The absence of the hyperphosphorylated EF-1δ in cells infected with the UL13 deletion mutant was not due to failure of posttranslational modification of infected-cell protein 22 (ICP22)/US1.5 or of interaction with ICP0, inasmuch as preferential accumulation of hyperphosphorylated EF-1δ was observed in cells infected with viruses from which the genes encoding ICP22/US1.5 or ICP0 had been deleted. (iii) Both forms of EF-1δ were labeled by 32Pi in vivo, but the prevalence of the hyperphosphorylated EF-1δ was dependent on the presence of the UL13 protein. (iv) EF-1δ immunoprecipitated from uninfected Vero cells was phosphorylated by UL13 precipitated by the anti-UL13 antibody from lysates of wild-type virus-infected cells, but not by complexes formed by the interaction of the UL13 antibody with lysates of cells infected with a mutant lacking the UL13 gene. This is the first evidence that a viral protein kinase targets a cellular protein. Together with evidence that ICP0 also interacts with EF-1δ reported in the paper cited above, these data indicate that herpes simplex virus 1 has evolved a complex strategy for optimization of infected-cell protein synthesis.
Viruses are totally dependent on the synthetic machinery of host cells for their replication, and as a consequence, viral gene products interact with and modify to activate, suppress, or redirect the functions of cellular proteins. Of the strategies for attaining this objective, phosphorylation of proteins by protein kinases could be expected to be among the most potent, since this is a major strategy employed by eukaryotic cells to regulate cellular functions (5). Curiously, only the large DNA viruses encode kinases (21, 34), and except for cellular oncogenes transduced into retroviruses (e.g., v-src and v-erb) (23), there is a dearth of information on employment, modification, and redirection of cellular protein kinases by viruses to modify cellular substrates. Even among the DNA viruses which encode their own kinases, most of the available data are on specific viral substrates. In this report, we show that the translation elongation factor 1δ (EF-1δ) is phosphorylated during infection by the product of the UL13 gene of herpes simplex virus 1 (HSV-1). Although this protein has long been thought to be a protein kinase, only recently has the UL13 gene product been purified and shown to be a kinase (3). In this report, we present the first evidence that a viral kinase phosphorylates a cellular protein. Relevant to this report are the following observations.
(i) HSV-1 encodes at least 84 different proteins expressed in three major coordinately regulated, sequentially ordered groups designated α, β, and γ (10, 11, 33, 34). The expression of α genes, the first set of genes to be expressed, is enhanced by α-trans-inducing factor (34). The expression of β genes requires functional α proteins, and both α and β proteins and viral DNA synthesis mediated by β proteins are required for optimal expression of γ genes that encode largely virion structural proteins (34). Six α proteins termed infected-cell protein 0 (ICP0), ICP4, ICP22, ICP27, ICP47, and US1.5 have been identified, and for the most part, they function as regulatory proteins that affect or alter viral gene expression or alter the function of cellular proteins (1, 34).
(ii) ICP0, one of the α proteins, has been shown to be a positive, promiscuous transactivator of genes introduced into cells by infection or transfection (Fig. 1) (8). Although the mechanism by which ICP0 acts in viral replication is unclear, it is likely that ICP0 is a multifunctional protein which interacts with a variety of cellular proteins and that the function of ICP0 in virus replication results from the sum of these interactions. For instance, it has been reported that ICP0 interacts with at least three different cellular proteins, the translation factor EF-1δ (13); a cell cycle regulator, cyclin D3 (14); and a ubiquitin-specific protease, HAUSP (7). Deletions of the regions of ICP0 which interact with these cellular proteins or are involved in the ability of promiscuous transactivation impair viral lytic growth (8). Therefore, ICP0 may modulate many key cellular functions, including translation, cell cycle regulation, the protein degradation pathway, and, possibly, transcription of host cells to confer a growth advantage to the virus.
FIG. 1.
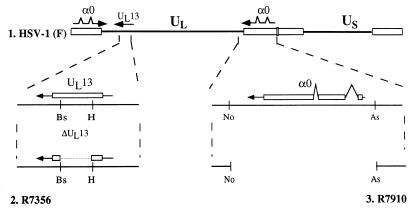
Schematic diagram of the sequence arrangement of the HSV-1 genome showing the location of the UL13 and α0 genes. Line 1 is a linear representation of the HSV-1(F) genome. The thin lines represent the unique long (UL) and unique short (US) sequences. The terminal repeats flanking the unique sequences are shown as open rectangles. Line 2 is an expanded section of the coding domain of the UL13 gene. The transcript is represented by a line capped by an arrowhead, and the coding domain of UL13 is represented by an open rectangle. R7356 contains a deletion spanning HindIII to BstEII restriction endonuclease sites within the UL13 coding domain. Line 3 is an expanded section of the coding domain of the α0 gene. The transcript and coding regions are shown for only one copy of the α0 gene. A second, identical copy is located in the terminal inverted repeat flanking UL. R7910 contains deletions of the entire coding regions of α0 genes spanning AseI to NotI sites. Bs, BstEII; H, HindIII; No, NotI; As, AseI.
(iii) An earlier report from this laboratory showed that ICP0 interacts with EF-1δ and that the domain of ICP0 which interacts with EF-1δ affects translational efficiency in vitro (13). These observations suggest that ICP0 may regulate viral gene expression at the translational level. In the course of the studies, we also found that HSV-1 infection modifies EF-1δ (13).
EF-1δ plays an important role in regulation of protein synthesis. EF-1δ is a subunit of EF-1, a complex of proteins which mediate the elongation of polypeptide chains during translation of mRNA (18, 20, 32, 36). EF-1α transports aminoacyl tRNA for binding to ribosomes concurrent with hydrolysis of GTP, whereas EF-1δ is a component of the EF-1βγδ complex responsible for GDP-GTP exchange on EF-1α (18, 20, 32, 36). EF-1δ is phosphorylated by several cellular kinases, including casein kinase II (26), cdc2 kinase (22), and protein kinase C (38). The studies on the phosphorylation of EF-1δ suggest that the hyperphosphorylation of the protein alters translational efficiency (19, 22, 31, 37–39).
(iv) HSV encodes two major protein kinases expressed by the UL13 and US3 genes, whose amino acid sequences contain motifs common to known protein kinases (2, 17, 34, 35). Whereas the amino acid sequence that encodes UL13 protein kinase is conserved in members of all subfamilies of the family Herpesviridae, that of US3 is conserved only in the Alphaherpesvirinae subfamily (2, 17, 34, 35). The known substrate of the US3 protein kinase is ICP22 and an essential viral membrane protein encoded by UL34 (29, 30). Recently it has been reported that US3 is required for protection from apoptosis induced by HSV-1 infection (16).
The UL13 protein kinase mediates the phosphorylation of several viral proteins, such as ICP22 (29), ICP0 (25), and viral glycoprotein E (24). Unlike US3, the UL13 is packaged into the virion (34). Studies with UL13 mutants showed that the viral protein kinase affects the accumulation of ICP0 and a subset of γ proteins, suggesting that the activity of UL13 plays a role in viral gene expression (28). We report here that UL13 hyperphosphorylates EF-1δ.
MATERIALS AND METHODS
Cells and viruses.
Vero and rabbit skin cells were originally obtained from the American Type Culture Collection and J. McClaren, respectively. The cell lines were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% newborn calf serum. An ICP0-expressing cell line, N3 (14), was grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. HSV-1(F), a limited-passage isolate, is the prototype strain used in this laboratory (6). The constructions of HSV-1 recombinant viruses HSV-1(F)Δ305, R7355, R7356, R7358, R7041, and R325 were reported previously (27–30). Construction of an ICP0 deletion mutant virus, R7910, is described below. Table 1 lists the genotypes of all of the viruses used in this study. All viruses except R7910 were propagated in Vero cells. The R7910 recombinant was grown in the ICP0-expressing cell line N3. All titrations of infectivity were done on Vero cells.
TABLE 1.
Genotype and phenotype of the viruses used in this study
| Virus | Genotypea | Relevant phenotype |
|---|---|---|
| HSV-1(F) | Wild type | Wild type |
| HSV-1(F) | Δ305 ΔUL23/ΔUL24 | TK− |
| R7041 | UL23R/UL24R ΔUS3 | US3− |
| R7355 | ΔUL223R/ΔUL24R ΔUL13 | TK− UL13− |
| R7356 | UL23R/UL24R ΔUL13 | UL13− |
| R7358 | ΔUL23/ΔUL24 UL13R | TK− |
| R325(TK−) | ΔUL23/ΔUL24 Δα22 | TK− ICP22− |
| R7910 | Δα0 | ICP0− |
Δ, deletion in gene; R, repair of gene; TK, thymidine kinase. A superscript minus sign indicates the function is not expressed.
Antibody and immunoblotting.
The rabbit polyclonal antibody to EF-1δ, ICP0, and UL13 was generated as described elsewhere (13, 14, 24, 25). The sera for EF-1δ used in immunoblotting and immunoprecipitation were collected 4 and 10 weeks after the first immunization, respectively. The electrophoretically separated proteins transferred to nitrocellulose sheets were reacted with the antibody to EF-1δ or ICP0 as described previously (13).
Cosmids.
Cosmids pBC1006, pBC1007, pBC1012, and pBC1013 were constructed as described elsewhere (14). Cosmid pBC1015 was constructed by cloning the product of an XbaI C fragment of HSV-1(F) viral DNA into the SpeI site of the cosmid vector pRB78 as described elsewhere (14).
Construction of α0 deletion mutant virus R7910.
The recombinant virus R7910 was constructed by transfection of cosmids pBC1006, pBC1007, pBC1012, pBC1013, and pBC1015 and plasmid pRB5163 (14) into ICP0-expressing N3 cells as described elsewhere (14). Viruses isolated from individual plaques were plaque purified on N3 cells.
Metabolic labeling and immunoprecipitation.
Replicate cell cultures of Vero cells in 25-cm2 flasks were infected with 10 PFU of the appropriate virus per cell. At 6 h after infection, the cells were incubated for 1 h in Eagle’s medium without phosphate. Cells were then labeled with 100 μCi of 32Pi (New England Nuclear, Boston, Mass.) in the phosphate-free medium for 5 h. The labeled cells were scraped and washed with phosphate-buffered saline and lysed in 500 μl of lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 50 mM NaF, 0.1 mM sodium orthovanadate, 10 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM TLCK [Nα-p-tosyl-l-lysine chloromethyl ketone], 0.1 mM TPCK [tolylsulfonylphenylalanyl chloromethyl ketone]).
Immunoprecipitations were done as described elsewhere (14). Briefly, supernatant fluids obtained after centrifugation of cell lysates were precleared by mixing with protein A-conjugated Sepharose beads (Sigma, St. Louis, Mo.) for 30 min and were reacted with 2 μl of EF-1δ for 2 h at 4°C. Protein A-Sepharose beads were then added and allowed to react for an additional hour. Immunoprecipitates were collected by brief centrifugation, rinsed four times with the lysis buffer, and either subjected to electrophoresis on polyacrylamide gel containing 9% sodium dodecyl sulfate (SDS) or used for in vitro kinase assays as described below.
In vitro kinase assays.
To assay the kinase activity of UL13, Vero cells were harvested 24 h after infection with either HSV-1(F) or R7356, rinsed with PBS, and solubilized in the lysis buffer as described above. The infected-cell lysates were reacted with 5 μl of normal rabbit sera for 30 min and then with 30 μl of a 50% slurry of protein A-Sepharose beads twice for 30 min. The precleared lysates were then used for immunoprecipitation with UL13 antibody as described above. The immune complexes harvested on the protein A-conjugated Sepharose beads were rinsed twice with kinase buffer (50 mM Tris-HCl [pH 8.0], 200 mM NaCl, 50 mM MgCl2, 0.1% Nonidet P-40, 1 mM dithiothreitol) and subjected to the kinase assay. To obtain EF-1δ as a substrate in kinase assays, precleared lysates of normal Vero cells with the rabbit normal sera and protein A-Sepharose were used for immunoprecipitation with EF-1δ antibody as described above. The immunoprecipitates were rinsed twice with kinase buffer and subjected to the kinase assay. The reactions were done with the mixtures of immunoprecipitates with the antibodies to EF-1δ and UL13 at 30°C for 30 min in a total volume of 70 μl of kinase buffer containing 6.5 μM ATP and 68 μCi of [γ-32P]ATP. After incubation, the beads were extensively washed with lysis buffer, and the phosphorylated proteins were resolved on a 9% polyacrylamide gel containing SDS. The gel was subjected to either Coomassie blue staining or immunoblotting with EF-1δ antibody and exposed to X-ray film.
RESULTS
The posttranslational modification of EF-1δ in HSV-1-infected cells is dependent on the presence of viral protein kinase specified by UL13 and not on the presence of another viral protein kinase specified by US3.
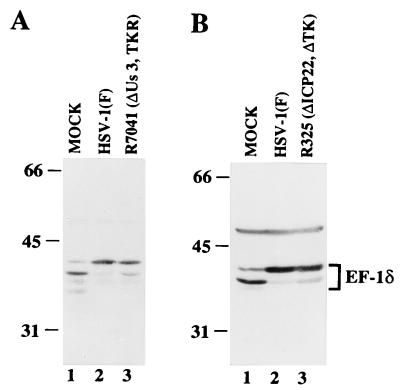
The objective of these studies was to identify the factor or factors which modify EF-1δ during HSV-1 infection. Of the known HSV-1 proteins, two protein kinases specified by UL13 and US3 were the most prominent candidates for this function. To test this hypothesis, Vero cells were harvested at 20 h after mock infection or infection with 10 PFU of wild-type or mutant viruses per cell, solubilized, electrophoretically separated in denaturing gels, electrophoretically transferred to nitrocellulose sheets, and reacted with the rabbit polyclonal antibody to EF-1δ. The results (Fig. 2 and 3A) were as follows.
FIG. 2.
Photograph of an immunoblot of electrophoretically separated lysates of Vero cells mock infected or infected with 10 PFU of the indicated virus per cell. The infected cells were harvested at 20 h after infection, solubilized, subjected to electrophoresis on an SDS–9% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the antibody to EF-1δ. Molecular weights (in thousands) are shown on the left. ΔTK, thymidine kinase gene deleted; TKR, thymidine kinase gene repaired.
FIG. 3.
Photograph of immunoblots of electrophoretically separated lysates of Vero cells mock infected or infected with 10 PFU of the indicated virus per cells. Experiments were done exactly as described in the legend to Fig. 1. ΔTK, thymidine kinase gene deleted; TKR, thymidine kinase gene repaired.
(i) EF-1δ harvested from mammalian cells forms two predominant bands migrating with apparent Mrs of 38,000 and 40,000 in denaturing gels (13). As reported previously (13) and shown in Fig. 2 (lanes 1, 2 and 4), in mock-infected cells, the fast-migrating band is the dominant one, whereas the relative amounts of protein in the slow-migrating band dramatically increased after infection with wild-type viruses.
(ii) In cells infected with UL13 deletion mutant viruses (ΔUL13), R7355 and R7356 (Fig. 2, lanes 3 and 5, respectively), the increase in the abundance of EF-1δ protein in the slow-migrating band was not observed and the fast-migrating band was still dominant, similar to what has been observed in mock-infected cells (Fig. 2, lane 1). The wild-type virus phenotype was restored in cells infected with the recombinant R7358, in which the UL13 sequence was repaired (Fig. 2, lane 5). Furthermore, the amount of the modified form of EF-1δ did not increase in R7356 (ΔUL13) virus-infected cells, even at 36 h after infection (data not shown).
(iii) The pattern of electrophoretic mobilities of EF-1δ from cells infected with the ΔUS3 mutant virus, R7041 (Fig. 3A, lane 3), could not be differentiated from that of EF-1δ extracted from cells infected with the wild-type virus (Fig. 3A, lane 2).
These results indicate that viral protein kinase UL13, but not US3, is required for the posttranslational modification of EF-1δ during HSV-1 infection.
Posttranslational modification of EF-1δ is mediated by UL13 protein and not by ICP22.
Earlier studies from this laboratory have shown that the phenotype of the ΔUL13 mutant cannot be differentiated from that of the ΔICP22/ΔUS1.5 mutant with respect to accumulation of ICP0 and of a subset of late proteins (28). To determine whether modification of EF-1δ is mediated by ICP22/US1.5 proteins, Vero cells were harvested 20 h after mock infection or infection with 10 PFU of HSV-1(F) or R325 (Δα22/ΔUS1.5) virus per cells. The electrophoretically separated infected-cell proteins transferred to a nitrocellulose sheet were reacted with antibody to EF-1δ. As shown in Fig. 3B, the electrophoretic pattern of EF-1δ cannot be differentiated from that observed for EF-1δ extracted from wild-type virus-infected cells. The results indicate that the posttranslational modification of EF-1δ is not mediated by ICP22.
ICP0 is not required for the posttranslational modification of EF-1δ.
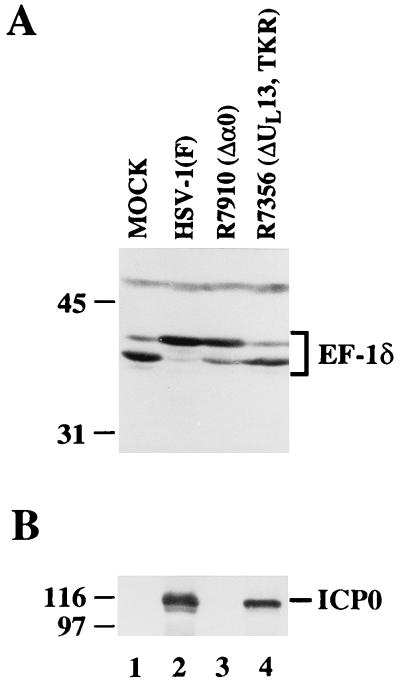
The observation that UL13 is required for the modification of EF-1δ during HSV-1 infection raises the possibility that ICP0, which has shown to interact with EF-1δ, is a cofactor of the modification of EF-1δ. Inasmuch as ICP0 is posttranslationally modified by UL13 and also binds EF-1δ (25), the hypothesis to be tested is that ICP0 binds to and brings UL13 in apposition to EF-1δ. To address this question, we constructed the α0 null mutant R7910, as described in Materials and Methods, and examined the level of modification of EF-1δ in Vero cells mock infected or infected with HSV-1(F), R7356 (ΔUL13), or R7910 (Δα0). As shown in Fig. 4, EF-1δ extracted from HSV-1(F)-infected cells cannot be differentiated from that of cells infected with R7910 (Δα0) but is readily differentiated from that of cells infected with R7356 (ΔUL13).
FIG. 4.
Photographs of immunoblots of electrophoretically separated lysates of Vero cells mock infected or infected with 1 PFU of the indicated virus per cell. Experiments were done exactly as described in the legend to Fig. 2, except that the nitrocellulose sheet was probed with EF-1δ (A) and then with ICP0 (B). TKR, thymidine kinase gene repaired.
UL13 is required for phosphorylation of EF-1δ in vivo.
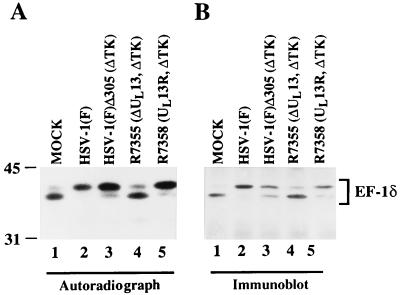
The EF-1δ from Xenopus oocytes is phosphorylated by several cellular kinases. A consequence of this phosphorylation is a decrease in the electrophoretic mobility of the protein on electrophoresis in denaturing gels similar to that observed in HSV-1-infected cells (22). Although the decrease in electrophoretic mobility of EF-1δ requires the presence of the protein kinase encoded by UL13, it is not known whether the modification of EF-1δ observed in HSV-1-infected cells is associated with the phosphorylation of the protein. The objectives of the experiments described in this section were to determine whether modification of EF-1δ during the HSV-1 infection is due to phosphorylation, and if this is the case, whether UL13 is required for the phosphorylation of EF-1δ. Vero cells were mock infected or infected with 10 PFU of HSV-1(F), HSV-1(F)Δ305 (ΔUL23/ΔU24), R7355 (ΔUL13), or R7358 (UL13R/ΔUL23/ΔUL24) virus per cell and labeled with 32Pi from 7 to 12 h after infection. EF-1δ immunoprecipitated from the infected cell lysates as described in Materials and Methods was then solubilized, electrophoretically separated on a denaturing gel, electrophoretically transferred to a nitrocellulose sheet, and subjected to autoradiography (Fig. 5A) and also reacted with the antibody to EF-1δ (Fig. 5B).
FIG. 5.
Autoradiographic and photographic images of 32P-radiolabeled infected-cell lysate immunoprecipitated by the antibody to EF-1δ, subjected to autoradiography, and then reacted with antibody to EF-1δ. Vero cells were mock infected or infected with the indicated virus. At 7 h after infection, the cells were labeled with 32Pi for 5 h and then harvested, solubilized, immunoprecipitated with the antibody to EF-1δ, electrophoretically separated in an SDS–9% polyacrylamide gel, transferred to a nitrocellulose sheet, and subjected to autoradiography (A) and then reacted with the antibody to EF-1δ (B). ΔTK, thymidine kinase gene deleted; ΔUL13, UL13 gene deleted; UL13R, UL13 gene repaired.
As previously reported for the Xenopus oocyte EF-1δ (22), both forms of EF-1δ with Mrs of 38,000 and 40,000 are labeled with 32Pi in mock-infected cells (Fig. 5A, lane 1). In these cells as in cells infected with the virus lacking the UL13 gene (R7355), the predominant form of EF-1δ was the fast-migrating form (Fig. 5A, lanes 1 and 4). In contrast, in cells infected with the parent viruses [HSV-1(F) (Fig. 5A, lane 2) or HSV-1(F)Δ305 (Fig. 5A, lane 3)], or with the mutant in which the UL13 virus was repaired, R7258 (Fig. 5A, lane 5), the predominant form of 32P-labeled EF-1δ was the slow-migrating type. Figure 5B shows the results of immunoblotting of the protein shown in Fig. 5A with antibody to EF-1δ. The results indicate that (i) phosphorylated proteins shown in Fig. 5A correspond to EF-1δ, (ii) the changes in radiolabeling profiles correspond to changes in the electrophoretic mobility of EF-1δ, and (iii) the UL13 protein kinase is required for hyperphosphorylation of EF-1δ in HSV-1-infected cells.
The kinase activity in UL13 immunoprecipitates phosphorylates EF-1δ in vitro.
Although the data shown above strongly suggest that EF-1δ is a substrate for the UL13 protein kinase, the experiments reported in this section were done to establish a direct link between UL13 and phosphorylation of EF-1δ. Specifically, immunoprecipitates of EF-1δ derived from uninfected Vero cells were mixed with those of UL13 from the cells infected with HSV-1(F) or R7356 (ΔUL13), reacted in kinase buffer containing [γ-32P]ATP, separated on a denaturing gel, electrophoretically transferred to a nitrocellulose sheet or stained with Coomassie blue (not shown), and subjected to autoradiography, and they were also reacted with the EF-1δ antibody. As reported previously, the immune complex obtained with the UL13 antibody from HSV-1(F)-infected Vero cells contains a specific protein kinase activity attributed to the UL13 gene (3, 24, 25). The results (Fig. 6) were as follows.
FIG. 6.
Autoradiogram and photographs of a Coomassie blue-stained gel and immunoblots of proteins subjected to in vitro kinase assay. Vero cells were infected with HSV-1(F) or R7536, harvested at 24 h after infection, solubilized, precleared with normal rabbit sera and protein A-Sepharose, and then subjected to immunoprecipitation (I.P.) with UL13 antibody (Ab). (A) The immune complex with the antibody to UL13 from HSV-1(F)-infected cells was incubated in the kinase buffer containing [γ-32P]ATP for 30 min at 30°C, separated on a denaturing gel, stained with Coomassie blue, and subjected to autoradiography. (B) The immunoprecipitated EF-1δ from normal Vero cells was incubated with the immune complex with the antibody to UL13 from R7536 (ΔUL13) (lane 1)- or HSV-1(F) (lane 2)-infected cells in kinase buffer containing [γ-32P]ATP, separated on a denaturing gel, transferred to a nitrocellulose sheet, and subjected to autoradiography. (C) The nitrocellulose sheet corresponding to that in panel B was reacted with the antibody to EF-1δ.
(i) Electrophoretically separated immune complex containing the UL13 protein kinase which was allowed to react by itself in the kinase buffer did not contain labeled protein bands with an apparent Mr corresponding to that of EF-1δ (Fig. 6A, lane 1). On the other hand, in the autoradiographic images of the same immune complex reacted with immunoprecipitated EF-1δ, only the slow-migrating form of EF-1δ with an apparent Mr of 40,000 was phosphorylated (Fig. 6B, lane 2). The identity of the radiolabeled band of EF-1δ was verified in the immunoblot shown in Fig. 5C.
(ii) No proteins were labeled by the reaction of mixtures containing mixtures of immune complex formed by antibody to UL13 with lysates of cells infected with R7356 (ΔUL13) and the immune complex containing EF-1δ obtained from uninfected cells (Fig. 6B, lane 1). This result indicates that the kinase activity observed in Fig. 6B, lane 2, was derived from UL13 and not from nonspecific kinase brought down by the polyclonal rabbit sera to EF-1δ or to UL13. Figure 6C shows that EF-1δ was present in both lane 1 of Fig. 6B, in which it was not phosphorylated, and in lane 2 of Fig. 6B, in which only the slow-migrating protein was phosphorylated.
These results indicate that the immune complex with the UL13 antibody from HSV-1(F)-infected cells possesses a kinase activity for EF-1δ, and we conclude that either UL13 or a complex containing UL13 as a necessary component phosphorylates EF-1δ in vitro.
DISCUSSION
The key finding reported here is that viral protein kinase encoded by HSV-1 UL13 phosphorylates the cellular translational regulatory protein EF-1δ, which plays a key role in protein synthesis. This is the first identification of a cellular protein target for virally encoded protein kinases of herpesviruses. The salient features of this study that support our conclusion are as follows.
(i) A hyperphosphorylated form of EF-1δ accumulates in Vero cells infected with wild-type viruses or UL13-repaired virus, whereas in mock-infected cells or cells infected with the UL13 deletion mutant, the predominant form of EF-1δ is a faster-migrating, hypophosphorylated form. These results indicate that UL13 is required for the hyperphosphorylation of EF-1δ during HSV-1 infection in infected cells.
(ii) Earlier studies have shown that the phenotype in ΔUL13 mutant-infected cells cannot be differentiated from that of Δα22 mutant-infected cells in that the accumulation of ICP0 and of a subset of late proteins is reduced relative to those of wild-type virus-infected cells (28). In this report, we show that EF-1δ in Δα22 mutant virus-infected cells is modified to the same extent as in wild-type-infected cells, eliminating the possibility that ICP22/US1.5 proteins are required for modification of EF-1δ.
(iii) The in vitro kinase assays showed that EF-1δ was phosphorylated in vitro by the mixture of independently derived immune complexes formed by lysates of cells infected with wild-type virus and the antibody to UL13 and those of uninfected cells and antibody, whereas substitution in the mixture of the immune complex containing UL13 protein with that formed by anti-UL13 protein and lysates of ΔUL13 mutant-infected cells failed to phosphorylate EF-1δ.
Taken together, these results support our conclusion that EF-1δ is phosphorylated by UL13 protein kinase and that this form comigrates with the hyperphosphorylated form of the protein during HSV-1 infection. The relevant issues are as follows.
(i) Several lines of evidence listed below suggest that the phosphorylation of EF-1δ is involved in enhancement of the activity for protein synthesis. First, phosphorylation of EF-1δ by cellular cdc2 kinase is correlated with an increase in the activity of protein synthesis (19, 22, 31, 39). Second, EF-1δ activity is enhanced by phosphorylation of EF-1δ in vivo with phorbol esters or in vitro phosphorylation of EF-1δ with cellular protein kinase C (37, 38). Third, phosphorylation of translation initiation factor 2B (eIF-2B), which appears to have the same function as the EF-1βδγ complex in the initiation step of translation, causes an increase in GDP-GTP exchange activity of the protein (4).
(ii) Although ICP0 interacts with EF-1δ (13), it is not involved in the modification of EF-1δ. Thus, earlier this laboratory reported that ICP0 interacts physically in yeast with EF-1δ and that the sequence which binds EF-1δ fused to glutathione S-transferase (GST) interferes with protein synthesis, whereas GST alone had no effect (13). In this report, we show that EF-1δ is hyperphosphorylated by UL13 protein kinase. These observations suggest that HSV-1 evolved two distinct pathways for modulating the function of EF-1δ and that EF-1δ plays an important role in efficient replication of HSV-1.
(iii) Viruses have evolved a variety of mechanisms to modulate the host cell translational apparatus to aid in their replication. In most cases reported so far, viruses have targeted proteins designed to maintain the integrity of translation initiation (15). For example, most viruses activate a double-stranded RNA-dependent kinase which phosphorylates the α subunit of eIF-2 and completely turns off protein synthesis during their replication (15). Many viruses encode proteins which are involved in blocking the phosphorylation of eIF2-α (12, 15). Viruses also change host cell translational machinery to enable preferential translation of viral mRNAs; these changes include host mRNA degradation and a change in translational specificity (15). HSV-1 has evolved both mechanisms: it encodes the γ134.5 protein which interacts with protein phosphatase 1α to dephosphorylate eIF2α and to preclude the shutoff of protein synthesis by double-stranded RNA-dependent protein kinase R (9, 34). HSV-1 also encodes two proteins, virion host shutoff protein (vhs gene) encoded by UL41, and ICP27, encoded by α27. The vhs-encoded protein degrades host mRNAs, and ICP27 down-regulates expression of cellular mRNA by inhibiting splicing to cause preferential translation of viral mRNA (15, 34).
(iv) Although the physiologic role of hyperphosphorylation of EF-1δ is not known at present, it is conceivable that UL13 protein kinase replaces a cellular function which is called upon during specific cell cycle stages or morphogenetic events to activate a higher level of protein synthesis in the infected cell. It is noteworthy that in its human host, HSV-1 causes lesions preeminently in nondividing cells. Furthermore, the infected cells used in our studies are not synchronized with respect to the cell cycle stage at which they are infected. HSV-1 may have evolved this function to compensate for a cellular function degraded during infection in order to upregulate translation to a level characteristic of dividing cells. It is noteworthy that the UL13 gene is conserved in members of all herpesvirus subfamilies (2, 35), raising the interesting possibility that modification of EF-1δ is a conserved herpesvirus function.
ACKNOWLEDGMENTS
We thank W. O. Ogle for making available to us the antibody to UL13.
These studies were aided by Public Health Service grants from the National Cancer Institute (CA47451). Y.K. is the recipient of a Japan Society for Promotion of Science Postdoctoral Fellowship for Research Abroad. C.V.S. is a predoctoral trainee aided by a grant from National Institute of General Medical Sciences (GM07183-22).
REFERENCES
- 1.Carter K L, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J Virol. 1996;70:172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chee M S, Lawrence G L, Barrell B G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 3.Daikoku T, Shibata S, Goshim F, Oshima S, Tsurumi T, Yamada H, Yamashita Y, Nishiyama Y. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology. 1997;235:82–93. doi: 10.1006/viro.1997.8653. [DOI] [PubMed] [Google Scholar]
- 4.Dholakia J N, Wahba A J. Phosphorylation of the guanine nucleotide exchange factor from rabbit reticulocytes regulates its activity in polypeptide chain initiation. Proc Natl Acad Sci USA. 1988;85:51–54. doi: 10.1073/pnas.85.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelman A M, Blumenthal D K, Krebs E G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 6.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effect on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 7.Everett R D, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:566–577. doi: 10.1093/emboj/16.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett R D, Preston C M, Stow N D. Functional and genetic analysis of the role of Vmw 110 in herpes simplex virus replication. In: Wagner E K, editor. Herpesvirus transcription and its regulation. Boston, Mass: CRC Press; 1991. pp. 50–76. [Google Scholar]
- 9.He B, Gross M, Roizman B. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptide. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katze M G. Regulation of the interferon induced-PKR: can viruses cope? Trends Microbiol. 1995;3:75–78. doi: 10.1016/s0966-842x(00)88880-0. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knipe D K. Virus-host cell interactions. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 273–299. [Google Scholar]
- 16.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeoch D J, Davison A J. Alphaherpesviruses possess a gene homologous to protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986;14:1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrick W C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minella O, Cormier P, Morales J, Poulhe R, Belle R, Mulner-Lorillon O. cdc2 kinase sets a memory phosphorylation signal on elongation factor EF-1δ during meiotic cell division, which perdures in early development. Cell Mol Biol. 1994;40:521–525. [PubMed] [Google Scholar]
- 20.Morales J, Cormier P, Mulner-Lorillon O, Poulhe R, Belle R. Molecular cloning of a new guanine-nucleotide exchange protein, EF-1δ. Nucleic Acids Res. 1992;20:4091. doi: 10.1093/nar/20.15.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2637–2671. [Google Scholar]
- 22.Mulner-Lorillon O, Minella O, Cormier P, Capony J-P, Cavadore J-C, Morales J, Poulhe R, Belle R. Elongation factor EF-1δ, a new target for maturation-promoting factor in Xenopus oocytes. J Biol Chem. 1994;269:20201–20207. [PubMed] [Google Scholar]
- 23.Nevins J R, Vogt P K. Cell transformation by viruses. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 301–343. [Google Scholar]
- 24.Ng T I, Ogle W O, Roizman B. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology. 1998;241:39–48. doi: 10.1006/viro.1997.8963. [DOI] [PubMed] [Google Scholar]
- 25.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology, in press. [DOI] [PubMed]
- 26.Palen E, Venema R C, Chang Y-W E, Traugh J A. GDP as a regulator of phosphorylation of elongation factor 1 by casein kinase II. Biochemistry. 1994;33:8515–8520. doi: 10.1021/bi00194a016. [DOI] [PubMed] [Google Scholar]
- 27.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 28.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purves F C, Spector D, Roizman B. Herpes simplex virus 1 protein kinase encoded by US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter J D, Wasserman W J, Smith L D. The mechanism for increased protein synthesis during Xenopus oocyte maturation. Dev Biol. 1982;89:159–167. doi: 10.1016/0012-1606(82)90304-9. [DOI] [PubMed] [Google Scholar]
- 32.Riis B, Rattan S I S, Clark B F C, Merrick W C. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990;15:420–424. doi: 10.1016/0968-0004(90)90279-k. [DOI] [PubMed] [Google Scholar]
- 33.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roizman B, Sears A E. The replication of herpes simplex viruses. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2231–2295. [Google Scholar]
- 35.Smith R F, Smith T F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Damme H T F, Amons R, Karssies R, Timmers C J, Janssen G M C, Moller W. Elongation factor 1β of artemia: localization of functional sites and homology to elongation factor 1δ. Biochim Biophys Acta. 1990;1050:241–247. doi: 10.1016/0167-4781(90)90174-z. [DOI] [PubMed] [Google Scholar]
- 37.Venema R C, Peters H I, Traugh J A. Phosphorylation of valyl-tRNA synthetase and elongation factor 1 in response to phorbol esters is associated with stimulation of both activities. J Biol Chem. 1991;266:11993–11998. [PubMed] [Google Scholar]
- 38.Venema R C, Peters H I, Traugh J A. Phosphorylation of elongation factor 1 (EF-1) and valyl-tRNA synthetase by protein kinase C and stimulation of EF-1 activity. J Biol Chem. 1991;266:12574–12580. [PubMed] [Google Scholar]
- 39.Wasserman R C, Richter J D, Smith L D. Protein synthesis during maturation promoting factor- and progesterone-induced maturation in Xenopus oocytes. Dev Biol. 1982;89:152–158. doi: 10.1016/0012-1606(82)90303-7. [DOI] [PubMed] [Google Scholar]