Abstract
Background
Studies in collegiate athletes have demonstrated a prevalence of cardiac involvement between 0.5 and 3 % after SARS-CoV-2 infection. When post-COVID cardiac involvement occurs in athletes, the ideal return to play timeline and many possible long-term sequela or complications are unknown.
Case summary
A 20 yo female collegiate athlete tested positive for SARS-CoV-2 and underwent routine cardiac screening prior to her return to play (RTP). Evaluation demonstrated an elevated high-sensitivity troponin-I and an ECG showed some mild T wave changes. She had a normal transthoracic echocardiogram, and her Cardiac magnetic imaging (CMR) met Lake Louise Criteria for acute myocarditis. She was diagnosed with acute myocarditis and restricted from sports. CMR was repeated at 3.5 months after normalization of troponin I HS and demonstrated continued active inflammation. She continued to be restricted from exertion. A third CMR was obtained at 6.5 months and showed resolution of active inflammation but a small area of fibrosis, and the remainder of her cardiac testing was normal. She was allowed to slowly progress back into sport and returned to competition at 9 months and successfully completed her season.
Discussion
CMR is not typically repeated prior to RTP after a diagnosis of myocarditis in athletes, but in this case, repeat CMR at 3.5 months initially demonstrated continued active inflammation, and a second repeat CMR at 6.5 months demonstrated abnormal cardiac fibrosis. This may suggest utility in repeating CMR and raises questions about possible long-term implications of cardiac fibrosis once the acute inflammation of myocarditis has resolved.
Keywords: Myocarditis, COVID-19, Athletes, Return to play (RTP)
1. Introduction
Coronavirus disease of 2019 (COVID-19) caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a global pandemic by the World Health Organization (WHO) on March 11th, 2020. There were initial reports of serious cardiac events in hospitalized patients with COVID-19 [1]. These reports caused concern for the risk of cardiac complications such as myocarditis in athletes after developing COVID and questions arose about the best way to safely return athletes to activity as they recovered from the disease. Various screening methodologies were proposed including using serum biomarkers, electrocardiogram (ECG), transthoracic echocardiogram (TTE), cardiac MRI (CMR), and exercise stress tests, in isolation or various combinations, to assess athletes for cardiac complications from COVID and to allow safe Return to Play (RTP). Over time, data demonstrated the rate of cardiac involvement in athletes returning from COVID infection was between 0.5 % to 3 % [[2], [3], [4]].
2. Case presentation
A 20 yo female collegiate athlete presented for RTP clearance 10 days after a symptomatic SARS-CoV-2 infection which had been confirmed with a positive polymerase chain reaction (PCR) test. This was prior to her being eligible for a vaccine, and at the time of infection, her institution's sports health program required routine cardiac screening with a troponin I HS, 12 lead ECG, and TTE before returning to exertion. She had a past medical history of asthma, and cough was a significant component of her COVID symptoms. During her illness she had been advised to increase her steroid and albuterol inhalers. These measures had helped her symptoms and by day 10, she was feeling much better and denied chest pain, dyspnea, or palpitations.
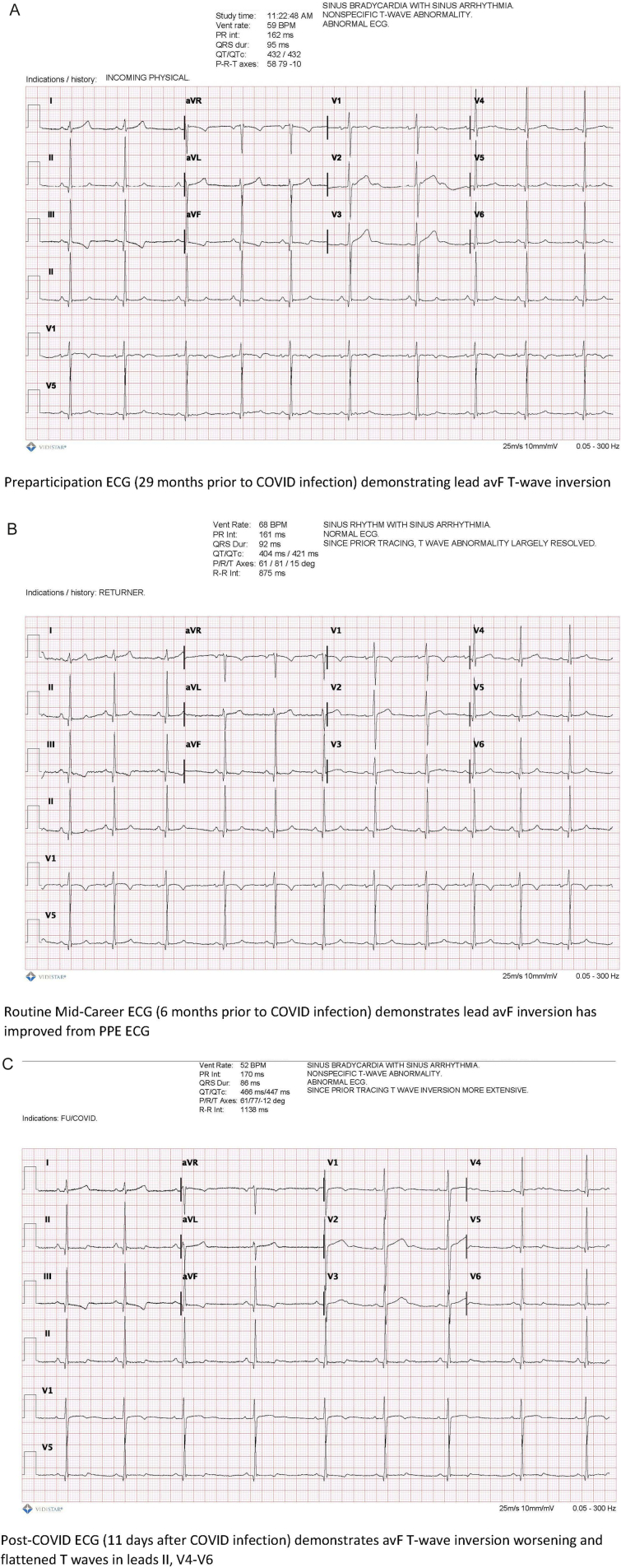
On Day 11 she had a troponin I HS of 211 pg/ml (normal <15 pg/ml) and her ECG showed a flattening/inversion of the T waves in leads II and V4–6 (Fig. 1) which was more extensive than previous routine screening ECGs. Her troponin I HS was repeated on Day 12 to ensure it was not an error and it returned still elevated at 179. It was repeated again on Day 13 with a value of 165, along with a high-sensitivity C-reactive protein (hsCRP) and B-type natriuretic peptide (BNP) both of which were normal. She was restricted from all physical activity while further workup was obtained.
Fig. 1.
ECG changes post-COVID as compared to two previous ECGs.
Upon reflection after these abnormal screening tests, the athlete reported she had felt some irregular heart beats for a few days between days 11 and 18 which would last about 20 s and occur several times per day. They always happened when she was at rest. A 72-hour Holter monitor was placed on Day 15. Over the next week she began noticing increasing palpitations with exertion (such as walking up or down the stairs in her apartment).
A TTE obtained on Day 18 was normal. A CMR was obtained on Day 19. It demonstrated normal systolic function with no regional wall motion abnormalities and a left ventricular ejection fraction (LVEF) of 56 %, but an abnormal late gadolinium enhancement (LGE) pattern consistent with myocarditis (subepicardial LGE involving the midventricular to apical inferior and inferolateral walls), and mildly elevated T2 values consistent with mild edema/inflammation in the inferolateral regions and normal elsewhere (Fig. 2). Repeat MR imaging was recommended after 3–4 months to assess for resolution of findings.
Fig. 2.
CMR1.
The Holter monitor results showed isolated Premature Ventricular Contractions (PVCs) at 2 %. There were two distinct PVC morphologies, with 95 % being the same morphology and felt to be non-malignant. She continued to be restricted from physical activity. Her palpitations significantly improved by 2 months post-infection. Aspirin 81 mg was started empirically at 2.5 months post-infection to provide an anti-inflammatory effect and counteract potential hypercoagulability which had been reported in some post-COVID patients.
Troponin I HS was repeated every 1–2 weeks until it normalized which occurred 3 months post-infection when its value decreased to 13 pg/ml (normal <15 pg/ml).
At week 13, the athlete was allowed to begin very small amounts of slow technique work for her sport, keeping her heart rate low (no more strenuous than a walk around campus). She had no symptoms with this exertion. A repeat Holter monitor showed 0.3 % PVCs, mostly isolated with a few couplets.
The CMR was repeated at 3.5 months post infection and continued to show normal function with an LVEF of 56 %. There was still an abnormal LGE pattern with subepicardial LGE in the inferior and inferolateral wall but the wall thickness percentage of LGE decreased from about 50 % to about 25 % compared to previous study (Fig. 3). There was still mildly elevated T2 values, essentially unchanged from previous, which suggested continued inflammation.
Fig. 3.
CMR 2.
She was allowed to continue the slow technique work and remained asymptomatic and noted this level of activity helped her mentally.
A third CMR was obtained at 6.5 months post infection and again showed normal function, with a LVEF of 59 % which was a 3 % increase from initial two post-covid CMRs. It demonstrated a slightly smaller focal area of mid-myocardial/subepicardial LGE involving basal to midventricular inferolateral wall and the basal inferior wall. T2 value was normal and CMR was without evidence of inflammation or edema. There was a mildly elevated T1 time in the ventricle, greatest in the inferolateral wall suggesting occult fibrosis (Fig. 4). A 14-day Holter monitor at this time revealed normal sinus rhythm, symptomatic PVCs <1 % total beats. A cardiac stress test was negative with rare isolated PVCs (total of 3) and stopped during stage 5 due to fatigue.
Fig. 4.
CMR3.
She was fully cleared for her sport at that time and one month into training (7.5 months from infection) was up to full practices and feeling good with no palpitations or symptoms. However, 3 weeks later (8.25 months from infection) she began reporting increasing palpitations. An Event monitor was placed and blood work ordered. Her thyroid studies were abnormally low but improved from a previously obtained test and she saw endocrinology who felt this was most likely due to resolving sick euthyroid syndrome. Her Event monitor did not show any concerning arrythmias. An Implantable Event monitor was placed to avoid it interfering with participation in her sport to get a better idea of what was occurring. There were no clinically relevant arrhythmias picked up on the implantable monitor over 6 months. Her palpitations resolved and she returned to competition 9 months after infection. She was able to complete her senior season successfully, although she did not improve on her performances prior to her myocarditis.
3. Discussion
Myocarditis is a known risk factor for sudden cardiac death (SCD) and is one of the leading causes of SCD in athletes [1,5,6]. The American College of Cardiology/American Heart Association (ACC/AHA) sports eligibility guidelines for myocarditis [5] from 2015, and more recent post-COVID-19 expert recommendations all advocate for a 3–6 month range of exercise abstinence after a diagnosis of myocarditis; and until systolic function is normal, serum makers of cardiac injury have normalized, and relevant arrhythmias are absent on both Holter monitor and graded exercise ECG [5,7,8]. Based on only these cardiac tests, our athlete would have been cleared to return around 3.5 months. While the guidelines do recommend using CMR findings in the diagnosis of myocarditis, they do not advocate using CMR in the cardiac testing recommended for RTP decisions [5,[7], [8], [9]]. Currently, it is also unresolved whether myocarditis-related LGE should be required to be normalized before return to play [5,7]. Given the unknowns surrounding the COVID-19 virus and at the recommendation of the CMR reading physician, we did repeat the CMR in our athlete and it demonstrated continued inflammation despite normalization of the other standard testing at the 3.5 month mark, which then resolved upon repeat CMR at 6.5 months. A more recent review, published after the clinical decision making in this case had occurred, does advocate for a follow up CMR at 3 months to identify residual inflammation or high-risk LGE [10].
The use of CMR for follow-up imaging of athletes diagnosed with myocarditis, based on previous abnormal CMR findings, may be preferred to relying on normalization of biomarkers, LV function, and the absence of significant rhythm disturbances as RTP criteria. In this case, if we had cleared her to return without getting the CMR which demonstrated active inflammation, she might have been at an increased risk for an adverse cardiac event, given the strong association of myocarditis with sudden cardiac death [5]. More data is needed on CMR findings and resolution after viral myocarditis before further recommendations can be made.
Persistent LGE following clinical recovery of myocarditis has unclear implications and might increase the risk for arrhythmia [5], but improvement of inflammatory findings on CMR after 3–6 months of exercise restriction is reassuring [1].
The addition of parametric T1 and T2 mapping techniques in the updated 2018 Lake Louise Criteria for myocarditis has been shown to improve the diagnostic accuracy of CMR for acute myocarditis [1,11]. In general, abnormal T2 values are felt to represent active inflammation/edema, while T1 represents more permanent changes/fibrosis [10]. The ACC/AHA sports eligibility guidelines for myocarditis were last updated in 2015 prior to the updated Lake Louise Criteria and before CMR became as widely available. It was also well before the COVID pandemic which has affected a large part of the population, and for which long-term sequela are still unknown.
4. Conclusion
This case highlights COVID myocarditis diagnosis in a Division-1 college athlete and the RTP decisions involved in allowing safe participation. It also demonstrates a need for more longitudinal data about long term implications on cardiac health and exercise after having sustained a cardiac complication from COVID, as well as more data on the utility of using CMR in RTP decisions.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
The authors wish to thank Monica Towns and Joyce Golden for their help compiling Fig. 1 and Dr. Mohammed Al-Ani for his help compiling Fig. 2, 3, &4.
References
- 1.Symanski J.D., Tso J.V., Phelan D.M., Kim J.H. Myocarditis in the athlete: a focus on COVID-19 sequelae. Clin. Sports Med. 2022;41(3):455–472. doi: 10.1016/j.csm.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulson N., Petek B.J., Drezner J.A., Harmon K.G., Kliethermes S.A., Patel M.R., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez M.W., Tucker A.M., Bloom O.J., Green G., DiFiori J.P., Solomon G., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maestrini V., Filomena D., Birtolo L.I., Serdoz A., Fiore R., Tatangelo M., et al. Systematic cardiovascular screening in olympic athletes before and after SARS-CoV-2 infection. J. Clin. Med. 2022;11(12) doi: 10.3390/jcm11123499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron B.J., Udelson J.E., Bonow R.O., Nishimura R.A., Ackerman M.J., Estes N.A.M., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, Arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015;66(21):2362–2371. doi: 10.1016/j.jacc.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Harmon K.G., Asif I.M., Maleszewski J.J., Owens D.S., Prutkin J.M., Salerno J.C., et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association Athletes: a decade in review. Circulation. 2015;132(1):10–19. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma G., Blumenthal R.S., Martinez M.W. COVID-19, myocarditis, and cardiac MRI in athletes: distinguishing signal from noise. Am. Coll. Cardiol. 2021 (www.acc.org) [Google Scholar]
- 8.Gluckman T.J., Bhave N.M., Allen L.A., Chung E.H., Spatz E.S., Ammirati E., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution set Oversight Committee. J. Am. Coll. Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chukumerije M. ACC expert consensus decision pathway on COVID-19: return-to-play take home points. Am. Coll. Cardiol. 2022;2022 (www.acc.org) [Google Scholar]
- 10.Eichhorn C., Greulich S., Bucciarelli-Ducci C., Sznitman R., Kwong R.Y., Gräni C. Multiparametric cardiovascular magnetic resonance approach in diagnosing, monitoring, and prognostication of myocarditis. JACC Cardiovasc. Imaging. 2022;15(7):1325–1338. doi: 10.1016/j.jcmg.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Cardim N., Oliveira A.G., Longo S., Ferreira T., Pereira A., Reis R.P., et al. Doppler tissue imaging: regional myocardial function in hypertrophic cardiomyopathy and in athlete’s heart. J. Am. Soc. Echocardiogr. 2003;16(3):223–232. doi: 10.1067/mje.2003.13. [DOI] [PubMed] [Google Scholar]