Abstract
Introduction: Recent studies have proposed various COVID-19 vaccines to control the disease and protect susceptible individuals. However, immunogenicity and safety of COVID-19 vaccines in various populations are not well identified yet. Therefore, this study aimed to elucidate the efficacy and safety of the BBIBP-CorV (Sinopharm) and ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccines in healthy subjects and patients with autoimmune diseases.
Methods: Study population included 121 healthy subjects and 100 patients with autoimmune diseases. Immunization was performed based on the national vaccination protocols. Of the 221 volunteers, 201 subjects received Sinopharm and 20 cases were vaccinated with Oxford-AstraZeneca. During a 1-year follow-up, the immunogenicity was measured by ELISA before primary vaccination and 1 to 3 months after secondary immunization. Side effects were studied before entering the study and 1 week after the second dose.
Results: Vaccination had a positive impact on the induction of immunogenic response (p < .0001). The rates of seropositive vaccine responses were 80% and 75% in subjects vaccinated with the Sinopharm and Oxford-AstraZeneca, respectively. The neutralizing antibody values were significantly higher in subjects with autoimmune diseases than those without autoimmunity (p < .05). The rate of adverse events were 38% and 42% in subjects vaccinated with the Sinopharm and Oxford-AstraZeneca, respectively. The rates of immunogenic responses induced with the Sinopharm and Oxford-AstraZeneca were, respectively, 76% and 81.5% in seropositive subjects, while they were 63.8% and 79.1% in seronegative subjects vaccinated with the Sinopharm and Oxford-AstraZeneca, respectively. Individuals previously infected with SARS-CoV-2 showed a significant reduction in the value of SARS-CoV-2 neutralizing antibodies compared with seronegative subjects (p < .01–.05). Seropositive individuals vaccinated with the Sinopharm had significantly higher the percentages of vaccine-related adverse events than seronegative persons (p < .05). There was no significant difference between seronegative and seropositive individuals vaccinated with the Oxford-AstraZeneca.
Conclusion: Our findings revealed that the Sinopharm and Oxford-AstraZeneca vaccines are effective in the production of neutralizing antibodies in healthy subjects and patients with autoimmune disorders undergoing immunosuppressive therapies without considerable reactogenicity.
Keywords: immunogenicity, adverse events, Oxford-AstraZeneca vaccine, Sinopharm vaccine, patients with autoimmune diseases
Introduction
On March 11, 2020, COVID-19 was considered as a global pandemic by the World Health Organization (WHO). 1 The Ministry of Health announced the spread of COVID-19 in Iran on February 18, 2020, and the virus quickly spread to every province in the country. 2 There is currently no solid evidence for the efficacy of current antiviral medications. Thus, additional studies are needed to confirm their efficacy and potential.3–5
Today, vaccination has been proposed as the most effective approach for controlling the disease, especially in patients with autoimmune diseases.6–9 These patients are usually more susceptible to infectious diseases and experience COVID-19 with more severity, possibly due to immunomodulatory impacts of immunosuppressive treatments. Thus, SARS-CoV-2 vaccination and follow-up in patients with autoimmune disorders are critical. 10
Until now, a number of temporarily licensed COVID-19 vaccines is available to prevent the disease through producing safe and effective responses. 11 The most frequent vaccines proposed to prevent COVID‐19 are included mRNA vaccines (Moderna and Pfizer-BioNTech), viral vector vaccines (Oxford-AstraZeneca, Sputnik V, Convidecia, and Johnson & Johnson), and inactivated vaccines (CoronaVac, BBIBP-CorV, Covaxin, CoviVac, and WIBP-CorV). 12
Despite a great number of proposed vaccines, several investigations have highlighted the discrepancies in the immunogenicity and adverse effects of the COVID-19 vaccination in different populations and various diseases.13–17 Side effects of the immune responses following vaccination are possible. These potential post-vaccine side effects are considered as the major reason for vaccine reluctance in the general population. 18 Improvement of vaccination acceptability is required to increase public awareness of vaccine efficacy and its negative effects. 19 Therefore, further research is necessary to determine vaccine side effects and its effectiveness during general vaccination.
The genetic diversity of the world’s populations is considered as a main reason for variations in the immunogenicity and adverse effects of various vaccines in different populations.20,21 It is indicated that different genetic polymorphisms and HLA variations affect virus pathogenicity and host immunity, which may have important implications for comprehending and illustrating the issue of genetics in SARS-CoV-2 infection and for providing individualized integrative medical care according to population research.22–24
Although various studies have been carried out to investigate the immunogenicity and reactogenicity of COVID-19 vaccines in various populations,25–27 the efficacy and adverse events of these vaccines in the Iranian population are not well identified so far. Therefore, the aim of this study was to investigate whether the Oxford-AstraZeneca- and Sinopharm vaccines, as the most frequent vaccines used in the national vaccination protocols, had immunogenicity and side effects in healthy individuals and patients with autoimmune disorders in Kashan, Iran.
Materials and methods
Study population
A retrospective cohort study included 221 individuals who vaccinated with two doses of COVID-19 vaccines. Participants were randomized according to a permuted block randomization scheme with a block size of four and selected from the western, north, east, south, and central districts of Kashan (Iran). Notably, Kashan University of Medical Sciences extended healthcare facilities to these above-mentioned regions. The electronic national vaccination registration system was used to collect data from individuals. This system was established before the start of COVID-19 vaccination to monitor the uptake of the second dose. The database included other electronic health records of individuals received vaccinations and was updated after offering health services, such as vaccination. This information is available at the level of the University of Medical Sciences and Ministry of Health Sciences. The data from the Kashan University of Medical Sciences (KAUMS) branch of this system was used to obtain the vaccination information of participants. The study was conducted from January 2021 to February 2022. All experiments and protocols were approved by the Ethics Committee of the Kashan University of Medical Sciences (IR.KAUMS.MEDNT.REC.1400.217). This study was performed according to the Helsinki Declaration. Inclusion criteria were: 1) negative for pregnancy and allergic reactions after vaccination; 2) the healthy subjects and patients without health problems and other diseases influencing immune responses; and 3) vaccination with two doses of COVID-19 vaccines. Exclusion criteria included: 1) the healthy volunteers undergoing immunosuppressive therapy; and 2) the presences of health problems, malignancy and other disorders affecting the immune system and antibodies production. All participants gave the written informed consent prior to study initiation.
Disease diagnosis
Patients with autoimmune disorders were referred to an internal medicine clinic of Shahid Beheshti hospital, Kashan, Iran. RA, SLE, AS, and SSc were, respectively, diagnosed by the internal medicine specialist according to ACR/EULAR criteria, 28 Systemic Lupus International Collaborating Clinics classification criteria, 29 Spondyloarthritis International Society (ASAS) classification criteria,30,31 and the 2013 ACR/EULAR classification criteria. 32
Vaccination procedure
All participants received the two-dose regimen of the COVID-19 vaccines (Sinopharm and Oxford-AstraZeneca) based on national protocols. Vaccination was done by trained health staff in the hospitals or other government health care services. Of the 221 volunteers, 201 subjects received the Sinopharm and 20 cases were vaccinated with the Oxford-AstraZeneca. Some subjects previously infected with SARS-CoV-2 had lower values of antibodies than the threshold (2.5 µg/ml) for positive detection of SARS-CoV-2 neutralizing antibodies prior to the entering study. Each dose of COVID-19 vaccines was given as an intramuscular injection in the deltoid muscle.
Subjects received one of two types of vaccines (Sinopharm or Oxford-AstraZeneca). The first and second doses of the Sinopharm and Oxford-AstraZeneca vaccines were, respectively, administered at 4-weeks and 12-weeks intervals.
The immunogenicity and safety of COVID-19 vaccines
To assess the immunogenicity of COVID-19 vaccines, the serum samples (2 mL) of individuals were collected before primary immunization and 1–3 months after secondary immunization. The serum levels of SARS-CoV-2 neutralizing antibodies were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Ideal Tashkhis, Iran) according to the manufacturer’s protocol. Adverse events were studied 7 days after secondary immunization. A questionnaire was used to collect the demographic characteristics and other information, including type of used vaccines, history of previous SARS-CoV-2 infection, adverse effects of vaccination, and other reported variables.
Statistical analysis
The data were analyzed by GraphPad Prism 6 (GraphPad, USA) and presented as the mean ± standard error of the mean (SEM) and mean ± standard deviation (SD). The normal distribution of data was determined by Kolmogrov–Smirnov test. The results with the normal distribution were analyzed by one-way analysis of variance (ANOVA) and unpaired t-tests, while those with non-normal distribution were analyzed by Kruskal–Wallis and Mann–Whitney tests. Correlation coefficients of the data were studied using the Spearman’s and Pearson’s tests in case of non-normal and normal distribution of data, respectively. The comparison of the ratios was evaluated by Fishers exact and Chi-square tests. p values ≤0.05 were considered statistically significant.
Results
Description of subjects
A total of 221 COVID-19 vaccine recipients (83 males and 138 females, mean age ± SD of 43.6 ± 9, aged 18–90 years) were enrolled for assessing immunogenicity and reactogenicity of the vaccination. Of 201 subjects vaccinated with the Sinopharm, 70 cases were male and 131 were female. Of 20 individuals received the Oxford-AstraZeneca, 13 subjects were male and seven cases were female. The mean age ±SD of subjects vaccinated with the Sinopharm was 44.21 ± 12.37, while it was 38.34 ± 14.51 for individuals received the Oxford-AstraZeneca. Of the 221 recipients, 38 had rheumatoid arthritis (RA), 26 had systemic lupus erythematosus (SLE), 15 had systemic sclerosis (SSc), 21 had ankylosing spondylitis (AS), 19 had smoking history, nine had diabetes, eight had hypertension and hyperlipidemia, four had other metabolic disorders, and one had anemia. After a 6-month follow-up, 25 subjects immunized with the Sinopharm experienced the mild and moderate forms of COVID-19, while two individuals vaccinated with the Oxford-AstraZeneca experienced the mild form of the disease. Of 27 recipients infected with SARS-CoV-2, none of the subjects did need hospitalization. Table 1 shows the clinical characteristics and demographic information of participants.
Table 1.
Demographic and clinical findings of volunteers received the Oxford-AstraZeneca and Sinopharm vaccines (n = 221).
| Healthy subjects (n = 121) | Patients with SLE (n = 26) | Patients with SSc (n = 15) | Patients with as (n = 21) | Patients with RA (n = 38) | |
|---|---|---|---|---|---|
| Age, years, median (range) | 46.11 ± 12.49 (19-75) | 49.93 ± 9.5 (35–65) | 31.7 ± 6.7 (19–63) | 42.15 ± 12.29 (21–65) | 52.62 ± 11.98 (25–75) |
| Gender, female, n (%) | 63 (52 %) | 24 (96%) | 15 (100%) | 4 (15.3%) | 32 (84.2%) |
| Smoking history | 9 (7.43%) | 2 (8%) | 0 (0.0%) | 4 (15.3%) | 4 (10.52%) |
| Disease duration, years, median (range) | - | 8.07 ± 3.8 (2–18) | 6.5 ± 3.8 (4–14) | 4.9 ± 2.3(3–10) | 8.2 ± 4.16 (3–18) |
| Background diseases | - | Diabetes: 4 | - | ||
| Hypertension:2 | Diabetes: 2 | Diabetes: 3 | |||
| Hyperlipidemia: 2 | Other | Hypertension: 6 | |||
| Anemia: 1 | metabolic disorders: 1 | Hyperlipidemia: 6 | |||
| Other metabolic disorders: 2 | Other metabolic disorders: 1 | ||||
| Myalgia | 20 (16.52%) | 7 (26.9%) | 0 (0.0%) | 3 (14.2%) | 6 (15.7%) |
| Fever | 8 (6.61%) | 2 (7.69%) | 1 (6.6%) | 3 (14.2%) | 2 (5.23%) |
| Headache | 13 (0.0%) | 2 (7.69%) | 0 (0.0%) | 2 (13.3%) | 4 (10.46%) |
| Natural history of COVID-19 | 46 (38.01%) | 17 (65.38%) | 4 (26.6%) | 7 (33.3%) | 21(55.26%) |
| Skin lesions | 1 (0.8%) | 1 (3.84%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Concomitant immunosuppressive medications, n (%) | |||||
| Methotrexate | 0 (0.0%) | 0 (0.0%) | 12 (80%) | 0 (0.0%) | 30 (78.90%) |
| Glucocorticoid | 0 (0.0%) | 26 (100%) | 14 (93.3%) | 0 (0.0%) | 38 (100%) |
| Mycophenolate mofetil | 0 (0.0%) | 23 (88.4%) | 13 (86.6%) | 0 (0.0%) | 0 (0.0%) |
| Hydroxyl chloroquine | 0 (0.0%) | 24 (92.3%) | 0 (0.0%) | 0 (0.0%) | 30 (78.90%) |
| Sulfasalazine | 0 (0.0%) | 4 (15.3%) | 5 (33.3 %) | 18 (85.7%) | 3 (7.8%) |
| Anti-TNF | 0 (0.0%) | 3 (11.5%) | 1 (6.6%) | 20 (95.2%) | 0 (0.0%) |
SLE: systemic lupus erythematosus; SSc: systemic sclerosis; AS: ankylosing spondylitis; RA, rheumatoid arthritis.
The immunogenicity of COVID-19 vaccines
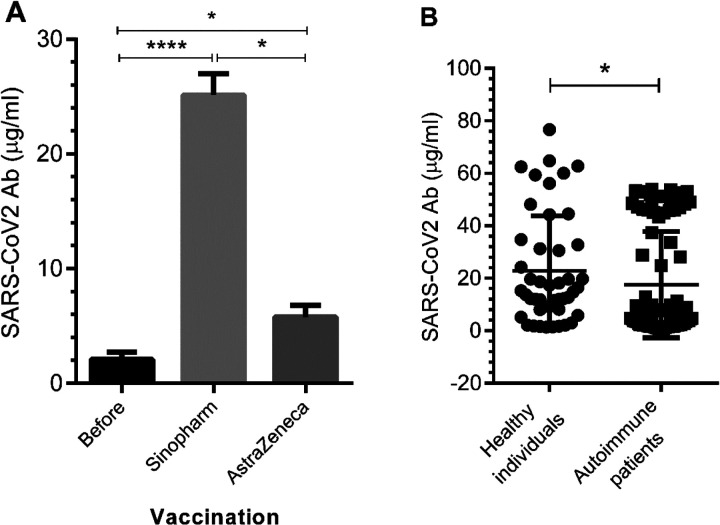
To clarify the efficiency of COVID-19 vaccines, the values of SARS-CoV-2 neutralizing antibodies were studied. Vaccination had a positive impact on the induction of immunogenic response (p < .0001, Figure 1(a)). The rates of SARS-CoV-2 neutralizing antibody responses to COVID-19 vaccines were 80% in subjects vaccinated with the Sinopharm, while it was 75% in those received the Oxford-AstraZeneca. Other data revealed that there was a significant difference in the level of neutralizing antibody between subjects with and without autoimmunity (SLE, RA, SSc, and AS) (p < .05, Figure 1(b)). The means ± SD of neutralizing antibody values observed in subjects with and without autoimmunity were 17.58 ± 20.2 and 22.78 ± 20.9, respectively (Figure 1(b)). To support this data, natural history of COVID-19 showed an increase in patients with autoimmune diseases compared to healthy individuals, although it was not statistically significant.
Figure 1.
The immunogenicity of the Sinopharm and AstraZeneca vaccines in healthy subjects and patients with autoimmune diseases. SARS-CoV-2 neutralizing antibody values in subjects were measured by ELISA. A and B) The depicted results are representative of 100 independent experiments for healthy individuals and 221 independent experiments for patients with autoimmune disorders (26 SLE subjects, 38 RA subjects, 15 SSc subjects, and 21 AS subjects). The results are shown as mean ± SD. *p < .05, **p < .01.
The safety of COVID-19 vaccines in participants
To determine the safety of COVID-19 vaccines, adverse events were documented 7 days after secondary immunization. Side effect rate was 38% in subjects vaccinated with the Sinopharm, while it was 42% in those received the Oxford-AstraZeneca. Of 221 cases, 76 and 9 cases suffered from adverse events of the Sinopharm and Oxford-AstraZeneca, respectively. The most common side effects of the COVID-19 vaccines among participants were fever, headache, and myalgia. Other adverse events of COVID-19 vaccination were joint swelling, breathing problems, hypoglycemia, skin lesions, gastrointestinal disorders, lethargy, which were observed in one case. After a 1-year follow-up, mucosal membrane pemphigoid (MMP) was reported in one case vaccinated with the Sinopharm. Table 2 indicates adverse events of the Oxford-AstraZeneca and Sinopharm in participants.
Table 2.
Side effects of the COVID-19 vaccines were documented 7 days after secondary immunization.
| Healthy subjects (n = 121) | Patients with SLE (n = 26) | Patients with SSc (n = 15) | Patients with as (n = 21) | Patients with RA (n = 38) | |
|---|---|---|---|---|---|
| Fever ≥38.0°C, n (%) | 12 (9.91%) | 2 (7.5%) | 3 (20%) | 4 (23.8%) | 1 (2.63%) |
| Myalgia, n (%) | 8 (6.61%) | 5 (20.9%) | 0 (0.0%) | 4 (23.8%) | 7 (19.7%) |
| Headache, n (%) | 14 (11.57%) | 2 (7.5%) | 0 (0.0%) | 2 (13.3%) | 6 (17.78%) |
| Joint swelling, n (%) | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.63%) |
| Shortness of breath, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.63%) |
| Hypoglycemia, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.63%) |
| Skin lesions, n (%) | 0 (0.0%) | 1 (3.84%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Gastrointestinal disorders, n (%) | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Lethargy, n (%) | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
SLE: systemic lupus erythematosus; SSc: systemic sclerosis; AS: ankylosing spondylitis; RA, rheumatoid arthritis.
The immunogenicity and reactogenicity of COVID-19 vaccines in SARS-CoV-2 seropositive and seronegative subjects
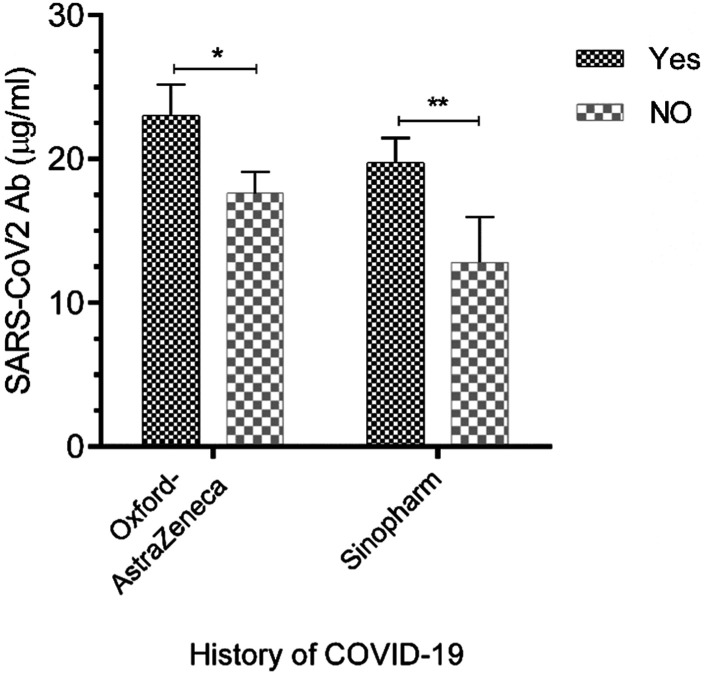
The efficiency of COVID-19 vaccines was investigated in SARS-CoV-2-non infected and-infected individuals. The rate of Sinopharm-induced positive immunogenic response was 76% in subjects with a history of SARS-CoV-2 infection, while it was 81.5% for Oxford-AstraZeneca-induced positive immunogenic response in subjects previously infected with SARS-CoV-2. These rates in seronegative subjects vaccinated with the Sinopharm and Oxford-AstraZeneca were 63.8% versus 79.1%, respectively. The titers of SARS-CoV-2 neutralizing antibodies were significantly higher in healthy subjects and patients with autoimmune disorders, who had a SARS-CoV-2-induced positive immunogenic response, than seronegative subjects (Figure 2, P < .01-.05).
Figure 2.
The immunogenicity of COVID-19 vaccines in seropositive and seronegative persons. SARS-CoV-2 neutralizing antibody titers in seropositive and seronegative subjects were assessed by ELISA. Data are representative of 126 independent experiments for subjects previously infected with SARS-CoV-2 and 95 independent experiments for those without a history of COVID-19. All data are shown as mean ± SD. *p < .05, **p < .01.
In the next step, vaccine reactogenicity was evaluated in seronegative and seropositive individuals. Seropositive individuals vaccinated with the Sinopharm showed a significant enhancement in the frequency of vaccine-related side effects compared to those without a history of COVID-19 (p < .05, 95% confidence interval: 0.250 to 0.9543, odds ratio: 0.3660), while there was no significant difference between seronegative and seropositive individuals vaccinated with the Oxford-AstraZeneca.
Discussion
Although there are several studies showing the efficacy and reactogenicity of COVID-19 vaccines in diverse populations, the immunogenicity and reactogenicity of COVID-19 vaccination in the Iranian populations are not well understood yet. The current study was therefore focused on illustrating whether the two-dose Sinopharm and Oxford-AstraZeneca vaccination regimens, as the most frequent vaccines used in the COVID-19 vaccination protocols in Iran, are effective in producing SARS-CoV-2 neutralizing antibodies with acceptable reactogenicity in healthy individuals and subjects with autoimmune diseases.
In the current study, immunization of Iranian subjects with the Sinopharm and Oxford-AstraZeneca vaccines provided sufficient immunity against SARS-CoV-2 infection. The result revealed that the Sinopharm had more the ability to produce neutralizing antibodies than the Oxford-AstraZeneca, which is in contrast with previous studies. 33 In a study conducted in Argentina, the antibody responses of volunteers were investigated at day 0 (primary immunization) and at 21–25 days after the second doses of the Oxford–AstraZeneca and Sinopharm injections. The detection of antibody (IgG) against the Spike protein and Receptor Binding Domain (RBD) indicated that the administration of the second dose of the vaccines induced considerable humoral responses in the majority of the immunized individuals. 33 After secondary immunization, the percentage of immunization was 100% for the AstraZeneca, while it was 88% in case of the Sinopharm. 33 Another study conducted in Vietnam, the rate of Oxford-AstraZeneca-induced positive immunogenic response was reported 98.1%, 14 days after secondary immunization. 34 In addition to these findings, the second dose of the Oxford-AstraZeneca increased safety against COVID-19 among United Kingdom people from 65% to 70%. 35 This discrepancy observed between previous reports and our study could be attributed to low sample size used to determine the immunogenicity of the Oxford-AstraZeneca vaccine in the present study.
Given that some participants suffered from autoimmunity, the immunogenicity of COVID-19 vaccines was assessed in these individuals. Patients with autoimmune diseases showed a significant reduction in the titer of neutralizing antibodies compared to healthy subjects, although they had suitable level of neutralizing antibodies. In line with the efficiency of COVID-19 vaccines in producing neutralizing antibodies, few reports have indicated that mRNA SARS-CoV-2 vaccines are unable to induce an adequate immune response in patients with autoimmune diseases undergoing immunosuppressive therapy. 36 These findings are consistent with other studies indicating the immunogenicity of COVID-19 vaccines against SARS-CoV-2 in these patients.9,37 It is reported that immunization with SARS-CoV-2 mRNA and inactivated CoronaVac vaccines provided a suitable titer of neutralizing antibody in the majority of patients with autoimmune diseases, however, the patients had a reduction in antibody response compared to the healthy individuals.9,38,39 These observations propose that the reduction in production of SARS-CoV-2 neutralizing antibodies may correlate to the undesirable effects of immunosuppressive treatments on the immunogenicity of COVID-19 vaccines.
Unlike the immunogenicity of the Oxford-AstraZeneca which was lower than immunization with the Sinopharm, our results indicated that the two-dose Oxford-AstraZeneca vaccination regimen had higher occurrence of side effects (42%) than the Sinopharm (38%). This prevalence is similar to those reported for a Phase 1/2 clinical trial conducted on the Sinopharm (39.0%) and other previous studies.40,41 Furthermore, a recent meta-analysis study on inactivated vaccines has shown lower incidence of side effects of the Sinopharm than SARS-CoV-2 mRNA vaccines. 42 In regard to adverse events of COVID-19 vaccines, there are some studies pointing to higher prevalence of side effects of COVID-19 vaccines than our study. A study conducted in Jordan revealed that the rates of adverse reactions of the Oxford-AstraZeneca and Sinopharm vaccines were 98.3% and 52.8%, respectively. 40 It is reported that 67.0% of Ethiopian healthcare professionals experienced side effects after the second dose of the Oxford-AstraZeneca. 43 Others have reported that 86% of the population of the United Arab Emirates had adverse events after receiving the second dose of the Sinopharm. 44 Moreover, adverse effect rate of the Sinopharm was 61.1% in the Iraqi population. 45 The discrepancy observed between this study and others may be attributed to sample size, study design, population heterogeneity, and ethnicity. 46
In our study, the most frequent adverse events of the Oxford-AstraZeneca and Sinopharm were fever, headache, and myalgia after secondary vaccination which are consistent with the findings of a large-scale study undertaken in the United Kingdom showing headache, weariness, and fever as the most prevalent systemic side effects of COVID-19 vaccines. 47 Zhu FC et al. reported that over half of subjects suffered from headache following receiving the second dose of a recombinant adenovirus type-5-vectored COVID-19 vaccine. 48 Taken together, the Sinopharm and Oxford-AstraZeneca vaccines had the ability to induce immune responses against SARS-CoV-2 which may be accompanied by systemic and local adverse events.
Although the results of the present study aligns with other studies suggesting the lower incidence of side effects of the Sinopharm than the Oxford-AstraZeneca, a 1-year follow-up demonstrated that one person vaccinated with the Sinopharm developed MMP after 1 year. This observation proposes further studies to illustrate the vaccination with COVID-19 vaccines, especially the Sinopharm, may act as a risk factor in long-term development of autoimmunity.
In the next step, the impacts of COVID-19 vaccination on inducing immune response in seronegative and seropositive subjects were investigated. Our data revealed that persons previously infected with SARS-CoV-2 had more success in enhancing the titer of SARS-CoV-2 neutralizing antibodies than seronegative people. This finding is consistent with some reports showing the first dose of mRNA vaccine produced antibody responses in seropositive subjects, which were sometimes exceeded titers observed in seronegative subjects received two doses of the mRNA vaccines.9,49 It is shown that the BNT162b2 SARS‐CoV‐2 vaccine had the ability to induce IgG level in both seropositive and seronegative individuals; however, only individuals with pre‐existing immunity showed the increased levels of IgM and IgA after primary vaccination. In line with the impacts of the Oxford-AstraZeneca, subjects already contracted SARS-CoV-2 produced neutralizing antibodies against alpha, D614 G, beta, delta, and gamma variants.50,51 It was indicated that history of COVID-19 infection played a fundamental role in enhancing IgG titer in Iraqi people vaccinated with the Oxford-AstraZeneca and Sinopharm. Furthermore, this study demonstrated that the Oxford-AstraZeneca had more effective in raising IgG in seropositive subjects than the Sinopharm. 52 This phenomenon may be correlated to hybrid immunity. It is suggested that antibodies produced by previous SARS-CoV-2 infection combine with post-vaccination antibodies to develop “hybrid immunity.”53,54 Thus, combination of acquired natural immunity to SARS-CoV-2 with vaccine-generated protection can provide a larger-than-expected immunological response. 55 These findings propose that pre‐existing immunity investigation before vaccination is needed to obtain a satisfactory antibody response against COVID-19.
Conclusions
Overall, vaccination with the Oxford-AstraZeneca and Sinopharm vaccines can provide a humoral response with an acceptable safety in the majority of healthy subjects and patients with autoimmune diseases undergoing immunosuppressive therapy. These findings may be reassurance for administering the Oxford-AstraZeneca and Sinopharm vaccines with the short-term immunogenicity and acceptable safety in the Iranian population. However, there are several limitations for this study, for example, the relatively small sample size of subjects vaccinated with the Oxford-AstraZeneca, which may not exactly represent the immunogenicity and safety in overall population. Moreover, adverse events could be investigated after primary immunization. Finally, cellular immunity was not investigated, which can offer more evidence in regard to immune responses, especially in seronegative subjects who had already contracted SARS-CoV-2. Therefore, these limitations should be addressed in future studies.
Acknowledgments
The authors would like to thank all subjects who participated in the study.
Footnotes
Author contributions: Amin Moradi Hasan-Abad participated in the study design and carried out some of the experiments. Mohsen Arbabi and Hamidreza Gilasi participated in the design of the experiments and performed statistical analysis. Hossein Motedayyen obtained funding for the work, participated in the study design, and drafted the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by Kashan University of Medical Sciences (Grant No.: 400,165).
Ethical statement
Ethical approval
The work was approved by the Ethics Committee of Kashan University of Medical Sciences (IR.KAUMS.MEDNT.REC.1400.217).
Consent to participate
The written informed consent to participate and publish the study was collected before entering in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.
References
- 1.Sohrabi C, Alsafi Z, O’Neill N, et al. (2020) World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). International Journal of Surgery, 76, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raoofi A, Takian A, Akbari Sari A, et al. (2020) COVID-19 pandemic and comparative health policy learning in Iran. Arch Iran Med 23(4): 220–234. [DOI] [PubMed] [Google Scholar]
- 3.Wu R, Wang L, Kuo H-CD, et al. (2020) An update on current therapeutic drugs treating COVID-19. Current pharmacology reports 6(3): 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starshinova Anna, Malkova Anna, Zinchenko Ulia, et al. (2021) Efficacy of Different Types of Therapy for COVID-19: A Comprehensive Review. Switzerland: Multidisciplinary Digital Publishing Institute (MDPI). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim SS, Devnarain N. (2022) Time to Stop using Ineffective Covid-19 Drugs. Mass Medical Soc 6: 654–655. [DOI] [PubMed] [Google Scholar]
- 6.Razai MS, Chaudhry UA, Doerholt K, et al. (2021) Covid-19 vaccination hesitancy. Bmj 9: 373. [DOI] [PubMed] [Google Scholar]
- 7.Schaffer DeRoo S, Pudalov NJ, Fu LY. (2020) Planning for a COVID-19 vaccination program. JAMA 323(24): 2458–2459. [DOI] [PubMed] [Google Scholar]
- 8.El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. (2021) Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med 14: 1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamani B, Moradi Hasan‐Abad A, Piroozmand A, et al. (2023) Immunogenicity and safety of the BBIBP‐CorV vaccine in patients with autoimmune inflammatory rheumatic diseases undergoing immunosuppressive therapy in a monocentric cohort. Immunity, Inflammation and Disease 11(5): e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furer V, Eviatar T, Zisman D, et al. (2021) Predictors of an immunogenic response to the BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune Inflammatory rheumatic diseases treated with Rituximab: a Multicenter study. Switzerland: Multidisciplinary Digital Publishing Institute (MDPI). [Google Scholar]
- 11.Forni G, Mantovani A, COVID-19 Commission of Accademia Nazionale dei Lincei, Rome (2021) COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ 28(2): 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndwandwe D, Wiysonge CS. (2021) COVID-19 vaccines. Curr Opin Immunol 71: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiasi N, Valizadeh R, Arabsorkhi M, et al. (2021) Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathologia Persa 7: 31. [Google Scholar]
- 14.Bernal JL, Andrews N, Gower C, et al. (2021) Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. Bmj 9: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton-Chubb D, Schneider D, Freeman E, et al. (2021) Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol 75(5): 1249–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havla J, Schultz Y, Zimmermann H, et al. (2022) First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol 269(1): 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javadinia SA, Ariamanesh M, Nabavifard M, et al. (2022) Multicenter study of antibody seroprevalence against COVID-19 in patients presenting to iranian cancer centers after one year of the COVID-19 pandemic. Cancer Invest 40(2): 115–123. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman T, Shiroma K, Fleischmann KR, et al. (2023) Misinformation and COVID-19 vaccine hesitancy. Vaccine 41(1): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhazmi A, Alamer E, Daws D, et al. (2021) Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines 9(6): 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riad A, Pokorná A, Attia S, et al. (2021) Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med 10(7): 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delshad M, Sanaei M-J, Pourbagheri-Sigaroodi A, et al. (2022) Host genetic diversity and genetic variations of SARS-CoV-2 in COVID-19 pathogenesis and the effectiveness of vaccination. Int Immunopharm 111: 109128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adli A, Rahimi M, Khodaie R, et al. (2022) Role of genetic variants and host polymorphisms on COVID‐19: from viral entrance mechanisms to immunological reactions. J Med Virol 94(5): 1846–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolday D, Fung CYJ, Morgan G, et al. (2023) HLA variation and SARS-CoV-2 Specific antibody response. Viruses 15(4): 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Made CI, Netea MG, van der Veerdonk FL, et al. (2022) Clinical implications of host genetic variation and susceptibility to severe or critical COVID-19. Genome Med 14(1): 96–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zare H, Rezapour H, Mahmoodzadeh S, et al. (2021) Prevalence of COVID-19 vaccines (Sputnik V, AZD-1222, and Covaxin) side effects among healthcare workers in Birjand city, Iran. Int Immunopharm 101: 108351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babamahmoodi F, Saeedi M, Alizadeh-Navaei R, et al. (2021) Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci Rep 11(1): 21464–21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pourakbari B, Mirbeyk M, Mahmoudi S, et al. (2022) Evaluation of response to different COVID‐19 vaccines in vaccinated healthcare workers in a single center in Iran. J Med Virol 94: 5669–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen S, Emery P. (2010) The American College of Rheumatology/European League against Rheumatism criteria for the classification of rheumatoid arthritis: a game changer. UK: BMJ Publishing Group Ltd, 1575–1576. [DOI] [PubMed] [Google Scholar]
- 29.Petri M, Orbai AM, Alarcón GS, et al. (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8): 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akkoc N, Khan MA. (2016) ASAS classification criteria for axial spondyloarthritis: time to modify. Clin Rheumatol 35(6): 1415–1423. [DOI] [PubMed] [Google Scholar]
- 31.Hayward RJ, Machado PM. (2020) Classification criteria in axial spondyloarthritis: what have we learned; where are we going? Rheumatic diseases clinics of North America 46(2): 259–274. [DOI] [PubMed] [Google Scholar]
- 32.van den Hoogen F, Khanna D, Fransen J, et al. (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65(11): 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giai C, Salassa BN, Zarelli VE, et al. (2023) Comparative analysis of humoral immune response upon the three first vaccines applied in Argentina: IgG production and neutralizing capacity against SARS-CoV-2. Heliyon 9(5): e15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau NVV, Nguyet LA, Truong NT, et al. (2022) Immunogenicity of Oxford-AstraZeneca COVID-19 vaccine in Vietnamese health-care workers. Am J Trop Med Hyg 106(2): 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchard E, Matthews PC, Stoesser N, et al. (2021) Impact of vaccination on SARS-CoV-2 cases in the community: a population-based study using the UK’s COVID-19 Infection. Survey. medRxiv 19: 2021. [Google Scholar]
- 36.Sieiro Santos C, Calleja Antolin S, Moriano Morales C, et al. (2022) Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open 8(1): e001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furer V, Eviatar T, Zisman D, et al. (2021) Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Annals of the rheumatic diseases 80(10): 1330–1338. [DOI] [PubMed] [Google Scholar]
- 38.Kian W, Zemel M, Kestenbaum EH, et al. (2022) Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: a retrospective single-center study. Medicine 101(2): e28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batıbay S, Ulucaköy RK, Günendi Z, et al. (2022) Immunogenicity and safety of the CoronaVac and BNT162b2 Covid-19 vaccine in patients with inflammatory rheumatic diseases and healthy adults: comparison of different vaccines. Inflammopharmacology 30(6): 2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Hammad O, Alduraidi H, Abu-Hammad S, et al. (2021) Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines 9(6): 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia S, Zhang Y, Wang Y, et al. (2021) Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 21(1): 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kouhpayeh H, Ansari H. (2022) Adverse events following COVID-19 vaccination: a systematic review and meta-analysis. Int Immunopharm 109: 108906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desalegn M, Garoma G, Tamrat H, et al. (2022) The prevalence of AstraZeneca COVID-19 vaccine side effects among Nigist Eleni Mohammed memorial comprehensive specialized hospital health workers. Cross sectional survey. PLoS One 17(6): e0265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeed BQ, Al-Shahrabi R, Alhaj SS, et al. (2021) Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis: IJID: official publication of the International Society for Infectious Diseases 111: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almufty HB, Mohammed SA, Abdullah AM, et al. (2021) Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metabol Syndr 15(5): 102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tequare MH, Abraha HE, Adhana MT, et al. (2021) Adverse events of Oxford/AstraZeneca's COVID-19 vaccine among health care workers of Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia. IJID Regions 1: 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menni C, Klaser K, May A, et al. (2021) Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 21(7): 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu F-C, Guan X-H, Li Y-H, et al. (2020) Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England) 396(10249): 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krammer F, Srivastava K, Alshammary H, et al. (2021) Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384(14): 1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadesalingam A, Cantoni D, Wells DA, et al. (2021) Paucity and discordance of neutralising antibody responses to SARS-CoV-2 VOCs in vaccinated immunodeficient patients and health-care workers in the UK. The Lancet. Microbe 2(9): e416–e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Folegatti PM, Ewer KJ, Aley PK, et al. (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet (London, England) 396(10249): 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibraheem ZK, AL-Azzawy RH. (2022) Comparative study of Immunoglobulin G and Gender between COVID-19 patients and vaccinated Iraqi individuals with Pfizer, AstraZeneca and Sinopharm vaccine. The Egyptian Journal of Hospital Medicine 89(1): 5758–5763. [Google Scholar]
- 53.Crotty S. (2021) Hybrid immunity. Science 372(6549): 1392–1393. [Google Scholar]
- 54.Gazit S, Shlezinger R, Perez G, et al. (2022) The incidence of SARS-CoV-2 reinfection in persons with naturally acquired immunity with and without subsequent receipt of a single dose of BNT162b2 vaccine: a retrospective cohort study. Ann Intern Med 175(5): 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamatatos L, Czartoski J, Wan Y-H, et al. (2021) mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 372(6549): 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.