Abstract
Background
To establish a nomogram to predict the probability of survival of patients with stage II/III gastric cancer (GC) who received incomplete peri-operative adjuvant chemotherapy (PAC).
Methods
The medical records of stage II/III GC patients who received curative resection and 1 to 5 cycles of PAC from two tertiary hospitals were retrospectively reviewed. Patients were randomly classified into either a training group or validation group at a ratio of 7:3. The nomogram was constructed based on various prognostic factors using Cox regression analysis in the training cohort, and was validated by the validation group. Concordance index and calibration curves were used to evaluate the discrimination and calibration of the nomogram. Additionally, decision curve analysis (DCA) was used to compare the net clinical benefits of the nomogram and eighth version of TNM staging system.
Results
A total of 1,070 consecutive patients were included and 749 patients were enrolled into the training group. Lower body mass index (< 18.5 kg/m2), total gastrectomy, stage III disease and fewer cycles of PAC were identified to be independent predictors for poorer survival. The area under the curve (AUC) values of receiver operating characteristics (ROC) curve predicting 5-year survival probabilities and C-index were 0.768 and 0.742, 0.700 (95%CI: 0.674–0.726) and 0.689 (95%CI: 0.646–0.732) in the training and validation groups, respectively. The calibration curves in the validation cohort showed good agreement between the prediction and observation of 1-, 3- and 5-year survival probabilities. Furthermore, DCA showed that our model has a better net benefit than that of TNM staging system.
Conclusions
The findings emphasize the value of completing PAC. The nomogram which was established to predict survival probability in patients with stage II/III GC receiving radical gastrectomy and incomplete PAC had good accuracy and was verified through both internal and external validation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12103-1.
Keywords: Gastric cancer, Adjuvant chemotherapy, Survival, Nomogram, Validation
Introduction
Gastric cancer (GC), with an estimated incidence of over one million new cases in 2020, is one of the most common malignancies and gastrectomy offers the only possible curative treatment to date [1]. Unfortunately, only about a quarter of GC patients were diagnosed at an early stage in the West and China. For those patients with locally advanced gastric cancer (LAGC), long term outcomes remain unsatisfactory even after curative resection, with nearly 40% of patients experiencing relapse within 2 years after their operations [2]. To reduce the recurrence rate, peri-operative adjuvant chemotherapy (PAC), including neo-adjuvant chemotherapy (NAC) and/or adjuvant chemotherapy (AC), is a well-established management regimen, in addition to curative resection for stage II/III GC [3–4]. Unfortunately, few patients received sufficient cycles of PAC in clinical practice, even during recent prospective large scale phase 3 research trials [5–7]. The possible explanations include the poor general conditions of patients, those suffering from complications after their operation, or severe toxicity induced by chemotherapy.
Although several predictive models have been established to assess the survival probability of GC patients, in most of these patients were usually roughly divided into those who received or did not receive AC [8–11]. Unfortunately, the exact cycles of PAC have rarely been clearly reported in the literature. Given that the relative dose intensity has been shown to be significantly associated with prognosis in several types of malignancies [12–14], it is reasonable to hypothesize that the potential predictors may be different in stage II/III GC patients who had received inadequate or adequate PAC. Moreover, the exact cycles of PAC may also be related to the long term outcomes for these patients. Therefore, in this retrospective study conducted in two tertiary hospitals in China, for the first time, we investigated whether the exact cycles of PAC significantly impacted overall survival (OS) of these patients. In addition, the aim was to develop a novel nomogram to predict survival probability of patients with stage II or III GC who had received curative resection but inadequate PAC.
Methods
Patients
The medical records of 2,805 consecutive patients with pathologically confirmed stage II or III gastric adenocarcinoma who underwent curative resection (plus D2 lymphadenectomy) in two tertiary hospitals from China between November 2010 and December 2020 were retrospectively reviewed. The demographic and clinicopathological data, operative variables, exact cycles of PAC and follow-up data were collected and carefully analyzed. The well-known CLASSIC study and some of our previous studies confirmed that patients receiving ≥ 6 cycles of chemotherapy had a significantly better prognosis [14–17]. Thus, patients undergoing 1 to 5 cycles of chemotherapy were considered to have incomplete PAC. The number of cycles of PAC was calculated as the sum of the number of cycles of NAC and AC. Patients who received no PAC, complete PAC (≥ 6 cycles), recurrent GC, with other synchronous malignancies, with missing essential medical data, lost to follow-up or died within 3 months after surgery were excluded from the study. This study was approved by the ethics committee of our institution (No. 114 in 2022). Written informed consent for operation and using their clinicopathological data was obtained from all patients prior to surgery.
Peri-operative management and follow-up
Surgeons with sufficient experience performed or supervised all of the operations in accordance with the guidelines [4] and staged basing on the eighth edition of the Tumor-Node-Metastasis (TNM) system [18]. The overwhelming majority of stage II/III GC patients in our institutions received resection and AC, using XELOX or SOX regimens [14, 19]. Some patients with stage II diseases or those with stage III diseases but poor condition, single regimen (S-1 alone) were sometimes performed. About 15% with cT3-4/N + diseases underwent 1–5 cycles of NAC involving fluorouracil- and platinum-based regimens, such as ECF, FLOT, XELOX or SOX combinations [3, 7]. AC was usually started about 1 month after surgery and lasted for circa 6 months [14–15, 20].
Post-operative complications were diagnosed and classified according to the Clavien-Dindo system [21]. Patients were followed-up every 3 months in the initial 2 years, and then every 6 months from the 3rd to 5th year, and once a year thereafter, until December 2021. OS was calculated as months from the time of surgery to death or the last follow-up day, whichever occurred first.
Statistical analysis
The entire cohort of patients were randomized in a 7:3 ratio into a training group or a validation group with a random seed. A χ2 test or Fisher’s exact test was used to compare categorical variables between groups and Student’s t-test was employed to test for differences between continuous variables. Kaplan-Meier curves and a log rank test were used to identify differences in OS. Data analyses were performed using R software (ver. 3.5.1, R Foundation for Statistical Computing) or SPSS ver. 24.0 software (IBM Corporation, NY, USA). All tests were bilateral and a P-value < 0.05 was considered to be a significant finding.
Nomogram development and validation
The nomogram was built using the variables confirmed by multivariate Cox regression analyses in the training cohort. For each patient, the first line shows the exact point endowed for each factor loading on the predictor axis. After calculating the sum of the points, we could show the survival probability. Receiver operating characteristic (ROC) curves for the nomogram was generated basing on the area under the curve (AUC). The concordance index (C-index) was calculated to assess the performance of nomogram on training and validation groups, respectively. Calibration curves (1,000 bootstrap resamples) were plotted to assess the predictive power of the nomogram in the validation group. In addition, the decision curve analysis (DCA) was utilized to compare the clinical practicability between the nomogram and eighth version of TNM staging system.
Results
Baseline characteristics
As shown in Table 1, 1,070 consecutive patients were included in the present study. More than half the patients were male (66.1%), with stage III disease (72.4%), who underwent distal subtotal gastrectomy (67.1%) by an open procedure (75.2%). The mean body mass index (BMI) was 21.87 kg/m2 (range 13.84–36.63), mean age was 55.9 years (range 24–84), and the mean post-operative hospital stay was 11.8 days (range 4–79). A total of 101 patients (9.4%) developed ≥ grade II of post-operative complications. Patients received 1 to 5 cycles of PAC, with a median of 3 cycles. After randomization at a ratio of 7:3, 749 and 321 patients were separately enrolled into the training cohort or the validation cohort. As shown in Table 1, variables were all comparable between the 2 groups, except for the BMI. Patients in the training group had significantly higher BMI values than the validation group (21.98 vs. 21.60 kg/m2, P = 0.046).
Table 1.
Clinicopathological characteristics of the training and validation cohort for patients with stage II/III gastric cancer (n = 1070)
| Variables | Training cohort (n = 749 ) | Validation cohort (n = 321)` | P value |
|---|---|---|---|
| Gender (males) | 501 (66.9%) | 206 (64.2%) | 0.390 |
| Age (years) | 55.73 ± 10.05 | 56.03 ± 10.73 | 0.661 |
| Body Mass Index (kg/m2) | 21.98 ± 2.90 | 21.60 ± 2.82 | 0.046 |
| Any comorbidities | 217 (29.0%) | 99 (30.8%) | 0.539 |
| Neo-adjuvant chemotherapy | 68 (9.1%) | 34 (10.6%) | 0.440 |
| Pre-operative albumin concentration (g/L) | 38.73 ± 4.57 | 38.68 ± 4.98 | 0.868 |
| Pre-operative lymphocyte count (×10 9/L) | 1.77 ± 0.71 | 1.79 ± 0.74 | 0.785 |
| Pre-operative hemoglobin (g/L) | 117.90 ± 25.00 | 118.07 ± 25.88 | 0.922 |
| Operation method | 0.699 | ||
| Open | 561 (74.9%) | 244 (76.0%) | |
| Laparoscopy | 188 (25.1%) | 77 (24.0%) | |
| Type of resection | 0.618 | ||
| Distal subtotal gastrectomy | 506 (67.6%) | 212 (66.0%) | |
| Proximal subtotal gastrectomy | 18 (2.4%) | 11 (3.4%) | |
| Total gastrectomy | 225 (30.0%) | 98 (30.5%) | |
| T stage* | 0.899 | ||
| T1 | 22 (2.9%) | 6 (1.9%) | |
| T2 | 79 (10.5%) | 31 (9.7%) | |
| T3 | 100 (13.4%) | 39 (12.1%) | |
| T4 | 548 (73.2%) | 242 (75.4%) | |
| N stage* | 0.415 | ||
| N0 | 167 (22.3%) | 61 (19.0%) | |
| N1 | 141 (18.8%) | 68 (21.2%) | |
| N2 | 196 (26.2%) | 77 (24.0%) | |
| N3 | 245 (32.7%) | 115 (35.8%) | |
| pTNM stage* | 0.086 | ||
| II | 218 (29.1%) | 77 (24.0%) | |
| III | 531 (70.9%) | 244 (76.0%) | |
| Intra-operative blood loss (mL) | 215.55 ± 145.25 | 208.03 ± 130.70 | 0.425 |
| Operation time (min) | 200.44 ± 52.78 | 204.29 ± 52.49 | 0.278 |
| Peri-operative blood transfusion | 0.655 | ||
| Yes | 156 (20.8%) | 63 (19.6%) | |
| No | 593 (79.2%) | 258 (80.4%) | |
| Post-operative complications † | 0.600 | ||
| Yes | 73 (9.7%) | 28 (8.7%) | |
| No | 676 (90.3%) | 293 (91.3%) | |
| Post-operative hospital stays (days) | 11.67 ± 5.00 | 12.09 ± 5.63 | 0.171 |
| Peri-operative chemotherapy | 0.880 | ||
| 1 cycles | 133 (17.8%) | 62 (19.3%) | |
| 2 cycles | 141 (18.8%) | 66 (20.6%) | |
| 3 cycles | 148 (19.8%) | 63 (19.6%) | |
| 4 cycles | 213 (28.4%) | 84 (26.2%) | |
| 5 cycles | 114 (15.2%) | 64 (19.9%) |
Data are presented as mean ± SD or n (%).
* Tumor stages are based on 8th edition of the Union for International Cancer Control TNM classification.
† Defined as Clavien-Dindo grade II or greater.
Univariate and multivariate analyses
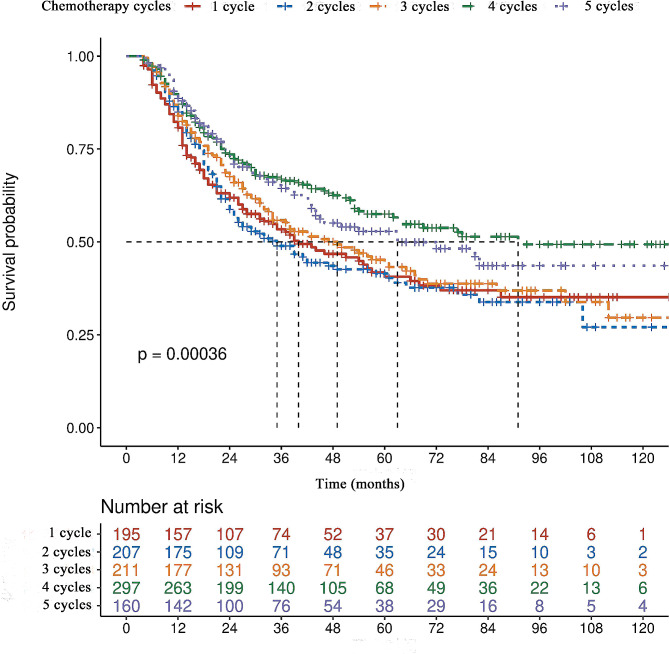
The median duration of follow-up was 30 months (range, 4–132) in the training group and 29 months (range, 4–128) in the validation group. As presented in Table 2, univariate analyses revealed that a lower BMI (< 18.5 kg/m2), higher intra-operative blood loss (≥ 300 mL), total gastrectomy, pTNM stage III disease, peri-operative blood transfusion and fewer cycles of PAC were all potential adverse factors related to poorer prognosis in the training cohort (all P < 0.05). After taking all of the factors with a P-value < 0.05 from univariate analyses in multivariate Cox regression analyses, only a lower BMI, total gastrectomy, stage III disease and fewer cycles of PAC were confirmed to be independent predictors for poorer survival. With respect to the number of cycles of PAC, the median OS in patients who received only 1 cycle was 37 months, which was comparable with that of 35 months and 45 months for patients who underwent 2 or 3 cycles, but significantly shorter than that of 91 months and 72 months for those who received 4 or 5 cycles of PAC, respectively (Fig. 1).
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival following radical gastrectomy of stage II/III gastric cancer in the training cohort (n = 749)
| Variables | N | Median OS time (months) |
UV P value |
MV HR (95% CI) |
MV P value |
|---|---|---|---|---|---|
| Gender | 0.287 | ||||
| Male | 501 | 54.0 | |||
| Female | 248 | 47.0 | |||
| Age (years) | 0.177 | ||||
| ≥ 65 | 147 | 45.0 | |||
| < 65 | 602 | 56.0 | |||
| Body mass index (kg/m2) | < 0.001 | 0.003 | |||
| ≥ 25.0 | 108 | 77.0 | Reference | ||
| 18.5–24.9 | 566 | 53.0 | 1.296 (0.926–1.814) | ||
| < 18.5 | 75 | 29.0 | 2.086 (1.356–3.208) | ||
| ASA score | 0.993 | ||||
| ≥ 3 | 58 | 56.0 | |||
| < 3 | 691 | 53.0 | |||
| Comorbidities | 0.326 | ||||
| Yes | 217 | 67.0 | |||
| No | 532 | 49.0 | |||
| Hemoglobin (g/L) | 0.524 | ||||
| ≥ 100 | 581 | 54.0 | |||
| < 100 | 168 | 50.0 | |||
| Albumin level (g/L) | 0.745 | ||||
| ≥ 35 | 608 | 56.0 | |||
| < 35 | 139 | 47.0 | |||
| Lymphocyte count (×10 9/L) | 0.744 | ||||
| ≥ 1.5 | 507 | 56.0 | |||
| < 1.5 | 242 | 52.0 | |||
| Operation procedure | 0.152 | ||||
| Open | 561 | 50.0 | |||
| Laparoscopy | 188 | 68.0 | |||
| Operation time (min) | 0.080 | ||||
| ≥ 240 | 157 | 35.0 | |||
| < 240 | 568 | 56.0 | |||
| Intra-operative blood loss (mL) | 0.003 | 0.128 | |||
| ≥ 300 | 170 | 34.0 | |||
| < 300 | 579 | 63.0 | |||
| Type of resection | < 0.001 | < 0.001 | |||
| Sub-total gastrectomy | 524 | 82.0 | Reference | ||
| Total gastrectomy | 225 | 23.0 | 1.921 (1.545–2.389) | ||
| pTNM stage † | < 0.001 | < 0.001 | |||
| II | 218 | NA* | Reference | ||
| III | 531 | 32.0 | 2.970 (2.215–3.982) | ||
| Peri-operative blood transfusion | 0.036 | 0.945 | |||
| Yes | 156 | 37.0 | |||
| No | 593 | 56.0 | |||
| Post-operative complication ‡ | 0.314 | ||||
| Yes | 73 | 39.0 | |||
| No | 676 | 56.0 | |||
| Peri-operative adjuvant chemotherapy | 0.001 | 0.001 | |||
| 1 cycles | 133 | 37.0 | Reference | ||
| 2 cycles | 141 | 35.0 | 1.103 (0.733–1.401) | ||
| 3 cycles | 148 | 45.0 | 0.995 (0.722–1.373) | ||
| 4 cycles | 213 | 91.0 | 0.549 (0.398–0.758) | ||
| 5 cycles | 114 | 72.0 | 0.609 (0.422–0.880) |
ASA, American Society of Anesthesiologists; OS, overall survival; CI, confidence interval; HR, hazard ratio; UV, univariate analysis; MV, multivariate analysis; NA, not available.
* The median overall survival time has not reached during the follow-up.
† Tumor stages are based on 8th edition of AJCC TNM classification.
‡ Defined as Clavien-Dindo grade II or greater.
Fig. 1.
Overall survival curves of the entire 1070 patients who underwent curative resection for stage II/III gastric cancer stratified by the received cycles of peri-operative adjuvant chemotherapy (Compared by log rank test)
Subgroup analyses
Among the 102 patients who were performed NAC, The median OS for patients receiving 1 cycle (n = 3), 2 cycles (n = 12), 3 cycles (n = 16), 4 cycles (n = 40) and 5 cycles (n = 31) of peri-operative adjuvant chemotherapy were 12, 17, 29, not available and 19 months, respectively. It seemed that patients underwent 4 cycles of chemotherapy had the best prognosis, and patients with 1 cycle of chemotherapy had the worst prognosis. But the difference was not statistically significant (P = 0.162, Supplementary Fig. 1).
Survival predictive probability and accuracy
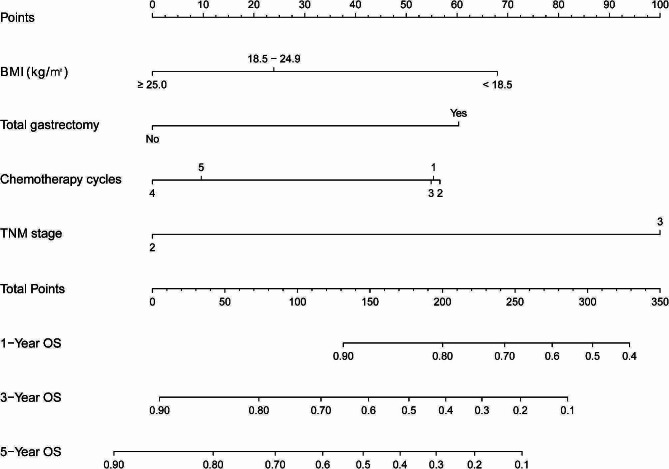
According to the findings of multivariate Cox regression analyses performed in the training group, 4 independent predictors (BMI, total gastrectomy, TNM stage and PAC) were utilized to develop a nomogram to estimate the probability of survival (Fig. 2).
Fig. 2.
A nomogram for 1-, 3- and 5-year overall survival for stage II/III gastric cancer who underwent curative resection and incomplete peri-operative adjuvant chemotherapy (1–5 cycles)
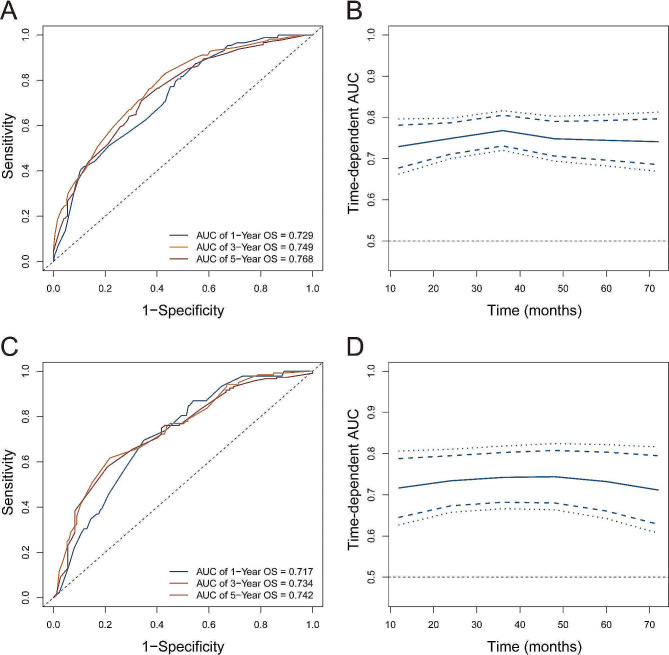
As shown in Fig. 3, in the training group, the AUC values to predict the 1- 3-, and 5-year survival probabilities were 0.729, 0.749, and 0.768, respectively, whereas the AUC values were 0.717, 0.734 and 0.742 in the validation group, separately. As a result, the developed nomogram using the 4 factors showed good predictive ability both for the training group and validation group. The C-index in the training and validation groups were 0.700 (95%CI: 0.674–0.726) and 0.689 (95%CI: 0.646–0.732), respectively.
Fig. 3.
Receiver operating characteristic area under the curves of nomogram for predicting overall survival in the training (A and B) and validation (C and D) cohort
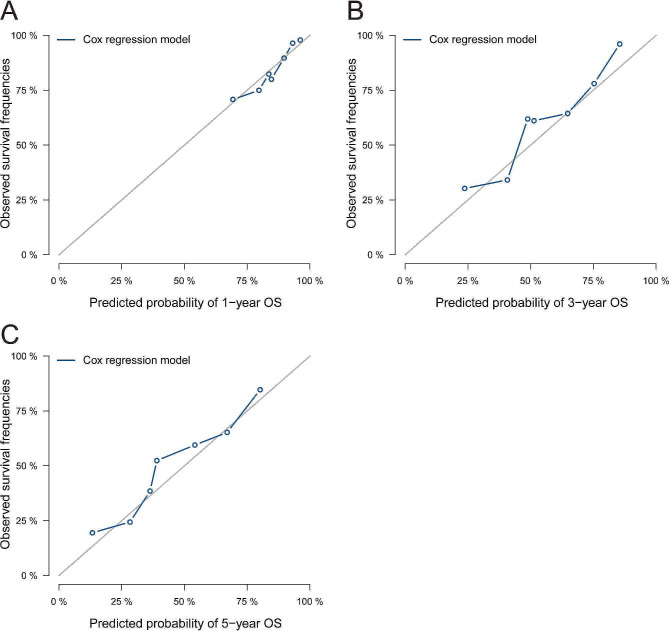
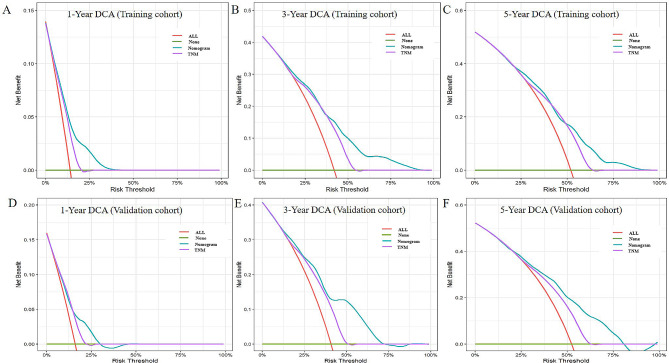
Thereafter, the calibration curve was used to assess the discriminate the ability of the nomogram. Fig. 4 shows that the calibration curves revealed satisfactory consistence between the predicted and observed 1-, 3- and 5-year survival probabilities in the validation group. Additionally, DCA of the nomogram show a greater range of death risks within the net benefit than the eighth version of TNM staging system both in the training and validation groups (Fig. 5).
Fig. 4.
Calibration curves for the nomogram to predict (A)1-, (B)3- and (C)5- year overall survival for stage II/III gastric cancer who underwent curative resection and incomplete peri-operative adjuvant chemotherapy (1–5 cycles) in the validation cohort
Fig. 5.
Decision curve analysis (DCA) of the nomogram. The DCAs of the nomogram in the training group (A-C) and the validation group (D-E) were plotted basing on the 1-, 3- and 5-year overall survival, respectively
Discussion
Although several nomograms have been established to forecast recurrence or the survival probability of GC patients [8, 10–11], none have focused on patients who received incomplete PAC (defined as 1–5 cycles in the present study). Moreover, whether the exact cycles of PAC was independently related to long-term outcomes has not previously been clarified. In this retrospective study of 1,070 consecutive patients with stage II/III GC who underwent radical gastrectomy and incomplete PAC in two tertiary hospitals in China, we confirmed that the number of chemotherapy cycles, along with BMI, total gastrectomy and stage III disease were significantly linked with prognosis. Thereafter, for the first time, a nomogram was constructed to predict survival probability, exploring the influence of the number of chemotherapy cycles in these patients. Further analyses established that the nomogram had satisfactory calibration and good discrimination to predict the 1-, 3- and 5-year survival probabilities. It seemed to be more consistent with clinical practice than the eighth version of TNM staging system.
PAC has been established as the most effective adjuvant management for LAGC patients [3–4], but it is common to encounter patients who could not complete all of the planned dose intensity. In one of our previous studies [16], we reported that among the entire 2,510 patients with LAGC, 546 patients (21.8%) underwent no AC and another 1,044 patients (41.6%) received 1 to 5 cycles of PAC. Only 920 patients (36.7%) underwent ≥ 6 cycles of PAC. Not surprisingly, patients with adequate PAC (≥ 6 cycles) had significantly better prognosis. It was echoed by Lu and colleagues [9], who reported that 425 patients (25.6%) among a cohort of 1,657 did not receive even one cycle of AC in a retrospective multicenter study. Further analyses confirmed that AC significantly decreased recurrence but the exact cycles of AC were not reported. More importantly, in the well-known CLASSIC study [14], 174 of 520 patients (33.5%) with stage II-IIIB GC could not complete the planned 8 cycles of adjuvant oxaliplatin and capecitabine therapy. Post hoc analysis identified that patients who received at least 6 cycles had significantly prolonged survival than those with low dose intensity. Another of our previous studies also confirmed that patients who were given ≥ 6 cycles of PAC had significantly better cancer-specific survival (CSS) [15]. Thus, patients undergoing 1–5 cycles were defined to have incomplete PAC in the present study. As shown in Table 2, the prognosis was comparable in patients undergoing 1, 2 or 3 cycles of PAC, but significantly worse than those who underwent 4 or 5 cycles of PAC. Consistence with previous studies, our findings confirmed the relationship between dose intensity and prognosis, and thus, emphasized the importance of completing as many PAC cycles as possible in stage II/III GC patients [9, 14–16].
The inclusion criteria, investigated variables and conclusions differed significantly in predictive models for GC patients. Liu et al. [8] established a nomogram to predict CSS in 688 stage II or III patients who had received more than 4 cycles of AC after resection. They concluded that inflammatory, nutritional and tumor markers, tumor location and the stage were significant predictors for CSS. The C-index of their nomogram based on these factors was 0.714, which was higher than the TNM stage (0.630, P < 0.001). Park and colleagues [10] developed a new staging system and a nomogram for advanced GC patients without adjuvant managements. They confirmed that post-operative morbidity, age and American Society of Anesthesiologists (ASA) score were independent predictors for OS. However, it should be noted that only 185 patients were included in the training group. In another retrospective study that included 639 stage I to III GC patients (except T1a) who underwent ≥ 2 cycles of AC within 2 months following their operations, single chemotherapy regimens were identified to be associated with a poorer prognosis compared to multiple chemotherapy regimens [11]. Unfortunately, the exact number of cycles of AC was not given. In addition, there has been increasing evidence favoring PAC instead of AC for the treatment of locally advanced GC, even in Eastern countries [7, 19]. It is noteworthy that almost all previous similar studies excluded those involving the administration of NAC, which inevitably harmed the generalizability of the conclusions [8–9, 11].
The prognostic factors and recurrence patterns might be different among patients given different dose intensities. In the study presented by Kanda et al. [21], 70 pairs of stage II/III patients were analyzed after propensity score matching. The authors identified that stage III, pT4, vessel invasion, total gastrectomy and carcinoembryonic antigen levels ≥ 5 ng/mL were independent predictors for long term survival in patients given no AC, whereas only a macroscopic tumor size ≥ 5 cm was significantly associated with survival in patients who received adjuvant S-1. In our previous studies, we have confirmed that complete PAC (≥ 6 cycles) could cancel out the adverse influence of a low prognostic nutritional index, low BMI, peri-operative blood transfusion and/or infections on survival in stage II or III GC patients [15–17]. Given that PAC could impact recurrence patterns and modify the predictive factors for survival in GC patients [22–23], we must cite the previous conclusions with caution and a new nomogram was clearly needed in patients treated incompletely with PAC.
Consistent with our previous findings, we also revealed that a poor nutritional status, late TNM stage and total gastrectomy were significantly related to poor prognosis [8–9, 11, 16–17, 22]. In addition, the exact dose intensity was confirmed to be an independent predictor of prognosis for the first time. Previous studies revealed that poor patient condition (age ≥ 65 years, ASA score ≥ 3), a poor nutritional and immune status (serum albumin level < 35 g/L, prognostic nutritional index < 43.9, BMI < 20.3 kg/m2), body weight loss and post-operative infection complications adversely affected compliance with AC [15, 24–27]. The possible explanations included that poor patient condition and nutritional status increased chemotherapy-related adverse events [28]. Post-discharge oral nutritional supplements significantly decreased weight loss and chemotherapy modifications after 3 months of invention [29]. On the other hand, NAC was confirmed to be a protective factor for patients to complete PAC, especially in those at a high risk of experiencing post-operative complications [16, 30]. Taken together, the findings suggest that in order to increase the completeness of PAC and improve prognosis, performing NAC and nutritional intervention are feasible strategies. But further prospective studies are still needed.
The present study had several limitations. First, it was a retrospective study and some important variables, such as tumour markers, the type of chemotherapy regimen, the exact time to initiate treatment, the exact number of patients having dose de-escalation, changing chemotherapy regimen or were put on single agent treatment was not collected and analyzed, which might also act as confounders and impact the reliability of our conclusions. Second, given that it was a long study period over 10 years, several chemotherapy regimens combinations were used in our institutions, such as S-1 alone, ECF, FLOT, XELOX and SOX [17]. The convenience and safety of different regimens might also impact the completion of PAC. Third, the median follow-up of 30 months of the entire cohort seemed not long enough to analyze later relapse and the deaths of patients. Fourth, for the 102 patients (9.5%) undergoing NAC, the pre-treatment clinical TNM stage was used, which might be inconsistent with the pathological TNM stage. Fifth, given that only a small proportion of patients underwent laparoscopic surgery, whether the conclusions were applicable to these patients still needs further investigation. Last but not least, our findings still need external validation, especially in patients from Western countries, where NAC is recommended as standard treatment for LAGC and the management strategy differed significantly from our institutions [3, 30].
Conclusions
A nomogram has been constructed to predict the survival probability of patients with stage II or III GC who underwent curative resection and incomplete PAC, using a large sample size of patients from two tertiary hospitals in China. We also explored the influence of exact cycles of PAC and confirmed that the dose intensity could have a significant impact on long term outcomes. Further analyses showed that the nomogram incorporating BMI, total gastrectomy, TNM stage and PAC variables produced a satisfactory calibration and good discrimination. Our findings should assist clinicians to evaluate the survival probability of patients receiving incomplete PAC, and encourage patients to complete all of the planned chemotherapy regimens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Hua Xiao, Yanping Xiao, Dian Liu and Hu Quan contributed to the conception and design. Hua Xiao, Dian Liu, Hu Quan, Min Ma, Huijun Zhou, Xiaolin Yang, Zhengchun Wu and Jia Luo contributed to data collection and analysis. Hua Xiao wrote the manuscript. Yanping Xiao reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Hunan Provincial Natural Science Foundation of China (No. 2023JJ60036 and 2022JJ40251), Health Research Project of Hunan Provincial Health Commission (No. 202203035248), Changsha Municipal Natural Science Foundation (No. Kq2208151) and Hunan Cancer Hospital Climb Plan (No. 2020NSFC-A004).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Hunan Cancer Hospital (N0. 114 in 2022). Written informed consent for operation and using their clinicopathological data was obtained from all patients prior to surgery in our institutions.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dian Liu and Hu Quan contributed equally to this work.
Contributor Information
Hua Xiao, Email: huakexh2010@163.com.
Yanping Xiao, Email: 44135478@qq.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15(8):886–93. doi: 10.1016/S1470-2045(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–28. doi: 10.1016/S1470-2045(18)30132-3. [DOI] [PubMed] [Google Scholar]
- 7.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Wu Z, Lin E, et al. Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr. 2019;38(4):1853–60. doi: 10.1016/j.clnu.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Xu BB, Zheng CH, et al. Development and external validation of a nomogram to predict recurrence-free survival after R0 resection for stage II/III gastric cancer: an international multicenter study. Front Oncol. 2020;10:574611. doi: 10.3389/fonc.2020.574611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KB, Jun KH, Song KY, et al. Development of a staging system and survival prediction model for advanced gastric cancer patients without adjuvant treatment after curative gastrectomy: a retrospective multicenter cohort study. Int J Surg. 2022;101:106629. doi: 10.1016/j.ijsu.2022.106629. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, Chen G, Wang D, et al. A nomogram to predict survival probability of gastric cancer patients undergoing radical surgery and adjuvant chemotherapy. Front Oncol. 2022;12:893998. doi: 10.3389/fonc.2022.893998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris M, Platell C, Fritschi L, lacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96(5):701–7. doi: 10.1038/sj.bjc.6603627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto I, Tanaka M, Shirakawa S, et al. Postoperative serum albumin level is a marker of incomplete adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2015;22(7):2408–24. doi: 10.1245/s10434-014-4280-7. [DOI] [PubMed] [Google Scholar]
- 14.Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomized phase 3 trial. Lancet Oncol. 2014;15(12):1389–96. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Zhou H, Zhang P, et al. Association among the prognostic nutritional index, completion of adjuvant chemotherapy, and cancer-specific survival after curative resection of stage II/III gastric cancer. Eur J Clin Nutr. 2020;74(4):555–64. doi: 10.1038/s41430-019-0502-1. [DOI] [PubMed] [Google Scholar]
- 16.Peng W, Dai J, Liu CC, et al. Body mass index and prognosis of patients with stage II/III gastric cancer after curative gastrectomy: completion of perioperative adjuvant chemotherapy may be a confounding factor. Front Oncol. 2022;12:899677. doi: 10.3389/fonc.2022.899677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao H, Xiao Y, Chen P, et al. Association among blood transfusion, postoperative infectious complications, and cancer-specific survival in patients with stage II/III gastric cancer after radical gastrectomy: emphasizing benefit from adjuvant chemotherapy. Ann Surg Oncol. 2021;28(4):2394–404. doi: 10.1245/s10434-020-09102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Zheng CH, Cao LL, et al. The effectiveness of the 8th American Joint Committee on Cancer TNM classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 7th and 8th editions. Eur J Surg Oncol. 2017;43(12):2349–56. doi: 10.1016/j.ejso.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda M, Murotani K, Kobayashi D, et al. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: a propensity score matching analysis. Surgery. 2015;158(6):1573–80. doi: 10.1016/j.surg.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Hosoda K, Ushiku H, Katada C, et al. Preoperative chemotherapy could modify recurrence patterns through postoperative complications in patients with gastric cancer. Langenbecks Arch Surg. 2021;406(4):1045–55. doi: 10.1007/s00423-021-02153-5. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20(6):2000–6. doi: 10.1245/s10434-012-2776-6. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K, Kurokawa Y, Yamamoto K, et al. Risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24(9):2639–45. doi: 10.1245/s10434-017-5923-2. [DOI] [PubMed] [Google Scholar]
- 26.Jang SH, Jung YJ, Kim MG, Kwon SJ. The prognostic significance of compliance with postoperative adjuvant chemotherapy in patients with stage III gastric cancer: an observational study. J Gastric Cancer. 2018;18(1):48–57. doi: 10.5230/jgc.2018.18.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoyama T, Kawabe T, Fujikawa H, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015;22(8):2560–6. doi: 10.1245/s10434-014-4296-z. [DOI] [PubMed] [Google Scholar]
- 28.Seo SH, Kim SE, Kang YK, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. 2016;16(1):900. doi: 10.1186/s12885-016-2934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr. 2021;40(1):40–6. doi: 10.1016/j.clnu.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Li SS, Udelsman BV, Parikh A, Klempner SJ, Clark JW, Roeland EJ, et al. Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J Am Coll Surg. 2020;230(6):912–24. doi: 10.1016/j.jamcollsurg.2019.12.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.