Abstract
It has been reported previously that cells expressing a truncated form of CD4 which lacks the cytoplasmic tail of the molecule (truncation at position 402) were not sensitive to human immunodeficiency virus type 1 (HIV-1)-induced apoptosis in an acute-phase model of infection (J. Corbeil, M. Tremblay, and D. D. Richman, J. Exp. Med. 183:39–48, 1996). The role played by the cytoplasmic domain of CD4 in HIV-1-induced apoptosis was reexamined here with clones of A2.01 cells expressing different forms of CD4 and the DNA intercalant YOPRO-1 assay. Six days after virus exposure, we found evidence of apoptosis in A2.01 cells expressing the wild-type CD4 (A2.01/CD4), whereas enhanced apoptosis remained absent in cultures of A2.01/CD4.401 and A2.01/CD4.403 cells (A2.01 cells which express CD4.401 and CD4.403 molecules with truncations at positions 401 and 403, respectively). However, cell death by apoptosis measured with YOPRO-1 was found in cultures of A2.01/CD4.401 and A2.01/CD4.403 cells 15 days after virus exposure. This result was confirmed with a terminal dUTP nick end-labeling assay and propidium iodide staining. The long lag time postinfection required for apoptosis to be observed in cultures of infected cells expressing truncated forms of CD4 was due to the delayed viral replication in these cells, as shown by monitoring of the viral reverse transcriptase activity and HIV-1 p24gag antigen expression. These results emphasize the relationship between virus replication and cell death by apoptosis.
Gradual depletion of CD4+ T lymphocytes is one of the major consequences of human immunodeficiency virus type 1 (HIV-1) infection (29). Among the mechanisms contributing to CD4 cell depletion (2, 17, 21, 31), the cytopathic effects resulting from the infection of CD4+ T cells by HIV-1 are considered to play an important role. Cytopathic effects may be ascribed to gp120-induced syncytium formation and HIV-1-induced single-cell killing by apoptosis. It has been proposed that HIV-1-induced apoptosis requires an initial round of virus gene expression followed by engagement of expressed gp120 with CD4 to complete the process (13, 22, 23).
Although the CD4 molecule is involved in virus binding and postbinding events leading to membrane fusion, its role during the latter stages of the HIV-1 replication cycle is not fully understood (11). It is generally admitted that the cytoplasmic tail of CD4 is not necessary for HIV-1 binding and subsequent internalization of virions but plays a role in controlling different steps of the virus replication cycle (4, 32). However, experiments aimed at investigating this role have given rise to divergent conclusions: cells expressing a CD4 molecule lacking the cytoplasmic tail can form syncytia, whereas cells expressing a CD4-CD8 hybrid remained insensitive to HIV-1-induced syncytium formation (28); however, if cultured for a longer time, A2.01/CD4-CD8 cells would form syncytia (15). We have also observed syncytium formation in infected cultures of A2.01/CD4-CD8, A2.01/CD4.401, and A2.01/CD4.403 cells associated with high virus production (16a).
Recently, cells expressing a truncated form of CD4 lacking the cytoplasmic tail (truncation at position 402) were used to assess the involvement of the cytoplasmic tail in HIV-1-induced apoptosis. A2.01/CD4.402 cells were reported not to be susceptible to HIV-1-induced apoptosis 3 days after exposure to a high virus concentration (14). Since we have demonstrated previously a significant delay in HIV-1 replication in cells expressing a truncated cytoplasmic domain of CD4 that resulted from the inability of the receptor to stimulate the nuclear expression of NF-κB (4), we reinvestigated HIV-1-induced apoptosis in A2.01/CD4.401 and A2.01/CD4.403 cells, taking into account the delayed replication parameter.
In this study, we found that cells expressing truncated forms of CD4 lacking the cytoplasmic tail are sensitive to HIV-1-induced apoptosis, but only after a long lag time. Early stages (binding, infection, and retrotranscription) of the HIV-1 replicative cycle occur at the same rate in those cells compared to cells expressing the wild-type CD4, but despite this, virus gene expression and virus particle production are delayed (4). Altogether, our results suggest that the long lag time that passed between virus exposure and cell death by apoptosis is due to a delayed virus gene expression and emphasize the relationship that exists between viral load, virus replication, and cell death by apoptosis.
MATERIALS AND METHODS
MAb and reagents.
Purified anti-CD4 IOT4A/13B8.2 monoclonal antibody (MAb) was kindly provided by M. Hirn (Immunotech S.A., Marseille, France). Anti-Fas (CH-11) immunoglobulin M (IgM) MAb, anti-Fas (ZB4) IgG MAb, fluorescein isothiocyanate (FITC)-labeled anti-Fas (UB2) MAb, and FITC-labeled F(ab′)2 goat anti-mouse Ig reagent were purchased from Immunotech. An FITC-labeled anti-p24gag MAb (KC57-FITC) was purchased from Coulter Corp. (Margency, France). An anti-p56lck MAb (3A5) was purchased from TEBU (Le Perray en Yvelines, France). The T4-4 rabbit anti-human CD4 antiserum (33) was provided by M. Benkirane (National Institute of Allergy and Infectious Diseases, Bethesda, Md.). DNA intercalant dye YOPRO-1 was purchased from Molecular Probes (Eugene, Oreg.). Geneticin G-418, used at 1 mg/ml, was purchased from Gibco-Life Technologies (Eragny, France).
Cells and viruses.
The CD4+ lymphoblastoid CEM T-cell line was obtained from the American Type Culture Collection (Bethesda, Md.). The A2.01 (a CD4− lymphoblastoid T-cell line derived from the CD4+ T-cell line A3.01), A2.01/CD4 (A2.01 expressing the wild-type CD4), A2.01/CD4.401 (A2.01 expressing a mutant form of CD4 truncated at position 401), and A2.01/CD4.403 (A2.01 expressing a mutant form of CD4 truncated at position 403) cell clones have been previously described (3, 4) and were provided by D. R. Littman (New York Medical College, New York, N.Y.). Cells were cultured in RPMI 1640 medium supplemented with a 1% penicillin–streptomycin antibiotic mixture, 1% GlutaMAX, and 10% fetal calf serum (Gibco-Life Technologies) to a density of 5 × 105 cells/ml in a 5% CO2 atmosphere. The culture medium of transfected cells was supplemented with 1 mg of G-418 per ml. Viral stocks of HIV-1Lai were prepared from chronically infected CEM cell supernatants, as previously described (12), and kept frozen at −80°C until used.
Assays for HIV-1 infection.
Cells (5 × 105) were incubated for 30 min at 4°C in flat-bottom, 96-microwell plates (TPP, Beyneix, Marseille, France) with 100 μl of HIV-1 at a concentration of 1,000 × 50% tissue culture infective dose (TCID50) per ml. Thereafter, cells were washed five times and cultured in 24-microwell plates (TPP). The amount of HIV-1 produced by CEM cells was monitored twice a week by measuring reverse transcriptase (RT) activity in 1 ml of cell-free culture supernatant with a synthetic template primer, as previously described (12).
Flow cytometry.
Cells (106) were incubated for 60 min at 4°C with saturating concentrations of anti-CD4 MAb or medium alone as control. After three washes with phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin, bound MAb was detected by addition of 50 μl of a 1/50 dilution of fluoresceinated goat anti-mouse Ig (Immunotech). After 60 min of staining, cells were washed with PBS-bovine serum albumin, and fluorescence intensity was measured on an EPICS XL4C cytofluorometer (Coulter, Coultronics, Margency, France). For the measurement of HIV-1 p24gag expression, the cells were fixed and permeabilized with methanol. HIV-1 p24gag antigen expression was monitored by direct immunofluorescence with MAb KC57-FITC (Coulter).
Detection of apoptosis.
Different assays were used for the detection of HIV-1-induced apoptosis. First, the percentage of apoptotic cells was assessed by flow cytometry analysis with the impermeant DNA intercalant YOPRO-1 (10 mM) (excitation maximum/emission maximum 491/509 nm) as described previously (18). Second, the level of apoptotic cells in cultures was determined with an ApopDETEK kit (Enzo Diagnostics, Farmingdale, N.Y.) for the terminal dUTP nick end-labeling (TUNEL) assay, which allows detection of double-stranded DNA (dsDNA) breaks by flow cytometry. Briefly, cells (2 × 106 cells/sample) were fixed in 1% paraformaldehyde in PBS (pH 7.4) containing 0.3% saponin for 15 min on ice. After being washed in PBS, cells were incubated at 37°C for 60 min with terminal deoxynucleotide transferase and biotin-16–dUTP. After being washed, cells were resuspended in 500 μl of PBS containing streptavidin-FITC. After 30 min of incubation at 37°C, cells were washed and fluorescence intensity was measured. Third, to relate apoptosis to the cell cycle, cells (3 × 105 cells/sample) were washed in PBS and resuspended for 2 h at 20°C in a solution containing 0.1% Triton X-100, 0.1% sodium citrate, and 50 μg of propidium iodide per ml. Cell cycle analysis based on DNA content per cell was performed with the MultiCycle AV version 3.0 program of the EPICS XL4C cytofluorometer. Apoptotic nuclei appeared as a broad hypodiploid DNA peak easily discriminable from the normal (diploid) DNA peak.
Western blot assay.
Cells (5 × 106) were washed twice in PBS. The cell pellets were resuspended in 100 μl of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 8], 100 mM NaCl, 10 mM EDTA, 1% Triton X-100, 1 mM MgCl2, 2 mM benzamidine, 2 μg of leupeptin per ml, 1 mM (phenylmethylsulfonyl fluoride) and lysed for 20 min at 4°C. After a 15-min centrifugation (10,000 rpm; Biofuge 13; Heraeus Instruments) at 4°C in a microcentrifuge, supernatants were harvested, and the protein concentration was measured. Cellular lysates were electrophoresed onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) (SDS-PAGE) and blotted to polyvinylidene difluoride (PVDF) membrane (Millipore). The blots were then incubated for 1 h at room temperature with a blocking solution (PBS containing 10% milk and 0.05% Tween 20) prior to addition of anti-p56lck MAb. After 1 h at 20°C, blots were washed three times with PBS–0.05% Tween 20 and incubated for 30 min with a 1:3,000 dilution of sheep anti-mouse Ig–peroxidase conjugate (Immunotech). After three washes, bound MAb was detected by incubation of the membrane for 1 min with enhanced chemiluminescence (ECL) reagent (Amersham). The membrane was then exposed for 0.5 to 5 min to hyperfilms-ECL (Amersham).
Coimmunoprecipitation of CD4 and p56lck.
Cell lysates (see above for details) were submitted to a round of preclearing with 30 μl of Sepharose-protein A (Pharmacia CL-4B) for 60 min at 4°C. The samples were microcentrifuged (10,000 rpm) for 1 min at 4°C, and supernatants (1 ml each) were incubated for 16 h at 4°C with 2 μg of anti-CD4 antibody (13B8-2 and BL4) and 30 μl of Sepharose-protein A. After three washes in radioimmunoprecipitation assay buffer, precipitates were electrophoresed in a 6.5% polyacrylamide gel and blotted to PVDF membrane. CD4 and p56lck antigens were detected by addition of T4-4 antiserum and mAb 3A5, respectively.
RESULTS
Absence of enhanced apoptosis in cells expressing truncated forms of CD4 6 days after infection by HIV-1.
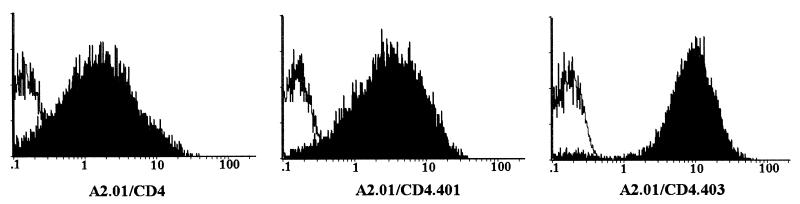
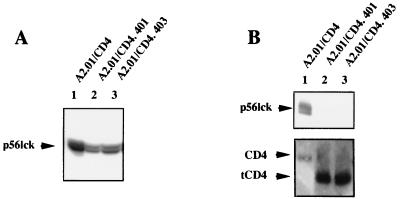
The role played by the cytoplasmic domain of CD4 in HIV-1-induced apoptosis was investigated with different clones of A2.01 cells, a CD4− derivative of the human T-cell leukemic line A3.01, expressing different CD4 constructs (3). After transfection, the clones of A2.01 cells expressing either the wild-type CD4 molecule (A2.01/CD4) or truncated forms of the CD4 receptor lacking the cytoplasmic domain of the molecule (A2.01/CD4.401 and A2.01/CD4.403) were selected and shown to be fully infectable by HIV-1 (3, 4, 28). Delayed virus production was previously reported for cells expressing the truncated forms of CD4 (3, 4, 28). In contrast to wild-type CD4, the truncated forms of CD4 are predicted to be unable to deliver signals through association with p56lck or other cytoplasmic signaling proteins because of an inability of the receptor to stimulate nuclear translocation of transcription factors after HIV-1 binding to its receptor (4). Cell surface expression of the different CD4 molecules, the presence of cytoplasmic p56lck, and the ability of p56lck to associate with CD4 were assessed. As shown in Fig. 1, cell surface expression of CD4, CD4.401, and CD4.403 was high in all of the clones (although it was slightly lower for the wild-type CD4). The clones were also found to express similar amounts of p56lck protein (Fig. 2A). p56lck was coimmunoprecipitated with CD4 in A2.01/CD4 cell lysates but was absent from immunoprecipitates utilizing A2.01/CD4.401 and A2.01/CD4.403 cell lysates (Fig. 2B).
FIG. 1.
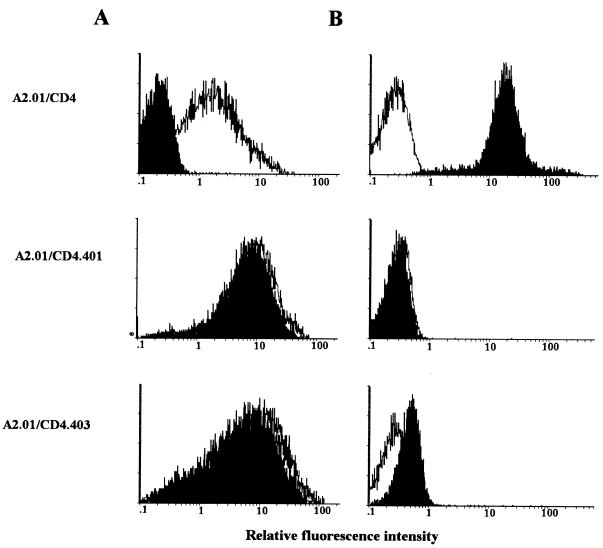
Expression of the different forms of CD4 at the surface of transfected A2.01 cells. A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells were incubated with saturating concentrations of anti-CD4 (13B8-2) MAb (black areas) or medium alone (white areas) and fluoresceinated goat anti-mouse Ig. The fluorescence intensity was measured on an EPICS XL4C cytofluorometer.
FIG. 2.
Expression of p56lck in transfected A2.01 cells expressing different forms of CD4. (A) Western blot assay of p56lck expressed in A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells. Cell lysates (50 μg) were electrophoresed onto an SDS-PAGE (10% polyacrylamide) gel and blotted to a PVDF membrane. The blots were incubated with an anti-p56lck antibody, and bound MAbs were reacted with sheep anti-mouse Ig–peroxidase conjugate–ECL reagent. (B) Coimmunoprecipitation of CD4 and p56lck. Cell lysates were incubated with a mixture containing 2 μg of anti-CD4 antibody (13B8-2 and BL4) and 30 μl of Sepharose-protein A. After being washed, precipitates were electrophoresed in a 6.5% polyacrylamide gel and blotted to the PVDF membrane. CD4 (lower panel) and p56lck (upper panel) antigens were detected by addition of T4-4 antiserum and MAb 3A5, respectively. tCD4, truncated forms of CD4.
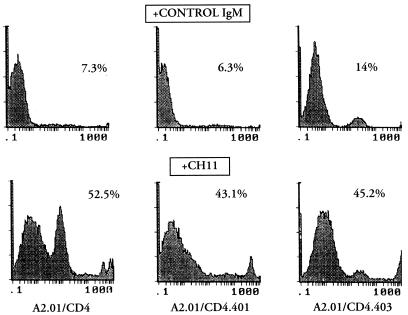
We verified the ability of a known inducer of apoptosis (anti-Fas IgM) to activate the signal transduction machinery controlling cell death in A2.01 cells expressing different forms of CD4. The cells were treated with the CH-11 anti-Fas IgM MAb or medium alone for 16 h before being stained with DNA intercalant YOPRO-1 in order to estimate the extent of apoptosis. As shown in Fig. 3, apoptosis was found in cultures of all clones treated with the CH-11 MAb, including A2.01/CD4 (52.5% cell death), A2.01/CD4.401 (43.1% cell death), and A2.01/CD4.403 (45.2% cell death) compared with that in cells treated with an isotype-matched control IgM, indicating that the signal transduction machinery that controls cell death by apoptosis can be recruited by the Fas signal in cells expressing truncated forms of CD4.
FIG. 3.
Anti-Fas IgM-induced apoptosis of CD4-transfected clones. Cells were cultured in medium supplemented with 1 μg of anti-Fas IgM MAb (CH-11) or an isotype-matched (IgM) MAb per ml. The percentage of apoptotic cells in cell cultures was assessed by flow cytometry analysis with YOPRO-1 at 16 h of culture. We provisionally included the peak number of dead cells in the calculation of the percentage of cells undergoing apoptosis.
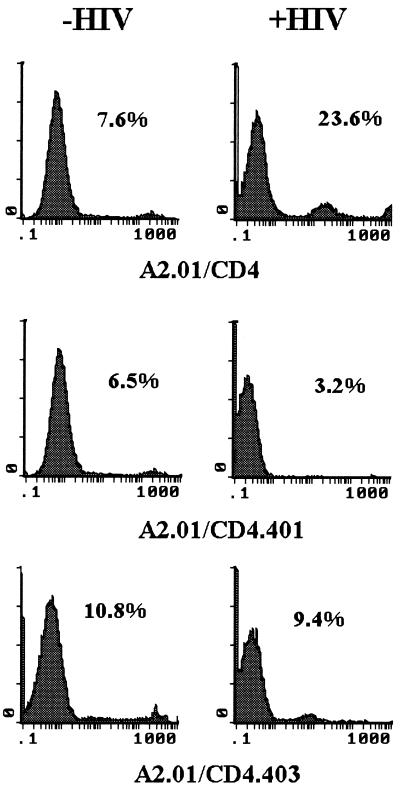
To determine the susceptibility of A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells to HIV-1-induced apoptosis, cells were exposed to 100 μl of HIV-1Lai (at 1,000 × TCID50/ml) and apoptosis was analyzed by cytofluorometry 6 days after virus exposure with YOPRO-1. As shown in Fig. 4, a background amount of apoptosis (ranging from 5 to 10%) occurred in uninfected control cultures (Fig. 4, left panel). Enhanced apoptosis was detected in A2.01/CD4 cells exposed to infectious particles (23.6% cell death compared to 7.6% cell death in the control culture). In contrast, HIV-1 exposure did not stimulate apoptosis in A2.01/CD4.401 and A2.01/CD4.403 cells at this time point. The CD4 phenotype of the cells and their ability to express the HIV-1 gene were investigated. Although the presence of HIV-1 DNA can be detected in truncated CD4 cells 24 h after HIV-1 exposure under these experimental conditions (data not shown and reference 4), cell surface expression of CD4 was strongly modulated in A2.01/CD4 cells but not in A2.01/CD4.401 and A2.01/CD4.403 cells (Fig. 5A). As expected, the extent of CD4 modulation was directly related to HIV-1 gene expression, as evaluated by p24gag antigen detection (Fig. 5B).
FIG. 4.
Measurement of apoptotic cell death in A2.01-transfected clones 3 days after virus exposure. A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells were exposed to 100 μl of virus suspension containing 1,000 × TCID50 of HIV-1Lai per ml, washed, and cultured for 3 days in medium alone. The percentage of apoptotic cells in cell cultures was assessed by flow cytometry analysis with the impermeant DNA intercalant YOPRO-1.
FIG. 5.
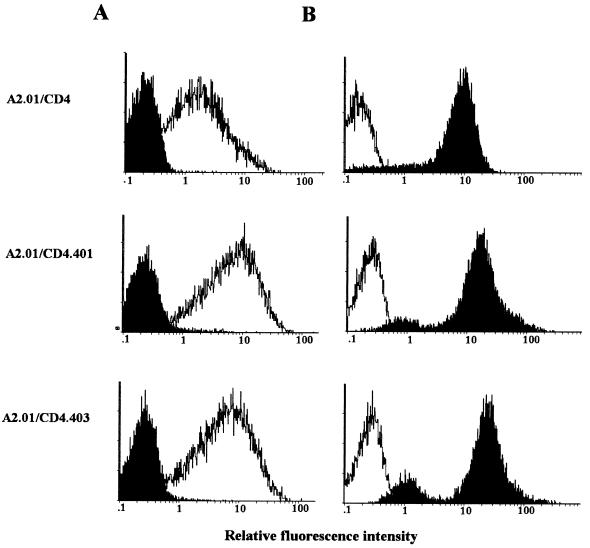
Effect of infection on CD4 antigen modulation and p24gag antigen expression in A2.01 transfectant cells. A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells exposed to HIV-1Lai were cultured for 3 days in medium alone. (A) The percentage of cells showing detectable expression of surface CD4 (black areas) was measured by indirect immunofluorescence with the anti-CD4 MAb 13B8.2 and an FITC-conjugated goat antimouse antibody probe. (B) HIV-1 p24gag antigen expression (black areas) was monitored on methanol-permeabilized cells by direct immunofluorescence with the MAb KC57-FITC. Levels of expression of CD4 and HIV-1 p24gag antigen in uninfected cells (white areas) are shown as a control.
Our results indicate that HIV-1-induced apoptosis was not found in A2.01/CD4.401 and A2.01/CD4.403 cells 6 days after virus exposure, although all clones turned out to be readily infected (also see the results presented below). These observations corroborate previously published results by Corbeil and coworkers (14).
Delayed HIV-1-induced apoptosis in culture of cells expressing truncated forms of CD4.
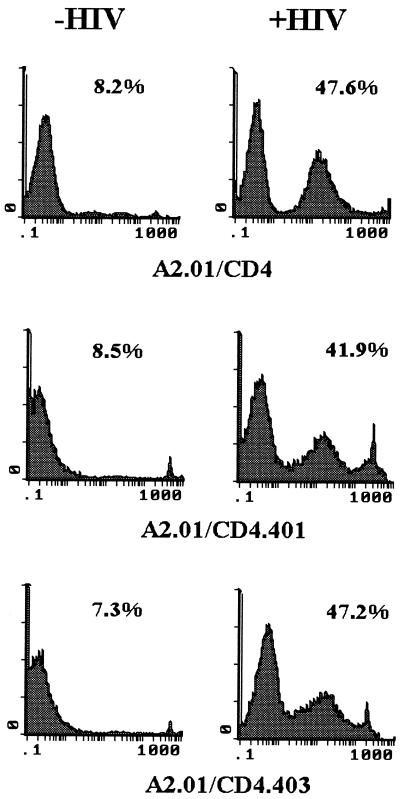
Previous studies have indicated that virion production was delayed in cells expressing truncated forms of CD4 which lack the cytoplasmic domain, although the kinetics of HIV-1 binding, fusion, and retrotranscription are similar in A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells (3, 4, 28). By running time course experiments aimed at investigating the kinetics of HIV-1-multispliced mRNA expression in those cells, we previously reported that HIV-1 gene expression was observed on day 3 after exposure to 100 μl of HIV-1Lai at 1,000 × TCID50/ml in A2.01/CD4 cells, whereas it was detected after day 10 in A2.01/CD4.401 cells (4). Figure 6A illustrates virus production in infected-cell cultures as measured by RT activity assay. In cells exposed to HIV-1Lai at 1,000 × TCID50/ml, virus production was detected 4 days after virus exposure and reached a plateau within 7 days of infection in A2.01/CD4 cells, whereas it was detected in A2.01/CD4.401 and A2.01/CD4.403 cells only after 10 days of culture. Cell death associated with virus replication was also delayed in A2.01/CD4.401 and A2.01/CD4.403 cells compared to that in A2.01/CD4 cells (Fig. 6B). Similar results were obtained when cells were exposed to 100 μl of HIV-1Lai at 10,000 × TCID50/ml (Fig. 6C and D). To confirm the HIV-1 induction of apoptosis in A2.01/CD4.401 and A2.01/CD4.403 cells, a time course experiment was performed in which apoptosis was monitored with the YOPRO-1 assay. Figure 7 illustrates a representative experiment run 15 days postinfection; under such experimental conditions, we found that HIV-1 triggers apoptosis in the two clones that expressed truncated forms of CD4. The percentages of cell death by apoptosis were 41.9 and 47.2% for infected A2.01/CD4.401 and A2.01/CD4.403 cells, respectively, whereas the background amounts of apoptosis in uninfected control cultures were 8.5 and 7.3% respectively. Enhanced apoptosis was also present in A2.01/CD4 cells (47.6% cell death compared to 8.2% cell death in the control culture). CD4 cell surface analysis indicated that all infected clones had downregulated surface expression of CD4 on day 15 postinfection (Fig. 8A). Moreover, levels of p24gag antigen synthesis were found to be equal in all clones on day 15 postinfection (Fig. 8B).
FIG. 6.
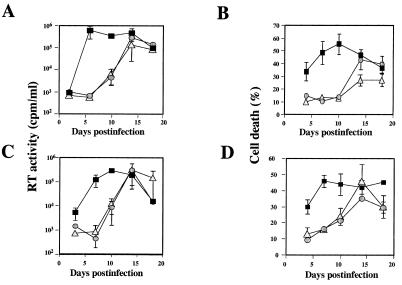
Kinetics of virus production and cell death. A2.01/CD4 (▪), A2.01/CD4.401 (○), and A2.01/CD4.403 (▵) cells were exposed to 100 μl of virus suspension containing either 1,000 × TCID50 of HIV-1Lai per ml (A and B) or 10,000 × TCID50 of HIV-1Lai per ml (C and D) washed and cultured in medium alone. Virus production was monitored by measuring RT activity (A and C). The percentage of cell death in cell cultures was assessed by trypan blue exclusion (B and D). Values represent means ± standard deviations (n = 3).
FIG. 7.
Measurement of apoptotic cell death in A2.01-transfected clones 15 days after virus exposure by YOPRO-1 staining. A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells were exposed to HIV-1Lai, and the percentage of apoptotic cells in cell cultures was assessed by flow cytometry analysis by YOPRO-1 labeling 15 days postinfection.
FIG. 8.
Effect of infection on CD4 antigen modulation and p24gag antigen expression in A2.01 transfectant cells. A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells exposed to HIV-1Lai were cultured for 15 days in medium alone. The percentages of cells showing detectable expression of surface CD4 (A) and HIV-1 p24gag antigen (B) were monitored as described in the legend to Fig. 5.
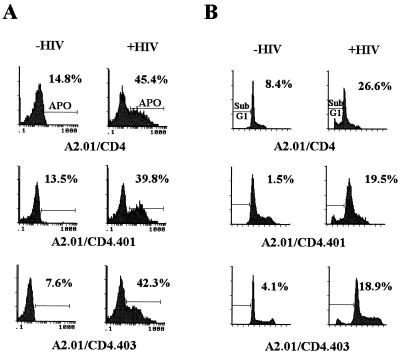
To make an unambiguous demonstration that HIV-1-induced apoptosis occurs in cells expressing mutated CD4 molecules lacking a cytoplasmic tail, we studied apoptosis by using two additional techniques. First, the early stages of the chromatin breakdown process were studied with TUNEL assay, which allows detection of dsDNA breaks by incorporation of biotin-labeled deoxynucleotides on the 3′-OH termini. Under these experimental conditions, we found chromatin damage in 39.8 and 42.3% of infected A2.01/CD4.401 and A2.01/CD4.403 cells, respectively, whereas the background levels of apoptosis in uninfected control cultures were 13.5 and 7.6%, respectively (Fig. 9A). These results agree with the percentage of apoptosis measured with YOPRO-1. Second, HIV-1 induction of apoptotic nuclei was studied by counting sub-G1-phase cells stained by propidium iodide. The numbers of A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells infected by HIV-1 showing a broad hypodiploid DNA peak were 26.6, 19.5, and 18.9%, respectively, whereas the background levels were 8.4, 1.5, and 4.1%, respectively (Fig. 9B). It is worth noting that an important proportion of apoptotic cells may not be considered by this method, based on DNA content per cell, and this probably explains why the percentages of apoptosis measured by propidium iodide staining were lower than those measured by the YOPRO-1 and TUNEL assays.
FIG. 9.
Measurement of apoptotic cell death in A2.01-transfected clones 15 days after virus exposure with the TUNEL assay (A) and propidium iodide staining (B). (A) A2.01/CD4, A2.01/CD4.401, and A2.01/CD4.403 cells were exposed to HIV-1Lai, and the percentage of cells in cultures showing DNA strand breakage was measured at day 15 postinfection by flow cytometry analysis (TUNEL assay). (B) To relate apoptosis to the cell cycle position, cells exposed to HIV-1Lai and control cultures were stained with propidium iodide. Sub-G1-phase cells were counted by flow cytometry analysis.
Altogether, our results indicate that A2.01/CD4.401 and A2.01/CD4.403 cells are sensitive to HIV-1-induced apoptosis, the sensitivity being directly related to HIV-1 gene expression.
DISCUSSION
CD4+ T cells undergo apoptosis when infected by HIV-1. The aim of the present study was to investigate the role played by the CD4 cytoplasmic tail in HIV-1-induced apoptosis. In agreement with results previously reported by Corbeil and coworkers (14), we found that cells expressing truncated forms of CD4 which lack the cytoplasmic domain did not undergo cell death early after virus exposure. However, when cultures were kept for over 2 weeks, we found HIV-1-induced apoptosis. The long lag time that passed between virus exposure and the first evidence for HIV-1-induced apoptosis in cultures of A2.01/CD4.401 and A2.01/CD4.403 cells was directly linked to delayed HIV-1 gene expression in these cells. Finally, the observation that the cytoplasmic tail of CD4 is not required to mediate HIV-1-induced apoptosis suggests that the interaction between CD4 and p56lck is not essential for HIV-1-induced apoptosis.
The role played by the cytoplasmic tail of CD4 in virus binding, virus internalization, retrotranscription of the virus genome, expression of virus genes, and virus envelope-dependent syncytium formation has been studied in depth. The cytoplasmic tail of CD4 is necessary neither for binding and subsequent internalization of HIV-1 virions (3, 4, 28, 32) nor for syncytium formation induced by the HIV-1 envelope (15, 16a). Although the cytoplasmic tail of CD4 is considered to be required for signal transduction via CD4 after HIV-1 binding (4, 5, 32), the results obtained by different laboratories have led to divergent conclusions which can probably be ascribed either to variations in technical approaches or to variations among the transfected cell clones used. It is worth noting that several reports describe activation by HIV-1 of cells expressing a wild-type CD4 (4, 6, 9, 10, 20, 27). Indeed, our previous studies have indicated that the cytoplasmic tail of CD4 is required for activation of signal transduction pathways, resulting in the nuclear translocation of transcription factors such as NF-κB, after HIV-1 binding to CD4+ cells (4). Similar conclusions can be drawn from the results by Merzouki and coworkers (25), indicating that HIV-1 envelope glycoproteins expressed at the surface of stimulating cells induced activation of the HIV-1 promoter in CD4-positive p56lck-positive cells, whereas induction was not observed in CD4-positive p56lck-negative cells. These observations strengthen the hypothesis that the CD4 cytoplasmic tail, which is known for its ability to interact with p56lck, is involved in transduction of an activation signal consecutive to HIV-1 binding to the extracellular portion of the molecule. Consequently, a lack of signal transduction could explain the delayed virus production observed in cells expressing a truncated form of CD4 (3, 4, 7, 28).
Concerning HIV-1-induced apoptosis, the role played by the cytoplasmic tail of CD4 remains obscure. A study recently reported by Goldman and coworkers (16) suggested that CD4 ligation by gp120–anti-gp120 complexes uncoupled p56lck from CD4. This phenomenon possibly induces anergy, a first step toward apoptosis. According to Corbeil and coworkers (14), the expression of p56lck is not absolutely required for HIV-1 induction of apoptosis, but it may be required to prolong the cell surface expression of CD4, thereby permitting the delivery of the apoptotic signal. These authors have also reported that A2.01/CD4.418 cells (expressing a truncated mutant of CD4 at position 418 which does not associate with p56lck) and A2.01/CD4mut (expressing a dicysteine C420A, C422A mutant form of CD4 that disrupts CD4-p56lck association) underwent apoptosis upon HIV-1 infection (26). However, they did not find apoptosis in A2.01/CD4.402 cells (expressing a truncated mutant of CD4 at position 402), and therefore have suggested that transduction of the apoptotic signal involves amino acids located between positions 402 and 418 of CD4.
Here, we have reinvestigated the putative role played by the cytoplasmic tail of CD4 in HIV-1-induced apoptosis by using an experimental model system similar to that described above, which consisted of cells expressing truncated mutants of CD4 at positions 401 and 403, respectively. These truncated forms of CD4 have lost the ability to interact with p56lck; therefore, the dissociation described by Goldman et al. (16) is not possible. Moreover, if these cells undergo apoptosis, the apoptotic signals cannot be transduced via the CD4 cytoplasmic tail. Although we could not find evidence for HIV-1-induced apoptosis in the A2.01/CD4.401 and A2.01/CD4.403 cells early after virus exposure, apoptosis was noticed 15 days after virus exposure (Fig. 7). It is worth noting that p24gag antigen was clearly detected in A2.01/CD4.401 and A2.01/CD4.403 cells 15 days after virus exposure (Fig. 8B) and that expression of CD4 was modulated in those cells (Fig. 8A). Most likely, downregulation of truncated forms of CD4 involves the formation of Env-CD4 complexes trapped within the endoplasmic reticulum (24), whereas several mechanisms, including those that involve the HIV-1 regulatory gene products (Nef and Vpu), probably contribute to the modulation of the surface expression of wild-type CD4 (8, 30). The long lag time that passed between virus exposure and the first evidence for HIV-1-induced apoptosis in cultures of A2.01/CD4.401 and A2.01/CD4.403 cells was directly linked to delayed HIV-1 gene expression in these cells (Fig. 6). Therefore, it is not clear at present why HIV-1-infected A2.01/CD4.402 cells were not found susceptible to HIV-1 apoptosis in the experiments reported by Corbeil and coworkers (14). These authors used much more virus (0.5 < multiplicity of infection <1) than we did (we used 100 μl of virus at 1,000 × TCID50/ml to 10,000 × TCID50/ml, concentrations of HIV-1 that correspond to 0.002 < multiplicity of infection <0.02), had a different infection protocol (3 h at 37°C instead of 30 min at 4°C), and investigated apoptosis within 3 days after infection by using propidium iodide, which is less sensitive than YOPRO-1 for quantification of apototic events. A possible explanation is that apoptosis of A2.01/CD4.402 cells induced by HIV-1 was slightly delayed compared to viral production (the reasons for delayed apoptosis are discussed below). Alternatively, it remains possible that the requirement for CD4 signaling differs between low- and high-viral-load models of infection. Although this problem remains to be elucidated, it can be concluded from our results that truncations of CD4 at position 401 or 403 do not abrogate HIV-1-induced apoptosis. This conclusion is strengthened by the work from Jacotot and coworkers (19), who have very recently reported apoptosis of A2.01/CD4.402 cells induced by HIV-1 infection or by coculture with chronically HIV-1-infected H9 cells. In their experiments, these authors have measured the occurrence of apoptosis in A2.01/CD4.402 cells by using a sensitive assay which consists of analysis of the presence of nucleosomal histones (H2A, H2B, H3, and H4) in the nucleoplasm of cells by quantitative densitometry of stained histones.
In agreement with Corbeil’s proposal, our results confirm that the interaction between p56lck and CD4 is not necessary to induce apoptosis, but rather may accelerate the transmission of a cell death signal. It is worth noting that virus production begins 3 days before significant apoptosis can be detected in culture. Previous experiments by Corbeil and Richman (13) suggested that at least reverse transcription and possibly production of viral proteins must occur to render the cells susceptible to apoptosis. Using a different experimental model system, we have recently observed that HIV-1-induced apoptosis of CEM cells was abrogated by addition to the cell culture medium 6 h postinfection of an antibody that inhibits HIV-1 transcription (16b). This result leads us to conclude that HIV-1-induced apoptosis requires HIV-1 gene expression. It has been hypothesized (13) that target cells would require to be infected and then would be resignaled at the cell surface to undergo apoptosis. The fact that the present study demonstrates that the CD4 cytoplasmic tail is not required for delivery of the apoptotic signals indicates that the second step of the process does not involve recruitment of a signaling cascade through the cytoplasmic tail of CD4 but rather acts through another mechanism that remains to be elucidated. An interesting hypothesis has been recently proposed by Jacotot and coworkers (19), who suggest that the HIV-1 fusion cofactor CXCR4/fusin may be implicated in HIV-1-induced apoptosis by allowing optimal interactions between the gp120-gp41 and the CD4-CXCR4 complexes and/or by transducing specific heterotrimeric protein G-dependent signals during these interactions.
Uncontrolled and chronic immune activation triggered by HIV-1 (4, 6, 19, 25) is probably the primary mechanism responsible for the collapse of the immune system observed in AIDS patients (1, 16). It should involve transcription factors stimulating cell activation and virus replication but also triggering activation-induced apoptosis. It is admitted that the CD4 molecule plays a role in HIV-1-induced apoptosis, yet many aspects of the molecular interactions triggering this process remain to be elucidated. Although the situation in the primary CD4+ T cell is fairly different from that in the lymphoblastoid T-cell line, the present study demonstrates that the cytoplasmic tail of CD4 is not required in this process.
ACKNOWLEDGMENTS
We thank Michel Hirn (Coulter-Immunotech, Marseille, France) and Dan R. Littman (New York Medical College, New York, N.Y.) for providing reagents and cells. We also thank Jacques Corbeil (University of California—San Diego, San Diego, Calif.) and Nelly Noraz (IGMM, Montpellier, France) for critical readings of the manuscript.
This work was supported by institutional funds from the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), and a grant (to C.D.) from the Agence Nationale de Recherches sur le SIDA (ANRS). C.G. and N.C. are fellows of the ANRS.
REFERENCES
- 1.Ameisen J C. Programmed cell death (apoptosis) and cell survival regulation: relevance to AIDS and cancer. AIDS. 1994;8:1197–1213. doi: 10.1097/00002030-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen J-C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 3.Bedinger P, Moriarty A, von Borstel II R C, Donovan N J, Steinmer K S, Littman D R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane M, Jeang K-T, Devaux C. The cytoplasmic domain of CD4 plays a critical role during the early stages of HIV infection in T-cells. EMBO J. 1994;13:5559–5569. doi: 10.1002/j.1460-2075.1994.tb06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bérubé P, Barbeau B, Cantin R, Sékaly R-P, Tremblay M. Repression of human immunodeficiency virus type 1 long terminal repeat-driven gene expression by binding of the virus to its primary cellular receptor, the CD4 molecule. J Virol. 1996;70:4009–4016. doi: 10.1128/jvi.70.6.4009-4016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briant L, Coudronnière N, Robert-Hebmann V, Benkirane M, Devaux C. Binding of HIV-1 virions or gp120-anti-gp120 immune complexes to HIV-1-infected quiescent peripheral blood mononuclear cells reveals latent infection. J Immunol. 1996;156:3994–4004. [PubMed] [Google Scholar]
- 7.Briant L, Signoret N, Gaubin M, Robert-Hebmann V, Zhang X, Murali R, Greene M I, Piatier-Tonneau D, Devaux C. Transduction of activation signal that follows HIV-1 binding to CD4 and CD4 dimerization involve the immunoglobulin CDR3-like region in domain 1 of CD4. J Biol Chem. 1997;272:19441–19450. doi: 10.1074/jbc.272.31.19441. [DOI] [PubMed] [Google Scholar]
- 8.Chen M-Y, Maldarelli F, Karczewski M K, Willey R L, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J Virol. 1993;67:3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirmule N, Goonewardena H, Pahwa S, Pasieka R, Kalyanaraman V S, Pahwa S. HIV-1 envelope glycoproteins induce activation of activated protein-1 in CD4+ T cells. J Biol Chem. 1995;270:19364–19369. doi: 10.1074/jbc.270.33.19364. [DOI] [PubMed] [Google Scholar]
- 10.Chirmule N, Kalyanaraman V S, Pahwa S. Signal transduced through the CD4 molecule on T lymphocytes activates NF-κB. Biochem Biophys Res Commun. 1994;203:498–505. doi: 10.1006/bbrc.1994.2210. [DOI] [PubMed] [Google Scholar]
- 11.Chirmule N, Pahwa S. Envelope glycoproteins of human immunodeficiency virus type 1: profound influences on immune functions. Microbiol Rev. 1996;60:386–406. doi: 10.1128/mr.60.2.386-406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbeau P, Devaux C, Kourilsky F, Chermann J-C. An early postinfection signal mediated by monoclonal anti-β2 microglobulin antibody is responsible for delayed production of human immunodeficiency virus type 1 in peripheral blood mononuclear cells. J Virol. 1990;64:1459–1464. doi: 10.1128/jvi.64.4.1459-1464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbeil J, Richman D D. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J Gen Virol. 1995;76:681–690. doi: 10.1099/0022-1317-76-3-681. [DOI] [PubMed] [Google Scholar]
- 14.Corbeil J, Tremblay M, Richman D D. HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J Exp Med. 1996;183:39–48. doi: 10.1084/jem.183.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golding H, Blumenthal R, Manischewitz J, Littman D R, Dimitrov D S. Cell fusion mediated by interaction of a hybrid CD4.CD8 molecule with the human immunodeficiency virus type 1 envelope glycoprotein does occur after a long lag time. J Virol. 1993;67:6469–6475. doi: 10.1128/jvi.67.11.6469-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman F, Crabtree J, Hollenback C, Koretzky G. Sequestration of p56lck by gp120, a model for TCR desensitization. J Immunol. 1997;158:2017–2024. [PubMed] [Google Scholar]
- 16a.Guillerm, C., and C. Devaux. Unpublished observations.
- 16b.Guillerm, C., V. Robert-Hebmann, U. Hibner, M. Hirn, and C. Devaux. Submitted for publication. [DOI] [PubMed]
- 17.Hovanessian A G. Apoptosis in HIV infection: the role of extracellular and transmembrane glycoproteins. In: Tomei L D, Cope F O, editors. Apoptosis II: the molecular basis of apoptosis in disease. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 21–42. [Google Scholar]
- 18.Idziorek T, Estaquier J, de Bels F, Ameisen J C. YOPRO-1 permits cytofluorometric analysis of apoptosis without interfering with cell viability. J Immunol Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 19.Jacotot E, Krust B, Callebaut C, Laurent-Crawford A G, Blanco J, Hovanessian A G. HIV-1 envelope glycoproteins-mediated apoptosis is regulated by CD4 dependent and independent mechanisms. Apoptosis. 1997;2:47–60. doi: 10.1023/a:1026435625144. [DOI] [PubMed] [Google Scholar]
- 20.Kornfeld H, Cruikshank W W, Pyle S W, Berman J S, Center D M. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988;335:445–448. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- 21.Laurent-Crawford A G, Krust B, Muller S, Rivière Y, Rey-Cuille M-A, Bechet J-M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 22.Laurent-Crawford A G, Krust B, Rivière Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 23.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin R A, Nayak D P. Membrane anchorage of gp160 is necessary and sufficient to prevent CD4 transport to the cell surface. Virology. 1996;220:473–479. doi: 10.1006/viro.1996.0334. [DOI] [PubMed] [Google Scholar]
- 25.Merzouki A, Patel P, Cassol S, Ennaji M, Tailor P, Turcotte F R, O’Shaughnessy M, Arella M. HIV-1 gp120/160 expressing cells upregulate HIV-1 LTR directed gene expression in a cell line transfected with HIV-1 LTR-reporter gene constructs. Cell Mol Biol. 1995;41:445–452. [PubMed] [Google Scholar]
- 26.Moutouh L, Richman D D, Corbeil J. HIV-induced apoptosis requires the CD4 cytoplasmic tail and is not Fas-dependent in A2.01 cell line expressing wild type and mutants of the CD4 receptor. In: Hugues S H, Coffin J M, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. p. 283. [Google Scholar]
- 27.Popik W, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulin L, Evans L A, Tang S, Barboza A, Legg H, Littman D R, Levy J A. Several CD4 domains can play a role in human immunodeficiency virus infection of cells. J Virol. 1991;65:4893–4901. doi: 10.1128/jvi.65.9.4893-4901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg Z F, Fauci A S. Immunopathogenesis of HIV infection. FASEB J. 1991;5:2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz O, Rivière Y, Heard J-M, Danos O. Reduced cell surface expression of processed human immunodeficiency virus type 1 envelope glycoprotein in the presence of Nef. J Virol. 1993;67:3274–3280. doi: 10.1128/jvi.67.6.3274-3280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terai C, Kornbluth R S, Pauza C D, Richman D D, Carson D A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremblay M, Meloche S, Gratton S, Wainberg M A, Sékaly R P. Association of p56lck with the cytoplasmic domain of CD4 modulates HIV-1 expression. EMBO J. 1994;13:774–783. doi: 10.1002/j.1460-2075.1994.tb06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J Virol. 1992;66:226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]