Abstract
Background:
Fatty liver disease comprises a wide range of related liver disorders affecting mainly people who drink no or minimal amounts of alcohol. Silymarin is a member of the Carduus marianum family that has been used for centuries to treat different diseases. There is little evidence supporting its efficacy in humans.
Objectives:
To evaluate the effects of Silymarin in patients with non alcoholic fatty liver disease (NAFLD) or recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD).
Methods:
We searched PubMed, SCOPUS, Web of Science, and Cochrane Library for relevant clinical trials assessing the use of silymarin in patients with NAFLD. A risk of bias assessment was performed using Cochrane's risk of bias tool. We included the following outcomes: alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) (mg/dL), degree of fibrosis resolution, low-density lipoprotein (LDL), and HOMA-IR. We analyzed continuous data using mean difference (MD) and relative 95% confidence interval (CI).
Results:
We included nine clinical trials. We found that silymarin significantly reduced the levels of ALT (MD= -17.12 [-28.81, -4.43]), (P < 0.004), AST (MD= -12.56 [-19.02, -6.10]), (P < 0.0001) and TG (MD = −22.60 [−23.83, −21.38]) (p < 0.00001). It also improved HDL (MD= 2.13 [1.60, 2.66]), (P < 0.01)). There was no significant difference regarding GGT (P=o.07), TC (P= 0.52), LDL (P= 0.06), HOMA-IR (P= 0.06) and BMI (p=0.1).One study reported significant improvement in the degree of fibrosis (P = 0.023).
Conclusion:
Silymarin treatment significantly reduces biochemical and transaminase levels in patients with MASLD.
Keywords: MASLD, NAFLD, silymarin
Introduction
Non-alcoholic fatty liver disease (NAFLD) is when excess fat is stored in the liver (1). This fat buildup is not caused by heavy alcohol use (2). NAFLD is the most common cause of chronic liver disease as it affects up to 30% of the general population (3). NAFLD is commonly associated with metabolic syndrome, obesity, diabetes, and hyperlipidemia (4).
Recntly, the experts advocated renaming nonalcoholic fatty liver disease (NAFLD) metabolic dysfunction-associated steatotic liver disease (MASLD) (5). Nearly 80% of patients with metabolic syndrome have MASLD (6). MASLD is classified into two types: simple fatty liver and non-alcoholic steatohepatitis (NASH) (7). People only develop one type of MASLD, although sometimes people with one form are later diagnosed with the other form of MASLD (8). NASH can lead to liver cirrhosis and liver cancer (9). MASLD is one of the cardiovascular risk factors, which also contributes to mortality in these patients (10). Both environmental and genetic factors are playing an important role in the development of MASLD and its progression. First-degree relatives of patients with MASLD are at higher risk than the general population (11). MASLD can affect people of any age, including children. About 10% of united states children ages 2 to 19 years have MASLD. However, MASLD may be found in people of any age (12).
Laboratory liver tests are defined as tests used in the evaluation of patients with hepatic dysfunction (13). ALT is found in the kidney, heart, muscle, and at a higher concentration in the liver compared with other tissues of the body (14). Marked elevations of ALT levels are observed most often in diseases that primarily affect hepatocytes, such as viral hepatitis, ischemic liver injury, and toxin-induced liver damage (15). AST is found in the highest concentration in the heart compared with other tissues of the body (14). Elevated mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction and also in chronic liver diseases (16).
Silymarin is a member of the Carduus marianum family that has been used for centuries for the treatment of different diseases (17). Silymarin has been used as a hepatoprotective drug. Studies in rodents have confirmed that silymarin has very low toxicity, which supports its history as a safe medication in hepatic diseases (18). Silymarin has antifibrotic, immunomodulating, anti-inflammatory effects, and anti-oxidant properties by scavenging free radicals and increasing the glutathione concentrations (19). Silymarin can be used in hepatitis and hepatic cirrhosis, and mushroom poisoning because of its action (20).
There no sufficient data regarding the effect of Silymarin on MASLD. Therefore, we performed this systemic review and meta-analysis to evaluate the effect of silymarin on biopsy-proven MASLD.
Methods
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21) and the guidelines reported in the Cochrane Handbook for Systematic Reviews of Interventions (22).
Literature search
We searched four databases: Web of Science, SCOPUS, Cochrane CENTRAL, and PubMed, from inception until October 2022. We followed this search strategy with no restriction on time or languages: (silymarin OR marianum OR legalon OR silibinin OR silybin) AND (steatohepatitis).
Eligibility criteria
We included all the studies that have the following criteria: (i) Population: patients with non-alcoholic fatty liver diseases, (ii) Intervention: Silymarin regardless of the dose and the mode of administration, (iii) Comparator: any control, (iv) Outcomes: Alanine transferase (IU/L) (ALT), aspartate transferase (AST) (IU/L), and γ-glutamyl transferase(GGT) (IU/L) as primary outcomes. The secondary outcomes was body mass index (BMI) (kg/m2), total cholesterol (TC) (mg/dL), triglyceride (TG) (mg/dL), high-density lipoprotein (HDL) (mg/dL), low-density lipoprotein (LDL) (mg/dL), and HOMA-IR. (v) Study design: We included clinical trials characteristics mentioned above. Our exclusion criteria were: (i) studies that did not report data or measures for our selected outcomes, or (ii) that with no available full-text.
Screening of results
We exported the results of the search into Endnote X8.0.1 (Build 1044), with the removal of duplicates automatically by computer. After that, we screened the studies manually in two steps; first, title and abstract screening, then full-text screening for the preliminary included studies in the first step.
Data extraction and analysis
After the screening step, we extracted the data from the selected studies and categorized the data into three main groups: (1) baseline and demographic data of patients in each study, including age, sample size, gender, BMI, levels of ALT, AST, GGT, TC, TG, HDL, LDL, and HOMA-IR score. (2) Data for analysis including outcome values of alanine aminotransferase (ALT) (IU/L), aspartate aminotransferase (AST) (IU/L), γ-glutamyl transferase (GGT) (IU/L), body mass index (BMI) (kg/m2), total cholesterol (mg/dL), triglyceride (mg/dL), HDL (mg/dL), LDL (mg/dL), degree of fibrosis resolution and HOMA-IR. In addition to the previous two categories, we extracted the data about the seven domains assessing the risk of bias according to Cochrane's risk of bias (23).
Data analysis
We used Review Manager Software (RevMan 5.4.1) to perform our analysis. We conducted the analysis of continuous outcomes using mean difference (MD) and standard deviations (SD), and relative 95% confidence interval (CI) under the Inverse variance method.
Quality assessment
We evaluated the quality of this systematic review and meta-analysis using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (24). According to the Cochrane risk of bias (ROB) tool for clinical trials, we performed the risk of bias (ROB) for the included studies. The tool depends on the following domains for assessment of the risk of bias: (1) proper randomization, (2) blinding allocation of the included patients into each group, (3) blinding of patients only (single-blinding), blinding of both personnel and participants (double-blinding), or not blinding at all, (4) attrition bias, (5) selection bias (outcomes reported matches with that of the protocol or not), (6) awareness of the outcome assessor (whether blinded or not), (7) other bias. We assessed the total risk of bias for the studies as well.
Results
Summary of included studies
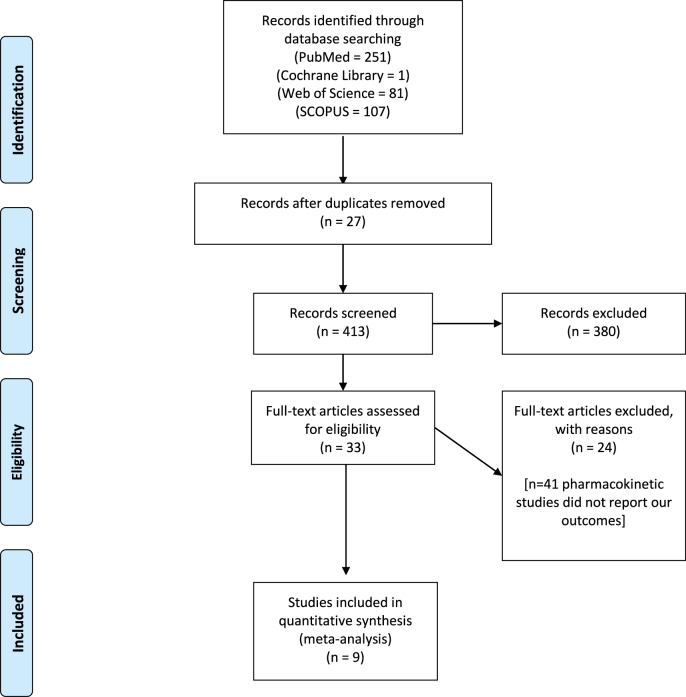
Figure 1 shows a PRISMA flow diagram of our literature search. In our study, we performed an analysis of 820 patients from nine studies (25–33). A total of 412 patients were allocated to receive silymarin, and 408 patients entered the control group. The mean age of the percipient in the treatment group was 44.8 ± 8.38 years, while that of the control group was 44.2 ± 8.09. The mean body mass index of the patients in the silymarin group was 27.888 ± 1.955, while that of the control group was 28.27 ± 2.815. Table 1–3 shows a detailed summary of the included participants, their demographic data, liver enzymes and complete lipid profile.
Figure 1:
PRISMA flow diagram of our literature search
Table 1:
Shows a detailed summary of the included participants, their demographic data. BMI, age, and male number
| Study ID | BMI (kg/m2) | Age (years) | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Number | ||
| Silymarin | Control | Silymarin | Control | Silymarin | Control | |||||
| Wah Kheong 2017 | 30 | 0.4 | 31 | 4.6 | 49.6 | 12.7 | 50.1 | 10.2 | 24 | 22 |
| Cacciapuoti 2013 | 26.7 | 1.67 | 26.7 | 1.67 | 44 | 3.2 | 44 | 3.2 | 40 | 40 |
| Hajiaghamohammadi 2012 | 27.44 | 1.65 | 27.44 | 1.65 | 32.62 | 6.4 | 32.62 | 6.4 | 14 | 14 |
| Hashemi 2009 | 26.75 | 2.65 | 27.8 | 3.75 | 39.28 | 11.117 | 39 | 10.7 | 28 | 29 |
| Masoodi 2013 | 29.04 | 3.66 | 29.18 | 3.32 | 48.42 | 6.75 | 48.32 | 5.45 | 31 | 31 |
| Jelodar 2015 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Solhi 2014 | 27.4 | 1.7 | 27.5 | 1.9 | 43.6 | 8.3 | 39.36 | 10.5 | 19 | 19 |
| Frățilă 2019 | NR | NR | NR | NR | NR | 10.2 | 56.08 | 10.2 | 25 | 25 |
| Taghvaei | NR | NR | NR | NR | NR | NR | NR | NR | 14 | 13 |
Data are represented as mean (SD). BMI = body mass index; NR = not reported.
Table 3:
Shows a detailed summary of the included participant's TC, TG, HDL, and LDL
| Study ID | Total cholesterol (TC) (mg/dL) | Triglyceride (TG) (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | MEAN | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| silymarin | control | silymarin | control | silymarin | control | silymarin | control | |||||||||
| Wah Kheong 2017 | 204.95 | 42.54 | 181.75 | 34.8 | 159.43 | 35.43 | 124 | 53.14 | 46.4 | 11.6 | 46.4 | 11.6 | 123.74 | 46.4 | 112.14 | 34.8 |
| Cacciapuoti 2013 | 205.7 | 9.3 | 205.7 | 9.3 | 178.4 | 4.1 | 178.4 | 4.1 | 43.6 | 2.1 | 43.6 | 2.1 | 157.4 | 4.3 | 157.4 | 4.3 |
| Hajiaghamohammadi 2012 | 191.68 | 33.81 | 191.68 | 33.81 | 254.18 | 56.04 | 254.18 | 56.04 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hashemi 2009 | 235.18 | 59.25 | 216.18 | 52.12 | 281.48 | 116.66 | 261.32 | 102.02 | 40.58 | 5.19 | 41.06 | 6 | 163.3 | 49.69 | 135.36 | 47.45 |
| Masoodi 2013 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Jelodar 2015 | 242.88 | 16.2 | 242.88 | 16.2 | 232.75 | 48.09 | 232.75 | 48.09 | NR | NR | NR | NR | NR | NR | NR | NR |
| Solhi 2014 | 195.7 | 34.4 | 193 | 35.7 | 252.2 | 52.8 | 248.4 | 53.2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Frățilă 2019 | 181.75 | 45.14 | 181.75 | 45.14 | 125 | 56.35 | 125 | 56.35 | NR | NR | NR | NR | NR | NR | NR | NR |
| Taghvaei | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Data are represented as mean(SD). HDL = high density lipoprotein; LDL = low density lipoprotein; TC = total cholesterol; TG = triglyceride; NR = not reported
Table 2:
Shows a detailed summary of the included participant's ALT, AST, GGT levels
| Study ID | Alanine aminotransferase (ALT) (IU/L) | Aspartate aminotransferase (AST) (IU/L) | GGT (U/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Silymarin | Control | Silymarin | Control | Silymarin | Control | |||||||
| Wah Kheong 2017 | 93.3 | 53.3 | 80.3 | 54.8 | 55.3 | 31.1 | 49 | 28.9 | 98.7 | 52.6 | 87.7 | 70.4 |
| Cacciapuoti 2013 | 109.48 | 4.4 | 109.48 | 4.4 | 72.39 | 8.4 | 72.39 | 8.4 | 45.51 | 1.2 | 45.51 | 1.2 |
| Hajiaghamohammadi 2012 | 78.73 | 19.71 | 78.37 | 19.71 | 56 | 11.07 | 56 | 11.07 | NR | NR | NR | NR |
| Hashemi 2009 | 113.54 | 50.92 | 104.54 | 41.82 | 71.42 | 66.5 | 73.02 | 39.62 | NR | NR | NR | NR |
| Masoodi 2013 | 84.06 | 6.65 | 74.48 | 4.45 | 71.94 | 4.56 | 62.94 | 4.45 | NR | NR | NR | NR |
| Jelodar 2015 | 52.12 | 6.46 | 52.12 | 6.46 | 48.73 | 7.09 | 48.73 | 7.09 | NR | NR | NR | NR |
| Solhi 2014 | 91.3 | 21.3 | 84.6 | 23.3 | 62.8 | 10.5 | 70.4 | 18.9 | NR | NR | NR | NR |
| Frățilă 2019 | 67.35 | 58.83 | 67.35 | 58.83 | 75.22 | 58.13 | 75.22 | 58.13 | 150.75 | 113.78 | 150.75 | 11378 |
| Taghvaei | 69.9 | 27.06 | 88.55 | 23.58 | 56.67 | 30.5 | 60.2 | 27.8 | NR | NR | NR | NR |
Data are represented as mean (SD). ALT = alanine aminotransferase, AST = aspartate aminotransferase, NR = not reported
Results of risk of bias assessment
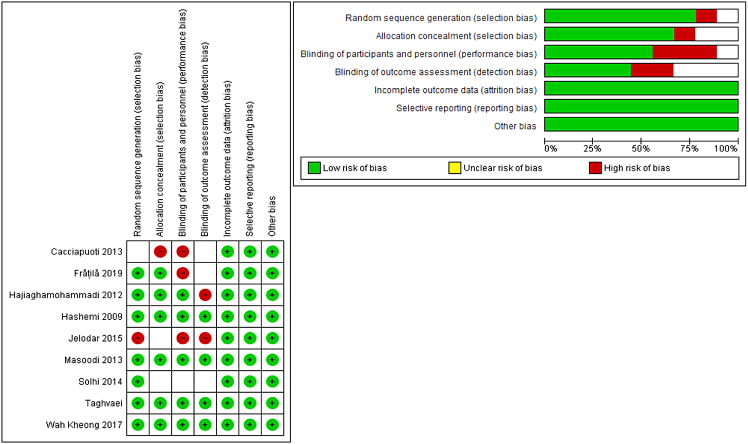
The result of the risk of bias assessments yielded an overall low risk of bias, according to Cochrane's tool. Regarding randomization, all studies were at low risk of randomization, except Kheong et al. and Masoodi et al. (25,30) were non-randomized trials. As for the allocation concealment, six studies (24,26–29,32) reported adequate allocation concealment; therefore, they were put to a low risk of bias. Two studies (25,31) did not report enough data about allocation concealment, thus put to an unclear risk of bias. One study (30) reported no allocation concealment. The majority of the included studies were blinded, and four studies (25,30–32) were not blinded to the participants and personnel. Four studies (24,26,27,29) were at low risk of blinding of outcome assessment. Two studies (25,28) were not blinded to outcome assessment, and three studies (30–32) did not report enough data about blinding of outcome assessment, thus put to an unclear risk of bias. The remaining domains of the Cochrane tool were all at low risk of bias. A summarized illustration of the risk of bias of included trials, Figure 2.
Figure 2:
Risk of bias graph and summary
Analysis of Outcomes
ALT
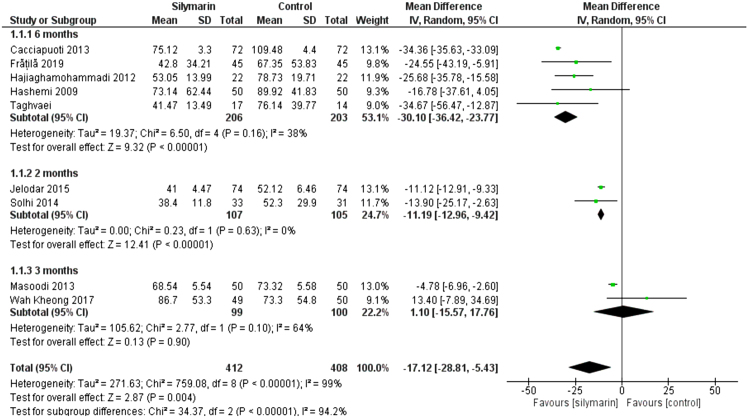
All studies (24–32) reported the ALT. The overall mean difference showed that there was a significant difference between both groups (MD= -17.12 [-28.81, -4.43]), (P < 0.004). Pooled analysis was heterogeneous (P < 0.00001); I2 = 99% as shown in Figure 3. We solved the heterogeneity by performing a subgroup analysis according to the duration of drug administration.
Figure 3:
Analysis of ALT outcome
The first subgroup (6 months) included five studies (26–28,30,32), the mean difference showed a significant difference between both groups (MD = −30.12 [−36.42, −23.77], (p < 0.01). Pooled analysis was homogeneous (p = 0.16); I2 = 38% as shown in Figure 3.
The second subgroup (2 months) included two studies (25,31) the mean difference showed that there was a significant difference between both groups (MD = −11.19 [−12.96, −9.42]), (p < 0.01). Pooled analysis was homogeneous (p = 0.63); I2 = 0% as shown in Figure 3C. The third subgroup (3 months) had two studies (24,29) the mean difference showed that there was no significant difference between both groups (MD = 1.10 [−15.57, 17.76]) (p < 0.90). Pooled analysis was homogeneous (p = 0.1); I2 = 64%, as shown in Figure 3.
AST
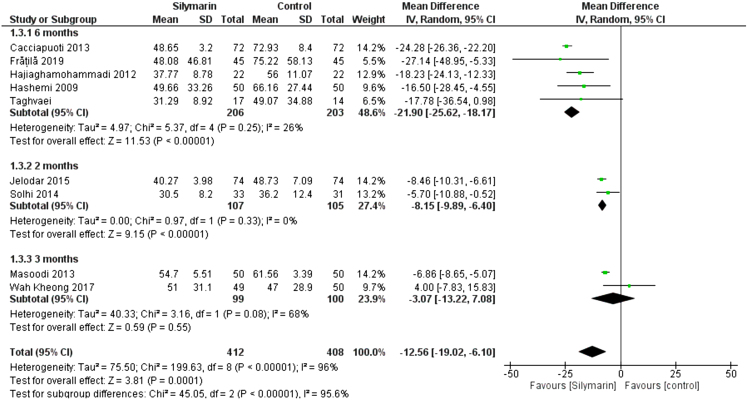
All studies (24–32) reported the AST. The overall mean difference showed that there was a significant difference between both groups (MD= -12.56 [-19.02, -6.10]), (P < 0.0001). Pooled analysis was heterogeneous (p < 0.00001); I2 = 96% as shown in Figure 4. We solved the heterogeneity by subgroup analysis according to the duration of drug administration. The first subgroup (6 months) contains five studies (26–28,30,32), the mean difference showed that there was a significant difference between both groups (MD = −21.90 [−25.62, −18.17]), (p < 0.00001). Pooled analysis was homogeneous (p = 0.25); I2 = 26% as shown in Figure 4. The second subgroup (2 months) contains two studies (25,31), the mean difference showed that there was a significant difference between both groups (MD = −8.15 [−9.89, −6.40]), (p < 0.00001). Pooled analysis was homogeneous (p = 0.33); I2 = 0% as shown in Figure 4. The third subgroup (3 months) contains two studies (24,29). The mean difference showed that there was no significant difference between both groups (MD = -3.07 [−13.22, −7.08]), (p = 0.55). Pooled analysis was heterogeneous (p = 0.08); I2 = 68%, as shown in Figure 4.
Figure 4:
Analysis of AST outcome
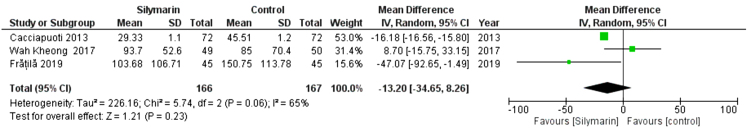
GGT
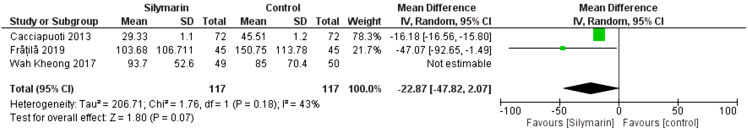
Three studies (29,30,32) reported the GGT. The overall mean difference showed that there was no significant difference between both groups (MD = −(MD= -13.20 [-34.65, - 80]), (P < 0.23). Pooled analysis was heterogeneous (p = 0.06; I2 = 65%) as shown in Figure 5A. We solved the heterogeneity by the exclusion of Wah Kheong 2017 et al. (29) (p = 0.18); I2 = 43%. The pooled analysis after exclusion showed that there was no significant difference between both groups (MD= -22.87 [-47.82, 2.07]) (P < 0. 07). Figure 5B illustrates the analysis after the exclusion of one study.
Figure 5A:
Analysis of GGT Outcome
Figure 5B:
Analysis of GGT Outcome – Rule one out analysis
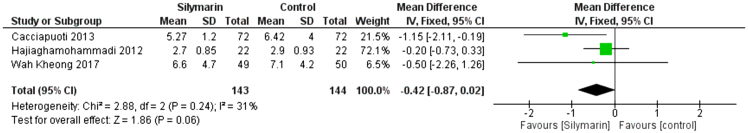
HOMA-IR
Three studies (28–30) reported the HOMA-IR score. The overall mean difference showed that there was no significant difference between both groups (MD= −0.42 [−0.87, 0.02]), (p = 0.06). Pooled analysis was homogeneous (p = 0.24); I2 = 31% as shown in Figure 6.
Figure 6:
Analysis of HOMA outcome
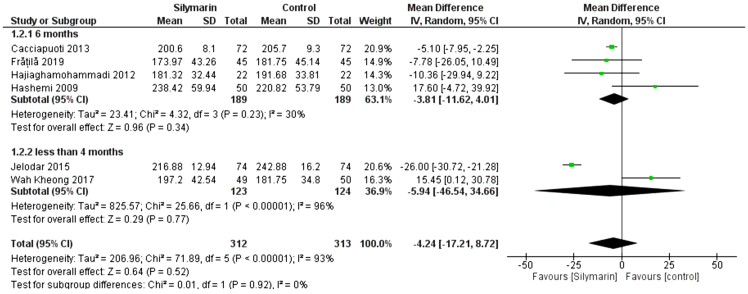
TC
Six studies (25,27–30,32) reported the TC. The overall mean difference showed that there was no significant difference between both groups (MD = −4.24 [−17.21, −8.72]), (p = 0.52). Pooled analysis was heterogeneous (p = 0.00001); I2 = 93% as shown in Figure 7. We solved the heterogeneity by subgroup analysis according to the duration of drug administration. The first subgroup (6 months) contains four studies (27,28,30,32). The mean difference showed that there was no significant difference between both groups (MD = −3.81 [−11.62, −4.01]), (p = 0.34). Pooled analysis was homogeneous (p = 0.22); I2 = 30% as shown in Figure 7. The second subgroup (less than 4 months) contains two studies (25,29). The mean difference showed that there was no significant difference between both groups (MD = −5.94 [−46.54, −34.66]), (p= 0.77). Pooled analysis was heterogeneous (p = 0.00001); I2 = 96% as shown in Figure 7.
Figure 7:
Analysis of TC outcome
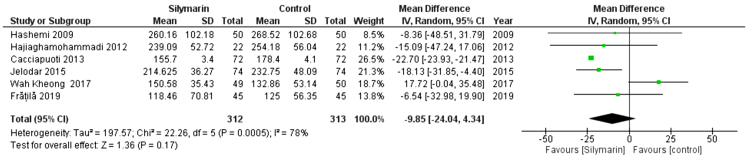
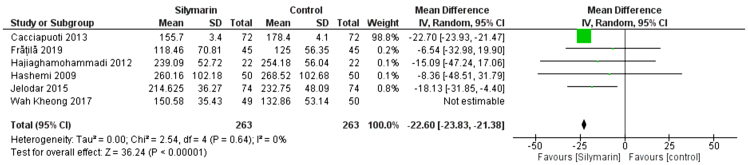
TG
Six studies (25,27–30,32) reported the TG. The overall mean difference showed that there was a non-significant difference between both groups (MD= -9.85 [-24.04, 19.90]), (P < 0.17). Pooled analysis was heterogeneous (P = 0.0005; I2 = 78%) as shown in Figure 8A. We solved the heterogeneity by the exclusion of Wah Kheong et al. (2017) (29) (p = 0.64); I2 = 0%. The pooled analysis after exclusion showed that there was a significant difference between both groups (MD = −22.60 [−23.83, −21.38]) (p < 0.00001). Figure 8B illustrates the analysis after the exclusion of one study.
Figure 8A:
Analysis of TG outcome
Figure 8B:
Analysis of TG outcome -Rule one out analysis
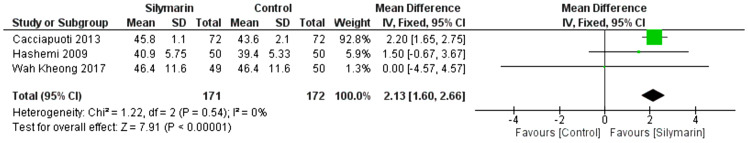
HDL
Three studies (27,29,30) reported the HDL. The overall mean difference showed that there was a significant difference between both groups (MD = 2.13 [1.60, 2.66]), (p < 0.01). Pooled analysis was homogeneous (p = 0.54); I2 = 0% as shown in Figure 9.
Figure 9:
Analysis of HDL outcome
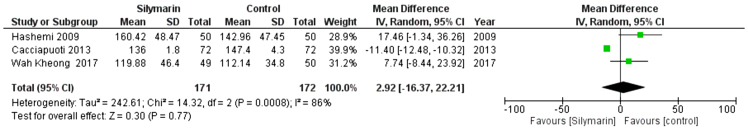
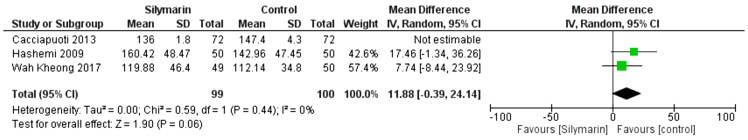
LDL
Three studies (27,29,30) reported the LDL. The overall mean difference showed that there was a non-significant difference between both groups (MD= -2.92 [-16.37, 22.21]), (P < 0.77). Pooled analysis was heterogeneous (p = 0.0008; I2 = 86%) as shown in Figure 10A. We solved the heterogeneity by the exclusion of Cacciapuoti et al. (30,31) (p = 0.44); I2 = 0%. The pooled analysis after exclusion showed that there was no significant difference between both groups (MD = 11.88 [−0.39, 24.14]) (p = 0.06). Figure 10B illustrates the analysis after the exclusion of one study.
Figure 10A:
Analysis of LDL outcome
Figure 10B:
Analysis of LDL outcome: Rule one out analysis
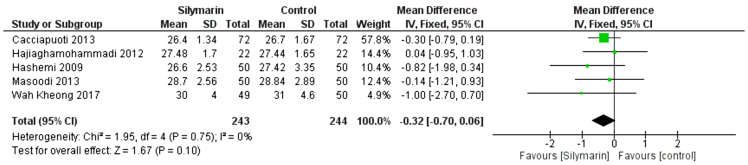
BMI
Five studies (24,27–30) reported the BMI. The overall mean difference of the BMI showed that there was no significant difference between either group (MD = −0.32 [−0.70, 0.06]), (p = 0.1). Pooled analysis was homogeneous (p = 0.75); I2 = 0% as shown in Figure 11.
Figure 11:
Analysis of BMI outcome
Degree of fibrosis resolution
Only one study (30) reported significant improvement in the degree of fibrosis resolution. Their results revealed that higher proportion of patients in the silymarin group had reductions in fibrosis based on histology (reductions of 1 point or more), (22.4%) than the placebo group (6.0%) (P = 0.023)
Discussion
In this meta-analysis, we included 412 patients treated with silymarin from nine clinical trials. We found that silymarin significantly reduced AST and ALT levels. The results also showed a significant reduction in the levels of triglyceride, and improvement in HDL. Subgroup analysis demonstrated that more duration of follow up was associated with more reduced levels of liver transaminases.
Overall, our analysis is more comprehensive and up to date than Kalopitas et al.'s study. It adds to the evidence for silymarin's positive effects on liver enzymes and metabolic variables in MASLD (34).
Elevated liver enzymes are the prevailing abnormality in patients with MASLD. MASLD is commonly associated with metabolic syndrome and insulin resistance, which in turn, may elevate the liver enzymes (35). Elevated transaminase levels correlate with the histological state of the liver. However, histological outcomes are relevant as biochemical outcomes in NAFLD for various reasons.
For this reason, liver enzymes could be a noninvasive clue to generalized liver injury. Silymarin, an insulin sensitizer, significantly reduced the liver enzymes (34). However, it had less significant results in restoring TG and TC levels back to normal. Recent trials performed by Kheong et al. (30), Cacciapuoti et al. (31), Hashemi et al. (28), Taghvaei et al. (27), Jelodar et al. (26), and Dahm et al. (24), found that silymarin showed a significant reduction in the levels of ALT and AST. Previous studies have already proved that reduction in transaminases is associated with reviving of liver function in MASLD patients (35,36). This also predicted a better prognosis and a lower incidence of progression to either liver cirrhosis or hepatocellular carcinoma. Silymarin was also associated with more reduction in ALT level than AST level because the original level of ALT was much higher than AST level in patients with MASLD.
A clinical review by Flora et al. (37) found that silymarin may be associated with a better clinical prognosis of both acute and chronic hepatitis, drugs, and alcohol-induced hepatitis. A trial by Kheong et al. (30) demonstrated similar results; they found that silymarin may be effective in reducing ultrasonographic changes and the biochemical induced by MASLD. A study by Loguercio et al. (38) found that silymarin added to vitamin E and phospholipids could be associated with improvement in the liver enzymes in patients with chronic liver damage.
Solhi et al. (32) proved that the combination of silymarin, Phyllanthus niruri, and choline was associated with better hepatoprotective effects than silymarin alone in hepatic disorders of different inflammation and destruction stages. This hepatoprotective combination had a smaller dose of silymarin with similar results, which may reduce the side effects produced by the drug. Hajiaghamohammadi et al. (29) found that silymarin 700 mg three times daily for 48 weeks, a well-tolerated and safe dose, was associated with greater fibrosis improvement compared with placebo. For this reason, silymarin seemed to be useful for fibrosis improvement in biopsy-proven NASH patients.
A trial by Masoodi et al. (25) found that silymarin extract in patients with MAFLD significantly reduced levels of plasma cholesterol, fasting glucose, triglyceride, AST, and ALT, while the plasma levels of folate and B12 did not change significantly. The major strength points of our analysis were that we only included clinical trials, which was an important strength point to ensure the highest evidence according to GRADE. Another strength point was that all the included studies showed low risk of bias. We also conducted the analysis of a good sample size. We tried to solve any inconsistency among studies using appropriate methodologies reported by the Cochrane's handbook (39) including mainly the leave-one-out and subgroup analysis.
Limitations and Recommendation
The heterogeneity in some outcomes was a major limitation; therefore, we tried to solve the heterogeneity by subgroup analysis. In addition, we have not registred a protocol for our study. Another limitation was that three of our included trials were not blinded, which might have affected the results of the analysis. We conclude that silymarin treatment seems to be significantly effective in the reduction of biochemical and transaminases levels in patients with MAFLD. In addition, a highly need for future well-designed studies to ensure our outcomes and to examine whether the reduction in transaminase levels corresponds to histological improvement.
Contributions:
N/A
Ethics Approval:
N/A
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Data Accessibility:
N/A
Funding:
N/A
Disclosures:
N/A
Peer Review:
This article has been peer reviewed.
Animal Studies:
N/A
References
- 1.Navarro VJ, Belle SH, D’Amato M, et al. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: a randomized, double-blind, placebo controlled trial. PLoS One. 2019;14(9):e0221683. 10.1371/journal.pone.0221683. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrieber SJ, Hawke RL, Wen Z, et al. Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos. 2011;39(12):2182–90. 10.1124/dmd.111.040212. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aller R, Izaola O, Gómez S, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19(16):3118–24. PMID: [PubMed] [Google Scholar]
- 4.Schiavo L, Busetto L, Cesaretti M, Zelber-Sagi S, Deutsch L, Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24(30):3330–46. 10.3748/wjg.v24.i30.3330. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De A, Bhagat N, Mehta M, Taneja S, Duseja A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J Hepatol. 2023:S0168-8278(23)05044–4. 10.1016/j.jhep.2023.07.031. PMID: [DOI] [PubMed] [Google Scholar]
- 6.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. 10.1038/s41572-018-0014-7. PMID: [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–74. 10.1111/j.1572-0241.1999.01377.x. PMID: [DOI] [PubMed] [Google Scholar]
- 8.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90(9):1233–46. 10.1016/j.mayocp.2015.06.013. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. 10.1002/hep.25762. PMID: [DOI] [PubMed] [Google Scholar]
- 10.Okosun IS, Chandra KMD, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960–2000. Prev Med. 2004;39(1):197–206. 10.1016/j.ypmed.2004.01.023. PMID: [DOI] [PubMed] [Google Scholar]
- 11.Townsend SA, Newsome PN. New treatments in non-alcoholic fatty liver disease. Aliment Pharmacol Therap. 2017;46(5):494–507. [DOI] [PubMed] [Google Scholar]
- 12.Pierantonelli I, Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103(1):e1–e13. 10.1097/TP.0000000000002480. PMID: [DOI] [PubMed] [Google Scholar]
- 13.Gowda S, Desai PB, Hull VV, Avinash AK, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. The Pan african medical journal. 2009; 3. Pan Afr Med J. 2009;3:17. PMID: [PMC free article] [PubMed] [Google Scholar]
- 14.Rifai N. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics-e-Book. Elsevier Health Sciences; 2017. [Google Scholar]
- 15.Kallai L, Hahn A, RÖDer V, ŽUpaniĆ V. Correlation between histological findings and serum Transaminase values in chronic diseases of the liver. Acta Med Scand. 1964;175:49–56. 10.1111/j.0954-6820.1964.tb00549.x. PMID: [DOI] [PubMed] [Google Scholar]
- 16.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74(7):663–71. 10.1007/s12098-007-0118-7. PMID: [DOI] [PubMed] [Google Scholar]
- 17.Křen V, Walterová D. Silybin and silymarin-new effects and applications. Biomed Papers. 2005;149(1):29–41. 10.5507/bp.2005.002. PMID: [DOI] [PubMed] [Google Scholar]
- 18.Hellerbrand C, Schattenberg JM, Peterburs P, Lechner A, Brignoli R. The potential of silymarin for the treatment of hepatic disorders. Clin Phytosci. 2016;2(1):7. 10.1186/s40816-016-0019-2 [DOI] [Google Scholar]
- 19.Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid Based Complement Alternat Med. 2005;2(3):383–6. 10.1093/ecam/neh103. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J-W, Lin L-C, Tsai T-H. Drug–drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol. 2009;121(2):185–93. 10.1016/j.jep.2008.10.036. PMID: [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane handbook for systematic reviews of interventions: Wiley; 2008. [Google Scholar]
- 23.Munder T, Barth J. Cochrane's risk of bias tool in the context of psychotherapy outcome research. Psychother Res. 2018;28(3):347–55. 10.1080/10503307.2017.1411628. PMID: [DOI] [PubMed] [Google Scholar]
- 24.Dahm P, Cleveland B, Lauwagie A, Gonzalez-Padilla DA. Adherence of the European Association of Urology Guidelines to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. Eur Urol. 2023:S0302-2838(23)02632–5. 10.1016/j.eururo.2023.02.023. PMID: [DOI] [PubMed] [Google Scholar]
- 25.Masoodi M, Rezadoost A, Panahian M, Vojdanian M. Effects of silymarin on reducing liver aminotransferases in patients with nonalcoholic fatty liver diseases. Govaresh. 2013;18(3):181–5. [Google Scholar]
- 26.Jelodar G, Rafiee B, Moosavi SH. Silymarine extract improved plasma homocysteine, lipids and liver enzymes in hyperhomocysteinemic non-alcoholic steatohepatitis. Physiol Pharmacol. 2015;19(2):139–45. [Google Scholar]
- 27.Taghvaei T, Bahar A, Hosseini V, Maleki I, Kasrai M. Efficacy of silymarin on treatment of nonalcoholic steatohepatitis. J Mazandaran Univ Med Sci. 2013;22(98):164–71. [Google Scholar]
- 28.Hashemi SJ, Hajiani E, Heydari SE. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. 2009;9(4):265–70. [Google Scholar]
- 29.Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12(8):e6099. 10.5812/hepatmon.6099. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kheong CW, Mustapha NRN, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940–9. 10.1016/j.cgh.2017.04.016. PMID: [DOI] [PubMed] [Google Scholar]
- 31.Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5(3):109–13. 10.4254/wjh.v5.i3.109. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solhi H, Ghahremani R, Kazemifar AM, Yazdi ZH. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med. 2014;5(1):9–12. PMID: [PMC free article] [PubMed] [Google Scholar]
- 33.Frățilă O, Mihele AI, Hodisan-Pap EF, Hocopan SC, Brata R, Iliaş T. Comparative hepatoprotective efficacy of silymarin-phyllanthus-choline combination versus silymarin alone in liver diseases with different destruction and inflammation stages. Farmacia. 2020;68(2):299–306. [Google Scholar]
- 34.Kalopitas G, Antza C, Doundoulakis I, et al. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutrition. 2021;83:111092. 10.1016/j.nut.2020.111092. PMID: [DOI] [PubMed] [Google Scholar]
- 35.Hajagha MA, Ziaei A, Rafiei R. The efficacy of silymarin in decreasing transaminase activities in non-alcoholic fatty liver disease: a randomized controlled clinical trial. Hepat Mon. 2008;8(3):191–5. [Google Scholar]
- 36.Velussi M, Cernigoi AM, Ariella DM, Dapas F, Caffau C, Zilli M. Long-term (23 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26(4):871–9. 10.1016/s0168-8278(97)80255-3. PMID: [DOI] [PubMed] [Google Scholar]
- 37.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139–43. 10.1111/j.1572-0241.1998.00139.x. PMID: [DOI] [PubMed] [Google Scholar]
- 38.Loguercio C, Federico A, Trappoliere M, et al. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52(9):2387–95. 10.1007/s10620-006-9703-2. PMID: [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A