Abstract
Background:
Nutrition and exercise are the mainstay of therapy for the prevention and treatment of frailty in cirrhosis. This pilot study assessed feasibility of the online delivery of an app-based semi-supervised nutrition and exercise intervention in this population.
Methods:
The 11-week pilot recruited adults with cirrhosis who owned internet-connected devices. Patients were encouraged to participate in exercise sessions 3× per week including a combination of online group exercise (weekly) and home-based follow-along exercise (biweekly). They also participated in group nutrition classes (five sessions) and one-to-one exercise and nutrition check-ins delivered through the app. Primary outcome measures pertained to program feasibility: recruitment, retention, adherence, and satisfaction. Exploratory measures included physical performance (liver frailty index [LFI], 6-minute walk test [6MWT]), health behaviour domains, and quality of life.
Results:
Twenty three patients completed baseline measures. Of these, 18 (72%) completed end of study measures (mean MELD-Na, 9.2; female, 44.4%). Over 70% of participants fulfilled 75% or more of the feasibility criteria. Satisfaction with the program was high (mean, 89%). Exercise program modifications were required for 17 patients to accommodate health events or abilities. Exploratory evaluation showed improvement in the LFI and the 6MWT by −0.58-units (95% CI: −0.91 to −0.25) and 46.0 m (95% CI: 22.7–69.3) respectively without changes in quality of life or health behaviour domains.
Conclusions:
Outcomes demonstrate feasibility of the app-based delivery of programming with promising exploratory impact on efficacy for physical performance. Findings can guide the design of a large-scale app-based randomized controlled trials in cirrhosis.
Keywords: liver disease, eHealth, self-management, semi-supervised
Lay summary: Nutrition and exercise are important for patient self-management in cirrhosis. We developed an online program to support these health behaviour changes using a combination of independent and group activities all of which were guided by health care experts. At the end of the 12-weeks, the program was acceptable to the 18 patients who were adherent. Physical improvements were also reported.
Introduction
Frailty in cirrhosis has been associated with worse health outcomes and increased mortality (1,2). Guideline based nutrition and exercise can modify physical frailty, especially in patients who are adherent to programming (3–5). Supervised, in-facility programs are generally associated with higher and more consistent rates of adherence to exercise relative to semi-supervised or unsupervised home-based programs 96 (6) both in cirrhosis (4,7) and in the general population (8). However, the need to travel to a facility presents several challenges for patients (e.g., transportation, location proximity, scheduling) (9), and can limit spread and scalability.
Application (app)-based, semi-supervised programs are a hybrid between on-site supervised and home-based unsupervised programming. This blended delivery has the potential to combine “the best of both worlds” with respect to patient convenience and access to expert-led programming and monitoring (3,4). The digital approach to program delivery has continued to gain traction, supported by the rapid adoption of virtual health in response to COVID-19 (10) and the widespread use of internet-connected digital technology (e.g., ∼310 million smartphone users in North America in 2021 (11)). The feasibility of an unsupervised, self-management app in cirrhosis has been demonstrated with the El-Fit app (3). The 25 patients enrolled accessed all the app's components, found areas for improvement, and most (85%) exceeded assigned daily step goals for one-third of the study. However, little is known about the feasibility of a semi-supervised delivery of app-based programming or impact on frailty.
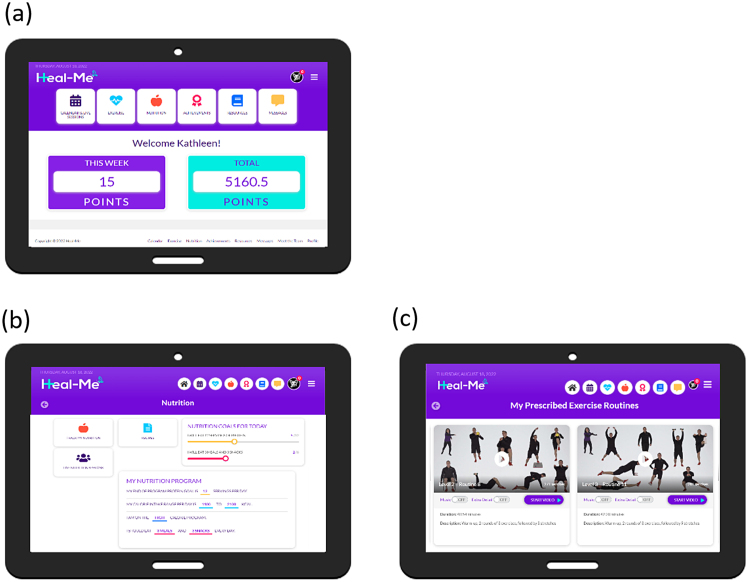
The Healthy Eating, Active Living - Mindful Energy (Heal-Me) app (Figure 1) was co-designed with patients and health care practitioners to deliver tailorable nutrition and exercise programs (12). The app supports interaction with health-care providers (e.g., dietitians, kinesiologists) via messaging and videoconferencing and hosts nutrition and exercise resources (e.g., high protein and low sodium recipes), and short instructional exercise videos.
Figure 1:
Heal-Me cirrhosis screenshots: (a) home page; (b) nutrition main page; (c) follow along exercise videos
Given the low rates of adherence seen in online exercise interventions in cirrhosis (4), and as a precursor to larger-scale prehabilitation RCTs, we conducted an open-label feasibility study to assess acceptability and feasibility of this intervention. For this 11-week study, we hypothesized: (i) ≥80% would find the nutrition and exercise activities acceptable, (ii) ≥70% of participants would adhere to 75% or more of the 5 nutrition and 10 exercise group sessions and (iii) ≥70% of patients would reach study completion at 11 weeks. The number of times a patient's exercise program was modified due to health changes or abilities was also recorded.
Methods
Setting
Eligible participants were recruited from outpatient hepatology clinics at two quaternary care hospitals in Edmonton, Alberta (ethics approval Pro00087451, ClinicalTrials.gov #NCT05033327).
Sample
Consecutive adults (≥18 years) were enrolled if they had: cirrhosis based on compatible imaging findings, medical history, or liver biopsy; MELD-Na <26; on guideline-based prophylaxis for high-risk gastroesophageal varices as required (13); English language proficiency; access to an internet-connected digital device in their homes; provided written informed consent. Ineligibility criteria: hepatocellular carcinoma (HCC) beyond the local liver transplant listing criteria (14); receiving treatment for non-HCC malignancy; end-stage renal disease on dialysis; liver transplant recipient; goals of care compassionate level (comfort care).
Intervention
The online nutrition and exercise interventions are summarized in Table 1 and the supplementary text. Targets adhered to guidelines (15). Briefly, for exercise, participants were asked to carry out three exercise sessions (one virtual group class + two follow along home exercise videos) per week and were encouraged to attend a weekly 10 minute 1-to-1 check-in with the exercise specialist to discuss exercise programming progress and any required modifications. For nutrition, participants were provided with a protein target (1.2–1.5 g/kg per day), were asked to attend five virtual nutrition group classes (topics in Table 1) and encouraged to attend 3x 30 minute 1-to-1 check-ins with the dietitian to discuss progress and modifications.
Table 1:
Overview of the program's content delivery for group classes (live stream video) and independent activities (data entry for tracking or videos)
| Study week | Nutrition (weeks 1–11) | Exercise (weeks 2–11) | ||||
|---|---|---|---|---|---|---|
Group classes—topics
|
Independent activities
|
Check ins* | Group classes
|
Independent activities
|
Check ins* | |
| 1 | Nutrition in cirrhosis | Protein tracking 3 days per week Access to high protein recipes, general cirrhosis nutrition education videos, and cooking videos. *3 x 30 min check-ins with program dietitian |
No exercise programming | |||
| 2 | X1 |
60-minute live exercise sessions once per week. Progress weekly with tailoring at the individual level as required. |
45–60 minute videos, twice per week. Progress weekly with tailoring at the individual level as required. *10 x 10 min check-ins with program exercise specialist |
X1 | ||
| 3 | X1 | |||||
| 4 | Plant-based proteins | X1 | ||||
| 5 | X1 | |||||
| 6 | Dairy proteins & protein-packed-drinks | X1 | X1 | |||
| 7 | X1 | |||||
| 8 | Sports nutrition | X1 | ||||
| 9 | X1 | |||||
| 10 | 4 Ps of healthy eating & reinventing leftovers | X1 | X1 | |||
| 11 | X1 | |||||
Completed as necessary.
Design and study flow
Following screening for eligibility and receipt of informed consent, in week 0 participants were sent online surveys via REDCap (16) and completed an in-person visit to screen for medical safety-to-exercise and carry out baseline physical function testing (Figure 2). They also participated in 20- to 60-minute app-onboarding videoconference sessions where they received an app walkthrough with training, a nutrition consultation, and safety assessment of their home workout space. The surveys and in-person visit were repeated at end of study (week 11). Participants started their nutrition programs during week 1 and added on their exercise programs in week 2.
Figure 2:
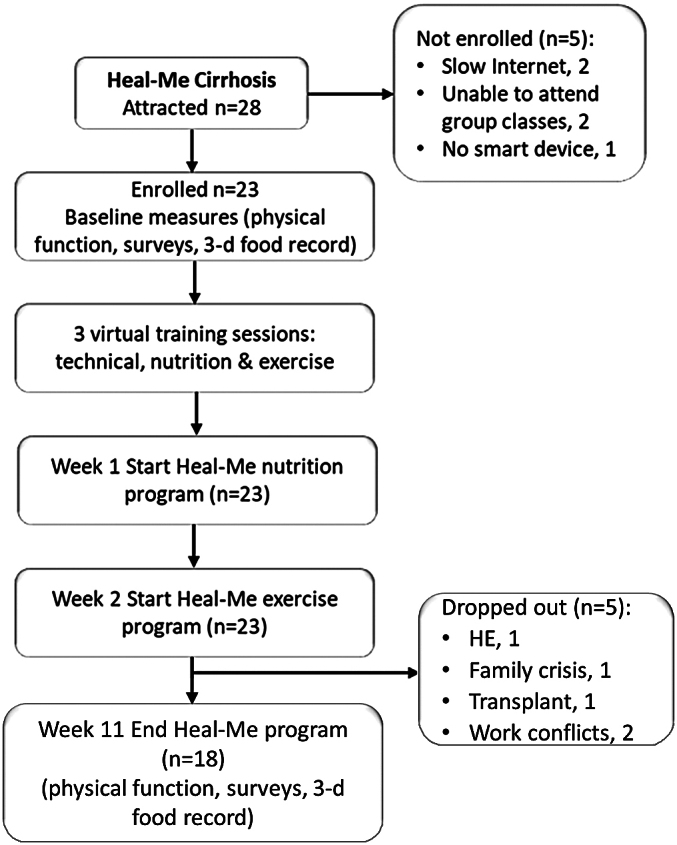
Study flow diagram
Measures
At baseline, participant characteristics were collected to describe the cohort (medical history, age, biological sex, socioeconomic information, and education). The Charlson Comorbidity Index was scored as described (17). The participant's proficiency with internet-connected digital technology was assessed for the device they most frequently used (18,19). Movement and capacity for exercise was assessed by the Physical Activity Readiness Questionnaires (PAR-Q+) (20). At 0 and 11 weeks, survey data were collected: 6-item COM-B (21) capturing behavioural information, quality of life (chronic liver disease questionnaire (CLDQ)) (22), EuroQol EQ-5D-5L, and visual analogue scale (VAS) (23). The EncephalApp Stroop test (24) and Animal Naming Test (25) tested for CHE. Three-day food records were analyzed using FoodProcessor® v11.7.1 (ESHA Research, OR, USA). In-person measures collected at 0 and 11 weeks were: weight and height (BMI), liver frailty index (LFI) (2), and 6-minute walk test (6MWT) (26). For the LFI, changes of 0.2 or 0.5 have indicated moderate or substantial clinical important differences, respectively (27). An activity tracker (Garmin Ltd., KS) was provided for feasibility of use. Program satisfaction was measured with questions, answered with a 0–10 Likert scale, at the end of the study.
Data analysis
Measures of study retention (target: ≥70% at 11 weeks), program adherence (target: ≥70% complete ≥75% of group classes, protein entry, and follow-along exercise videos), and satisfaction (target: ≥80% moderately or very satisfied) were collected. Descriptive statistics (e.g., means, standard deviations [SD], frequencies) were reported for all variables using SPSS v.20 (SPSS Inc., Il). For exploratory outcomes, paired sample t-tests (e.g., EQ-VAS), McNemar's test, or Wilcoxon signed rank test (COM-B) were used depending upon the type of variable and its distribution; Cohen's effect size was determined and 95% confidence intervals (CI) were reported instead of p-values given the exploratory nature. Analyses included correlations for parametric data (Pearson's r), Kendall's tau-b (τb) for non-parametric data. All coauthors had access to the study data and had reviewed and approved the final manuscript.
Results
Patient characteristics
Of 28 patients who expressed interest, 23 were enrolled within 3 months (Table 1) and all fulfilled PARQ+ criteria. Ten patients were clinically compensated while eight were decompensated (Table 2). All but two had a BMI in the overweight or obesity categories (mean BMI 32.1 SD 6.0 kg/m2). As measured by the digital technology proficiency questionnaires, the group had a median score of 96.8% (IQR: 81.3%, 100.0%). Three participants had scores below 70.0%. A total of 55.6% had post-secondary schooling. Household incomes ranged from $20,000 to >$100,000; two and eight participants came from households with incomes less than $39,999 (11.1%) and more than $80,000 (44.4%), respectively.
Table 2:
Participant (n = 18) characteristics
| Characteristics | Mean (SD) or proportion |
|---|---|
| Age (years) | 59.8 (11.8), range: 30.0–79.0 |
| Female sex, n (%) | 8 (44.4%) |
| Cirrhosis etiology, n (%): | |
| • NAFLD | 10 (55.6%) |
| • Alcohol related | 5 (27.9%) |
| • Autoimmune hepatitis | 1 (5.5%) |
| • Primary biliary cholangitis | 1 (5.5%) |
| • Hepatitis C | 1 (5.5%) |
| MELD-Na | 9.2 (2.3) |
| Child-Pugh A: B, n (%) | 12 (66.7%): 6 (33.3%) |
| Listed for liver transplant, n (%) | 2 (11.1%) |
| History of decompensation events or HCC: | |
| • Ascites, n (%) | 6 (33.3%) |
| • Overt HE requiring medication | 2 (11.1%) |
| • Variceal bleed | 2 (11.1%) |
| • HCC, n (%) | 1 (5.5%) |
| Charlson Comorbidity Index | 3.8 (2.2) |
| Device proficiency (CPQ or MDPQ) | 87.2% (19.0%) |
| Had post-secondary schooling, n (%) | 10 (55.6%) |
| Employed, full or part-time, n (%) | 9 (50%) |
| Household income ≥$80,000, n (%) | 8 (44.4%) |
| Marital status: | |
| • Married/common law, n (%) | 72.3%) |
| • Separated/divorced, n (%) | 5 (27.7%) |
CPQ = Computer Proficiency Questionnaire; COPD = chronic obstructive pulmonary disease; HCC = hepatocellular carcinoma; HE = hepatic encephalopathy; MDPQ = Mobile Device Proficiency Questionnaire; MELD-Na = model for end-stage liver disease—sodium
Primary study outcomes
Satisfaction
Participant (n = 17) satisfaction with the program was high with an average score of 89.2% (SD 11.2%). Most thought the extent of interaction with the trainers was satisfactory; 4 of 17 (24%) preferred fewer interactions. Time spent on program activities (4–5 hours per week) was acceptable to most, five reported it was too intensive, and two thought it was too little. Enjoyability of the nutrition and exercise programs were rated as either “quite a bit” or “very much“ by 100%.
Patient adherence and retention
The lowest rate of adherence was recorded for the follow along home exercise videos—10 (55.6%) participants completed ≥75% workout videos. Several participants experienced unrelated health events (i.e., broken foot, hospitalization for demand ischemia due to anemia, and pre-existing mobility issues) which disrupted program adherence for at least 3 or more days. A “one-size-fits-all” program was impossible, and, for 17 patients, the kinesiologist had to adjust the routines at least once during the 11-weeks to meet changing abilities. There were weak correlations between digital technology proficiency (CPQ/MDPQ scores) and adherence for group classes (τb = −0.14, p = 0.51), protein entry (τb = −0.17, p = 0.42), and follow-along exercise videos (τb = 0.09, p = 0.67).
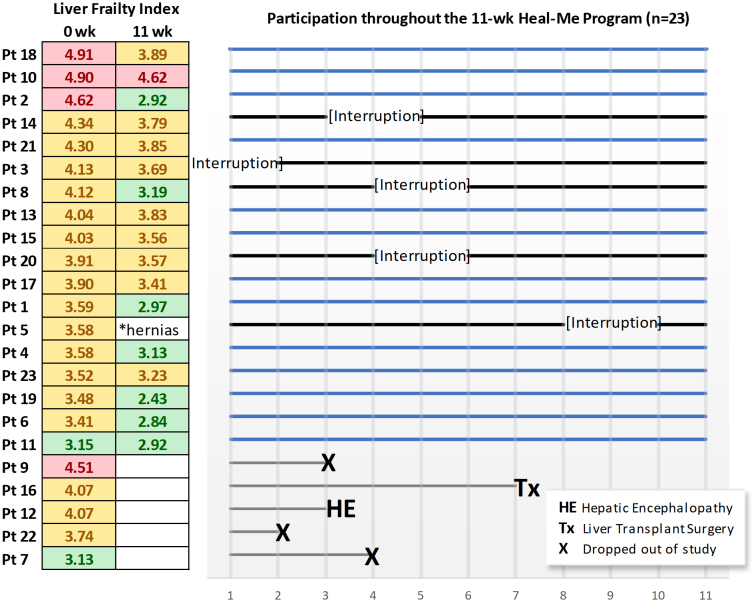
During the 11-week intervention, five participants dropped out: new hepatic encephalopathy (n = 1), time constraints (n = 2), family emergency (n = 1), and liver transplant (n = 1) (Figure 3). For the 18 remaining participants, 16 (88.9%) and 13 (72.2%) attended ≥75% of the group nutrition or exercise classes, respectively. Most participants (72.2%) entered their protein intake on the platform for ≥3 days per week for 11 weeks.
Figure 3:
Patient progression throughout the study. LFI score for robust <3.2, green; pre-frail 3.2–4.4, yellow; and frail >4.4. Solid blue lines indicate no study interruption
Exploratory study outcomes
Quality of Life: Although not powered for exploratory outcomes, the Heal-Me program did not have a significant impact on scores for the CLDQ, EQ-5D-5L subdomains, and EQ-VAS measures (Table 3).
Table 3:
Participant (n = 18) measures at baseline (0 week) and end of study (11 weeks)
| Characteristics | 0 week (SD) | 11 weeks (SD) | Mean difference (95%CI), Cohen's d |
|---|---|---|---|
| CLDQ | 4.46 (1.34) | 4.52 (1.28) | 0.05 (−0.26, 0.36), 0.09 |
| EQ-5D-5L index | 0.79 (0.15) | 0.79 (0.13) | 0.01 (−0.04, 0.05), 0.06 |
| EQ-5D VAS | 69.8 (16.50) | 71.6 (14.21) | 1.83 (−6.31, 9.98), 0.11 |
| LFI (n = 17)* | 3.98 (0.51) | 3.40 (0.53) | −0.58 (−0.91, −0.25), -0.91 |
| 6MWT (n = 17)† | 414.1 m (82.6) | 460.1 m (88.4) | 46.0 (22.7, 69.3), 1.01 |
| Daily step counts (n = 13) | 5,911 (4,329) | 5,242 (874) | −670 (−1,723, 384), -0.38 |
One participant excluded because of two inguinal hernias
One participant excluded due to mobility issues
LFI: Pre- and post-study LFI data were available in 17 of the 18 participants as one participant experienced had hernia pain at the end of the study and was unable to complete the chair sit-to-stand. If the participant was excluded from the analysis, the mean change in LFI was −0.58 (95% CI: -0.91, -0.25) and the proportion of patients with 0.2- and 0.5-unit changes in LFI were 10 (58.9%) and 7 (38.9%), respectively. Similar results were seen if the participant's baseline LFI was used as the end of study result (i.e., last observation carried forward), with a significant mean change in LFI at −0.56-units (95% CI: −0.23, −0.86). In Figure 3, the change in LFI had a strong positive association with the adherence rate of online group sessions (τb = 0.59, p = 0.004).
6MWT: Eighteen patients did not have a significant change in the distance walked (Table 3). However, the ambulation of one patient changed at the end of the study due to an arthritic flare in both knees and was unable to complete the test (0 m). If this patient was excluded, the mean difference was 46.0 m (95% CI: 22.7, 69.3). Using the last observation carried forward for the patient, the 6MWT mean difference was 41.3 m (95% CI: 20.9, 66.0). No association was found between the change in distance walked with adherence.
Nutrition: Of the 12 participants who provided a 3-day food record, all were incomplete and required follow-up calls. There was a trend (p = 0.054) to increased daily protein content reported between weeks 0 and 11 (74 g/d (SD 21.1) vs. 96.4 g/d (SD 30.8)). Three (25%) patients met or exceeded their daily protein goal of 1.2–1.5 g/kg body weight at baseline. By week 11, this had increased to 8 (67%) patients at week 11. None decreased their protein intake.
CHE: According to the EncephalApp Stroop test, nine patients tested positive for CHE at baseline versus eight patients at the end of study. The animal naming test identified one patient with CHE at baseline and none at end of study. At baseline, patients said an average of 23.1 (SD 6.2) animal names in 1 minute; at the end of the study, this changed to 22.2 (SD 5.3) names (mean difference was −0.94 (95% CI: −3.3, 1.4). Significant correlations were observed for the baseline scores between the EncephalApp Stroop test and the LFI (r = 0.535, p = 0.027) for 17 participants; one was excluded due to color blindness. Similarly, the baseline animal naming test values correlated with LFI scores (r = 0.666, p = 0.003). However, no correlations were found between measures of covert HE and digital technology proficiency, program adherence, or the ability to use the activity trackers.
COM-B: The COM-B constructs for nutrition or exercise did not change significantly by subdomains nor were they correlated with either the change in protein intake or LFI.
Activity tracker: Three patients did not want to use the study's activity tracker in preference of their own devices. Two patients could not get the activity tracker to connect to their home networks. The remaining 13 of 20 (65%) patients successfully created app accounts and synced the activity trackers with assistance from study staff. Within this group, two patients had issues with the wrist band sizing and two others could not read the screens. No significant correlations were found between the successful set up of the wearable and either baseline Stroop EncephalApp test or technology proficiency.
Discussion
In this feasibility study of an 11-week online nutrition and exercise program in cirrhosis, we present data on satisfaction, retention, and adherence. Patient satisfaction exceeded targets, and rates of retention and adherence also fulfilled a priori thresholds. In the cirrhosis population, the rapidity of study recruitment reflected a high interest for adjunctive non-pharmaceutical approaches to therapy as well as the desire to learn more about their condition. Throughout the study, moderate to high levels of support were required by the participants, primarily to adjust programming in response to unexpected health changes. The overall results demonstrate the feasibility of online, semi-supervised, interventions, such as Heal-Me, with exploratory efficacy data suggesting promising impact on functional outcomes.
Program adherence is the foundation to any behaviour change program (5). By investigating adherence to discrete program components, adherence was highest for supervised group exercise and nutrition classes. This finding is consistent with another study where healthy older adults commented that instructor-led Zoom sessions alleviated boredom and they appreciated receiving comments in real time (28). Specifically in cirrhosis, adherence rates for exercise programming have varied considerably (14% (4) to 100% (29)) over varying program durations between 8 and 14 weeks (30). A review of non-cirrhosis unsupervised apps (n = 99) for self-management reported user adherence rates of 56.0% (SD 24.4%) over an average of 111.4 days (31). More information is required about strategies to optimize adherence in this population. Given our findings and the findings of others, future interventions could consider emphasizing group-based, supervised programming.
Despite substantial support available from study personnel (exercise specialist: 10x 1-to-1 sessions; dietitian: 3x 1-to-1 sessions), and that 2/3 of our patients were clinically compensated, the attrition or loss to follow-up rate was 21.7%. There was also frequent health issues (7/23 or 30.4%) leading to program interruption and adjustment of the exercise prescription. Attrition rates in exercise RCTs in cirrhosis have ranged from 5% to 36% (30). These findings reinforce that compensated status alone does not negate the presence of additional comorbidities and thereby does not equate to low support needs during lifestyle programming. Given the extent of tailoring required, we would propose that “one size of programming does not fit all” in cirrhosis. Future studies may find this information useful to adjust sample size calculations for anticipated attrition as well as to ensure adequate staffing is available to accommodate tailoring of the exercise and nutrition interventions.
Overall, the participants found the Heal-Me app relatively easy to use, but there were some challenges with the activity tracker requiring the assistance of care partners or the study team. Covert HE was not associated with factors influencing adherence or technology proficiency. A systematic review of 24 studies similarly found that cognitive dysfunction in adults without cirrhosis did not prevent them from learning new apps (32). Regarding the difficulties with the trackers in our small sample, data showed that covert HE did not appear to be involved. In two published cirrhosis studies, 84% (3) and 81% 94 (4) were able to use activity trackers. The latter study however commented that patients “faced major challenges to the logistics of using personal activity trackers,” and that “management of the personal activity tracker was time-consuming and labor-intensive” (4). In follow on work, it is advisable that study staff are aware that additional support may be required for the successful set up and synchronization of these devices.
The feasibility of a 3-day food record was low; 12 patients submitted records at baseline and end-of study and all were incomplete. While a well-recognized measure of assessing food intake, 3-day food records are subject to bias, are time consuming to complete, require formal instruction, and attention to detail (33). With the reliance on memory recall, studies evaluating food-intake have often excluded people with cognitive impairment (34,35). Recognizing discrepancies in food and portion recall, food frequency questionnaires and nutrient biomarkers provide a more complete consumptive history (36). Potentially viable options for future studies include the use of a 24-hour food recall and other formats such as food photography (37). Nutrition guidelines in dementia (38) suggest that care partners often play a large role in the patient's nutrition and, in some cases, they may be best suited to complete the food surveys.
It is of interest that both a clinically and statistically significant change was seen in the LFI and the 6MWT. For the LFI, changes of 0.2 or 0.5 indicate moderate or substantial clinical important differences, respectively (27). This is relevant as frailty is one of the multiple factors that may impact a patient's ability to be listed for liver transplantation (15). In the largest RCT of exercise in cirrhosis, there was no significant difference seen in the LFI in the control group over 12 weeks (4). The change of almost 0.6 for the LFI seen in our exploratory analysis is clinically meaningful and should be evaluated in a larger RCT.
Behavioural constructs are relevant both for intervention design as well as understanding why an intervention did or did not work (5). Here, the COM-B assessed if the intervention influenced behavioral domains of capability, opportunity, and motivation. While objective measures showed significant differences, the nutrition and exercise results for the COM-B (39) questionnaire did not change. Likely, the study was underpowered to detect this change or, as previously identified, the baseline survey captured the participants’ optimism before exposure to the intervention (40). To explore the contribution of behavioral mediators to adherence and outcomes, future work may benefit from assessing COM-B constructs at multiple time points.
Limitations
Exploratory outcomes of effectiveness were underpowered and uncontrolled, and provided preliminary signals to guide the decision to advance to future RCTs. Other limitations included the intentional exclusion of patients without internet-connected digital devices which limits generalizability to this population. Ideally, future initiatives could make provisions for devices, Internet access, and device training to address the digital divide (41). Alternatives to the 3-day food record may need to be considered for future studies if a more complete estimate of nutrition intake is desired. User experience issues were identified, such as app-based nutrition entry tasks and issues with the wearable activity trackers that can be addressed.
In conclusion, this app-based semi-supervised program was feasible and acceptable to patients. Exploratory outcomes show promising improvements in physical frailty measures and protein intake. Future research in the area can build off these findings to further enhance acceptability and definitively assess efficacy, moving us closer to a time to where all patients with cirrhosis have access to lifestyle therapies.
Funding Statement
This study was funded by the Canadian Institutes of Health Research (app development 155456 and 164994), the Alberta Collaboration of Nutrition in Digestive Disease, Canadian National Transplant Research Program, and Nutricia Research Foundation. KPI was supported by Mitacs and unrestricted educational support from Lupin Pharma Canada and Astellas Canada.
Acknowledgements
The authors would like to acknowledge and thank Ben Vandermeer of the Alberta SPOR Support Unit Consultation and Research Services for his contribution to the biostatistics analysis and support for this study. Participant clinical measures were completed at the Human Nutrition Research Unit, Department of Agricultural, Food and Nutritional Science at the University of Alberta.
Contributions:
Conceptualization, P Tandon, ML McNeely, KP Ismond; Data curation, P Tandon, KP Ismond; Funding acquisition, P Tandon, ML McNeely, KP Ismond; Investigation, KP Ismond, C Cruz, ATL Miro; Methodology, P Tandon, ML McNeely, KP Ismond; Writing – original draft, KP Ismond; Writing – review & editing, all authors.
Ethics Approval:
Ethical approval was required for this article (Pro00087451).
Registry and the Registration No. of the Study/Trial:
ClinicalTrials.gov identifier: NCT05033327
Funding:
This study was funded by the Canadian Institutes of Health Research (app development 155456 and 164994), the Alberta Collaboration of Nutrition in Digestive Disease, Canadian National Transplant Research Program, and Nutricia Research Foundation. KPI was supported by Mitacs and unrestricted educational support from Lupin Pharma Canada and Astellas Canada.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
Supplemental Material
References
- 1.Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology. 2019;156(6):1675–82. 10.1053/j.gastro.2019.01.028. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–74. 10.1002/hep.29219. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte-Rojo A, Bloomer PM, Rogers RJ, et al. Introducing EL-FIT: a smartphone app to prehabilitate and monitor liver transplant candidates. Liver Transpl. 2021;27(4):502–12. 10.1002/lt.25950. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai JC, Dodge JL, Kappus MR, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: STRIVE. Am J Gastroenterol. 2021;116(4):717–22. 10.14309/ajg.0000000000001113. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kok B, McNeely ML, Watt M, et al. Exercise only works if you do it!. Am J Gastroenterol. 2021;116(4):673–4. 10.14309/ajg.0000000000001198 [DOI] [PubMed] [Google Scholar]
- 6.Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69(5):1164–77. 10.1016/j.jhep.2018.06.017. PMID: [DOI] [PubMed] [Google Scholar]
- 7.Kruger C, McNeely ML, Bailey RJ, et al. Home exercise training improves exercise capacity in cirrhosis patients. Sci Rep. 2018;8(1):99. 10.1038/s41598-017-18320-y. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergevi J, Andermo S, Woldamanuel Y, et al. User perceptions of eHealth and mHealth services promoting physical activity and healthy diets. JMIR Hum Factors. 2022;9(2):e34278. 10.2196/34278. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ney M, Gramlich L, Mathiesen V, et al. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol. 2017;23(2):97–104. 10.4103/1319-3767.203357. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik N, Hameed BMZ, Sooriyaperakasam N, et al. Transforming healthcare through a digital revolution. Front Digit Health. 2022;4:919985. 10.3389/fdgth.2022.919985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statista. Number of smartphone subscriptions; 2022. [cited 2023 February 25]. Available from: https://www.statista.com/.
- 12.Tandon P, Purdy G, Ismond KP, et al. Heal-me PiONEer: an RCT assessing 2 levels of app-based programming in individuals with chronic disease. Contemp Clin Trials. 2022;118:106791. 10.1016/j.cct.2022.106791. PMID: [DOI] [PubMed] [Google Scholar]
- 13.de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII-Renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–74. 10.1016/j.jhep.2021.12.022. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma. Hepatology. 2015;62(1):158–65. 10.1002/hep.27787. PMID: [DOI] [PubMed] [Google Scholar]
- 15.Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis. Hepatology. 2021;74(3):1611–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston MC, Marks A, Crilly MA, et al. Charlson index scores from administrative data and case-note review compared favourably in a renal disease cohort. Eur J Public Health. 2015;25(3):391–6. 10.1093/eurpub/cku238. PMID: [DOI] [PubMed] [Google Scholar]
- 18.Boot WR, Charness N, Czaja SJ, et al. Computer proficiency questionnaire. Gerontologist. 2015;55(3):404–11. 10.1093/geront/gnt117. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roque NA, Boot WR. A new tool for assessing mobile device proficiency in older adults. J Appl Gerontol. 2018;37(2):131–56. 10.1177/0733464816642582. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredin SS, Gledhill N, Jamnik VK, et al. PAR-Q+ and ePARmed-X+. Can Fam Physician. 2013;59(3):273–7. PMID: [PMC free article] [PubMed] [Google Scholar]
- 21.Keyworth C, Epton T, Goldthorpe J, et al. Acceptability, reliability, and validity of a brief measure of capabilities, opportunities, and motivations (”COM-B“). Br J Health Psychol. 2020;25(3):474–501. 10.1111/bjhp.12417. PMID: [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300. 10.1136/gut.45.2.295. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabin R, de Charro F. EQ-5D-a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. 10.3109/07853890109002087. PMID: [DOI] [PubMed] [Google Scholar]
- 24.Bajaj JS, Heuman DM, Sterling RK, et al. Validation of EncephalApp, Smartphone-based stroop test, for the diagnosis of Covert Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2015;13(10):1828–35.e1. 10.1016/j.cgh.2014.05.011. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campagna F, Montagnese S, Ridola L, et al. The animal naming test. Hepatology. 2017;66(1):198–208. [DOI] [PubMed] [Google Scholar]
- 26.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167(9):1287. [DOI] [PubMed] [Google Scholar]
- 27.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant. 2018;18(8):1986–94. 10.1111/ajt.14675. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz H, Har-Nir I, Wenhoda T, et al. Staying physically active during the COVID-19 quarantine. Transl Behav Med. 2021;11(2):314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida Y, Ide Y, Okada M, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47(3):E193–200. 10.1111/hepr.12748. PMID: [DOI] [PubMed] [Google Scholar]
- 30.Jamali T, Raasikh T, Bustamante G, et al. Outcomes of exercise interventions in patients with advanced liver disease. Am J Gastroenterol. 2022;117(10):1614–20. 10.14309/ajg.0000000000001883. PMID: [DOI] [PubMed] [Google Scholar]
- 31.Jakob R, Harperink S, Rudolf AM, et al. Factors influencing adherence to mhealth apps for prevention or management of noncommunicable diseases. J Med Internet Res. 2022;24(5):e35371. 10.2196/35371. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman DR, Srinivas B, Emmett TW, et al. Categorizing health outcomes and efficacy of mHealth Apps for persons with cognitive impairment. J Med Internet Res. 2017;19(8):e301. 10.2196/jmir.7814. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurinović M, Zeković M, Milešević J, et al. Nutritional assessment. In: Food Science. Elsevier; 2017. [Google Scholar]
- 34.Chen X, Liu Z, Sachdev PS, et al. Dietary patterns and cognitive health in older adults. J Nutr Health Aging. 2021;25(2):255–62. 10.1007/s12603-020-1536-8. PMID: [DOI] [PubMed] [Google Scholar]
- 35.Yu AD, Mumme KD, Conlon CA, et al. Relative validity and reproducibility of a semi-quantitative food frequency questionnaire for determining nutrient intake in older adults in New Zealand-REACH. Nutrients. 2022;14(3):519. 10.3390/nu14030519. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowman GL, Shannon J, Ho E, et al. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis Assoc Disord. 2011;25(1):49–57. 10.1097/WAD.0b013e3181f333d6. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Höchsmann C, Martin CK. Review of the validity and feasibility of image-assisted methods for dietary assessment. Int J Obes (Lond.) 2020;44(12):2358–71. 10.1038/s41366-020-00693-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkert D, Chourdakis M, Faxen-Irving G, et al. ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015;34(6):1052–73. 10.1016/j.clnu.2015.09.004. PMID: [DOI] [PubMed] [Google Scholar]
- 39.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. 10.1186/1748-5908-6-42. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spence JC, Burgess J, Rodgers W, et al. Effect of pretesting on intentions and behaviour. Psychol Health. 2009;24(7):777–89. 10.1080/08870440801989938. PMID: [DOI] [PubMed] [Google Scholar]
- 41.Gallegos-Rejas VM, Thomas EE, Kelly JT, et al. A multi-stakeholder approach is needed to reduce the digital divide and encourage equitable access to telehealth. J Telemed Telecare. 2023;29(1):73–8. 10.1177/1357633X221107995. PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.