Abstract
Compared to healthy volunteers, participants with post‐acute sequelae of SARS‐CoV‐2 infection (PASC) demonstrated increased plasma levels of the prothrombotic protein NEDD9, which associated inversely with indices of pulmonary vascular function. This suggests persistent pulmonary vascular dysfunction may play a role in the pathobiology of PASC.
Keywords: NEDD9, post‐acute sequelae of SARS‐CoV‐2 infection, pulmonary vascular dysfunction
INTRODUCTION
Pulmonary vascular abnormalities have been described in severe SARS‐CoV‐2 infection, 1 , 2 including increased pulmonary endothelial expression of the prothrombotic protein NEDD9. 3 However, it is not known if endothelial dysfunction is a prominent feature of non‐severe SARS‐CoV‐2 infection or post‐acute sequelae of SARS‐CoV‐2 infection (PASC). We recently performed dynamic contrast‐enhanced magnetic resonance imaging (DCE‐MRI) in participants with prior SARS‐CoV‐2 infection and healthy volunteers. 4 DCE‐MRI is a technique that can assess pulmonary microvascular abnormalities in chronic lung disease. 5 , 6 Using DCE‐MRI, we observed a slower rate of contrast arrival and a wider full width at half maximum in the posterior coronal lung region consistent with decreased pulmonary vascular perfusion in 10 participants with remote COVID‐19 compared to age‐similar healthy volunteers. 4 In this secondary analysis, we aimed to determine if (1) plasma biomarkers of inflammation, coagulation, and epithelial and endothelial injury differed between participants with prior SARS‐CoV‐2 infection and healthy volunteers and if (2) an association exists between selected plasma proteins and indices of pulmonary vascular dysfunction.
METHODS
Study details have been published previously. 4 Briefly, we recruited participants encountered in the Massachusetts General Hospital Coronavirus Recovery Clinical with a positive SARS‐CoV‐2 polymerase chain reaction (PCR) test within the preceding 3−12 months. Healthy volunteers were recruited through a different protocol and did not have known lung disease. Exclusion criteria for all subjects included: acute respiratory illness within 6 weeks, cigarette smoking within 6 months, gadolinium allergy, or contraindications to MRI. All subjects provided written informed consent.
Participants with antecedent COVID‐19 underwent detailed clinical phenotyping as previously reported. 4 Briefly, we collected demographics, severity of index SARS‐CoV‐2 infection, and results of pulmonary function testing, including diffusing capacity of the lung for carbon monoxide (DLCO), from the electronic medical record.
We performed DCE‐MRI according to previously published methods. 4 , 5 Briefly, we acquired serial volumetric images of the thorax before, during, and after the injection of 0.05 mmol/kg gadoterate meglumine (Guerbet) at a rate of 4 mL/s. We generated signal intensity versus time curves on a voxel‐by‐voxel basis throughout the lung. We performed a model‐free analysis to extract parameters reflective of pulmonary perfusion and microvascular function over the whole lung and in a posterior coronal region of interest. We measured the magnitude of peak enhancement, the rate of contrast arrival (kwashin), time to peak enhancement, and the full width at half maximum for the peak as a surrogate of contrast transit time. We also measured the rate of contrast washout (kwashout) as a measurement of the extravascular extracellular space.
We collected whole blood from all participants at the time of MRI and stored plasma aliquots at −80°C until use. We performed a previously validated NEDD9 enzyme‐linked immunosorbent assay (Aviva Systems Biology) to quantify NEDD9 levels (ng/mL) 7 and a Luminex® Multiplex Assay (R&D Systems) to quantify plasma levels of additional endothelial, epithelial, inflammatory, and coagulation proteins (pg/mL) based on their reported associations with PASC (Figure 1a). 8 Continuous data are presented as median (interquartile range [IQR]), unless otherwise specified. We assessed differences between groups using the Wilcoxon rank‐sum test. p Values < 0.003 were considered significant after adjusting for multiple comparisons by Bonferroni correction. We explored associations between plasma protein levels and both MRI parameters and DLCO using univariate linear regression. Statistical analyses were performed using Stata SE (version 17.0).
Figure 1.
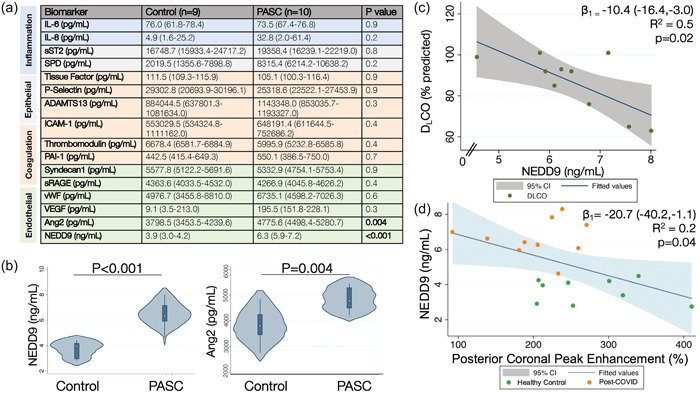
Plasma and imaging biomarkers of pulmonary vascular dysfunction in PASC. (a) Compared to the healthy volunteers, PASC participants had increased plasma levels of NEDD9 and angiopoeitin‐2 (Ang2), but there were no differences in the other measured inflammatory, epithelial, coagulation, or endothelial protein levels, including interleukin 6 (IL‐6) and interleukin 8 (IL‐8); soluble suppression of tumorigenicity 2 (sST2) and surfactant protein D (SPD); tissue factor, P‐selectin, a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13 (ADAMTS13), intercellular adhesion molecule 1 (ICAM‐1), thrombomodulin, and plasminogen activator inhibitor 1 (PAI‐1); and Syndecan‐1, soluble receptor of advanced glycation end‐products (sRAGE), von Willebrand factor (vWF), and vascular endothelial growth factor (VEGF), respectively. Data presented as median (interquartile range [IQR]). (b) Violin plots of plasma NEDD9 (ng/mL) and angiopoietin‐2 (pg/mL) levels are presented in which the white dot represents the median, the thick blue bar represents the IQR, the thin line represents the rest of the distribution, and the plot distribution depicts a kernel density estimation whereby wider sections represent a higher probability that members of the population will take on the given value. There was a significant inverse association between (c) NEDD9 levels and DLCO (% predicted) for the PASC participants and (d) NEDD9 levels and the magnitude of peak enhancement (%) in the posterior coronal region of interest on DCE‐MRI for all participants. Control, healthy volunteers; DCE‐MRI, dynamic contrast‐enhanced magnetic resonance imaging; DLCO, diffusing capacity of the lung for carbon monoxide; PASC, participants with post‐acute sequalae of SARS‐CoV‐2 infection.
RESULTS
All 10 participants with prior SARS‐CoV‐2 infection (mean age, 55.6 years; 50% female) and 9 (90%) healthy volunteers (mean age, 51.3 years; 56% female) had plasma available for analysis. At the time of their clinical evaluation, all participants with prior SARS‐CoV‐2 infection met criteria for PASC as defined according to the World Health Organization criteria, that is, new or persistent symptoms more than 3 months from SARS‐CoV‐2 infection symptom onset not attributable to another cause. 9 The median time from SARS‐CoV‐2 PCR to DCE‐MRI and plasma sample collection was 8 months (IQR 4−10 months) in the PASC cohort. The majority of PASC participants had non‐severe COVID‐19 with 30% requiring hospitalization, two of whom required supplemental oxygen (maximum flow rate of 4 L/min). All PASC participants underwent clinically indicated pulmonary function testing. DLCO was measured a median 80 days before DCE‐MRI (IQR 52.5−140 days). The median modified Medical Research Council (mMRC) dyspnea scale score obtained from PASC participants on the day of plasma sample collection was 1 (range 0−2), wherein a score of 0 indicates dyspnea only with strenuous activity, 1 indicates dyspnea when hurrying or walking up a slight hill, and 2 indicates stopping when walking at own pace or walking slower than contemporaries on the level because of dyspnea. 10 DLCO was not available for the healthy volunteers.
Compared to the healthy volunteers, PASC participants demonstrated increased median plasma levels of NEDD9 (3.9 [2.9−4.2] vs. 6.3 [5.9−7.2] ng/mL, p < 0.001) and angiopotein‐2 (3799 [3453−4240] vs. 4775.6 [4498−5281] pg/mL, p = 0.004), two biomarkers of endothelial dysfunction (Figure 1b). However, only differences in plasma NEDD9 met the Bonferroni adjusted threshold for statistical significance (p < 0.003). There were no significant differences between groups for the other measured endothelial, epithelial, inflammatory, or coagulation protein levels (Figure 1a).
In univariate linear regression, there was an association between NEDD9 and DLCO (predicted 10.4% DLCO decrease for every standard deviation increase in NEDD9, 95% CI: −16.4 to −3.0, R 2 = 0.5, p = 0.02) (Figure 1c) and peak enhancement on DCE‐MRI (predicted 20.7% peak enhancement decrease for every standard deviation increase in NEDD9, 95% CI: −40.2 to −1.1, R 2 = 0.2, p = 0.04) (Figure 1d), respectively.
DISCUSSION
In this secondary analysis of participants with PASC who underwent DCE‐MRI a median 8 months after index SARS‐CoV‐2 infection, we observed several key findings. First, the PASC cohort was distinguished by elevated plasma levels of NEDD9 and angiopoietin‐2 compared to the healthy volunteers. However, differences in biomarkers of systemic inflammation, epithelial injury, and activated coagulation between groups were not observed. Second, NEDD9 levels were inversely associated with both peak enhancement (a marker of impaired microvascular perfusion on DCE‐MRI) and DLCO (a marker of the efficiency of gas transfer in the lung impacted by the pulmonary capillary surface area available for gas exchange). Together the association between plasma NEDD9 and measurements of pulmonary vascular dysfunction suggests a possible mechanistic role of pulmonary endothelial dysfunction in SARS‐CoV‐2 infection and PASC.
NEDD9 is a scaffolding protein that organizes signaling complexes regulating several signal transduction pathways involved in cellular adhesion, motility, and proliferation. 11 We demonstrated previously that pulmonary endothelial NEDD9 is prothrombotic by binding activated platelets via P‐selectin to promote platelet‐endothelial adhesion, and plasma NEDD9 levels were increased in patients with pulmonary arterial hypertension and associated with adverse clinical outcomes. 7 , 12 Further, in decedents with acute respiratory distress syndrome (ARDS) due to SARS‐CoV‐2 infection, we observed increased pulmonary endothelial NEDD9 expression compared to other causes of fatal ARDS. 3 While there is no single unifying pathobiology underlying PASC, microvascular endothelial injury is one hypothesis underpinning some proportion of the clinical syndromes observed. 13 , 14 Our current results extend the pathophysiological relevance of NEDD9 to pulmonary vascular dysfunction in the recovery from SARS‐CoV‐2 infection, particularly patients with PASC.
There is a growing body of literature exploring diagnostic and prognostic biomarkers for PASC. However, progress has been hindered by nonuniform definitions of PASC, the heterogeneity of PASC clinical phenotypes, and the advent of novel treatments and viral variants that can alter the underlying pathobiology. A number of candidate biomarkers are purported to predict or diagnose PASC, including persistence of viral antigens, auto‐antibodies, dysregulated T/NK cell signaling, amyloid deposits (“microclots”), and endothelial proteins, such as angiopoietin‐2, but findings vary across cohort studies. 8 , 15 , 16 , 17 Here, there was no difference in plasma biomarkers of systemic inflammation, epithelial injury, or activated coagulation between groups, but we observed increased endothelial proteins, although only NEDD9 maintained significance when adjusting for multiple comparisons. Moreover, plasma NEDD9 associated with indices of abnormal pulmonary vascular function, providing a direct link between a circulating endothelial biomarker and an observable pathophenotype.
There are several limitations to our study. First, this is a small sample size from a single center and all participants were recruited from a PASC clinic which introduces selection bias. Second, all PASC participants developed SARS‐CoV‐2 before the Omicron variant, so we are unable to distinguish how novel variants affect the pulmonary vasculature. Third, we did not determine prior SARS‐CoV‐2 infection status for the healthy volunteers. Fourth, we were unable to compare convalescent cases of SARS‐CoV‐2 with and without PASC to ensure the elevated plasma NEDD9 levels and microvascular abnormalities observed are not just an epiphenomenon of prior SARS‐CoV‐2 infection. Finally, lung imaging and plasma collection occurred at a single time point that varied among participants. Thus, we are unable to comment on the persistence of these findings over time.
In conclusion, our results suggest microvascular perfusion abnormalities months following SARS‐CoV‐2 infection may be attributable to pulmonary vascular endothelial dysfunction, as evidenced by increased plasma NEDD9 levels. Given the relevance of NEDD9 to both aberrant vascular remodeling and thrombosis, ongoing research may assist in further elucidation of pathogenetic mechanisms. Pairing advanced lung imaging, such as fibrin‐targeted molecular imaging, 18 with proteomic profiling could help decipher the pathobiology of PASC with potential therapeutic implications.
AUTHOR CONTRIBUTIONS
George A. Alba and Sydney B. Montesi were responsible for conceptualization, data curation, data analysis, preparation of the original draft, and review and editing the final draft. Iris Y. Zhou, Molly Mascia, Michael Magaletta, Peter Caravan, and Sydney B. Montesi were responsible for the DCE‐MRI study data curation and analysis. George A. Alba, Leo C. Ginns, Iris Y. Zhou, Molly Mascia, and Sydney B. Montesi were responsible for the DCE‐MRI study participant recruitment. George A. Alba, Jehan W. Alladina, and Francesca L. Giacona conducted the plasma analyses. All authors contributed to reviewing and editing the final draft.
CONFLICT OF INTEREST STATEMENT
G. A. A. reports consulting fees from Minerva Biotechnologies and holds a patent related to NEDD9 (PCT/US2019/059890). P. C. holds equity in Reveal Pharmaceuticals and Collagen Medical, reports consulting fees from Collagen Medical, and receives research funding from Pliant Therapeutics, Takeda, Transcode Therapeutics, and Janssen. B. A. M. reports consulting fees from Actelion Pharmaceuticals, Deerfield Company, and Tenax Therapeutics and holds a patient related to NEDD9 (PCT/US2019/059890; PCT/US2020/066886; Provisional 2023‐152‐29618‐0438P01). S. B. M. reports research funding from Pliant Therapeutics, Merck, and Boehringer Ingelheim, consulting fees from DevPro Biopharma, Gilead Sciences, and Roche, advisory board fees from Pliant Therapeutics and APIE Therapeutics, royalties form Wolters Kluwer, and speaking fees from Cowen. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Mass General Brigham Institutional Review Board.
ACKNOWLEDGMENTS
We would like to acknowledge Staci Mangini, Demi Ajao, and members of the Athinoula A. Martinos Center for Biomedical Imaging MRI Core for their assistance with DCE‐MRI, and Sophia Zhao for her assistance with statistical review. This study was funded with support from the Massachusetts General Hospital Department of Medicine to G. A. A., NIH K23HL150331 to S. B. M., K25HL148837 to I. Y. Z., and R01HL153606 to P. C.
Alba GA, Zhou IY, Mascia M, Magaletta M, Alladina JW, Giacona FL, Ginns LC, Caravan P, Maron BA, Montesi SB. Plasma NEDD9 is increased following SARS‐CoV‐2 infection and associates with indices of pulmonary vascular dysfunction. Pulm Circ. 2024;14:e12356. 10.1002/pul2.12356
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–128. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villalba JA, Hilburn CF, Garlin MA, Elliott GA, Li Y, Kunitoki K, Poli S, Alba GA, Madrigal E, Taso M, Price MC, Aviles AJ, Araujo‐Medina M, Bonanno L, Boyraz B, Champion SN, Harris CK, Helland TL, Hutchison B, Jobbagy S, Marshall MS, Shepherd DJ, Barth JL, Hung YP, Ly A, Hariri LP, Turbett SE, Pierce VM, Branda JA, Rosenberg ES, Mendez‐Pena J, Chebib I, Rosales IA, Smith RN, Miller MA, Rosas IO, Hardin CC, Baden LR, Medoff BD, Colvin RB, Little BP, Stone JR, Mino‐Kenudson M, Shih AR. Vasculopathy and increased vascular congestion in fatal COVID‐19 and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2022;206:857–873. 10.1164/rccm.202109-2150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alba GA, Samokhin AO, Wang R‐S, Wertheim BM, Haley KJ, Padera RF, Vargas SO, Rosas IO, Hariri LP, Shih A, Thompson BT, Mitchell RN, Maron BA. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071. 10.1002/pul2.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou IY, Mascia M, Alba GA, Magaletta M, Ginns LC, Caravan P, Montesi SB. Dynamic contrast‐enhanced MRI demonstrates pulmonary microvascular abnormalities months after SARS‐CoV‐2 infection. Am J Respir Crit Care Med. 2023;207(12):1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montesi SB, Zhou IY, Liang LL, Digumarthy SR, Mercaldo S, Mercaldo N, Seethamraju RT, Rosen BR, Caravan P. Dynamic contrast‐enhanced magnetic resonance imaging of the lung reveals important pathobiology in idiopathic pulmonary fibrosis. ERJ Open Res. 2021;7(4):00907‐2020. 10.1183/23120541.00907-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weatherley ND, Eaden JA, Hughes PJC, Austin M, Smith L, Bray J, Marshall H, Renshaw S, Bianchi SM, Wild JM. Quantification of pulmonary perfusion in idiopathic pulmonary fibrosis with first pass dynamic contrast‐enhanced perfusion MRI. Thorax. 2021;76(2):144–151. 10.1136/thoraxjnl-2019-214375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samokhin AO, Hsu S, Yu PB, Waxman AB, Alba GA, Wertheim BM, Hopkins CD, Bowman F, Channick RN, Nikolic I, Faria‐Urbina M, Hassoun PM, Leopold JA, Tedford RJ, Ventetuolo CE, Leary PJ, Maron BA. Circulating NEDD9 is increased in pulmonary arterial hypertension: a multicenter, retrospective analysis. J Heart Lung Transplant. 2020;39(4):289–299. 10.1016/j.healun.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Espín E, Yang C, Shannon CP, Assadian S, He D, Tebbutt SJ. Cellular and molecular biomarkers of long COVID: a scoping review. EBioMedicine. 2023;91:104552. 10.1016/j.ebiom.2023.104552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post‐COVID‐19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 11. Nikonova AS, Gaponova AV, Kudinov AE, Golemis EA. CAS proteins in health and disease: an update: CAS proteins: recent developments. IUBMB Life. 2014;66(6):387–395. 10.1002/iub.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alba GA, Samokhin AO, Wang R‐S, Zhang YY, Wertheim BM, Arons E, Greenfield EA, Lundberg Slingsby MH, Ceglowski JR, Haley KJ, Bowman FP, Yu YR, Haney JC, Eng G, Mitchell RN, Sheets A, Vargas SO, Seo S, Channick RN, Leary PJ, Rajagopal S, Loscalzo J, Battinelli EM, Maron BA. NEDD9 is a novel and modifiable mediator of platelet‐endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533–1545. 10.1164/rccm.202003-0719OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB. Pathogenic mechanisms of post‐acute sequelae of SARS‐CoV‐2 infection (PASC). eLife. 2023;12:e86002. 10.7554/eLife.86002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahamed J, Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest. 2022;132(15):e161167. 10.1172/JCI161167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Talla A, Vasaikar SV, Szeto GL, Lemos MP, Czartoski JL, MacMillan H, Moodie Z, Cohen KW, Fleming LB, Thomson Z, Okada L, Becker LA, Coffey EM, De Rosa SC, Newell EW, Skene PJ, Li X, Bumol TF, Juliana McElrath M, Torgerson TR. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat Commun. 2023;14(1):3417. 10.1038/s41467-023-38682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, Li S, Hong S, Zhang R, Xie J, Kornilov SA, Scherler K, Pavlovitch‐Bedzyk AJ, Dong S, Lausted C, Lee I, Fallen S, Dai CL, Baloni P, Smith B, Duvvuri VR, Anderson KG, Li J, Yang F, Duncombe CJ, McCulloch DJ, Rostomily C, Troisch P, Zhou J, Mackay S, DeGottardi Q, May DH, Taniguchi R, Gittelman RM, Klinger M, Snyder TM, Roper R, Wojciechowska G, Murray K, Edmark R, Evans S, Jones L, Zhou Y, Rowen L, Liu R, Chour W, Algren HA, Berrington WR, Wallick JA, Cochran RA, Micikas ME, Wrin T, Petropoulos CJ, Cole HR, Fischer TD, Wei W, Hoon DSB, Price ND, Subramanian N, Hill JA, Hadlock J, Magis AT, Ribas A, Lanier LL, Boyd SD, Bluestone JA, Chu H, Hood L, Gottardo R, Greenberg PD, Davis MM, Goldman JD, Heath JR. Multiple early factors anticipate post‐acute COVID‐19 sequelae. Cell. 2022;185(5):881–895. 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izquierdo‐Garcia D, Désogère P, Philip AL, Mekkaoui C, Weiner RB, Catalano OA, Iris Chen YC, DeFaria Yeh D, Mansour M, Catana C, Caravan P, Sosnovik DE. Detection and characterization of thrombosis in humans using fibrin‐targeted positron emission tomography and magnetic resonance. JACC: Cardiovasc Imag. 2022;15(3):504–515. 10.1016/j.jcmg.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


