Abstract
Background
The mass occurrence of scyphozoan jellyfish severely affects marine ecosystems and coastal economies, and the study of blooming jellyfish population dynamics has emerged in response. However, traditional ecological survey methods required for such research have difficulties in detecting cryptic life stages and surveying population dynamics owing to high spatiotemporal variations in their occurrence. The environmental DNA (eDNA) technique is an effective tool for overcoming these limitations.
Results
In this study, we investigated the biodiversity and spatial distribution characteristics of blooming jellyfish in the Bohai Sea of China using an eDNA metabarcoding approach, which covered the surface, middle, and bottom seawater layers, and sediments. Six jellyfish taxa were identified, of which Aurelia coerulea, Nemopilema nomurai, and Cyanea nozakii were the most dominant. These three blooming jellyfish presented a marked vertical distribution pattern in the offshore regions. A. coerulea was mainly distributed in the surface layer, whereas C. nozakii and N. nomurai showed a upper-middle and middle-bottom aggregation, respectively. Horizontally, A. coerulea and C. nozakii were more abundant in the inshore regions, whereas N. nomurai was mainly distributed offshore. Spearman’s correlation analysis revealed a strong correlation between the eDNA of the three dominant blooming jellyfish species and temperature, salinity, and nutrients.
Conclusions
Our study confirms the applicability of the eDNA approach to both biodiverstiy evaluation of blooming jellyfish and investigating their spatial distribution, and it can be used as a supplementary tool to traditional survey methods.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12862-024-02224-3.
Keywords: Environmental DNA metabarcoding, Jellyfish bloom, Biodiversity, Spatial distribution
Background
Jellyfish, gelatinous cnidarians, are critical elements of marine ecosystems, and they greatly influence food webs and elemental fluxes [1]. Historically, jellyfish blooms have been considered occasional and episodic; however, in recent years, reports of such events have increased dramatically [2–4]. Mounting evidence indicates that these blooms cause losses in aquaculture and tourism, block nuclear power plants, and reduce fish catches [5, 6]. Therefore, increasing concerns about the population dynamics of gelatinous jellyfish have been raised [7–10]. However, jellyfish samples can be difficult to identify using traditional visual or plankton trawling methods, due to the fragility of their tissues, their metagenic life cycles, and the existence of cryptic species. In addition, the uneven vertical distribution of jellyfish in the water column poses a challenge to traditional field-based ecological surveys [11–13]. A fast, highly recognizable, and accurate technique is urgently needed as a supplement (or even alternative) to traditional approaches.
In this respect, environmental DNA (eDNA) metabarcoding, an emerging method, combines traditional ecological approaches with in-depth molecular methods and advanced computational tools to investigate biodiversity and biomass [14]. The term, eDNA is defined as the DNA fragments present in environmental samples, including whole cells, extracellular DNA, and potentially whole organisms [15, 16]. This technique has been effectively used in biodiversity surveys, endangered species tracking, invasive species detection, ancient ecosystem reconstruction, pollution prediction, and diet analyses [16–22]. However, its application in the analysis of targeted jellyfish taxa remains relatively limited. Nevertheless, several published studies have assessed and demonstrated the effectiveness of eDNA metabarcoding in jellyfish (Medusozoa) detection [23–25]. DNA metabarcoding analysis of fecal and intestinal samples from multiple species has been used to indirectly detect the presence of jellyfish, possibly reflecting their population dynamics [26, 27]. Furthermore, eDNA techniques have enormous potential for monitoring the vertical distribution patterns of jellyfish [13, 28]. Thus, using this powerful eDNA tool to conduct jellyfish taxa assessment in hotspots may provide a reliable foundation for the monitoring and prevention of jellyfish blooms.
The Bohai Sea, a semi-enclosed and marginal sea in China, is characterized by abundant fishery resources, intense human activity, and increasing ocean engineering. Recently, jellyfish assemblages and blooms have been frequently reported there, especially in the harbors and coastal waters [29–31]. These blooms block coastal nuclear power plants, cause an imbalance in marine ecosystems, and are associated with a decline in economic fishery production [2, 32]. This study aimed to develop an effectively indicative tool for investigating jellyfish, which will contribute to constructing monitoring systems of jellyfish blooms. In this respect, we investigated the diversity and distribution of blooming jellyfish during a periodic jellyfish outbreak in August 2022 in the Bohai Sea using eDNA metabarcoding based on the 16S rRNA gene, and then conducted a correlation analysis between the distribution of dominant jellyfish and environmental factors.
Methods
Sample collection
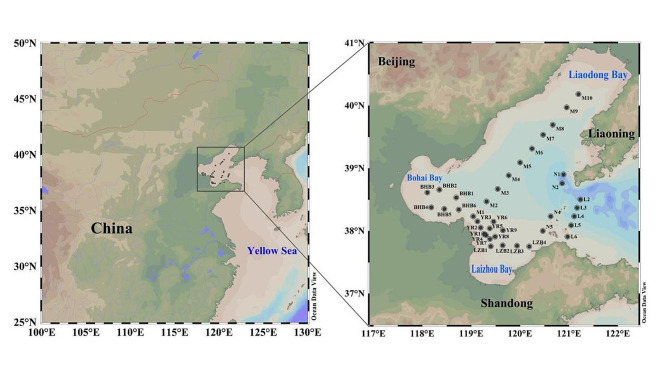
The investigation was conducted at 38 stations in the Bohai Sea in August 2022 (118°06.222′-121°13.915′ E, 37°45.278′-40°11.093′ N); six stations in the Bohai Bay (BHB1-6), four stations in the Laizhou Bay (LZB1-4), ten stations in the central Bohai Sea area (M1-10), nine stations at the Yellow River estuary (YR1-9), four stations near the Miaodao Archipelago (N1-2, N4-5), and five stations in the Bohai Strait (L2-6) (Fig. 1). We categorized those located in the Bohai Bay, the Laizhou Bay, the Yellow River estuary, and M1-3 in the central Bohai Sea as inshore stations; those in the Miaodao Archipelago, the Bohai Strait, and M4-10 in the central Bohai Sea were categorized as offshore stations. Seawater samples were collected from three depths (surface, middle, and bottom layers; the average sampling depths are shown in Table 1); sediment samples were collected separately. A total of 114 seawater and 24 sediment samples were obtained.
Fig. 1.
Maps of sampling stations in the Bohai Sea. Note The maps in the figure were obtained from Ocean Data View software (Reiner Schlitzer, Alfred Wegener Institute, Bremerhaven, Germany)
Table 1.
Environmental characteristics of the Bohai Sea (mean ± SE)
| Layer | PO43− (mg/L) | NH4+ (mg/L) | NO2− (mg/L) | SiO32− (mg/L) | NO3− (mg/L) | Chl a (µg/L) | Temp (T) (℃) | Salinity (S) (ppt) | Depth (D) (m) |
|---|---|---|---|---|---|---|---|---|---|
| Surface | 0.0038 ± 0.0017b | 0.0207 ± 0.0161b | 0.0296 ± 0.0286 | 0.2927 ± 0.2163 | 0.1188 ± 0.1336 | 3.95 ± 2.61 | 26.15 ± 1.41a | 28.27 ± 1.32 | 2.98 ± 0.38c |
| Middle | 0.0048 ± 0.0021a | 0.0289 ± 0.0161a | 0.0310 ± 0.0213 | 0.3131 ± 0.1704 | 0.1103 ± 0.0846 | 2.91 ± 2.73 | 24.35 ± 3.02b | 28.76 ± 1.32 | 10.97 ± 4.85b |
| Bottom | 0.0044 ± 0.0019ab | 0.0276 ± 0.0137ab | 0.0358 ± 0.0233 | 0.3103 ± 0.1765 | 0.1242 ± 0.1011 | 3.02 ± 2.53 | 24.17 ± 3.16b | 28.84 ± 1.28 | 21.24 ± 13.89a |
Note The superscript letters represent the statistical differences between environmental characteristics at different water depths
The seawater samples were collected from the surface, middle, and bottom seawater at each station using a water sampler integrated with a Sea-Bird conductivity-temperature-depth (CTD) profiler (Sea-Bird Scientific Inc., the United States). One liter of seawater was filtered through 0.7 μm GF/F (glass fiber filters) membranes (Whatman, Maidstone, UK), which were then stored in 2-mL sterile cryopreservation tubes (Beyotime, Shanghai, China). Sediment samples were collected using a bottom sampler and stored in 50-mL sterile centrifuge tubes with a sterile disposable syringe. All membrane and sediment samples were temporarily frozen in liquid nitrogen and quickly transferred to a -80 ℃ refrigerator when returned to the laboratory. To avoid cross-contamination between samples, all devices used for sample collection and filtration were bleached with 10% sodium hypochlorite and washed at least twice with Milli-Q water before sampling. In addition, a negative blank control was collected to examine for potential contamination, 1 L of distilled water was filtered at each station and the membrane was preserved.
The temperature (T), salinity (S), and depth (D) at different depths at each station were recorded using the CTD profiler. The seawater used to measure nutrients and chlorophyll a (Chl a) was obtained during the collection of the above seawater samples. Subsequently, 500 mL seawater was filtered through a 0.7 μm GF/F membrane (Whatman, Maidstone, UK) [12, 33] for the estimation of Chl a. Chl a was extracted with 90% acetone at -20 ℃ in the dark for 12 h, after which it was measured using a fluorophotometer (Trilogy, Turner Designs, USA) [34]. To measure the nutrient concentrations, 50 mL of seawater was filtered through a 0.45 μm polyethersulphone membrane. Phosphate (PO43−), silicate (SiO32−), nitrate (NO3−), nitrite (NO2−), and ammonia (NH4+) levels were determined using a QuAAtro automatic analyzer (SEAL Inc., Germany) [35].
eDNA extraction
eDNA on the GF/F filter membranes was extracted using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the protocol described in Takahashi et al. (2020) [33], with slight adjustments. A single membrane was placed in the suspended part of a Salivette tube (Sarstedt, Nümbrecht, Germany) and a 440 µL mixed solution containing 40 µL protease K and 400 µL AL lysis buffer was added to the membrane. After incubation for 1 h at 56 ℃, centrifugation was performed at 5000 × g for 3 min to collect the pyrolysis liquid. Subsequently, 200 µL TE buffer was added onto the membrane, followed by repeated centrifugation. A mixed solution consisting of 200 µL AL buffer and 600 µL anhydrous ethanol was then added to the collected liquid, and the mixture was transferred to a spin column, which was operated according to the manufacturer’s instructions. The eDNA was then eluted in 80 µL AE buffer and finally stored at -20 ℃. A blank filter membrane as a negative control was also subjected to all the steps mentioned above to detect possible contamination during extraction.
eDNA was extracted from the sediments using the DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany). Approximately 0.25 g of sediment per sample was weighed for extraction, and the extraction steps followed the manufacturer’s instructions. The eDNA was then eluted with 80 µL of Solution C6 and stored at -20 ℃. A 0.25 g Milli-Q water sample was included as the negative control to detect contamination during this process.
eDNA metabarcoding and sequence processing
The 16S rRNA gene fragments of extracted eDNA (n = 138) were amplified using cnidarian-universal primers: 16 S-L 5´-GACTGTTTACCAAAAACATA-3´ and 16 S-H 5´-CATAATTCAACATCGAGG-3´ [36]. PCR amplification was performed on an ABI GeneAmp® 9700 PCR instrument (Applied Biosystem, Waltham, Massachusetts), using a 20 µL reaction system of TransStart Fastpfu DNA Polymerase (TransGen AP221-02) that included 4 µL 5 × FastPfu Buffer, 2 µL 2.5 mM dNTPs, 0.8 µL each of forward and reverse primers with barcodes (5 µM), 0.4 µL FastPfu Polymerase, 0.2 µL BSA, 2 µL template DNA, and 9.8 µL double distilled H2O. The thermal conditions for the PCR reaction were as follows: initial denaturation at 95 ℃ for 3 min and 37 cycles of 95 ℃ for 30 s, 60 ℃ for 30 s, and 72 ℃ for 45 s, followed by a final extension executed at 72 ℃ for 10 min. Each eDNA sample was amplified three times, and three replicates from the same sample were mixed and analyzed using 2% (w/v) agarose gel electrophoresis. The PCR products recovered using the AxyPrepDNA gel recovery kit were quantified using a QuantiFluorTM-ST Blue Fluorescence Quantification System (Promega, Beijing, China) and normalized to equimolar amounts. Amplicon libraries were constructed using TruSeq™ DNA Sample Prep Kit (Illumina). Paired-end sequencing (2 × 300 bp) was performed using an Illumina MiSeq platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China).
Paired-end reads were merged with FLASH v 1.2.11 [37] to obtain spliced sequences. The spliced sequences were subjected to quality control and filtering to obtain high-quality clean reads using QIIME v 1.9.1 [38, 39]. Chimeric sequences were detected and removed using the UCHIME algorithm [40]. Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs), and the representative sequence for each OTU was screened for further annotation using the UPARSE software [41]. Taxonomic annotation was performed using the Nucleotide Sequence Database (nt_v20210917) from the NCBI database based on BLAST (E-value = 10− 5). Only OTUs classified as Metazoa were retained, and the sequence numbers were normalized using the standard of the sample with the fewest sequences.
Data processing and statistical analysis
Maps of the sampling stations were visualized using Ocean Data View (Reiner Schlitzer, Alfred Wegener Institute, Bremerhaven, Germany). One-way analysis of variance (ANOVA) was used to identify the significance of environmental factors and relative read abundance of jellyfish at different stations and water depths. The Student’s t test was performed to analyze the significance of the relative read abundance of jellyfish in the seawater and sediment samples. The neighbor-joining phylogenetic tree of the six jellyfish taxa detected in this study was constructed using MEGA v11.0.11. Spearman’s correlation analysis between the relative read abundance of jellyfish taxa and environmental factors in the Bohai Sea was performed using the Omicshare platform (https://www.omicshare.com/tools/).
Results
Environmental characteristics
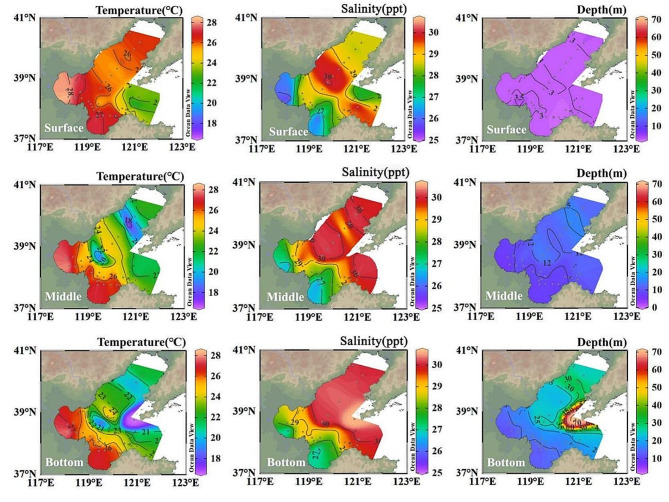
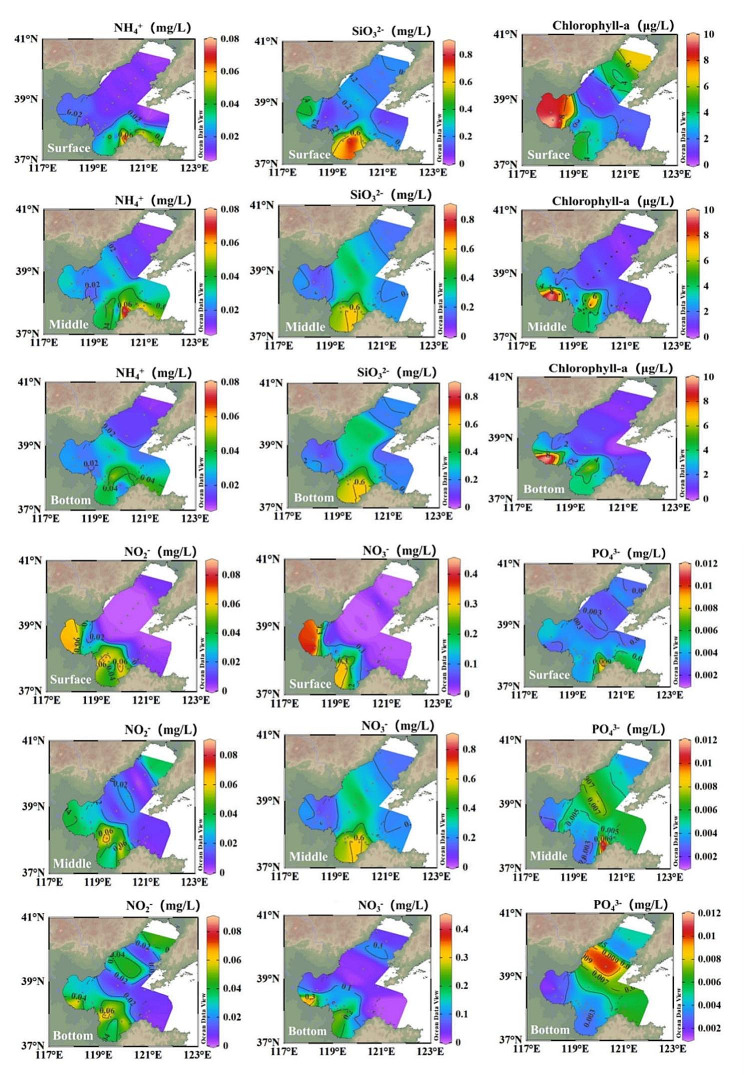
The temperature, salinity, Chl a, and nutrient concentration distributions at different depths at the 38 stations are shown in Table 1; Figs. 2 and 3. The sampling depth ranged from 2.45 to 70.90 m. The surface temperatures were significantly higher than those of the middle (ANOVA, P < 0.05) and bottom (ANOVA, P < 0.01) depths. The inshore seawater exhibited higher temperatures and lower salinity than those of the offshore seawater in the middle and bottom layers (ANOVA, P < 0.01), whereas those at sea surface were similar. Additionally, the inshore concentrations of NO3− and NO2− decreased with seawater depth (ANOVA, P < 0.05), whereas PO43− maintained a low concentration distribution at all three seawater depths. The concentration of PO43− increased from the surface to the bottom in the central Bohai Sea area (ANOVA, P < 0.05). Furthermore, the inshore concentrations of NH4+, SiO32−, and Chl a were significantly higher than those in the offshore seawater (ANOVA, P < 0.05).
Fig. 2.
Variations in temperature, salinity, and depth in the Bohai Sea in August 2022. Note The maps in the figure were obtained from Ocean Data View software (Reiner Schlitzer, Alfred Wegener Institute, Bremerhaven, Germany)
Fig. 3.
Variations in nutrient and chlorophyll-a concentrations in the Bohai Sea in August 2022. Note The maps in the figure were obtained from Ocean Data View software (Reiner Schlitzer, Alfred Wegener Institute, Bremerhaven, Germany)
eDNA metabarcoding for jellyfish biodiversity detection
Ninety-eight amplicon libraries, derived from 28 surface seawater, 28 middle seawater, 27 bottom seawater, and 15 sediment samples across 33 stations, were successfully constructed from the 138 eDNA samples using cnidarian universal primer pairs. The 16S rRNA gene amplicon sequencing generated 5,120,633 high-quality filtered sequences. In total, 29 OTUs were clustered in Metazoa with 97% sequence similarity. Sixteen of these were classified as the phylum Cnidaria, covering three classes, 13 orders, 13 families, and 13 genera. Six medusozoan taxa were identified, including the scyphozoans Aurelia coerulea, Nemopilema nomurai and Cyanea nozakii, and the hydrozoans Eirene ceylonensis, Ectopleura crocea, and Craspedacusta sowerbii (Table 2). The identification rate of medusozoan taxa was above 99.42%, confirming the reliability of the taxonomic annotation (Table 2). Subsequently, a neighbor-joining phylogenetic tree of the six medusozoan species was constructed (see Additional file 1), and the genetic relationships between the six species were revealed.
Table 2.
Summary of jellyfish taxa identified by eDNA metabarcoding based on 16S rRNA gene sequences
| Phylum | Class | Family | Species | Best match in NCBI | |
|---|---|---|---|---|---|
| Identity | Accession | ||||
| Cnidaria | Scyphozoa | Rhizostomatidae | Nemopilema nomurai | 100.00% | JX845343.1 |
| Ulmaridae | Aurelia coerulea | 100.00% | MZ061800.1 | ||
| 99.42% | OP458507.1 | ||||
| Cyaneidae | Cyanea nozakii | 100.00% | MW832752.1 | ||
| Hydrozoa | Olindiidae | Craspedacusta sowerbii | 100.00% | MK600507.1 | |
| Eirenidae | Eirene ceylonensis | 99.63% | HM053550.1 | ||
| Tubulariidae | Ectopleura crocea | 100.00% | MG811598.1 | ||
Spatial distribution pattern of the blooming jellyfish
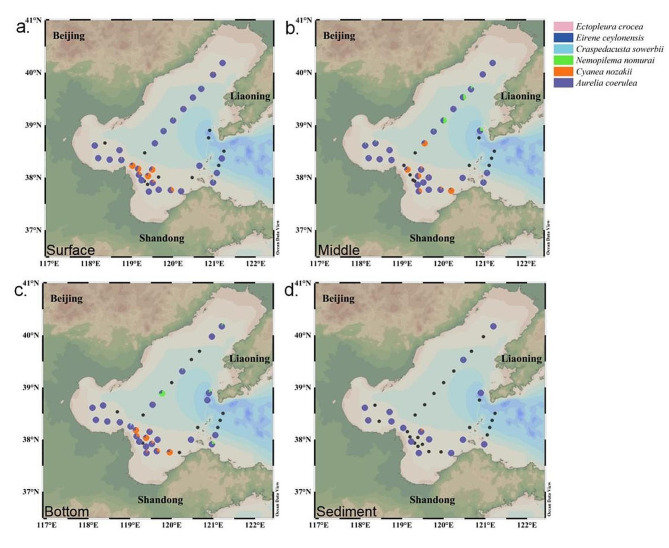
A. coerulea was detected in 98 eDNA samples from the Bohai Sea during the survey period and was the most abundant jellyfish species in 86 eDNA samples, with relative read abundance 54.48% to 100% (Fig. 4). Among 22 eDNA samples from 12 stations, A. coerulea was the only jellyfish species identified (Fig. 4). Ten of the eDNA samples were dominated by C. nozakii, and all relative read abundance were 57.52% or greater; the highest relative read abundance was detected in the middle seawater of LZB4 (88.20%) (Fig. 4). N. nomurai was detected in 42 eDNA samples and was the most abundant in the middle seawater of M5 (55.20%) and bottom seawater of M4 (74.03%) (Fig. 4). Interestingly, eDNA samples with high C. nozakii or N. nomurai abundance (relative read abundance > 10%) were not coincident, and there was a low overlap in the spatial distribution of these two jellyfish species (Fig. 4). Moreover, E. ceylonensis, E. crocea, and C. sowerbii, which are rare species, were only detected in three, one, and two eDNA samples, respectively, and with a low relative read abundance (relative read abundance ≦ 10%) (Fig. 4). Therefore, the spatial distribution pattern analysis of jellyfish mainly focused on the three dominant jellyfish taxa (A. coerulea, C. nozakii, and N. nomurai).
Fig. 4.
Spatial variations in the relative read abundance of six jellyfish taxa in the Bohai Sea in August 2022. Note a–d: Taxonomic composition and relative read abundance of jellyfish in eDNA from surface seawater (a), middle seawater (b), bottom seawater (c) and sediments (d). Jellyfish species represented by the distinct colors in the pie plots are described in the legend on the right. The black dots indicate that no jellyfish was detected in eDNA from the station. The maps in the figure were obtained from Ocean Data View software (Reiner Schlitzer, Alfred Wegener Institute, Bremerhaven, Germany)
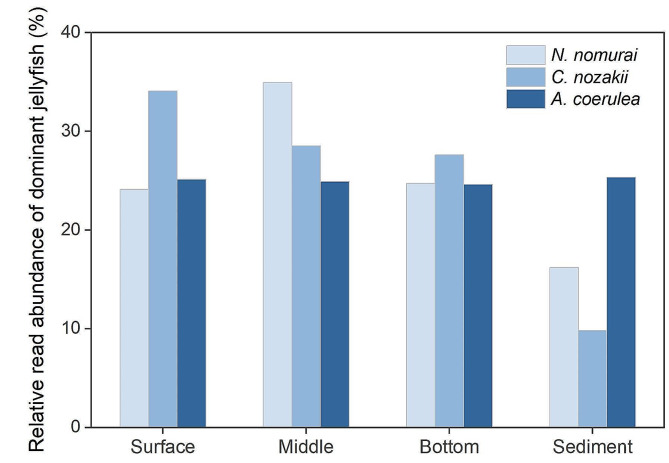
Overall, in the surveyed Bohai Sea, the relative distribution proportions of A. coerulea, C. nozakii, and N. nomurai at different seawater depths were generally uniform. The eDNA of C. nozakii was more abundant in the surface layer, whereas that of N. nomurai was relatively aggregated in the middle layers (Fig. 5). A. coerulea displayed no significant difference in relative read abundances between sediments and seawater, while the relative read abundances of C. nozakii and N. nomurai were higher in seawater than in sediments (Student’s t test, P < 0.01; Fig. 5). Furthermore, marked differences between three jellyfish taxa in a vertical direction were revealed in seawater samples of partial regions. The relative read abundance of A. coerulea in the surface layer was significantly higher than that in the middle and bottom layers of the offshore stations (ANOVA, P < 0.05; Fig. 4a-c). The relative read abundance of C. nozakii in the offshore regions peaked in the middle layer and was significantly higher than that in the surface layer (ANOVA, P < 0.05; Fig. 4a-c). The eDNA of N. nomurai presented a middle-bottom aggregation at several offshore stations with a relative read abundance > 10% (M4, M5, M7, M8, N1, and L6), and was significantly more abundant in the bottom layer than in the surface layer (ANOVA, P < 0.05; Fig. 4a-c). Conversely, the difference between the vertical abundance of A. coerulea, C. nozakii, and N. nomurai was not significant in the inshore seawater (ANOVA, P > 0.05; Fig. 4a-c). In sediments, A. coerulea was the absolute dominant species, with a mean relative read abundance of 98.74% (Fig. 4d).
Fig. 5.
Vertical variations in the relative read abundance of dominant jellyfish taxa
In terms of the horizontal distribution, the eDNA of A. coerulea was dominant in BHB, with a significantly higher abundance than in LZB, M, and YR (ANOVA, P < 0.05; Fig. 4). The eDNA of C. nozakii was mainly gathered in LZB and YR, as opposed to in BHB and the offshore seawater (ANOVA, P < 0.01; Fig. 4). The eDNA of N. nomurai was prevalent in M, where its relative read abundance was significantly higher than that in the inshore regions, including BHB, LZB, and YR (ANOVA, P < 0.05; Fig. 4).
Correlation analysis between blooming jellyfish and environmental factors
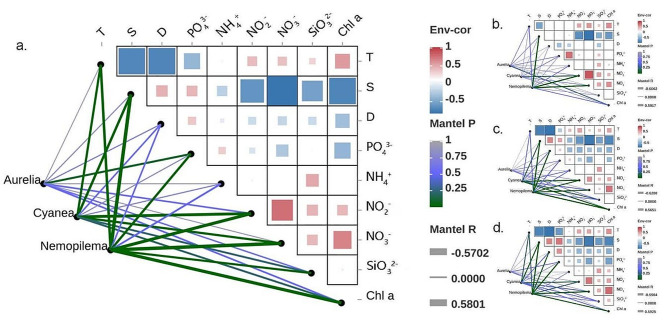
Spearman correlation analysis showed that the relative read abundance of A. coerulea was significantly negatively correlated with the PO43− content (P < 0.05; Fig. 6a). The relative read abundance of C. nozakii was positively correlated with NO3−, NO2−, T, SiO32−, and Chl a but negatively correlated with S (P < 0.05; Fig. 6a). Conversely, the relative read abundance of N. nomurai was negatively correlated with NO3−, NO2−, T, SiO32−, and Chl a but positively correlated with PO43− and S (P < 0.01; Fig. 6a). The concentration of NH4+ was also negatively correlated with the relative read abundance of N. nomurai (P < 0.05; Fig. 6a).
Fig. 6.
Spearman correlation analysis between the relative read abundance of dominant jellyfish taxa and environmental factors (a) in all 98 eDNA samples, and (b–d) in samples taken from the surface, middle, and bottom layers, respectively. Note A network heatmap of correlations between the relative read abundance of dominant jellyfish taxa and environmental factors. The color of the blocks in the right heatmap indicates a positive or negative correlation coefficient between different environmental factors, and the size of the blocks indicates the absolute value of correlation coefficients. The thickness of the lines indicates the strength of the correlations between the relative read abundances of the three dominant jellyfish species and environmental factors (Mental R), and the color of the lines indicates the degree of significance (Mental P)
Vertically, there were differences between the correlations of the three jellyfish species and environmental factors. A significantly positive correlation between the relative read abundance of A. coerulea and the Chl a concentration was detected in the surface seawater (P < 0.05; Fig. 6b). The relative read abundance of C. nozakii was positively correlated with NO2− content at all three seawater depths, but negatively correlated with S in the middle and bottom layers (P < 0.05; Fig. 6b–d). In the middle and bottom layers, the relative read abundance of N. nomurai was positively correlated with S and PO43−, but negatively correlated with NO3−, NO2− and Chl a (P < 0.05; Fig. 6b–d).
Discussion
As a new marine ecological survey tool, eDNA-based techniques are non-invasive, environmentally friendly, and accurate, and they are expected to play an essential role in optimizing ecological studies of jellyfish [42–44]. Recent studies have highlighted the great potential of eDNA-based techniques in biodiversity investigation, spatial distribution detection, and early life history stage monitoring of jellyfish [45–48]. Metabarcoding, as one of the major eDNA-based techniques, has obvious advantages in providing broad-scale distribution data for multiple species from a single analysis and detecting unknown biodiversity previously not recorded, including “unexpected” invasive or non-native species [21, 23, 28, 49]. Here, the eDNA metabarcoding was used as an indicator of the spatial distribution of jellyfish in the Bohai Sea. Six jellyfish species were identified in this study, of which A. coerulea, C. nozakii and N. nomurai were the dominant jellyfish taxa, which is consistent with the results of traditional ecological surveys [50–52].
Indicator of the spatial distribution of the blooming jellyfish by eDNA
It is important to determine the vertical distribution of medusae to study their trophic interactions, vertical migration behavior, and spatiotemporal changes under global changes [11, 53, 54]. However, traditional sampling using plankton nets cannot effectively demonstrate the vertical distribution of jellyfish in shallow sea due to the trawling depths employed, the limited collection efficiency, and the damage caused to the fragile bodies of gelatinous organisms [55, 56]. In recent years, underwater imaging and acoustic equipment have been used to monitor the vertical distribution of jellyfish [53, 54, 57, 58]; however, these depend on diving and require large and expensive devices. In the current study, the distribution of three dominant jellyfish species, A. coerulea, N. nomurai, and C. nozakii, in the typical layers were indicated by eDNA metabarcoding, the results of which were relatively easier to realize.
We found that more eDNA from A. coerulea was detected in the surface layer of offshore seawater than in the middle and bottom layers. N. nomurai and C. nozakii were preferentially aggregated in the middle-bottom and upper-middle layers, respectively. These results are consistent with previous findings obtained using traditional methods. There are seasonal variations in the vertical distribution of Aurelia sp., and the study of Barz et al. (2005) found that most A. aurita were distributed in the upper layer in August [59]. Kim et al. (2016) found that approximately 93% of N. nomurai individuals were distributed within a water depth of 10–40 m [57]. It is of note that in this survey, there was a significant difference in temperature between the surface and the middle and bottom layers in a vertical direction, which indicates the existence of a possible thermal stratification in the Bohai Sea. The existence of thermal stratification may thus affect the vertical diffusion range of eDNA. Littlefair et al. (2021) demonstrated that eDNA signals show strong seasonal stratification during summer that closely reflects the thermal preference of fishes [60]. Therefore, the vertical distribution patterns of eDNA may also reflect the thermal preference of these three blooming jellyfish.
Horizontally, A. coerulea and C. nozakii were more abundant in the inshore regions than in the offshore regions, and this result is consistent with those of several previous trawl surveys on these two species [61, 62]. N. nomurai aggregations were mainly distributed in offshore areas, and similar results also were detected in an ecological investigation [50].
The relative read abundance of the three dominant jellyfish taxa in the sediments were significantly different from those in the seawater, indicating a difference between the eDNA preservation of the two media. Due to the low degradation of DNA in sediments, it is speculated that sediment eDNA reflects resident organisms and historical events rather than transient events [63–65]. Therefore, the eDNA analysis of sediments and seawater may contribute to different research purposes.
Although possible interference from eDNA released by the decaying jellyfish sinking to the bottom cannot be completely excluded in this study, the eDNA collected in this study is more likely to come from live jellyfish rather than dead individuals considering that our sampling time is not autumn and winter when jellyfish decay events occur frequently in the Bohai Sea [66].
The response of blooming jellyfish to environmental factors in the Bohai Sea
The Spearman correlation analysis showed a significantly positive relationship between the relative read abundance of C. nozakii and both Chl a and every nutrient tested for. It also indicated a significantly positive relationship between the relative read abundance of N. nomurai and salinity, while negative correlations were determined between N. nomurai and NO3−, NO2−, temperature, SiO32−, and Chl a, and between C. nozakii and salinity (Fig. 6). These results verified the preference of C. nozakii in the rich nutrient, highly productive, and low salinity inshore water, and the preference of N. nomurai in low salinity and low nutrient offshore environments. Notably, the relative read abundance of N. nomurai was positively correlated with PO43−, which was likely the result of excretion of PO43− by N. nomurai themselves [67].
The relative read abundance of A. coerulea in the BHB was significantly higher than that in the LZB and other offshore regions, and showed less correlation with environmental factors in this study. Dong et al. (2012) also found that the spatial distribution of A. coerulea was less correlated with environmental variables in the Sishili Bay, and the intense construction of coastal aquaculture rafts may provide suitable habitats for their early growth [68]. In a survey of large jellyfish in the Bohai Sea in 2018, A. coerulea was found to peak in the BHB [69], particularly in an area that was used for marine utilization and human activities, including aquaculture and harbor engineering [70]. Therefore, the greater polyp habitat availability in the BHB could be associated with the high abundance of abundance of A. coerulea in this area, and water dynamics and currents may contribute to its patchy distribution in this region [71].
Conclusions
The biodiversity and vertical and horizontal distribution patterns of blooming jellyfish in the Bohai Sea were investigated using eDNA metabarcoding in August 2022. The eDNA-based results corresponded to traditional findings of previous studies, confirming the feasibility of the eDNA approach for blooming jellyfish monitoring and population surveys over a wide range of scales. The current study highlights the advantages of eDNA metabarcoding as a tool for further revealing the vertical distribution of blooming jellyfish. However, limited by the diffusivity and instability of eDNA, the combined use of eDNA-based techniques and traditional ecological survey tools would contribute to making precise estimates of biodiversity and biomass. Additionally, eDNA has been used to detect the locations of the benthic polyp stage [47]; therefore, future studies could be conducted using the eDNA method to investigate the potential polyp habitat in the Bohai Sea during winter when medusae are absent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- eDNA
Environmental DNA
- BHB
Bohai Bay
- LZB
Laizhou Bay
- M
The central Bohai Sea area
- YR
The Yellow River estuary
- N
The Miaodao Archipelago
- T
Temperature
- S
Salinity
- D
Depth
- SE
Standard Error
- CTD
Conductivity-temperature-depth
- GF/F
Glass fiber filters
- PO43−
Phosphates
- SiO32−
Silicates
- NO3−
Nitrates
- NO2−
Nitrites
- NH4+
Ammoniums
- Chl a
Chlorophyll-a
- PCR
Polymerase chain reaction
- FLASH
Fast length adjustment of short reads
- QIIME
Quantitative insights into Microbial Ecology
- OTUs
Operational taxonomic units
- NCBI
National Center of Biotechnology Information
- BLAST
Basic Local Alignment Search Tool
- ANOVA
Analysis of variation
- MEGA
Molecular Evolutionary Genetics Analysis
Author contributions
ZD conceptualized this experiment and provided the funding. LY and SP performed eDNA extraction experiments, analyzed the results and wrote the manuscript. LW and CZ conducted the investigation and collection works. XS determinated the nutrients and chlorophyll-a. ZD, WZ, YM, MY and JZ reviewed and edited. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2023YFC3108201), the National Science & Technology Fundamental Resources Investigation Program of China (2022FY100603), Key Project of the NSFC-Shandong Joint Fund (U2106208), National Natural Science Foundation of China (41876138), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA23050301), and Taishan Scholars Program (tsqn202211263).
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI database with the primary accession code PRJNA1034232.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijing Ye and Saijun Peng contributed equally to this work.
References
- 1.Hay S. Marine Ecology: Gelatinous bells May Ring Change in Marine ecosystems. Curr Biol. 2006;16:R679–82. doi: 10.1016/j.cub.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Dong Z, Liu D, Keesing JK. Jellyfish blooms in China: Dominant species, causes and consequences. Mar Pollut Bull. 2010;60:954–63. doi: 10.1016/j.marpolbul.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Kennerley A, Wood LE, Luisetti T, Ferrini S, Lorenzoni I. Economic impacts of jellyfish blooms on coastal recreation in a UK coastal town and potential management options. Ocean Coast Manage. 2022;227:106284. doi: 10.1016/j.ocecoaman.2022.106284. [DOI] [Google Scholar]
- 4.Reyes Suárez NC, Tirelli V, Ursella L, Ličer M, Celio M, Cardin V. Multi-platform study of the extreme bloom of the barrel jellyfish Rhizostoma pulmo (Cnidaria: Scyphozoa) in the northernmost gulf of the Mediterranean Sea (Gulf of Trieste) in April 2021. Ocean Sci. 2022;18:1321–37. doi: 10.5194/os-18-1321-2022. [DOI] [Google Scholar]
- 5.Palmieri MG, Barausse A, Luisetti T, Turner K. Jellyfish blooms in the Northern Adriatic Sea: fishermen’s perceptions and economic impacts on fisheries. Fish Res. 2014;155:51–8. doi: 10.1016/j.fishres.2014.02.021. [DOI] [Google Scholar]
- 6.Chiaverano LM, Robinson KL, Tam J, Ruzicka JJ, Quiñones J, Aleksa KT, et al. Evaluating the role of large jellyfish and forage fishes as energy pathways, and their interplay with fisheries, in the Northern Humboldt Current System. Prog Oceanogr. 2018;164:28–36. doi: 10.1016/j.pocean.2018.04.009. [DOI] [Google Scholar]
- 7.Hamel H, Lhoumeau S, Wahlberg M, Javidpour J. Using drones to measure jellyfish density in shallow estuaries. JMSE. 2021;9:659. doi: 10.3390/jmse9060659. [DOI] [Google Scholar]
- 8.Kingsford MJ, Schlaefer JA, Morrissey SJ. Population structures and levels of connectivity for Scyphozoan and Cubozoan jellyfish. Diversity. 2021;13:174. doi: 10.3390/d13040174. [DOI] [Google Scholar]
- 9.Dobson JY, Fonfría ES, Palacios R, Blasco E, Bordehore C. Citizen science effectively monitors biogeographical and phenological patterns of jellyfish. Ocean Coast Manage. 2023;242:106668. doi: 10.1016/j.ocecoaman.2023.106668. [DOI] [Google Scholar]
- 10.Zang W, Zhang F, Sun Y, Xu Z, Sun S. Benthic ecosystem determines jellyfish blooms by controlling the polyp colony development. Mar Pollut Bull. 2023;193:115232. doi: 10.1016/j.marpolbul.2023.115232. [DOI] [PubMed] [Google Scholar]
- 11.Malej A, Turk V, Lučić D, Benović A. Direct and indirect trophic interactions of Aurelia sp. (Scyphozoa) in a stratified marine environment (Mljet Lakes, Adriatic Sea) Mar Biol. 2007;151:827–41. doi: 10.1007/s00227-006-0503-1. [DOI] [Google Scholar]
- 12.Suzuki KS, Yasuda A, Murata Y, Kumakura E, Yamada S, Endo N, et al. Quantitative effects of pycnocline and dissolved oxygen on vertical distribution of moon jellyfish Aurelia aurita s.l.: a case study of Mikawa Bay, Japan. Hydrobiologia. 2016;766:151–63. doi: 10.1007/s10750-015-2451-6. [DOI] [Google Scholar]
- 13.Minamoto T, Fukuda M, Katsuhara KR, Fujiwara A, Hidaka S, Yamamoto S, et al. Environmental DNA reflects spatial and temporal jellyfish distribution. PLoS ONE. 2017;12:e0173073. doi: 10.1371/journal.pone.0173073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruppert KM, Kline RJ, Rahman MS. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: a systematic review in methods, monitoring, and applications of global eDNA. Glob Ecol Conserv. 2019;17:e00547. [Google Scholar]
- 15.Barnes MA, Turner CR. The ecology of environmental DNA and implications for conservation genetics. Conserv Genet. 2016;17:1–17. doi: 10.1007/s10592-015-0775-4. [DOI] [Google Scholar]
- 16.Deiner K, Fronhofer EA, Mächler E, Walser J-C, Altermatt F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat Commun. 2016;7:12544. doi: 10.1038/ncomms12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willerslev E, Davison J, Moora M, Zobel M, Coissac E, Edwards ME, et al. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature. 2014;506:47–51. doi: 10.1038/nature12921. [DOI] [PubMed] [Google Scholar]
- 18.Yoon T-H, Kang H-E, Lee SR, Lee J-B, Baeck GW, Park H, et al. Metabarcoding analysis of the stomach contents of the Antarctic Toothfish (Dissostichus mawsoni) collected in the Antarctic Ocean. PeerJ. 2017;5:e3977. doi: 10.7717/peerj.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper LR, Lawson Handley L, Hahn C, Boonham N, Rees HC, Gough KC, et al. Needle in a haystack? A comparison of eDNA metabarcoding and targeted qPCR for detection of the great crested newt (Triturus cristatus) Ecol Evol. 2018;8:6330–41. doi: 10.1002/ece3.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Peng Y, Fang W, Altermatt F, Xie Y, Yang J, et al. Application of environmental DNA metabarcoding for Predicting Anthropogenic Pollution in Rivers. Environ Sci Technol. 2018;52:11708–19. doi: 10.1021/acs.est.8b03869. [DOI] [PubMed] [Google Scholar]
- 21.Gargan LM, Brooks PR, Vye SR, Ironside JE, Jenkins SR, Crowe TP, et al. The use of environmental DNA metabarcoding and quantitative PCR for molecular detection of marine invasive non-native species associated with artificial structures. Biol Invasions. 2022;24:635–48. doi: 10.1007/s10530-021-02672-8. [DOI] [Google Scholar]
- 22.Leurs G, Verkuil YI, Hijner N, Saalmann F, Dos Santos L, Regalla A, et al. Addressing data-deficiency of threatened sharks and rays in a highly dynamic coastal ecosystem using environmental DNA. Ecol Indic. 2023;154:110795. doi: 10.1016/j.ecolind.2023.110795. [DOI] [Google Scholar]
- 23.Ames CL, Vora GJ. Fieldable Environmental DNA sequencing to assess Jellyfish Biodiversity in Nearshore Waters of the Florida Keys, United States. Front Mar Sci. 2021;8:640527. doi: 10.3389/fmars.2021.640527. [DOI] [Google Scholar]
- 24.Di Capua I, Piredda R, Mazzocchi MG, Zingone A. Metazoan diversity and seasonality through eDNA metabarcoding at a Mediterranean long-term ecological research site. ICES J Mar Sci. 2021;78:3303–16. doi: 10.1093/icesjms/fsab059. [DOI] [Google Scholar]
- 25.Blackman RC, Brantschen J, Walser J, Wüthrich R, Altermatt F. Monitoring invasive alien macroinvertebrate species with environmental DNA. River Res L. 2022;38:1400–12. doi: 10.1002/rra.3947. [DOI] [Google Scholar]
- 26.McInnes JC, Alderman R, Lea M-A, Raymond B, Deagle BE, Phillips RA, et al. High occurrence of jellyfish predation by black-browed and Campbell albatross identified by DNA metabarcoding. Mol Ecol. 2017;26:4831–45. doi: 10.1111/mec.14245. [DOI] [PubMed] [Google Scholar]
- 27.Urban P, Præbel K, Bhat S, Dierking J, Wangensteen OS. DNA metabarcoding reveals the importance of gelatinous zooplankton in the diet of Pandalus borealis, a keystone species in the Arctic. Mol Ecol. 2022;31:1562–76. doi: 10.1111/mec.16332. [DOI] [PubMed] [Google Scholar]
- 28.Govindarajan AF, Francolini RD, Jech JM, Lavery AC, Llopiz JK, Wiebe PH, et al. Exploring the use of environmental DNA (eDNA) to Detect Animal Taxa in the Mesopelagic Zone. Front Ecol Evol. 2021;9:574877. doi: 10.3389/fevo.2021.574877. [DOI] [Google Scholar]
- 29.Wang YT, Sun S. Population dynamics of Aurelia sp.1 ephyrae and medusae in Jiaozhou Bay, China. Hydrobiologia. 2015;754:147–55. doi: 10.1007/s10750-014-2021-3. [DOI] [Google Scholar]
- 30.Wu L, Wang J, Gao S, Zheng X, Huang R. An analysis of dynamical factors influencing 2013 giant jellyfish bloom near Qinhuangdao in the Bohai Sea, China. Estuar Coast Shelf S. 2017;185:141–51. doi: 10.1016/j.ecss.2016.12.010. [DOI] [Google Scholar]
- 31.Yoon W, Hahn K, Dong J, Chae J. Monthly geographical distribution of Nemopilema Nomurai (Scyphozoa: Rhizosotomeae) in the Bohai and North Yellow seas: a preliminary study using ships of opportunity. Ocean Sci J. 2018;53:699–706. doi: 10.1007/s12601-018-0050-y. [DOI] [Google Scholar]
- 32.Purcell J, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar Ecol Prog Ser. 2007;350:153–74. doi: 10.3354/meps07093. [DOI] [Google Scholar]
- 33.Takahashi S, Sakata MK, Minamoto T, Masuda R. Comparing the efficiency of open and enclosed filtration systems in environmental DNA quantification for fish and jellyfish. PLoS ONE. 2020;15:e0231718. doi: 10.1371/journal.pone.0231718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George JA, Lonsdale DJ, Merlo LR, Gobler CJ. The interactive roles of temperature, nutrients, and zooplankton grazing in controlling the winter-spring phytoplankton bloom in a temperate, coastal ecosystem, Long Island Sound: Long Island Sound winter-spring bloom. Limnol Oceanogr. 2015;60:110–26. doi: 10.1002/lno.10020. [DOI] [Google Scholar]
- 35.Zhang W, Dong Z, Zhang C, Sun X, Hou C, Liu Y, et al. Effects of physical-biochemical coupling processes on the Noctiluca scintillans and Mesodinium red tides in October 2019 in the Yantai nearshore, China. Mar Pollut Bull. 2020;160:111609. doi: 10.1016/j.marpolbul.2020.111609. [DOI] [PubMed] [Google Scholar]
- 36.Ender A, Schierwater B. Placozoa are not derived cnidarians: evidence from molecular morphology. Mol Biol Evol. 2003;20:130–4. doi: 10.1093/molbev/msg018. [DOI] [PubMed] [Google Scholar]
- 37.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57–9. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen PF, Møller PR, Sigsgaard EE, Knudsen SW, Jørgensen OA, Willerslev E. Environmental DNA from seawater samples correlate with Trawl catches of Subarctic, Deepwater fishes. PLoS ONE. 2016;11:e0165252. doi: 10.1371/journal.pone.0165252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans NT, Li Y, Renshaw MA, Olds BP, Deiner K, Turner CR, et al. Fish community assessment with eDNA metabarcoding: effects of sampling design and bioinformatic filtering. Can J Fish Aquat Sci. 2017;74:1362–74. doi: 10.1139/cjfas-2016-0306. [DOI] [Google Scholar]
- 44.Yamamoto S, Masuda R, Sato Y, Sado T, Araki H, Kondoh M, et al. Environmental DNA metabarcoding reveals local fish communities in a species-rich coastal sea. Sci Rep. 2017;7:40368. doi: 10.1038/srep40368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaynor JJ, Bologna PAX, Restaino DJ, Barry CL. qPCR detection of Early Life History Stage Chrysaora quinquecirrha (Sea Nettles) in Barnegat Bay, New Jersey. J Coastal Res. 2017;78:184–92. doi: 10.2112/SI78-014.1. [DOI] [Google Scholar]
- 46.Morrissey SJ, Jerry DR, Kingsford MJ. Genetic detection and a method to study the Ecology of Deadly Cubozoan Jellyfish. Diversity. 2022;14:1139. doi: 10.3390/d14121139. [DOI] [Google Scholar]
- 47.Morrissey SJ, Jerry DR, Kingsford MJ. Use of eDNA to test hypotheses on the ecology of Chironex fleckeri (Cubozoa) Mar Ecol Prog Ser. 2024;728:25–41. doi: 10.3354/meps14507. [DOI] [Google Scholar]
- 48.Bolte B, Goldsbury J, Huerlimann R, Jerry D, Kingsford M. Validation of eDNA as a viable method of detection for dangerous cubozoan jellyfish. Environ DNA. 2021;3:769–79. doi: 10.1002/edn3.181. [DOI] [Google Scholar]
- 49.Euclide PT, Lor Y, Spear MJ, Tajjioui T, Vander Zanden J, Larson WA, et al. Environmental DNA metabarcoding as a tool for biodiversity assessment and monitoring: reconstructing established fish communities of north-temperate lakes and rivers. Divers Distrib. 2021;27:1966–80. doi: 10.1111/ddi.13253. [DOI] [Google Scholar]
- 50.Guo D, Zhang F, Wang P, Sun S. Distribution patterns of large jellyfish and their effects on the Zooplankton Community in the Northern Chinese Coastal seas during the summer of 2021. Diversity. 2023;15:729. doi: 10.3390/d15060729. [DOI] [Google Scholar]
- 51.Feng S, Wang S, Sun S, Zhang F, Zhang G, Liu M, et al. Strobilation of three scyphozoans (Aurelia Coelurea, Nemopilema Nomurai, and Rhopilema esculentum) in the field at Jiaozhou Bay, China. Mar Ecol Prog Ser. 2018;591:141–53. doi: 10.3354/meps12276. [DOI] [Google Scholar]
- 52.Tang C, Sun S, Zhang F. Intraguild predation by polyps of three scyphozoan jellyfish: Nemopilema Nomurai, Aurelia coerulea, and Rhopilema esculentum. J Ocean Limnol. 2020;38:1755–61.
- 53.Moriarty P, Andrews K, Harvey C, Kawase M. Vertical and horizontal movement patterns of scyphozoan jellyfish in a fjord-like estuary. Mar Ecol Prog Ser. 2012;455:1–12. doi: 10.3354/meps09783. [DOI] [Google Scholar]
- 54.Maekakuchi M, Matsuno K, Yamamoto J, Abe Y, Yamaguchi A. Abundance, horizontal and vertical distribution of epipelagic ctenophores and scyphomedusae in the northern Bering Sea in summer 2017 and 2018: quantification by underwater video imaging analysis. Deep-Sea Res Pt II. 2020;181–2:104818.
- 55.Youngbluth MJ, Båmstedt U. Distribution, abundance, behavior and metabolism of Periphylla periphylla, a mesopelagic coronate medusa in a Norwegian fjord. In: Purcell JE, Graham WM, Dumont HJ, editors. Jellyfish blooms: ecological and societal importance. Volume 155. Dordrecht: Springer Netherlands; 2001;321–33.
- 56.Uye SI, Brodeur RD, Ishii H, Zavolokin A. Sampling considerations. In: Uye, SI, Brodeur, RD, editors. Report of Working Group 26 on Jellyfish Blooms around the North Pacific Rim: Causes and Consequences. PICES Sci Rep. 2017;51:29–29.
- 57.Kim S, Lee K, Yoon WD, Lee H, Hwang K. Vertical distribution of giant jellyfish, Nemopilema nomurai using acoustics and optics. Ocean Sci J. 2016;51:59–65. doi: 10.1007/s12601-016-0006-z. [DOI] [Google Scholar]
- 58.Lee K, Bae B-S, Kim I-O, Yoon W-D. Measurement of swimming speed of giant jellyfish Nemopilema nomurai using acoustics and visualization analysis. Fish Sci. 2010;76:893–9. doi: 10.1007/s12562-010-0294-7. [DOI] [Google Scholar]
- 59.Barz K, Hirche H-J. Seasonal development of scyphozoan medusae and the predatory impact of Aurelia aurita on the zooplankton community in the Bornholm Basin (central Baltic Sea) Mar Biol. 2005;147:465–76. doi: 10.1007/s00227-005-1572-2. [DOI] [Google Scholar]
- 60.Littlefair JE, Hrenchuk LE, Blanchfield PJ, Rennie MD, Cristescu ME. Thermal stratification and fish thermal preference explain vertical eDNA distributions in lakes. Mol Ecol. 2021;30:3083–96. doi: 10.1111/mec.15623. [DOI] [PubMed] [Google Scholar]
- 61.Pourjomeh F, Shokri MR, Rezai H, Rajabi-Maham H, Maghsoudlou E. The relationship among environmental variables, jellyfish and non-gelatinous zooplankton: a case study in the north of the Gulf of Oman. Mar Ecol. 2017;38:e12476. doi: 10.1111/maec.12476. [DOI] [Google Scholar]
- 62.Wang P, Zhang F, Liu M, Sun S, Xian H. Isotopic evidence for size-based dietary shifts in the jellyfish Cyanea nozakii in the northern East China Sea. J Plankton Res. 2020;42:689–701. [Google Scholar]
- 63.Gleason JE, Hanner RH, Cottenie K. Hidden diversity: DNA metabarcoding reveals hyper-diverse benthic invertebrate communities. BMC Ecol Evo. 2023;23:19. doi: 10.1186/s12862-023-02118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He W, Xu D, Liang Y, Ren L, Fang D. Using eDNA to assess the fish diversity and spatial characteristics in the Changjiang River-Shijiu Lake connected system. Ecol Indic. 2022;139:108968. doi: 10.1016/j.ecolind.2022.108968. [DOI] [Google Scholar]
- 65.Ogata M, Masuda R, Harino H, Sakata MK, Hatakeyama M, Yokoyama K, et al. Environmental DNA preserved in marine sediment for detecting jellyfish blooms after a tsunami. Sci Rep. 2021;11:16830. doi: 10.1038/s41598-021-94286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Song J, Ma Q, Li N, Yuan H, Duan L, Qu B. Experiments and evidences: jellyfish (Nemopilema Nomurai) decomposing and nutrients (nitrogen and phosphorus) released. Acta Oceanol Sin. 2015;34:1–12. [Google Scholar]
- 67.Xiao W, Zeng Y, Liu X, Huang X, Chiang K-P, Mi T, et al. The impact of giant jellyfish Nemopilema Nomurai blooms on plankton communities in a temperate marginal sea. Mar Pollut Bull. 2019;149:110507. doi: 10.1016/j.marpolbul.2019.110507. [DOI] [PubMed] [Google Scholar]
- 68.Dong Z, Liu D, Wang Y, Di B, Song X, Shi Y. A report on a Moon Jellyfish Aurelia aurita bloom in Sishili Bay, Northern Yellow Sea of China in 2009. Aquat Ecosyst Health. 2012;15:161–7. doi: 10.1080/14634988.2012.689583. [DOI] [Google Scholar]
- 69.Wang PP, Zhang F, Sun S, Tao Y. Distribution of giant jellyfish in the Bohai Sea in June 2018. Oceanol et Limno Sin. 2020;51:85–94. [Google Scholar]
- 70.Liu BJ. Comprehensive ecological stress evaluation of typical marine-related human activities on marine environment: a case study in the Bohai Sea and its adjacent areas[D]. Chin Acad Sci. 2021. (in Chinese).
- 71.Magome S, Yamashita T, Kohama T, Kaneda A, Hayami Y, Takahashi S, et al. Jellyfish patch formation investigated by aerial photography and drifter experiment. J Oceanogr. 2007;63:761–73. doi: 10.1007/s10872-007-0065-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the NCBI database with the primary accession code PRJNA1034232.