Abstract
Background
In clinical routine, voriconazole plasma trough levels (Cmin) out of target range are often observed with little knowledge about predisposing influences.
Objectives
To determine the distribution and influencing factors on voriconazole blood levels of patients treated on intensive- or intermediate care units (ICU/IMC).
Patients and methods
Data were collected retrospectively from patients with at least one voriconazole trough plasma level on ICU/IMC (n = 153) to determine the proportion of sub-, supra- or therapeutic plasma levels. Ordinal logistic regression analysis was used to assess factors hindering patients to reach voriconazole target range.
Results
Of 153 patients, only 71 (46%) reached the target range at the first therapeutic drug monitoring, whereas 66 (43%) patients experienced too-low and 16 (10%) too-high plasma levels. Ordinal logistic regression analysis identified the use of extra corporeal membrane oxygenation (ECMO), low international normalized ratio (INR) and aspartate-aminotransferase (AST) serum levels as predictors for too-low plasma levels.
Conclusion
Our data highlight an association of ECMO, INR and AST levels with voriconazole plasma levels, which should be considered in the care of critically ill patients to optimize antifungal therapy with voriconazole.
Introduction
Voriconazole, known as VFEND®, is a second-generation antifungal drug with a broad use spectrum for prophylaxis and therapy of fungal infections caused by Aspergillus spp. and Candida spp.1,2 Infections with fungal pathogens often affect patients hospitalized within ICUs due to their compromised immune status and lead to high rates of morbidity and mortality.3 Since voriconazole treatment influences availability of several drugs and is influenced as an inhibitor and a substrate of CYP2C19, CYP3A4 and CYP2C9, its administration has to be well-considered and critically examined.4 This impedes an appropriate adjustment of plasma levels, which is crucial to ensure therapeutical effects and avoid adverse clinical events.5,6 Inflammation, CYP2C19 genotype, variability in body weight measures and liver function also influence plasma concentration.7–9 Nevertheless, to ensure the desired clinical effect of therapy with voriconazole considering these numerous influencing factors, therapeutic drug monitoring (TDM) is required within 7 days after treatment initiation.10 Non-attainment of drug levels was associated with an increased risk of breakthrough infections.11,12
ICU patients represent a vulnerable population often requiring extracorporeal membrane oxygenation (ECMO) and/or renal replacement therapy. The use of ECMO has grown in the last decade also in relation to coronavirus disease pandemic but is still associated with high mortality.13 Fungal infections due to Candida and Aspergillus spp. represent a substantially complication in intensive- or intermediate care units (ICU/IMC) patients by increasing morbidity and mortality as well as prolonging ICU stay.14–16 Previous data described an influence of ECMO use on the plasma levels of voriconazole presumably due to its lipophilia and strong protein-binding properties. Thus, antifungal therapy needs to be monitored and adapted stringently to provide sufficient therapy success.17–21 In contrast to these results, there are also data suggesting no impact of ECMO on voriconazole plasma levels, which highlights the urgent need for further studies.22 Additionally, known studies are lacking the possible impact of renal replacement therapy as a renal replacement procedure in ICU patients treated with voriconazole alone or in combination with ECMO. For this reason, this study aims to further clarify this important clinical topic by comparing voriconazole trough levels in patients with/without ECMO and/or renal replacement therapy. These issues are also highlighted in transplanted patients, who represent an important part of the intensive care patients in our hospital, are particularly susceptible to infections due to immunosuppression and their immunosuppressive therapy often interacts with antifungals.
Patients and methods
This retrospective observational cohort study included adult patients (≥18 years) with at least one voriconazole plasma trough level (Cmin) during hospitalization between January 2020 and March 2023 from nine ICU/IMC belonging to surgical as well as internal medicine departments in Hanover Medical School. The following data were collected for each patient: sex, age, the use of ECMO and renal replacement therapy at the time of plasma level measurement, solid organ transplantation, stem cell transplantation or current systemic immunosuppression, treatment duration with voriconazole in days, co-medication having known influence on voriconazole plasma level,23 treatment indication, microbiologically detected invasive aspergillosis and application form (intravenous or oral). Additionally, international normalized ratio (INR), bilirubin and the Model for End-Stage Liver Disease (MELD) score {formula, 3.8[log serum bilirubin (mg/dL)] + 11.2 [log INR] + 9.6 [log serum creatinine (mg/dL)] + 6.4} were assessed. None of the included patients received medicaments directly affecting the INR. For every plasma voriconazole level, the time after treatment initiation was calculated. Solid organ and stem cell transplantation were combined as transplanted with further differentiation into the consecutively used immunosuppressive drugs tacrolimus, mycophenolate mofetil and prednisolone. Patients with systemic immunosuppression either suffered from an acquired immunodeficient disease (e.g. human immunodeficiency virus infection) or were treated with either high-dose prednisolone or rituximab. Empirical voriconazole treatment was initiated due to deterioration unless there was broad antibacterial therapy and/or laboratory evidence without pathogen detection. In case of therapeutic treatment, clinical, radiological and microbiological criteria for invasive aspergillosis according to Ullmann et al. were fulfilled.24 All analysed voriconazole plasma levels were trough levels and all blood samples were taken a maximum of 2 hours before the morning dose. Intravenously applied voriconazole was administered as an intermittent infusion every 12 h in all included patients. The initial loading dose was 6 mg/kg body weight (within the first 24 h) twice per day and a maintenance dose of 4 mg/kg body weight (after the first 24 h) twice per day. The initial dose for oral use was 400 mg every 12 hours in the first 24 hours, followed by a maintenance dose of 200 mg every 12 hours.10 Here, 1–5.5 mg/L was defined as target range for voriconazole trough levels, under or above was defined as too low or too high.25 All plasma levels were taken from day 3 after treatment initiation. For patients with available longitudinal course (at least two available plasma trough levels) the percentage of initial in-range plasma levels, the percentage of dose adjustment as well as the percentage of levels never in the target range was determined.
Sample preparation and measurement were carried out according to the instructions of the IVD-Ce certified kit Itraconazole, Posaconazole, Voriconazole in Serum/Plasma—HPLC (Chromsystems Instruments & Chemicals, Graefelfing, Germany). HPLC-fluorescence measurements were performed on an Agilent 1260 Infinity II HPLC (excitation 261 nm, emission 366 nm).
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethic committee of the Hannover Medical School (10778_BO_K_2023). All patients provided written informed consent allowing the use of their data for scientific research.
Data analysis was conducted using IBM SPSS Statistics v.28.0 (IBM Corp., Armonk, NY, USA) and R environment for statistical computing v.4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables are stated as numbers (n) and percentages (%) whereas continuous variables are shown as mean ± standard deviation (SD). For group comparisons, analysis of variance (ANOVA) or Chi-squared test were used, as appropriate. Ordinal logistic regression was conducted to assess the factors associated with subtherapeutic and supratherapeutic levels and consequently hindering patients reaching the therapeutic target range of voriconazole. All reported P values are two-sided and P < 0.05 were considered statistically significant.
Results
Patient characteristics
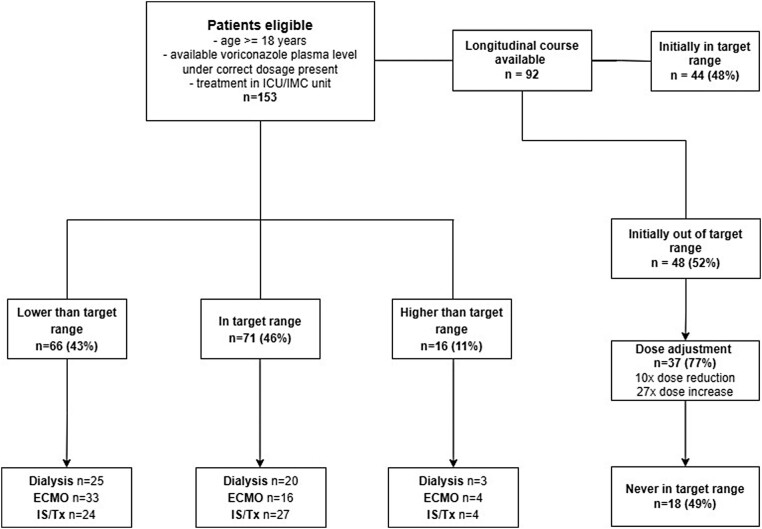
In total, 153 adult patients treated on ICU/IMC with at least one available voriconazole plasma trough level (Cmin) were included, of whom 66 were below the target range (1–5.5 mg/L), 71 within and 16 above with the first voriconazole plasma level. Of 92 patients of whom longitudinal course was available, 48 were not initially within the target range. Dose adjustment was subsequently performed in 37 patients (10 dose reductions, 27 dose increase), of whom 18 patients did not reach the target range during the entire course (Figure 1). All patients received the correct dose described in the Methods section.
Figure 1.
Flowchart of patient inclusion with consecutive subdivisions in patients with lower-than-target range, in-target range or higher-than-target range consecutive percentages of ECMO, renal replacement therapy and immunosuppression (IS)/transplantation (Tx) as well as description of the longitudinal course of therapy.
In this study, 52 patients (34%) received ECMO support, 49 patients (32%) renal replacement therapy, 38 patients (25%) were organ transplanted and 17 patients (11%) immunosuppressed without organ transplantation. In a following step, we compared characteristics between patients with too-low, too-high and in-range plasma levels in Table 1. Herein, patients with too-high serum levels exhibit significant higher INR values (1.2 versus 1.15 and 1.04, P = 0.001) whereas patients with too-low serum levels received more often ECMO support (P = 0.001) and oral drug application (P = 0.037). No significantly statistical differences were detected for demographic characteristics, therapy duration, use of renal replacement therapy, transplantation with its underlying immunosuppressive drugs, immunosuppression, bilirubin plasma levels, MELD score, inflammatory markers (leucocyte count, C-reactive protein, procalcitonin), transaminases and serum albumin (Table 1).
Table 1.
Patient characteristics at the time of the first voriconazole plasma level measurement
| All n = 153 |
Low (<1.0 mg/L) n = 66 |
In range (1.0–5.5 mg/L) n = 71 |
High (>5.5 mg/L) n = 16 |
P value | |
|---|---|---|---|---|---|
| Age, mean (±SD) | 54.6 ± 14.8 | 52.8 ± 13.9 | 55.4 ± 15.8 | 58.9 ± 13.1 | 0.271 |
| Female sex, n (%) | 98 (64.1%) | 46 (70%) | 44 (62%) | 8 (50%) | 0.298* |
| Therapy duration (days), mean (±SD) | 16.7 ± 10.9 | 16.1 ± 9.9 | 17.5 ± 12.1 | 15.1 ± 9.5 | 0.644 |
| Measurement after therapy initiation (days), mean (±SD) | 5.6 ± 3.3 | 6.4 ± 4.1 | 4.6 ± 2.1 | 6.4 ± 2.7 | 0.003 |
| ECMO, n (%) | 52 (34%) | 33 (50%) | 16 (23%) | 3 (19%) | 0.001* |
| Renal replacement therapy, n (%) | 49 (32%) | 25 (38%) | 20 (28%) | 4 (25%) | 0.389* |
| Tx, n (%) | 38 (25%) | 19 (29%) | 16 (23%) | 3 (19%) | 0.643* |
| —Tacrolimus, n (%) | 24 (16%) | 14 (21%) | 8 (11%) | 2 (13%) | 0.11* |
| — Mycophenolate mofetil, n (%) | 13 (9%) | 7 (11%) | 5 (7%) | 1 (6%) | 0.671* |
| —Prednisolone, n (%) | 42 (27%) | 20 (30%) | 19 (27%) | 3 (19%) | 0.442* |
| Immunosuppression, n (%) | 17 (11%) | 5 (16%) | 11 (16%) | 1 (6%) | 0.428* |
| Oral applicationa, n (%) | 7 (5%) | 6 (9%) | 0 (0%) | 1 (6%) | 0.037* |
| INR, mean (±SD) | 1.1 ± 0.2 | 1.04 ± 0.12 | 1.15 ± 0.24 | 1.2 ± 0.33 | 0.001 |
| Bilirubin (mmol/L), mean (±SD) | 41.9 ± 71.9 | 34.5 ± 69.1 | 44.2 ± 62.5 | 61.9 ± 118.4 | 0.383 |
| MELD score, mean (±SD) | 15.3 ± 7.9 | 14 ± 7.7 | 16.5 ± 7.9 | 16 ± 8.1 | 0.172 |
| Leucocytes (1.000/mL), mean (±SD) | 14.6 ± 23.8 | 14.4 ± 7.9 | 16.2 ± 32.9 | 7.7 ± 7.4 | 0.523 |
| PCT (µg/L), mean (±SD) | 5.8 ± 18.7 | 1.9 ± 3.5 | 8.7 ± 25.1 | 8.3 ± 16.7 | 0.130 |
| CRP (mg/L), mean (±SD) | 124.1 ± 95.5 | 109.5 ± 88.9 | 138.6 ± 100.2 | 112.8 ± 95 | 0.235 |
| ALT (U/L), mean (±SD) | 106.4 ± 232.7 | 68.1 ± 98 | 137.2 ± 299.9 | 114.4 ± 246.5 | 0.274 |
| AST (U/L), mean (±SD) | 183.8 ± 522.1 | 73.7 ± 96.8 | 286.8 ± 719.4 | 130.3 ± 250.6 | 0.081 |
| Serum albumin (g/L), mean (±SD) | 27.5 ± 7.8 | 29.4 ± 8.8 | 26 ± 6.8 | 27.1 ± 5.9 | 0.061 |
Continuous variables are stated as mean and SD and categorical variables are stated as n and percentages (%); tests were performed using ANOVA, tests marked with an asterisk * were conducted using the chi-squared test.
Statistically significant values are shown in bold.
Tx, transplanted; PCT, procalcitonin; CRP, C-reactive protein; ALT, alanine-aminotransferase.
aOthers intravenous.
Potential factors for not reaching voriconazole target range
A detailed comparison between patients with subtherapeutic, therapeutic and supratherapeutic voriconazole plasma level is presented in Table 1. To determine factors for subtherapeutic and supratherapeutic trough levels ordinal logistic regression was performed as presented in Table 2. The use of ECMO [OR 4.59 (1.73–12.2), P = 0.002], lower INR values [OR 0.6 (0.41–0.87), P = 0.007] as well as lower serum aspartate-aminotransferase (AST) levels [OR 0.99 (0.99–1), P = 0.019] exhibited significant association with subtherapeutic voriconazole plasma levels. Additionally, serum albumin levels were significantly associated with too-low voriconazole trough levels in univariate analysis [OR 1.89 (1.18–2.76), P = 0.008] but not in multivariate analysis [OR 1.67 (0.91–3.06), P = 0.098]. All further analysed factors did not show a significant impact. Results for supratherapeutic trough levels in a small subpopulation (n = 16) revealed significant association for leukocytes count [OR 0.91 (0.84–1), P = 0.049] but not in multivariate analysis [OR 0.92 (0.82–1.03), P = 0.144] without significant association for other examined factors (Table 2).
Table 2.
Ordinal logistic regression analysis assessing factors associated with voriconazole levels below or above the target range
| Below target range | Above target range | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate OR (95% CI) |
P value | Multivariate OR (95% CI) |
P value | Univariate OR (95% CI) |
P value | Multivariate OR (95% CI) |
P value | |
| Age | 0.99 (0.97–1.01) |
0.293 | 1.02 (0.98–1.06) |
0.379 | ||||
| Sex | 0.71 (0.35–1.45) |
0.342 | 1.63 (0.55–4.85) |
0.38 | ||||
| ECMO | 3.44 (1.65–7.18) |
0.001 | 4.59 (1.73–12.2) |
0.002 | 0.79 (0.2–3.12) |
0.741 | ||
| Renal replacement therapy | 1.55 (0.76–3.19) |
0.228 | 0.85 (0.25–2.95) |
0.798 | ||||
| Immunosuppression | 0.93 (0.47–1.86) |
0.84 | 0.54 (0.16–1.86) |
0.33 | ||||
| Tx | 1.39 (0.64–3.01) |
0.403 | 0.79 (0.2–3.13) |
0.741 | ||||
| —Tacrolimus | 2.1 (0.83–5.4) |
0.118 | 1.12 (0.22–5.88) |
0.889 | ||||
| —Mycophenolate mofetil | 1.56 (0.47–5.2) |
0.464 | 0.88 (0.1–8.1) |
0.910 | ||||
| —Prednisolone | 1.19 (0.57–2.5) |
0.646 | 0.63 (0.16–2.46) |
0.508 | ||||
| INRa | 0.68 (0.54–0.86) |
0.001 | 0.6 (0.41–0.87) |
0.007 | 1.08 (0.88–1.31) |
0.469 | ||
| Bilirubinb | 0.98 (0.93–1.03) |
0.417 | 1.02 (0.97–1.08) |
0.442 | ||||
| MELD score | 0.99 (0.98–1) |
0.674 | 0.99 (0.93–1.06) |
0.836 | ||||
| Leukocytes | 0.99 (0.98–1.01) |
0.896 | 0.91 (0.84–1) |
0.049 | 0.92 (0.82–1.03) |
0.144 | ||
| PCT | 0.93 (0.86–1.01) |
0.072 | 0.99 (0.97–1.03) |
0.968 | ||||
| CRP | 0.99 (0.99–1) |
0.066 | 0.99 (0.99–1) |
0.485 | ||||
| ALT | 0.99 (0.99–1) |
0.129 | 0.99 (0.99–1) |
0.697 | ||||
| AST | 0.99 (0.99–1) |
0.037 | 0.99 (0.99–1) |
0.019 | 0.99 (0.99–1) |
0.434 | ||
| Serum albumin | 1.89 (1.18–2.76) |
0.008 | 1.67 (0.91–3.06) |
0.098 | 1.35 (0.64–2.86) |
0.426 | ||
Statistically significant values are in bold.
Tx, transplanted; PCT, procalcitonin; CRP, C-reactive protein; ALT, alanine-aminotransferase.
aValue changes by 0.1.
bValue changes per 10 mmol/L.
Discussion
In this retrospective study, we identified a high percentage of critically ill patients with voriconazole trough levels out of target range. Additionally, our data revealed ECMO use as a factor for lower plasma levels, whereas lower INR values were associated with lower voriconazole levels.
Achieving an adequate plasma level is a major challenge to ensure both a sufficient therapeutic effect but also to prevent side effects due to voriconazole therapy. Despite the implementation of the recommendations for loading and maintenance dose, nearly half of our included patients initially did not reach the defined target range. Of note, despite dose adjustment, the target range was not reached in the course of voriconazole therapy in more than 10% of all included patients. Sebaaly et al. reported on a comparable percentage of analysed trough levels out of target range with a higher amount above than below target range and also an increasing percentage of in-range plasma levels in the course of voriconazole treatment.26 Conversely, further results observe this aspect only for oral but not intravenous application that in their study reliably ensured sufficient drug plasma levels.27 Several other studies have described a varying percentage between 20% and 50% of voriconazole trough levels not within the target range in different study populations thus—according to our data—highlighting a considerable proportion of inadequate voriconazole trough levels.28–31 The resulting need for TDM is corroborated by studies that observed poorer therapeutic effects at levels too low and increased side effects or therapy discontinuation at levels too high,25,32–34 whereas studies also doubt the benefit.29,35 Presumably, the benefit of TDM also varies between different subgroups and is influenced by their disease-specific factors, so further studies on these issues are needed. Owing to a notable number of patients out of target range even in the course of voriconazole therapy, further clarification about the effectiveness of dose adjustment and a potential need for an additional antifungal treatment are crucial.
In the past, an increasing number of ex vivo studies, case studies and clinical studies examined the impact of ECMO use on voriconazole plasma levels.18,21,36–39 Compared to our study, the results of the aforementioned studies are mainly based on non-clinical approaches or a proportionally small sample size. In a comparable study concerning patient cohort and design, Ye et al. published similar results but defined another target range as subtherapeutic.21 Thus, our study supports the potential influence of ECMO use independently of underlying target range. However, van Daele et al. did not detect any influence of ECMO on voriconazole plasma level.22 Nevertheless, the influences of ECMO on volume balance, organ perfusion, protein binding and drug elimination have been described in detail and are thus justified content for scientific discussion.40 It can be concluded that for these antifungal drugs clear results are missing with the urgent need for further studies. As for voriconazole, the cited data show increasing evidence of an impact of ECMO on trough levels. Some authors have already discussed an empirical dose adjustment, which in our opinion should be supplemented by another antifungal treatment in critically ill patients with invasive aspergillosis as a bridging measure because the therapeutic range has not been reached in many cases.
As another important aspect, our study detected a significant association between INR, AST and voriconazole levels. To our knowledge, the relation between voriconazole, AST and INR in patients in ICU has not been evaluated yet. Retrospectively analysed data about trough concentration of orally taken voriconazole in oncology patients identified a correlation with INR but not AST.41 However, results from different patient cohorts detected an association for AST and voriconazole levels.42–44 Additionally, several studies have focused on the INR–voriconazole relation in patients with underlying liver diseases. In patients with Child–Pugh scores of B and C, an influence of INR on voriconazole plasma levels was described as well as in a patient cohort after liver transplantation.43,45,46 Because of the retrospective design of our study, we were not able to assess the Child–Pugh score or to perform CYP2C19 genotyping. Through ordinal logistic regression analysis, we did not detect any correlation between bilirubin, MELD score and infection parameters, and INR or voriconazole levels. Thus, a reduced liver function assumed by elevated MELD score and higher bilirubin plasma levels cannot be used as a simple explanation for not reaching the voriconazole target range concerning our study results. Furthermore, bilirubin cannot be considered to be a sensitive or specific predictor of liver function, so results must always be interpretated carefully in an individual clinical context.47 On the basis of our results and data from the aforementioned studies, markers associated with liver dysfunction seem to be associated with voriconazole levels in different patient cohorts. Because in our cohort of ICU patients low INR and AST levels, and in the univariate analysis also increased albumin levels, are associated with too-low voriconazole levels, liver function seems to have at least a partial influence as well. Therefore, we suggest further studies to examine liver function parameters as risk factors for an azole therapy failure period.
The main advantage of our study is the large amount of voriconazole trough levels compared to recently published studies in a well-defined patient cohort of critically ill patients in ICU.
The limitations include the reduced validity of our study by its monocentric and retrospective design. Second, the main results were based on statistical correlations for the first analysed plasma level, and there was no proof of causal relation in ICU patients in which many as-yet unknown processes take place that can also potentially have an influence. Concerning longitudinal courses, there is no information about the reasons for non-execution of dose adjustment.
This study detected a wide dispersion of voriconazole plasma levels with a high proportion outside the target range in intensive care patients associated with albumin serum levels, INR and the use of ECMO as potential influencing factors. Further research is needed to establish these factors mentioned here as part of improved dosing along with continuous TDM in clinical practice to improve treatment success and prevent adverse clinical events of voriconazole.
Acknowledgements
We wish to thank all patients who participated in this project. We would also like to thank the clinical staff caring about the patients and last but not least the families of the authors who have given up a lot of time with their loved ones to make this research possible.
Contributor Information
Christopher Alexander Hinze, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany.
Jan Fuge, Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany.
Denis Grote-Koska, Institute of Clinical Chemistry, Hannover Medical School, Hannover, Germany.
Korbinian Brand, Institute of Clinical Chemistry, Hannover Medical School, Hannover, Germany.
Hortense Slevogt, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany; Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany; Respiratory Infection Dynamics Group, Helmholtz Centre for Infection Research, Braunschweig, Germany.
Markus Cornberg, Department of Gastroenterology, Hepatology, Infectious Diseases and Endocrinology, Hannover Medical School, Centre for Individualized Infection Medicine, Hannover, Germany; German Center for Infection Research (DZIF), partner-site Hannover-Braunschweig, Hannover, Germany.
Susanne Simon, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany.
Oana Joean, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany.
Tobias Welte, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany; Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany.
Jessica Rademacher, Department of Respiratory Medicine and Infectious Disease, Hannover Medical School, Hannover, Germany; Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany.
Funding
This study was carried out as part of our routine work.
Transparency declarations
C.A.H. declares no conflict of interest. J.F. received personal fees/speaker honoraria from AstraZeneca outside the submitted work. D.G.K. declares no conflict of interest. K.B. declares no conflict of interest. H.S. declares no conflict of interest. S.S. declares no conflict of interest. O.J. declares no conflict of interest. T.W. and/or his institution received grants advisory/lecture/clinical trial fees and non-financial support from the BMBF (German Ministry of Research and Education), Advanz Pharma, Basilea, MSD, Pfizer and Biotest outside the submitted work. M.C. received honoraria for lectures and consulting from AbbVie Deutschland GmbH & Co. KG, AiCuris AG, Falk Foundation e.V., Gilead Sciences GmbH, GSK Service Unlimited, MSD Sharp & Dohme GmbH, Novartis AG, Roche AG, Spring Bank Pharmaceuticals and Swedish Orphan Biovitrum AB (SOBI) outside the submitted work. J.R. received research grants from the BMBF, Innovationsfond, Infectopharm, Bayer, Grifols, Insmed, Novartis and personal fees/speaker honoraria AstraZeneca, Boehringer, GSK, Chiesi, Esanum, Novartis, ThermoFisher, MedUpdate, Berlin-Chemie, INSMED, MSD, Shionogi and GILEAD.
References
- 1. Limper AH, Knox KS, Sarosi GA et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 2011; 183: 96–128. 10.1164/rccm.2008-740ST [DOI] [PubMed] [Google Scholar]
- 2. Walsh TJ, Anaissie EJ, Denning DW et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46: 327–60. 10.1086/525258 [DOI] [PubMed] [Google Scholar]
- 3. Montagna MT, Caggiano G, Lovero G et al. Epidemiology of invasive fungal infections in the intensive care unit: results of a multicenter Italian survey (AURORA project). Infection 2013; 41: 645–53. 10.1007/s15010-013-0432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet 2006; 45: 649–63. 10.2165/00003088-200645070-00002 [DOI] [PubMed] [Google Scholar]
- 5. Dolton MJ, Ray JE, Chen SC et al. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56: 4793–9. 10.1128/AAC.00626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin H, Wang T, Falcione BA et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother 2016; 71: 1772–85. 10.1093/jac/dkw045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veringa A, Avest T, Span M et al. Voriconazole metabolism is influenced by severe inflammation: a prospective study. J Antimicrob Chemother 2017; 72: 261–7. 10.1093/jac/dkw349 [DOI] [PubMed] [Google Scholar]
- 8. Blanco-Dorado S, Maroñas O, Latorre-Pellicer A et al. Impact of CYP2C19 genotype and drug interactions on voriconazole plasma concentrations: a Spain pharmacogenetic-pharmacokinetic prospective multicenter study. Pharmacotherapy 2020; 40: 17–25. 10.1002/phar.2351 [DOI] [PubMed] [Google Scholar]
- 9. Shi C, Xiao Y, Mao Y et al. Voriconazole: a review of population pharmacokinetic analyses. Clin Pharmacokinet 2019; 58: 687–703. 10.1007/s40262-019-00735-7 [DOI] [PubMed] [Google Scholar]
- 10. Ashbee HR, Barnes RA, Johnson EM et al. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 2014; 69: 1162–76. 10.1093/jac/dkt508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Troke PF, Hockey HP, Hope WW. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother 2011; 55: 4782–8. 10.1128/AAC.01083-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ueda K, Nannya Y, Kumano K et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol 2009; 89: 592–9. 10.1007/s12185-009-0296-3 [DOI] [PubMed] [Google Scholar]
- 13. Bercker S, Petroff D, Polze N et al. ECMO use in Germany: an analysis of 29,929 ECMO runs. PLoS One 2021; 16: e0260324. 10.1371/journal.pone.0260324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vincent JL, Rello J, Marshall J et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–9. 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 15. Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 2006; 25: 419–25. 10.1007/s10096-006-0159-2 [DOI] [PubMed] [Google Scholar]
- 16. Olaechea PM, Palomar M, León-Gil C et al. Economic impact of Candida colonization and Candida infection in the critically ill patient. Eur J Clin Microbiol Infect Dis 2004; 23: 323–30. 10.1007/s10096-004-1104-x [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Hu H, Zhang Q et al. Effects of ex vivo extracorporeal membrane oxygenation circuits on sequestration of antimicrobial agents. Front Med (Lausanne) 2021; 8: 748769. 10.3389/fmed.2021.748769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raffaeli G, Cavallaro G, Allegaert K et al. Sequestration of voriconazole and vancomycin into contemporary extracorporeal membrane oxygenation circuits: an in vitro study. Front Pediatr 2020; 8: 468. 10.3389/fped.2020.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cies JJ, Moore WS II, Giliam N et al. Oxygenator impact on voriconazole in extracorporeal membrane oxygenation circuits. Perfusion 2020; 35: 529–33. 10.1177/0267659120937906 [DOI] [PubMed] [Google Scholar]
- 20. Mehta NM, Halwick DR, Dodson BL et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 2007; 33: 1018–24. 10.1007/s00134-007-0606-2 [DOI] [PubMed] [Google Scholar]
- 21. Ye Q, Yu X, Chen W et al. Impact of extracorporeal membrane oxygenation on voriconazole plasma concentrations: a retrospective study. Front Pharmacol 2022; 13: 972585. 10.3389/fphar.2022.972585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Daele R, Bekkers B, Lindfors M et al. A large retrospective assessment of voriconazole exposure in patients treated with extracorporeal membrane oxygenation. Microorganisms 2021; 9: 1543. 10.3390/microorganisms10010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection 2017; 45: 737–79. 10.1007/s15010-017-1042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ullmann AJ, Aguado JM, Arikan-Akdagli S et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24(Suppl 1): e1–38. 10.1016/j.cmi.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 25. Park WB, Kim NH, Kim KH et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis 2012; 55: 1080–7. 10.1093/cid/cis599 [DOI] [PubMed] [Google Scholar]
- 26. Sebaaly JC, MacVane SH, Hassig TB. Voriconazole concentration monitoring at an academic medical center. Am J Health Syst Pharm 2016; 73: S14–21. 10.2146/ajhp150372 [DOI] [PubMed] [Google Scholar]
- 27. Yi WM, Schoeppler KE, Jaeger J et al. Voriconazole and posaconazole therapeutic drug monitoring: a retrospective study. Ann Clin Microbiol Antimicrob 2017; 16: 60. 10.1186/s12941-017-0235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitsani D, Nguyen MH, Shields RK et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob Agents Chemother 2012; 56: 2371–7. 10.1128/AAC.05219-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chu HY, Jain R, Xie H et al. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis 2013; 13: 105. 10.1186/1471-2334-13-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guinea J, Escribano P, Marcos-Zambrano LJ et al. Therapeutic drug monitoring of voriconazole helps to decrease the percentage of patients with off-target trough serum levels. Med Mycol 2016; 54: 353–60. 10.1093/mmy/myv099 [DOI] [PubMed] [Google Scholar]
- 31. van Wanrooy MJ, Rodgers MG, Span LF et al. Voriconazole therapeutic drug monitoring practices in intensive care. Ther Drug Monit 2016; 38: 313–8. 10.1097/FTD.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Yim DS, Choi SM et al. Voriconazole-related severe adverse events: clinical application of therapeutic drug monitoring in Korean patients. Int J Infect Dis 2011; 15: e753–8. 10.1016/j.ijid.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 33. Cabral-Galeano E, Ruiz-Camps I, Len-Abad O et al. Clinical usefulness of therapeutic drug monitoring of voriconazole in a university hospital. Enferm Infecc Microbiol Clin 2015; 33: 298–302. 10.1016/j.eimc.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 34. Abdul-Aziz MH, Alffenaar JC, Bassetti M et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 2020; 46: 1127–53. 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veringa A, Brüggemann RJ, Span LFR et al. Therapeutic drug monitoring-guided treatment versus standard dosing of voriconazole for invasive aspergillosis in haematological patients: a multicentre, prospective, cluster randomised, crossover clinical trial. Int J Antimicrob Agents 2023; 61: 106711. 10.1016/j.ijantimicag.2023.106711 [DOI] [PubMed] [Google Scholar]
- 36. Vu T, Feih J, Juul J. Fluctuating voriconazole concentrations during extracorporeal membrane oxygenation. J Pharm Pract 2023; 36: 998–1001. 10.1177/08971900211060959 [DOI] [PubMed] [Google Scholar]
- 37. Lin XB, Hu XG, Xia YZ et al. Voriconazole pharmacokinetics in a critically ill patient during extracorporeal membrane oxygenation. J Chemother 2022; 34: 272–6. 10.1080/1120009X.2021.2014725 [DOI] [PubMed] [Google Scholar]
- 38. Mathieu A, Thiboutot Z, Ferreira V et al. Voriconazole sequestration during extracorporeal membrane oxygenation for invasive lung aspergillosis: a case report. ASAIO J 2022; 68: e56–8. 10.1097/MAT.0000000000001427 [DOI] [PubMed] [Google Scholar]
- 39. Ronda M, Llop-Talaveron JM, Fuset M et al. Voriconazole pharmacokinetics in critically ill patients and extracorporeal membrane oxygenation support: a retrospective comparative case-control study. Antibiotics (Basel) 2023; 12: 1100. 10.3390/antibiotics12071100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lyster H, Pitt T, Maunz O et al. Variable sequestration of antifungals in an extracorporeal membrane oxygenation circuit. ASAIO J 2023; 69: 309–14. 10.1097/MAT.0000000000001802 [DOI] [PubMed] [Google Scholar]
- 41. Lombardi LR, Miano TA, Davis JL et al. A retrospective analysis of the effect of patient-specific factors on voriconazole concentrations in oncology patients. J Oncol Pharm Pract 2012; 18: 3–9. 10.1177/1078155210397963 [DOI] [PubMed] [Google Scholar]
- 42. Trifilio S, Ortiz R, Pennick G et al. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2005; 35: 509–13. 10.1038/sj.bmt.1704828 [DOI] [PubMed] [Google Scholar]
- 43. Johnson HJ, Han K, Capitano B et al. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrob Agents Chemother 2010; 54: 852–9. 10.1128/AAC.00429-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng L, Zhao Y, Liang Z et al. Prediction of plasma trough concentration of voriconazole in adult patients using machine learning. Eur J Pharm Sci 2023; 188: 106506. 10.1016/j.ejps.2023.106506 [DOI] [PubMed] [Google Scholar]
- 45. Zhao Y, Hou J, Xiao Y et al. Predictors of voriconazole trough concentrations in patients with Child-Pugh class C cirrhosis: a prospective study. Antibiotics (Basel) 2021; 10: 1130. 10.3390/antibiotics10091130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang T, Yan M, Tang D et al. Therapeutic drug monitoring and safety of voriconazole therapy in patients with Child-Pugh class B and C cirrhosis: a multicenter study. Int J Infect Dis 2018; 72: 49–54. 10.1016/j.ijid.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 47. Guerra Ruiz AR, Crespo J, López Martínez RM et al. Measurement and clinical usefulness of bilirubin in liver disease. Adv Lab Med 2021; 2: 352–72. 10.1515/almed-2021-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]