Abstract
Background
Clinical studies have shown that programmed cell death-1 (PD-1) inhibitors can activate T cells and inhibit cancer growth. Therefore, the use of a PD-1 inhibitor plus chemotherapy as neoadjuvant chemotherapy for locally advanced esophageal cancer is worth further exploration.
Methods
Patients with locally advanced esophageal squamous cell carcinoma were enrolled in this study to receive two cycles of a preoperative combination of toripalimab, paclitaxel, and cisplatin. Efficacy was evaluated after two treatment cycles. The patients’ postoperative pathological staging was analyzed and compared. Surgery was performed within 42 days of the start date of the last chemotherapy cycle.
Results
Neoadjuvant immunochemotherapy achieved a high pathologic complete response (pCR) rate (29.0%), major pathological response rate (41.9%), and objective response rate (80.6%) and demonstrated statistically significant downstaging after neoadjuvant therapy (P < .05) with manageable treatment-related adverse effects. No significant association was found between PD-L1 level and pCR (P = .365). In addition, R0 resection was achieved in all 31 (100%) patients during surgery. For all the included patients, the one-year progression-free survival rate was 87.1% (95% CI: 75.3%-98.9%), the one-year overall survival (OS) rate was 96.8% (95% CI: 79.8%-95.9%), and the two-year OS rate was 83.9% (95% CI: 71.6%-92.2%).
Conclusions
Our findings indicate that this combination may be a potential neoadjuvant therapy regimen in this setting.
Keywords: neoadjuvant immunochemotherapy, esophageal squamous cell carcinoma, thoracic surgery, programmed death 1, pathologic complete response, OVERALL SURVIVAL, progression-free survival
Introduction
Esophageal cancer is a common fatal disease with the seventh highest incidence rate and the sixth highest mortality rate globally. 1 The two main types of esophageal cancer are esophageal squamous cell carcinoma (ESCC) and adenocarcinoma. The incidence of ESCC is the highest in Southeast and Central Asia, where it accounts for 79% of all reported ESCC cases. 2 Locally advanced esophageal cancer refers to tumors that invade regional lymph nodes (N1-3) or local structures (T4 disease).3,4 Patients in this category may be resectable, inoperable because of medical reasons, or technically unresectable because of local tumor extension. Currently, surgical resection is the primary treatment for locally advanced esophageal cancer. Most patients are already at the locally advanced stage when they are diagnosed; thus, the surgical resection and long-term survival rates are low, and the 5-year survival rate is only 20%. Preoperative neoadjuvant therapy can enhance the surgical resection rate. According to the 2012 CROSS study data (the Netherlands), compared with surgery alone, neoadjuvant chemoradiotherapy plus surgery can improve overall survival (OS) with acceptable adverse-event rates among patients with potentially curable esophageal or esophagogastric junction cancer. 5 Preoperative chemotherapy with cisplatin plus 5-fluorouracil is the current standard treatment for locally advanced esophageal cancer in Japan. 6 As a standard treatment for patients diagnosed with locally advanced ESCC, surgery after preoperative chemotherapy has a considerably higher OS rate than surgery alone. 7 However, 31%–39% of patients still relapse within 3–5 years after treatment.8,9
Recently, a monoclonal antibody that inhibits programmed cell death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) has been developed. It triggers remarkable therapeutic responses in various malignancies, including ESCC. 10 PD-1/PD-L1 inhibitors have been used to treat many cancer types, such as melanoma, lung cancer, renal cell carcinoma, and esophageal cancer, 11 because they can activate T cells and suppress tumor growth. 12 Moreover, related studies have shown that the effects of PD-1/PD-L1 inhibitors may be associated with tumor mutational burden (TMB).13,14 TMB is a genetic characteristic of tumorous tissue that can be informative to cancer treatment. 15 In clinical practice, a certain level of TMB expression has been found in patients with esophageal cancer.16,17 Therefore, the combination of the PD-1 inhibitor and chemotherapy as neoadjuvant therapy for patients with esophageal cancer is expected to reduce TMB and achieve downstaging.
According to the National Comprehensive Cancer Network Guidelines, first-line chemotherapy for ESCC is based on platinum and combined with fluorouracil or paclitaxel. 16 Preoperative neoadjuvant chemotherapy has become the standard preoperative esophageal cancer treatment; however, no uniform criteria for using neoadjuvant therapy have been established. Previous clinical data have confirmed that the combination of docetaxel, cisplatin, and 5-fluorouracil (DCF) as neoadjuvant chemotherapy alone achieves a 10% postoperative pathologic complete response (pCR) rate in patients with locally advanced ESCC, suggesting that neoadjuvant chemotherapy alone has a limited local control rate of esophageal cancer. Several studies have revealed that PD-1 inhibitors exert therapeutic effects on esophageal cancer as first- or second-line treatments.18–22 Meanwhile, several small-sample exploratory studies have reported the use of PD-1 monoclonal antibody combined with chemotherapy for the neoadjuvant treatment of esophageal cancer. Therefore, we employed immunotherapy combined with chemotherapy as a neoadjuvant treatment for locally advanced ESCC to improve R0 resection and pCR rates and enhance patient prognosis.
Toripalimab, a monoclonal antibody specific for humans PD-1, has been approved for the treatment of several malignancies.23–26 Recent studies have demonstrated its efficacy and safety in treating esophageal carcinoma. 27 During the planning period of the present study, no data existed on large-scale clinical trials that used toripalimab and chemotherapy as neoadjuvant chemotherapy for locally advanced ESCC. Furthermore, preoperative chemoradiation has been reported to increase the risk of bleeding or the difficulty of surgery. 28 In addition, most patients prefer surgery when their cancer diagnosis is confirmed. Therefore, this study did not apply radiotherapy in the neoadjuvant setting. This prospective study was conducted to evaluate the efficacy and safety of toripalimab and chemotherapy as neoadjuvant therapy for locally advanced ESCC.
Materials and Methods
Patient Selection
Patients with locally advanced ESCC (cT3N0-xM0, cT2NxM0; based on the eighth edition of the tumor/node/metastasis [TNM] classification for esophageal cancer made by the American Joint Committee on Cancer [AJCC]) were recruited. The study protocol was approved by the Research Ethics Board of Research Ethical Board of Nanjing Medical University (approval # 2019-005). All included patients signed a consent form, and we de-identified all patient details.
The inclusion criteria were as follows: (I) patients aged between 18 and 75 years with an Eastern Cooperative Oncology Group performance status score of 0–1 29 ; (II) patients with ESCC diagnosed by pathological or histological examinations before neoadjuvant therapy; (III) patients whose cardiopulmonary function and tumor staging were judged as tolerable for surgical resection after a multidisciplinary team discussion; and (IV) patients whose clinical staging was confirmed via positron emission tomography–computed tomography, contrast-enhanced computed tomography (CT), or magnetic resonance imaging. The main exclusion criteria were as follows: (I) patients who received any other antitumor therapy before admission, (II) patients with distant metastases, and (III) patients with other severe malignant tumors.
Neoadjuvant Regimen
The patients were administered a fixed dose of the PD-1 inhibitor (toripalimab, 240 mg) in combination with an intravenous infusion of paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) on the first day of the cycle. One cycle was for three weeks. The patients underwent surgery after two treatment cycles. Blood routine and renal, hepatic, and thyroid functions were reviewed every three weeks before each chemotherapy session to assess the toxicity of the patients. The doses of paclitaxel and cisplatin were reduced by 20% in the subsequent course when grade 4 neutropenia, anemia, or thrombocytopenia was observed.
Surgery
The patients received two immunotherapy cycles plus chemotherapy, followed by esophagectomy. After two neoadjuvant therapy cycles, the patients underwent CT reexaminations to evaluate the efficacy of the treatment. The surgeon selected the surgical method in accordance with the location of the lesion.
The patients underwent esophagectomy 6–8 weeks after neoadjuvant therapy. All resected lymph nodes were pathologically examined and classified on the basis of their anatomical position using the numbering system of the Mountain–Dresler modification originally proposed by the American Thoracic Society. 30 In addition, the patients’ surgical indicators (eg, surgical time, estimated blood loss, complete resection rate [R0], and lymph node dissection), pathologic response, and postoperative TNM staging were recorded.
Outcomes
The primary outcome was the pCR rate. pCR was defined as the absence of residual tumor cells in the completely resected tumor specimen and all sampled regional lymph nodes.31,32 The objective response to neoadjuvant therapy was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. 33 Pathologists with at least five years of working experience assessed the objective response on the basis of the percentage of the measured residual viable tumor cells in the resected primary tumor. Moreover, the degree of histomorphologic tumor regression was classified into four categories: Grade 0 (no residual cancer cells; pCR), Grade 1 (single cells or small groups of cancer cells), Grade 2 (residual cancer cells outgrown by fibrosis), and Grade 3 (minimal or no therapeutic effect).34,35
The secondary outcomes included the objective response rate (ORR), major pathological response (MPR), R0 resection rate, postoperative downstaging rate, safety of the neoadjuvant therapy, one-year progression-free survival (PFS), one-year OS, two-year OS, and post-neoadjuvant therapy response rates. MPR was defined as the presence of 10% of residual tumor cells in the primary tumor bed after surgery.31,32 The R0 resection rate was determined based on the following criteria: no tumor was visible to the naked eye at the surgical cut edge and no tumor cells were present within 1 mm of the surgical cut edge under a microscope. 36 For postoperative downstaging, judgment was made in accordance with the eighth edition of the AJCC staging of epithelial cancers of the esophagus and esophagogastric junction, which classified the staging diagnosis as clinical, pathologic, and post-neoadjuvant. 37 Postoperative downstaging was defined as decreased T, N, or TNM stage before and after neoadjuvant therapy. The toxic effects were closely monitored in patients who received neoadjuvant therapy on the basis of the National Cancer Institute's Common Terminology Criteria for Adverse Events version 5.0. PFS was defined as the time from therapy to disease progression from any cause, and OS was defined as the time from therapy to death from any cause. 38 The definitions and measurements of the response rates (ie, complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD]) were consistent with the standards stated in RECIST 1.1. 33
To determine if the PD-L1 level was associated with immunotherapy efficacy, we used streptavidin peroxidase solutions in the immunohistochemistry method to determine the PD-L1 of all biological tissues before neoadjuvant therapy. The combined positive score (CPS) was calculated for each patient in accordance with the assessment results, ie, CPS = the total number of PD-L1-staining cells (tumor and immune cells) / the total number of viable tumor cells × 100. In addition, CPS < 10 signified low PD-L1 expression. 39
Statistical Analysis
Data were analyzed using SPSS version 26.0 (IBM Corporation; Armonk, New York). Qualitative variables are expressed as frequency and percentage, and quantitative variables are presented as mean ± standard deviation (SD) or median and range (minimum–maximum). Wilcoxon's signed-rank test was used to determine the difference between pre-operative and postoperative TNM stages. Survival curves were estimated using the Kaplan–Meier method. P < .05 was considered statistically significant. Additionally, the differences in pCR between patients whose PD-L1 CPS ≥ 10 and CPS < 10 were analyzed through the χ² test. This prospective study followed the relevant Equator guidelines. 40
Results
Patient Characteristics
Thirty-three patients with locally advanced ESCC treated at Jiangsu Cancer Hospital from May 2019 to August 2021 were included in this study. Two patients withdrew their consent before receiving treatment, and 31 patients underwent surgery after two neoadjuvant therapy cycles. The baseline characteristics of the 31 patients met the study inclusion criteria (Table 1). The median age of the included patients was 65 years, and most of the patients were male (80.6%). The dominant cancer location was the middle third of the esophagus (61.3%), and approximately half of the patients (48%) were at Stage III.
Table 1.
Baseline Characteristics of the Patients.
| Characteristics | All patients, n (%) * (N = 31) |
|---|---|
| Age (years), median (range) | 65 (49-74) |
| Sex, n (%) | |
| male | 25 (81) |
| Female | 6 (19) |
| Tumor location, n (%) | |
| Proximal third | 3 (10) |
| Middle third | 19 (61) |
| Distal third | 9 (29) |
| Esophagogastric junction | 0 (0) |
| ECOG PS, n (%) | |
| 0 | 17 (55) |
| 1 | 14 (45) |
| Clinical T stage, n (%) | |
| T1 | 0 (0) |
| T2 | 18 (58) |
| T3 | 13 (42) |
| T4 | 0 (0) |
| Clinical N stage, n (%) | |
| N0 | 6 (19) |
| N1 | 9 (29) |
| N2 | 14 (45) |
| N3 | 2 (7) |
| Clinical M stage, n (%) | |
| M0 | 31 (100) |
| M1 | 0 (0) |
| Clinical TNM stage, n (%) | |
| Stage II | 3 (10) |
| Stage III | 15 (48) |
| Stage IVA | 13 (42) |
| PD-L1 status, n (%) | |
| CPS<10 | 16 (52) |
| CPS ≥ 10 | 15 (48) |
*Or specified.
Primary Outcome
The postoperative pathology revealed that Grade 0 postoperative pCR was achieved in nine patients (29.0%). The mean number of resected lymph nodes was 17 (range of 7-32) in 31 patients. Six (19.0%), nine (29.0%), and seven (23.0%) patients belonged to grades 1, 2, and 3, respectively. Seven patients (23.0%) did not respond to neoadjuvant therapy.
Secondary Outcomes
All patients underwent preoperative CT examination to evaluate the therapeutic effects of neoadjuvant therapy. We found PR in 25 (80.6%) patients and SD in 6 (19.4%) patients. No patient had CR or PD. Therefore, the ORR rate was 80.6%. In addition, 22, 7, and 2 cases underwent McKeown esophagectomy, Ivor Lewis esophagectomy, and Sweet esophagectomy, respectively, 41 and MPR was achieved in 13 patients (41.9%). R0 resection was achieved in all 31 (100%) patients during surgery. The median operation time was 242 min (IQR, 221-260 min), and the median intraoperative blood loss was 120 mL (IQR, 110-140 mL). No patient experienced in-hospital death. Pulmonary infection anastomotic (n = 9, 29.0%) was the most common complication in the postoperative period (Table 2).
Table 2.
Operative Details and Complications.
| TNM stages | No. of patients | % |
|---|---|---|
| Surgical approach | ||
| McKeown esophagectomy | 22 | 70.9 |
| Ivor Lewis esophagectomy | 7 | 22.6 |
| Sweet esophagectomy | 2 | 6.5 |
| The type of resection | ||
| R0 | 31 | 100 |
| R1 | 0 | 0 |
| Postoperative complications | ||
| Anastomotic fistula | 4 | 12.9 |
| Pulmonary infection anastomotic | 9 | 29.0 |
| Anastomotic hemorrhage | 7 | 22.6 |
| Hoarseness | 5 | 16.1 |
Downgrading was achieved via neoadjuvant chemotherapy in 20 (64.5%) of the 31 patients. Postoperative pathological analysis revealed that neoadjuvant therapy had a considerable downstaging effect, and the patients’ conditions were effectively controlled. A significant difference was observed between pre-neoadjuvant therapy and postoperative TNM staging (P < .05; Table 3). However, no significant differences in pCR were found between patients whose CPS ≥ 10 and CPS < 10 (P = .365).
Table 3.
Changes in TNM Stages Between Pretreatment and Postoperative Patients with Locally Advanced Esophageal Squamous Cell Carcinoma.
| TNM stages | Pretreatment clinical stage TNM, n (%) (N = 31) | post-operation pathologic stage TNM, n (%) (N = 31) | P value |
|---|---|---|---|
| T stage | <.001 | ||
| T0 | 0 | 11 (35.5) | |
| T1 | 0 | 3 (9.7) | |
| T2 | 18 (58.1) | 8 (25.8) | |
| T3 | 13 (41.9) | 9 (29.0) | |
| N stage | .001 | ||
| N0 | 6 (19.4) | 21 (67.7) | |
| N1 | 9 (29.0) | 6 (19.4) | |
| N2 | 14 (45.2) | 3 (9.7) | |
| N3 | 2 (6.5) | 1 (3.2) |
All patients completed two cycles of PD-1 inhibitor plus chemotherapy, and none experienced intolerable toxic side effects or disease progression during the treatment. Among the 31 patients, 30 (96.8%) experienced treatment-related adverse events (Table 4), with neutropenia as the most common grade 3-4 adverse event; it was eliminated after treatment with the granulocyte-colony stimulating factor (G-CSF). Moreover, 64.5% (n = 20) of the patients showed leukopenia, which improved after treatment with G-CSF, and 38.7% (n = 12) showed thrombocytopenia, with grades 3-4 accounting for 9.7% (n = 3). Hemoglobin decreased in 29% of the patients and reached grades 3-4 (accounting for 6.5%), but it was restored to grades 1-2 after treatment via erythropoietin, iron, and blood transfusion. The other common chemotherapy drug-associated adverse events were weakness or fatigue in 27 patients (87.1%), nausea in 9 (29%), vomiting in 8 (25.8%), alopecia in 17 (54.8%), and myalgia or arthralgia in 19 (61.3%), as shown in Table 4. In addition, hypothyroidism occurred in four patients (12.9%), but it improved after oral administration of thyroxine tablets. One patient had immune dermatitis. We regarded hypothyroidism and immune dermatitis as immune-related adverse events.
Table 4.
Toxic and side Effects After Neoadjuvant Therapy.
| Grade 1-2, n (%) | Grade 3-4, n (%) | |
|---|---|---|
| Leukopenia | 13(41.9) | 7(22.6) |
| Neutropenia | 15(48.4) | 9(29.0) |
| Thrombocytopenia | 9(29.0) | 3 (9.7) |
| Anemia | 7(22.6) | 2 (6.5) |
| Nausea | 9(29.0) | 4(12.9) |
| Vomiting | 8(25.8) | 2 (6.5) |
| Alopecia | 15(48.4) | 2 (6.5) |
| Myalgia/Arthralgia | 16(51.6) | 3 (9.7) |
| Weakness or fatigue | 24(77.4) | 3 (9.7) |
| Immune dermatitis | 1(3.2) | 0 (0) |
| hypothyroidism | 4(12.9) | 0 (0) |
Survival
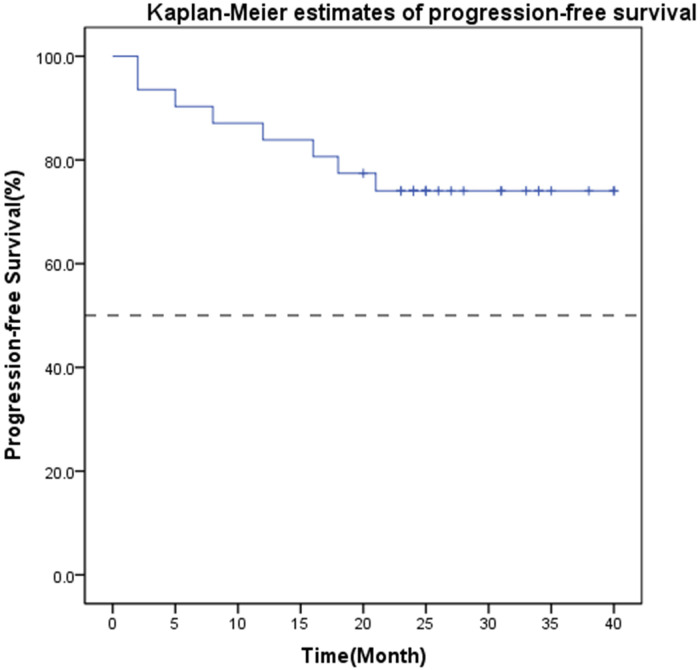
The median PFS was not reached after a median follow-up period of 33 months (range: 23-50 months). The one-year PFS rate was 87.1% (95% CI: 75.3%-98.9%), the one-year OS rate was 96.8% (95% CI: 79.8%-95.9%), and the two-year OS rate was 83.9% (95% CI: 71.6%-92.2%). During the follow-up, 10 patients progressed, and 6 died due to the disease. Among the 10 patients who progressed, 4 had recurrence and metastasis within 1 year. The Kaplan–Meier analysis of PFS is shown in Figure 1.
Figure 1.
Kaplan–Meier estimates of progression-free survival of all 31 included patients.
Discussion
In this study, we applied neoadjuvant immunochemotherapy (ie, to ripalimab plus paclitaxel and cisplatin) to patients with ESCC. The results revealed that this specific neoadjuvant immunochemotherapy achieved a high pCR rate (29.0%), MPR rate (41.9%), ORR (80.6%), and significant downstaging after neoadjuvant therapy (P < .05) with manageable treatment-related adverse effects. No significant association was found between PD-L1 level and pCR (P = .365). In addition, R0 resection was achieved in all 31 (100%) patients during surgery. For all included patients, the one-year PFS rate was 87.1% (95% CI: 75.3%-98.9%), the one-year OS rate was 96.8% (95% CI: 79.8%-95.9%), and the two-year OS rate was 83.9% (95% CI: 71.6%-92.2%). Immunotherapy with immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway has produced encouraging results in advanced patients with esophageal cancer.42–44 Several clinical trials conducted in 2019 have shown that immunotherapy combined with chemoradiotherapy/chemotherapy results in a neoadjuvant setting for locally advanced esophageal cancer.45–51 Our findings are consistent with recent findings on pCR in neoadjuvant immunochemotherapy for esophageal cancer (16.6%-45.4%).45–52 Specifically, two studies46,47 that examined the combination of toripalimab with chemotherapy obtained a lower pCR than that obtained in our study. Another study 53 reported that preoperative treatment combined with immunotherapy, radiotherapy, and chemotherapy can achieve high pCR (55.6%). Other studies on this specific neoadjuvant immunochemotherapy regimen are currently underway.47,54 In contrast, landmark trials, such as NEOCRTEC5010, 55 CROSS trial l, 5 and FFCD 9901, 56 that enrolled patients with clinical stage N0-1 disease administered chemoradiotherapy and obtained high pCR rates of 33.3%-49%, which may be attributed to improved local control with radiotherapy.
Given that pCR can demonstrate the absence of residual invasive cancer in tissues, it has been used to assess the efficacy of neoadjuvant therapies in oncology. 57 The Radiation Therapy Oncology Group (RTOG) trial 8911 showed that the prognosis for patients who achieve pCR via neoadjuvant therapy is improved remarkably compared with that for those who do not. 58 pCR has been proven to be an independent favorable prognostic predictor of esophageal cancer. 59 According to the OGSG1003 clinical trial data in 2017, 60 using the DCF combination alone as neoadjuvant chemotherapy achieves a 10% postoperative pCR rate in patients with locally advanced ESCC. 32 Other relevant studies have shown that the pCR rate of platinum-based neoadjuvant chemotherapy ranges from 0% to 7.7%.61–63 In this study, pCR was achieved in nine patients (29.0%) according to the postoperative pathological report, indicating that the neoadjuvant immunochemotherapy regimen may have better efficacy than chemotherapy alone.
Our results showed remarkable efficacy, including a 100% R0 resection rate, 64.5% downstaging rate, and ORR in 25 (80.6%) patients after two neoadjuvant chemotherapy cycles. Wu et al 64 reported that neoadjuvant therapy plus PD-1 does not delay surgery, and the R0 resection rate reaches 100%. Other studies65,66 have discovered that the R0 resection rates for neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy are 60% and 98%, respectively. In our study, the one-year PFS rate was 87.1% (95% CI: 75.3%-98.9%), the one-year OS rate was 96.8% (95% CI: 79.8%-95.9%), and the two-year OS rate was 83.9% (95% CI: 71.6%-92.2%). In the OEO2 study, 802 patients with esophageal cancer (66% with adenocarcinoma and 31% with ESCC) were randomly divided into PF neoadjuvant chemotherapy and surgery groups (two cycles), and the median survival time was 13.3 and 16.8 months, respectively; the two-year OS rates were 34% and 43%, respectively, 65 both of which are lower than the rate in our research. Meanwhile, a phase III clinical trial (JCOG9907) 67 confirmed that neoadjuvant chemotherapy can considerably improve the 5-year survival rate of resectable Stage II and III esophageal cancer compared with postoperative adjuvant chemotherapy (55% vs 43%). Given these results, preoperative chemotherapy has become the standard treatment for locally advanced esophageal cancer. A recent report 68 proved that the 2-year OS for neoadjuvant adebrelimab for locally advanced ESCC is 92%, which is similar to our result. The RTOG trial 8911 57 showed that the survival of patients undergoing R0 resection was significantly higher than that of patients undergoing R1, R2, or no resection (P < 0001). Several clinical trials69–71 have also revealed a considerable survival benefit for patients with tumor downstaging. Furthermore, the MRCOE02 study showed that OS was correlated with pathological lymph node status and primary tumor regression grade (TRG). Patients with TRG 1-3 tumors have a much longer OS than patients with TRG 4-5 tumors. 72
The results of our study indicate a potential considerable survival benefit. According to a recent review, although neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy followed by surgery is the standard treatment for patients with ESCC, with the gradual application of immunotherapy in esophageal cancer, neoadjuvant immunotherapy plays an important role. 29 Our findings prove that combining PD-1 inhibitors with chemotherapy as a neoadjuvant therapy may provide additional clinical benefits to patients with ESCC.
This study did not find a statistically significant association between the PD-L1 level and pCR, which may be due to the small sample size. However, previous research 58 has shown that the PD-L1 pathological level is not a reliable biomarker for stratifying patients for anti-PD-L1 therapy. The ATTRACTION-3 experiment 73 revealed that the use of nivolumab for the survival benefit of patients with esophageal cancer is unrelated to tumor PD-L1 expression. However, in the study by Yang et al, 74 the proportion of patients with high TMB and high expression of PD-L1 in the primary tumor was considerably higher in the PCR group than in the non-PCR group. Large-scale, long-term research is needed to clarify this phenomenon. Recently, TMB was proven to be an effective biomarker across many cancer types for the identification of patients who can benefit from immunotherapy.75–78 Over the last few years, many studies have mapped and characterized TMB variations across disease pathologies.79–81 These studies showed that the highest level of TMB is found in melanoma, followed by NSCLCs and other squamous carcinomas. Meanwhile, leukemias and pediatric tumors have the lowest TMB levels. Cancers, such as those of the breast, kidney, and ovary, display intermediate mutational load levels. In ESCC, the optimal critical value of TMB is 7.3 mutations/Mb. 82 Yuan et al suggested that TMB can be considered a prognostic marker in patients who have not received radiotherapy. 83
In this study, the dominant treatment-related adverse effects were leukopenia, neutropenia, thrombocytopenia, and nausea or vomiting. However, all were manageable. Compared with previous studies,84,85 this study found no new chemotherapy-related adverse effects. Given the small sample size of this study, large-scale clinical trials are needed to investigate the adverse effects of immunotherapy combined with chemotherapy. Nevertheless, the results of our study show that the combination of toripalimab plus paclitaxel and carboplatin is safe and feasible and exerts manageable treatment-related adverse effects on locally advanced ESCC.
This single-center clinical trial has some limitations. First, the sample size is small and needs to be enlarged in future studies. Second, all patients in this trial received neoadjuvant therapy followed by surgery for esophageal cancer and had a good prognosis. We will continue to follow up with these patients and provide findings on long-term efficacy, such as tumor recurrence, metastasis, and 5-year survival rate, in the future.
Conclusions
The use of toripalimab plus chemotherapy as preoperative neoadjuvant chemotherapy for ESCC showed optimal antitumor activity and produced a prognosis with manageable adverse effects, indicating that this combination may be a potential neoadjuvant therapy regimen in this setting. We did not find evidence that PD-L1 expression in pretreatment tissue can predict tumor responses. Further studies with large sample sizes are required to confirm this statement.
Contribution to the Field Statement
The incidence of ESCC is the highest in Southeast and Central Asia, where 79% of all ESCC cases are reported. Most patients are already at a locally advanced stage when they are diagnosed; thus, surgical resection and long-term survival rates are low. Preoperative neoadjuvant therapy can enhance the surgical resection rate. Currently, the standard neoadjuvant treatment for locally advanced esophageal cancer is platinum- and taxane-based chemotherapy or chemoradiation. Several studies have shown that the combination of PD-1 inhibitors and chemotherapy prolongs the survival of patients with advanced ESCC. In this study, we applied neoadjuvant immunochemotherapy (ie, to ripalimab plus paclitaxel and cisplatin) to patients with ESCC. This specific neoadjuvant immunochemotherapy achieved a considerable pCR rate (29.0%), ORR (80.6%), and statistically significant downstaging after neoadjuvant therapy (P < .05) with manageable treatment-related adverse effects. In addition, R0 resection was achieved in all 31 (100%) patients during surgery. For all included patients, the 1-year PFS rate was 87.1% (95% CI: 75.3%-98.9%). This study showed that toripalimab combined with chemotherapy is safe and may greatly benefit clinical outcomes. Therefore, this combination may be a potential neoadjuvant therapy regimen in this setting.
Abbreviation
- ESCC
esophageal squamous cell carcinoma.
Footnotes
Data Availability Statement: The data presented in this study are available upon request from the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study was approved by The Research Ethical Board of Nanjing Medical University (approval # 2019-005). All patients provided written informed consent prior to enrollment in the study.
Funding: This research was funded by the scientific research project of Jiangsu Cancer Hospital, grant number ZM202012; Natural Science Foundation of Jiangsu Province, China, grant number BK20201496; and Science and Technology Department Social Development-Clinical Frontier Technology Project of Jiangsu Provincial, China, grant number BE2017759.
ORCID iD: Ting Qian https://orcid.org/0009-0009-4479-3376
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381-387. [DOI] [PubMed] [Google Scholar]
- 3.Rahma OE, Greten TF, Duffy A. Locally advanced cancer of the esophagus, current treatment strategies, and future directions. Front Oncol. 2012;24(2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38(23):2677-2694. [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074-2084. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43(7):752-755. [DOI] [PubMed] [Google Scholar]
- 7.Boonstra JJ, Kok TC, Wijnhoven BP, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: Long-term results of a randomized controlled trial. BMC Cancer. 2011;19(11):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi M, Yang Y, Zhang L, et al. Multi-institutional analysis of recurrence and survival after neoadjuvant chemoradiotherapy of esophageal cancer: Impact of histology on recurrence patterns and outcomes. Ann Surg. 2019;269(4):663-670. [DOI] [PubMed] [Google Scholar]
- 9.Kong M, Shen J, Zhou C, et al. Prognostic factors for survival in esophageal squamous cell carcinoma (ESCC) patients with a complete regression of the primary tumor (ypT0) after neoadjuvant chemoradiotherapy (NCRT) followed by surgery. Ann Transl Med. 2020;8(18):1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baba Y, Nomoto D, Okadome K, et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111(9):3132-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chedgy EC, Black PC. Nivolumab: The new second line treatment for advanced renal-cell carcinoma commentary on: Nivolumab versus everolimus in advanced renal-cell carcinoma. Urology. 2016;5(89):8-9. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384. [DOI] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jardim DL, Goodman A, de Melo Gagliato D, et al. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajani JA, 'Amico D, Bentrem TA, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855-883. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li ZC, Sun YT, Lai MY, et al. Efficacy and safety of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal cancer: A systematic review and network meta-analysis. Int Immunopharmacol. 2022;8(109):108790. [DOI] [PubMed] [Google Scholar]
- 19.Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: Microsatellite instability vs. PD-L1. J Gastrointest Oncol. 2016;7(5):771-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ Book. 2017;37:292-300. [DOI] [PubMed] [Google Scholar]
- 21.Koemans WJ, Chalabi M, van Sandick JW, et al. Beyond the PD-L1 horizon: In search for a good biomarker to predict success of immunotherapy in gastric and esophageal adenocarcinoma. Cancer Lett. 2019;1(422):279-286. [DOI] [PubMed] [Google Scholar]
- 22.Sun J-M, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo controlled, phase 3 study. Lancet. 2021;398(10302):759-771. [DOI] [PubMed] [Google Scholar]
- 23.Sheng X, Chen H, Hu B, et al. Safety, efficacy, and biomarker analysis of toripalimab in patients with previously treated advanced urothelial carcinoma: Results from a multicenter phase II trial POLARIS-03Toripalimab in second-line metastatic urothelial carcinoma. Clin Cancer Res. 2022;28(3):489-497. [DOI] [PubMed] [Google Scholar]
- 24.Tang B, Chi Z, Chen Y, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: Results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res. 2020;26(16):4250-4259. [DOI] [PubMed] [Google Scholar]
- 25.Wang F-H, Wei X-L, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol. 2021;39(7):704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X-L, Ren C, Wang F-H, et al. A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors. Cancer Commun. 2020;40(8):345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40(3):277-288.e3. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Ma S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thorac Cancer. 2021;12(17):2293-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 30.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111(6):1718-1723. [DOI] [PubMed] [Google Scholar]
- 31.Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42-e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28(1):116-120. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 34.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680-2686. [DOI] [PubMed] [Google Scholar]
- 35.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347-1355. [DOI] [PubMed] [Google Scholar]
- 36.Depypere L, Moons J, Lerut T, et al. Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus. 2017;31(2):1-8. [DOI] [PubMed] [Google Scholar]
- 37.Rice TW, Patil DT, Blackstone EH. 8th Edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Tao J, Dai Z, et al. Progression-Free survival as early efficacy endpoint in resectable esophageal cancer treated with neoadjuvant therapy: A systematic review. Front Oncol. 2021;17(11):771546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei M, Siemers NO, Pandya D, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. 2021;27(14):3926-3935. [DOI] [PubMed] [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 41.Uzunoglu F, Reeh M, Kutup A, et al. Surgery of esophageal cancer. Langenbeck's Arch Surg. 2013;398(2):189-193. [DOI] [PubMed] [Google Scholar]
- 42.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138-4148. [DOI] [PubMed] [Google Scholar]
- 43.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506-1517. [DOI] [PubMed] [Google Scholar]
- 44.Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 2019;15(10):1057-1066. [DOI] [PubMed] [Google Scholar]
- 45.He W, Leng X, Mao T, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. Oncologist. 2022;27(1):e18-e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chengqiang L, Shengguang Z, Yuyan Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144(2):232-241. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(3):e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv H, Tian Y, Li J, et al. Neoadjuvant sintilimab plus chemotherapy in resectable locally advanced esophageal squamous cell carcinoma. Front Oncol. 2022;29(12):864533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah MA, Almhanna K, Iqbal S, et al. Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC). J Clin Oncol. 2021;39(15_suppl):4005. [Google Scholar]
- 50.Shen D, Chen Q, Wu J, et al. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang P, Zhou X, Yang X, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: A pilot study. World J Surg Oncol. 2021;19(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Ende T, de Clercq NC, van Berge Henegouwen MI, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res. 2021;27(12):3351-3359. [DOI] [PubMed] [Google Scholar]
- 53.Xing W, Zhao L, Fu X, et al. A phase II, single-centre trial of neoadjuvant toripalimab plus chemotherapy in locally advanced esophageal squamous cell carcinoma. J Thorac Dis. 2020;12(11):6861-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y, Liu XB, Sun HB, et al. A phase III study on neoadjuvant chemotherapy versus neoadjuvant toripalimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: Henan cancer hospital thoracic oncology group 1909 (HCHTOG1909). Ann Transl Med. 2021;9(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416-2422. [DOI] [PubMed] [Google Scholar]
- 57.Patil S, Agarwal V, Drupad HS. Significance of emerging clinical oncology endpoints in support of overall survival. Indian J Cancer. 2022;59(Supplement):S106-Ss18. [DOI] [PubMed] [Google Scholar]
- 58.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA intergroup 113): A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719-3725. [DOI] [PubMed] [Google Scholar]
- 59.Alnaji RM, Du W, Gabriel E, et al. Pathologic complete response is an independent predictor of improved survival following neoadjuvant chemoradiation for esophageal adenocarcinoma. J Gastrointest Surg. 2016;20(9):1541-1546. [DOI] [PubMed] [Google Scholar]
- 60.Wang YN, Lee HH, Wei Y, et al. An optimized protocol for PD-L1 pathological assessment with patient sample deglycosylation to improve correlation with therapeutic response. STAR Protoc. 2022;3(1):101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. [DOI] [PubMed] [Google Scholar]
- 62.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-1721. [DOI] [PubMed] [Google Scholar]
- 63.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z, Zheng Q, Chen H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. 2021;13(6):3518-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet. 2002;359(9319):1727-1733. doi: 10.1016/S0140-6736(02)08651-8 [DOI] [PubMed] [Google Scholar]
- 66.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2793-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68-74. doi: 10.1245/s10434-011-2049-9 [DOI] [PubMed] [Google Scholar]
- 68.Yin J, Yuan J, Li Y, et al. Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: A phase 1b trial. Nat Med. 2023;29(8):2068-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: Implications for response classification. Ann Surg. 2005;242(5):684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanoni A. Nodal downstaging in esophageal and esophagogastric junction cancer: More important than ever. J Thorac Dis. 2017;9(7):1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanoni A, Verlato G, Giacopuzzi S, et al. Ypn0: Does it matter how you get there? Nodal downstaging in esophageal cancer. Ann Surg Oncol. 2016;23(Suppl 5):998-1004. [DOI] [PubMed] [Google Scholar]
- 72.Davarzani N, Hutchins GGA, West NP, et al. Prognostic value of pathological lymph node status and primary tumour regression grading following neoadjuvant chemotherapy - results from the MRC OE02 oesophageal cancer trial. Histopathology. 2018;72(7):1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okada M, Kato K, Cho BC, et al. Three-year follow-up and response–survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin Cancer Res. 2022;28(15):3277-3286. doi: 10.1158/1078-0432.CCR-21-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(1):e003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in nonsmall cell lung cancer. Science. 2015;348(6230):124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100, 000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703-713. 10.1038/nm.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421. Nat Med 2017; 23(6): 703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greally M, Ku GY. Immune checkpoint inhibitors in esophagogastric adenocarcinoma: Do the results justify the hype? J Thorac Dis. 2018;10(12):6407-6411. doi: 10.21037/jtd.2018.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan C, Xiang L, Cao K, et al. The prognostic value of tumor mutational burden and immune cell infiltration in esophageal cancer patients with or without radiotherapy. Aging (Albany NY). 2020;12(5):4603-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Y, Chen J, Zhao L, et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat. 2021;53(1):172-183. [DOI] [PMC free article] [PubMed] [Google Scholar]