Abstract
Objective
Dipeptidase-1 (DPEP-1) is a recently discovered leucocyte adhesion receptor for neutrophils and monocytes in the lungs and kidneys and serves as a potential therapeutic target to attenuate inflammation in moderate-to-severe COVID-19. We aimed to evaluate the safety and efficacy of the DPEP-1 inhibitor, LSALT peptide, to prevent specific organ dysfunction in patients hospitalised with COVID-19.
Design
Phase 2a randomised, placebo-controlled, double-blinded, trial.
Setting
Hospitals in Canada, Turkey and the USA.
Participants
A total of 61 subjects with moderate-to-severe COVID-19.
Interventions
Randomisation to LSALT peptide 5 mg intravenously daily or placebo for up to 14 days.
Primary and secondary outcome measures
The primary endpoint was the proportion of subjects alive and free of respiratory failure and/or the need for renal replacement therapy (RRT). Numerous secondary and exploratory endpoints were assessed including ventilation-free days, and changes in kidney function or serum biomarkers.
Results
At 28 days, 27 (90.3%) and 28 (93.3%) of subjects in the placebo and LSALT groups were free of respiratory failure and the need for RRT (p=0.86). On days 14 and 28, the number of patients still requiring more intensive respiratory support (O2 ≥6 L/minute, non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation) was 6 (19.4%) and 3 (9.7%) in the placebo group versus 2 (6.7%) and 2 (6.7%) in the LSALT group, respectively (p=0.14; p=0.67). Unadjusted analysis of ventilation-free days demonstrated 22.8 days for the LSALT group compared with 20.9 in the placebo group (p=0.4). LSALT-treated subjects had a significant reduction in the fold expression from baseline to end of treatment of serum CXCL10 compared with placebo (p=0.02). Treatment-emergent adverse events were similar between groups.
Conclusion
In a Phase 2 study, LSALT peptide was demonstrated to be safe and tolerated in patients hospitalised with moderate-to-severe COVID-19.
Trial registration number
Keywords: COVID-19, Clinical Trial, INFECTIOUS DISEASES, Safety
Strengths and limitations of this study.
Phase 2 randomised double-blind placebo-controlled multi-centre design.
International study cohort to enhance generalisability.
Demonstrated safety and tolerance of LSALT peptide in persons with moderate-to-severe COVID-19 infection.
Underpowered to identify significant differences in clinical efficacy.
Summary boxes.
Section 1
Dipeptidase-1 is a newly identified leucocyte adhesion receptor in the lungs, kidneys and liver.
LSALT is a novel dipeptidase-1 targeting peptide that prevents lung inflammation and injury.
This is a first-in-human exploratory study to evaluate the efficacy and safety of LSALT peptide for patients with moderate-to-severe COVID-19.
Section 2
Targeting dipeptidase-1 with the LSALT peptide may reduce the need for intensive respiratory support and inflammation in patients with moderate-to-severe COVID-19.
Introduction
Early in the course of the COVID-19 pandemic, 17%–42% of hospitalised patients developed acute respiratory distress syndrome (ARDS) within 8 days of admission and almost half of those patients died while hospitalised.1 2 Since 2020, the medical treatment of patients with moderate-to-severe COVID-19 has substantially improved through medical experience and the introduction of effective antiviral and anti-inflammatory therapies.3 4 The standard of care for COVID-19 evolved to include targets for both viral replication as well as host inflammatory responses.5 6 Combined with vaccination, widespread natural infection and the emergence of less virulent SARS-CoV-2 variants, the severity of COVID-19 has decreased and outcomes have improved.7 However, SARS-CoV-2 has continued to circulate globally, but in contrast to the early waves of the pandemic, severe COVID-19 infection now primarily occurs in immunocompromised, comorbid and elderly patients.7–9
At the onset of the pandemic, there was an urgent need to devise approaches to complement antiviral therapies and vaccination to control the host immune/inflammatory response and attenuate disease severity and organ dysfunction such as ARDS in patients with established COVID-19. Dipeptidase-1 (DPEP-1) is a recently identified endothelial adhesion receptor for neutrophil and monocyte/macrophage recruitment in the lungs, liver and kidneys.10 Targeting DPEP-1 attenuates lung, liver and kidney inflammation and injury in preclinical models of sepsis and ischaemia–reperfusion injury.10 11 Given the prominent role for neutrophils in COVID-19 lung pathogenesis, DPEP-1 represents a viable therapeutic target to diminish lung inflammation in patients with moderate-to-severe disease. Similarly, leucocyte recruitment and inflammation play prominent roles in acute kidney injury (AKI),11 and DPEP-1 targeting may also prevent renal complications associated with COVID-19. LSALT is a novel peptide drug and non-enzymatic DPEP-1 inhibitor that inhibits neutrophil and monocyte/macrophage recruitment, inflammation and injury in the lung and kidney.10 11 LSALT peptide was isolated from a peptide phage-display library in the context of experimental sepsis and led to clinical development for the treatment of acute organ inflammation and injury in patients with critical illness.10 LSALT peptide demonstrated a favourable safety profile in a first-in-human Phase 1 study in 52 healthy volunteers (AB001, ClinicalTrials.gov identifier, NCT03772678). Based on its mechanism of action and proposed clinical indication, LSALT peptide was identified early in the pandemic as a potential therapeutic agent to target lung and kidney inflammation and attenuate ARDS and/or AKI during COVID-19, an effect that would be agnostic to SARS-CoV-2 variants. We report the results of a Phase 2 clinical trial testing the hypothesis that LSALT peptide was safe and could attenuate pulmonary and renal inflammation and injury in patients with moderate-to-severe COVID-19 infection.
Methods
Study design and patients
We conducted a randomised double-blind, placebo-controlled, proof of concept multi-centre Phase 2 study to explore the safety and efficacy of LSALT peptide versus placebo in hospitalised patients with confirmed COVID-19. This industry-funded study was conducted at six centres in the USA, Turkey and Canada. Patients were enrolled from 13 October 2020 through 28 April 2021. Informed written consent was obtained from all participants who met the prespecified protocol inclusion criteria. In the event the patient was unable to provide consent, a legally authorised representative provided written informed consent.
The original protocol was approved by the US Food and Drug Administration (FDA) on 15 June 2020 and registered on ClinicalTrials.gov (NCT04402957). The key inclusion criteria were: (1) hospitalised patients between 45 and 80 years of age; (2) clinical and laboratory-confirmed diagnosis of COVID-19 infection (based on Reverse Transcription Polymerase Chain Reaction testing) as well as at least two of the following three symptoms: fever, dyspnoea (≤2 on modified Medical Research Council (mMRC) Dyspnoea Scale) or non-productive cough; (3) moderate illness defined as evidence of lower respiratory disease by clinical assessment or imaging and an oxygen saturation (SpO2) >93% on room air at sea level or severe illness defined as a respiratory frequency >30 breaths per minute (bpm), SpO2 ≤93% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 or lung infiltrates >50%; (4) acute physiology, age, and chronic health evaluation (APACHE) II Score <20. The key exclusion criteria were: (1) known sensitivity, allergy or previous exposure to LSALT peptide; (2) exposure to any investigational drug or device <90 days prior to entry into study; (3) treatment with immunomodulators or immunosuppressant drugs, including but not limited to interleukin (IL)-6 inhibitors, tumour necrosis factor inhibitors, anti-IL-1 immunomodulators and Janus kinase (JAK) inhibitors prior to randomisation and throughout the study period; (4) uncontrolled or poorly treated active hepatitis B, hepatitis C or HIV infection; (5) participation in another drug or device study at any time during this study; (6) pregnancy; (7) any medical condition considered to be clinically significant and could potentially affect patient safety or study outcome, including but not limited to: acute or chronic kidney disease (stage-4 or stage-5 renal impairment; estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 or haemodialysis), end-stage malignancy undergoing treatment, immunocompromised patients or those with medical/surgical conditions (eg, solid organ transplantation) which require chronic immunosuppression, chronic haematological disease, acute liver injury with aspartate aminotransferase and/or alanine aminotransferase levels greater than 3× upper limit of normal, coagulopathy, end-stage lung disease, acute lung injury, severe chronic obstructive pulmonary disease or mechanical ventilation.
Treatment intervention
The study drug for intravenous infusion was supplied to each study site as an aqueous solution at 1 mg/mL in a glass vial stored at −20°C with access restricted to the site pharmacist. The drug product was prepared by the unblinded study-site pharmacist to maintain blinding for all study personnel and the patient. Once warmed to room temperature, LSALT peptide or an equivalent volume of 0.9% sodium chloride (normal saline) was diluted in normal saline to a volume of 100 mL in an intravenous mini bag. Postpreparation, the drug product and placebo were stored for no longer than 4 hours at room temperature (20–24°C) or no longer than 24 hours at 3–5°C prior to patient administration. The drug or placebo was administered to the patient via a peripheral intravenous catheter or through a central line over 2 hours and under the supervision of the study investigator blinded to treatment.
Participants underwent physical examination including vital signs, and standard blood and urine tests for baseline chemistry and haematological indices and documentation of baseline abnormalities. Randomisation of participants was done via a computer-generated schedule in blocks of six to either LSALT peptide 5 mg intravenously infused over 2 hours once daily (based on Phase 1 dose) or matching placebo (drug-free saline similar in appearance to active drug) in a 1:1 allocation ratio for a maximum duration of 14 days and followed for safety and efficacy to 28 days. One pharmacist at each site who was not blinded had access to the randomisation schema and the treatment kits that were distributed by central depots in the USA and Turkey.
Patients were monitored daily with vital signs and physical examinations. Blood chemistries, haematology, coagulation and urinalysis were assessed every second day. ECG was recorded at baseline and when clinically indicated. Chest x-ray was obtained at baseline, day 3, end of treatment (EOT) and end of study (EOS). Disease severity scores APACHE II, Sequential Organ Failure Assessment (SOFA), Berlin Definition and mMRC Dyspnoea Scale) were assessed at baseline, EOT, EOS and when clinically indicated. Patients were maintained on the standard of care per the institutional guidelines, including venous thromboembolism prophylaxis or treatment throughout the study.
The protocol underwent four amendments prior to and during the study in partnership with the regulatory agencies and investigators as the knowledge of COVID-19 changed. The major changes were as follows: (1) therapies that emerged during the pandemic as COVID-19 standard of care (eg, dexamethasone) were permitted (amendment 1, 15 July 2020); (2) inclusion criteria changed from 45–80 years of age to 18–80 years of age (amendment 3, 15 February 2021); (3) inclusion criteria changed to laboratory-confirmed SARS-CoV-2 infection or an existing complication secondary to SARS-CoV-2 infection confirmed within 2 weeks of entry into the study (amendment 3, 15 February 2021) and (4) a reduction in the frequency of clinical lab and biomarker testing (amendment 4, 17 March 2021). All amendments were filed with the regulatory agencies and approved by the individual ethics committees prior to implementation.
Endpoints
The primary endpoint was the proportion of subjects alive and free of respiratory failure (eg, need for non-invasive or invasive mechanical ventilation, high flow oxygen (≥6 L/minute) or extracorporeal membrane oxygenation (ECMO)) and free of the need for continued renal replacement therapy (RRT) on day 28. The need for continued RRT on day 28 was defined as either dialysis in the preceding 3 days or an eGFR on day 28 <10 mL/min/1.73 m2. Numerous secondary endpoints were also evaluated that included all-cause mortality, ventilation-free days (defined as respiratory support with non-invasive or invasive mechanical ventilation or high flow oxygen (≥6 L/minute)), the presence, severity and time to development of ARDS (clinician-defined diagnosis), length of ICU and hospital stay and change in liver and kidney function tests. Exploratory outcomes included the change in serum biomarkers (IL-1β, IL-1α, IL-1Ra, IL-6, IL-5, C-X-C motif chemokine ligand (CXCL) 10, C-C motif chemokine ligand (CCL) 7/human monocyte chemotactic protein-3 (MCP-3), CXCL8/IL-8 and ferritin). Biomarkers were measured using the Meso Scale Diagnostics (Rockville, Maryland, USA) multiplex biomarker assay as per the manufacturer’s protocol. In general, data on the secondary endpoints were collected at baseline prior to study drug administration, at EOT (day 14 or earlier as a post hoc measure) and at EOS (day 28). Biomarker blood samples were collected at baseline prior to the start of drug or placebo infusion on day 1, then at 1 hour and 2 hours after the start of the daily infusions on day 3 and at EOT.
Safety was assessed in all patients who received at least one dose (partial or complete) of the study medication, assigned to treatment according to the treatment received. Adverse events (AEs) regardless of attribution were collected from the initiation of treatment until 30 days following the final dose (within the time of residual drug effect to a maximum timeframe of day 44) tabulated by Overall, System Organ Class and Preferred Term using the MedDRA coding system. Treatment-emergent adverse events (TEAEs) were defined as any AE occurring after start of study medication and within the time of residual drug effect or a pretreatment AE or pre-existing medical condition that worsened in intensity after start of study medication in the same timeframe. A Data and Safety Monitoring Board evaluated patients on a continuing basis for primarily safety assessments throughout the study period.
Statistical analysis
Given a rapidly evolving new disease (COVID-19) and no prior clinical efficacy studies for the LSALT peptide, a sample size of 60 (LSALT peptide (N=30), placebo (N=30)) was determined for this exploratory Phase 2 study based on medical judgement and the objective to assess preliminary safety and drug efficacy in a limited number of patients prior to proceeding with a larger study. The Statistical Analysis Plan (SAP) was finalised prior to database lock and unblinding. Analyses for the primary and secondary endpoints were performed on the full analysis set (FAS) including all subjects randomised who received any part of at least one infusion of randomised treatment. Unless otherwise specified, all treatment comparisons were performed at the EOS visit, or over the whole study period, as applicable. Due to the small size of this study, no inferential subgroup analyses were planned however, some exploratory post hoc investigations within subgroups were performed.
To compare the proportion of patients meeting the primary endpoint between the treatment groups, the OR (LSALT peptide/placebo) was reported using the Cochran-Mantel-Haenszel test. In this analysis, patients who did not complete the study due to early withdrawal or death were assumed to have failed to meet the endpoint.
Secondary endpoints were analysed as described in the SAP. The number of ventilation-free days, ECMO-free days, number of hours on nasal cannula or oxygen mask were summarised by treatment separately. For each, a one-way analysis of covariance model was used with a continuous covariate of baseline oxygen status (PaO2/FiO2 ratio) to estimate the treatment difference. In the above analysis, the number of ventilation-free days and ECMO-free days assigned to patients who did not complete the study due to early withdrawal or death was set to zero. A similar analysis was performed for percentage of days on high flow O2, non-invasive/mechanical ventilation or both compared with low flow O2.
As an exploratory outcome of interest, serum biomarkers were analysed if >50% of samples were above the lower limit of quantitation (LLOQ) for the assay. Serum biomarkers (IL-1β, IL-1Ra, IL-6, IL-5, CXCL10, CCL7, CXCL8 and ferritin) were normalised to each individual patient by expressing as fold induction from baseline (predose day 1) to day 3 and EOT. Biomarker fold change was given a value of 1 (ie, no change) if both baseline and treatment samples were below LLOQ. Mean fold change in individual biomarkers was compared between LSALT peptide and placebo-treated patients using a two-sided Mann-Whitney test. Proportional change in the fold induction of all biomarkers was assessed using a two-sided Fisher’s exact test.
Patient and public involvement
Patients and the public were not involved in the original design, recruitment, conduct, reporting or dissemination plans of our research, but input from patients on procedures and design was incorporated in the amendments of the protocol.
Results
72 patients were screened for the study and 65 patients were randomised to receive LSALT peptide or placebo. Four patients discontinued the study prior to receiving their randomised treatment, two LSALT peptide-treated patients (physician/investigator decision and withdrawal of consent) and two placebo-treated patients (withdrawal of consent). 61 patients received at least one dose of treatment and were analysed in the FAS. Approximately 70% of the treated patients were enrolled at six sites in three countries: three sites (Canada (15 patients at one site), Turkey (29 patients at two sites]) and the remainder were enrolled in the USA (17 patients at three sites). Three patients discontinued the study: two in the placebo group (lost-to-follow-up and withdrawal of consent) and one in the LSALT peptide group (death). The remaining patients completed the study as per protocol.
Demographic and baseline characteristics of the study cohort are shown in table 1. The LSALT peptide and placebo groups were similar regarding sex (70.0% vs 64.5% male) and race (73.3% vs 83.9% white) but the LSALT peptide group was on average 5 years older compared with placebo (62.3±8.7 vs 57.3±10.2 years). The relative proportion of comorbidities was also similar between both groups (table 1). The mean number of treatment days was 5.8±3.72 for placebo and 7.2±3.3 for LSALT peptide groups.
Table 1.
Demographics and baseline characteristics
| Characteristics | Placebo (N=31) n (%) |
LSALT (N=30) n (%) |
| Female | 11 (35) | 9 (30) |
| Male | 20 (65) | 21 (70) |
| White or Caucasian | 26 (84) | 22 (73) |
| Black or African American | 2 (6.5) | 5 (16.7) |
| Age (years; mean±SD) | 57.3±10.2 | 62.3±8.7 |
| Weight (kg; mean±SD) | 95±34 | 96±19 |
| Height (cm; mean±SD) | 170±10 | 172±9 |
| BMI (kg/m2; mean±SD) | 32.3±10 | 31.7±6 |
| PaO2/FiO2 ratio (mean±SD)* | 248±94 | 247±111 |
| SOFA Score (mean±SD) | 2.4±0.9 | 2.4±1.5 |
| APACHE II Score (mean±SD) | 7.1±3.6 | 8.1±3.6 |
| mMRC Score (mean±SD) | 2.0±1.4 | 2.5±1.2 |
| Comorbidities, N (%) | ||
| ≥1 comorbidity | 24 (77.4) | 23 (76.7) |
| Age >65 years | 6 (19.4) | 12 (40.0) |
| Age >70 years | 3 (9.7) | 6 (20.0) |
| Diabetes (type 1 or 2) | 10 (32.3) | 13 (43.3) |
| Cardiovascular disease | 15 (48.4) | 18 (60.0) |
| Obesity | 4 (12.9) | 5 (16.7) |
| Pulmonary disease | 3 (9.7) | 5 (16.7) |
| Chronic renal disease | 1 (3.2) | 1 (3.3) |
| Chronic liver disease | 1 (3.2) | 1 (3.3) |
| SpO2 >95% | 13 (41.9) | 9 (30.0) |
| Advanced cancer | 1 (3.2) | 0 (0) |
*Subjects AB002-102-003 and AB002-102-004 both in the LSALT peptide group were excluded from analysis of PaO2/FiO2 ratios due to outlier values at baseline (9600 and 2800, respectively).
APACHE II, acute physiology, age, and chronic health evaluation II; BMI, Body Mass Index; mMRC, modified Medical Research Council; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; SOFA, Sequential Organ Failure Assessment; SpO2, oxygen saturation.
Primary outcome measure
At day 28, 27 (90.3%) and 28 (93.3%) subjects in the placebo and LSALT groups were free of respiratory failure and the need for RRT (p=0.86). Three patients in the placebo group and two patients in the LSALT peptide group developed respiratory failure in the study period. There were only three AKI events in the study period, and none required RRT at day 28.
Secondary outcome measures
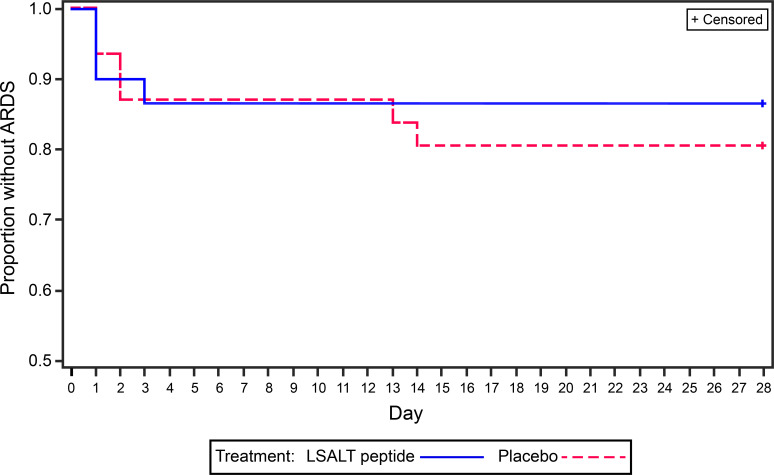
There were two deaths, one within and one after the study period, in the LSALT peptide group and have been detailed in the safety analysis section. No differences were seen in the development or severity of ARDS between groups with two instances of ARDS at day 28 in each of the LSALT peptide and placebo arms (figure 1; table 2). The LSALT group had 22.8 (95% CI 19.6 to 26) ventilation-free days compared with 20.9 (95% CI 17.7 to 24) in the placebo group at 28 days (p=0.4) (table 2). Other secondary outcomes were similar between placebo and LSALT peptide-treated groups when data were available (table 2).
Figure 1.
Kaplan-Meier plot for time to acute respiratory distress syndrome (ARDS).
Table 2.
Outcomes in secondary endpoints at day 28 (EOS)
| Parameter | Placebo (N=31) |
LSALT (N=30) |
Treatment difference LSALT−placebo (95% CI) |
P value |
| All-cause mortality (day 28) | 0 | 1* | −0.03 (−0.10 to 0.03) | 0.31 |
| Proportion with ARDS (day 28) | 2 (6.5%) | 2 (6.7%) | 0.00 (−0.13 to 0.13) | 1.00 |
| Time to ARDS | nd | nd | ||
| Ventilation and ECMO-free days | ||||
| Ventilation (mean) | 22.8 | 20.9 | 1.9 (−2.6 to 6.4) | 0.4 |
| ECMO (mean) | 25.1 | 23.7 | −1.4 (−8.7 to 5.8) | 0.69 |
| Time (hours) on nasal cannula or oxygen mask (mean) | 203.6 | 192.1 | −11.5 (203.5 to 180.5) | 0.9 |
| Length of stay in ICU | nd | nd | ||
| Median time to hospital discharge (days) | 7 | 7.5 | 0.89 (0.55 to 1.45) | 0.65 |
| Virological clearance rate | nd | nd | ||
| Worst PaO2/FiO2 ratio (mean) | −175.79 | −200.89 | −25.10 (−115 to 64.80) | 0.57 |
| Change in PaO2/FiO2 ratio (mean) | −25.7 | −141.2 | −115.5 (−287.3 to 56.3) | 0.18 |
| Vasopressor-free days | nd | nd | ||
| Radiographic change (CXR) | nd | nd | ||
| Change in baseline mMRC Score | −0.91 | −1.34 | −0.43 (−1.06 to 0.20) | 0.18 |
| Change in baseline APACHE II Score | −1.04 | −1.43 | −0.38 (−2.63 to 1.86) | 0.74 |
| Change in baseline SOFA Score | −1.28 | −0.59 | 0.68 (−0.27 to 1.64) | 0.16 |
| Change in liver function tests | ||||
| Alanine aminotransferase | −1.4 | −5.9 | −4.4 (−22.8 to 13.9) | 0.63 |
| Aspartate aminotransferase | −19.4 | −15.4 | 4.0 (−9.8 to 17.7) | 0.56 |
| Total bilirubin | nd | nd | ||
| Change in renal function | ||||
| BUN (mmol/L) | −2.6 | −2.5 | 0.1 (-2.60 to 2.88) | 0.92 |
| Creatinine (mmol/L) | −1.5 | −1.7 | −0.2 (-8.98 to 8.58) | 0.96 |
| eGFR | nd | nd | nd | nd |
| Change in hs-troponin levels | nd | nd | ||
| Change in coagulation indices | ||||
| APTT (s) | 2.23 | 1.47 | −0.8 (−5.17 to 3.64) | 0.73 |
| PT (s) | 0.10 | −0.77 | −0.9 (−2.34 to 0.60) | 0.24 |
| INR | 0.03 | −0.07 | −0.1 (−0.22 to 0.03) | 0.12 |
*There were two deaths, both in the LSALT peptide treatment group. One patient died on day 9 after 8 days of therapy and is included in this table. The other patient received 10 days of therapy, discontinued from treatment and died 21 days after the last dose of study drug and after the end of the 28-day study period.
APACHE II, acute physiology, age, and chronic health evaluation II; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; CXR, chest x-ray; eGFR, estimated glomerular filtration rate; EOS, end of study; hs, high sensitivity (in relation to troponin as it is hs-troponin); ICU, intensive care unit; INR, international normalised ratio; mMRC, modified Medical Research Council; nd, not determined due to insufficient events or data points; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PT, prothrombin time; SOFA, Sequential Organ Failure Assessment.
In post hoc analyses, adjusting for age, the mean difference in ventilation-free days was 2.9 days (95% CI −1.7 to 7.5; p=0.21) greater in the LSALT peptide group versus placebo and increased to 6.7 days when adjusted for age, Body Mass Index (BMI) and baseline PaO2/FiO2 ratio (24.6, 95% CI 17.7 to 31.4 vs 17.9, 95% CI 13.0 to 22.8; p=0.14) (table 3). At day 14, 6 (19.4%) and 2 (6.7%) in the placebo and LSALT arms required more intensive respiratory support with oxygen therapies that included high flow oxygen (≥6 L/minute), mechanical ventilation and ECMO with a greater number in the placebo arm requiring high-flow nasal oxygen (5 (16.1%) vs 1 (3.3%), p=0.14) (online supplemental table 1).
Table 3.
Treatment difference in ventilation-free days
| Ventilation-free days | Placebo | LSALT | LSALT−Placebo (95% CI) | P value |
| Unadjusted | 20.9 (n=31) | 22.8 (n=30) | 1.9 (−2.6 to 6.4) | 0.4 |
| Age adjustment | 20.4 (n=31) | 23.3 (n=30) | 2.9 (−1.7 to 7.5) | 0.21 |
| Age and BMI adjustment | 19.7 (n=28) | 23.5 (n=21) | 3.8 (−2.0 to 9.5) | 0.19 |
| Baseline P/F, age and BMI adjustment* | 17.9 (n=18) | 24.6 (n=10) | 6.7 (−2.2 to 15.6) | 0.14 |
*30 patients had P/F (PaO2/FiO2) ratios determined at baseline. Subjects 102-003 and 102-004 were excluded from the analysis of P/F ratios due to outlier values at baseline (9600 and 2800, respectively).
BMI, Body Mass Index; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen.
bmjopen-2023-076142supp002.pdf (150.6KB, pdf)
Serum biomarkers exploratory analysis
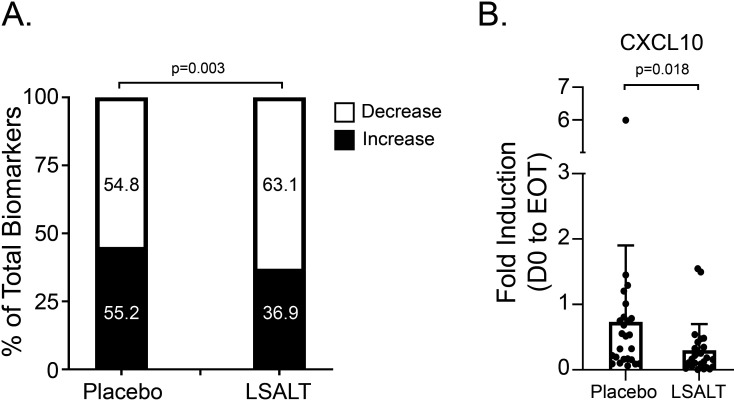
Patient sera were analysed at baseline, day 3 and EOT (samples drawn 1 hour and 2 hours during drug/placebo infusion) for IL-1β, IL-1α, IL-1Ra, IL-5, IL-6, CXCL8, CXCL10, CCL7 and ferritin. Biomarkers were below LLOQ in a small number of samples, especially for IL-1β and IL-5. Serum IL-1α was not detectable in any sample. Collectively, a greater proportion of the biomarkers decreased from baseline at day 3 and EOT in the LSALT peptide treatment group compared with placebo (63.1% vs 54.8%, OR 1.41 95% CI 1.13 to 1.75, p=0.003) (figure 2A, online supplemental table 2 and online supplemental figure 1). Individually, only changes in CXCL10 were significant at EOT as the mean fold change in CXCL10 between the placebo and LSALT peptide treatment groups was 0.83±1.19 versus 0.3±0.35 (1-hour sample, n=25 placebo, 25 LSALT peptide; p=0.02) and 0.73±1.17 versus 0.3±0.4 (2-hour sample, n=25 placebo, 25 LSALT peptide; p=0.02) (figure 2B).
Figure 2.
Inflammatory biomarker expression. (A) Proportion of inflammatory biomarkers increasing or decreasing from baseline in placebo and LSALT peptide-treated groups (fold induction of all biomarkers and all time points, n=635 placebo, n=686 LSALT peptide; Fisher’s exact test). (B) Mean fold induction of C-X-C motif chemokine ligand 10 (CXCL10) in 2-hour biomarker samples at end of treatment (EOT) (mean±SD; n=25 placebo, n=25 LSALT peptide; Mann-Whitney test).
bmjopen-2023-076142supp001.pdf (170.1KB, pdf)
Safety outcomes
There were no significant differences in the occurrence of serious or treatment-emergent AEs between the treatment groups (online supplemental table 3). Elevated liver enzymes were the most prevalent AE in both treatment groups (online supplemental table 4). A total of 22 (71%) placebo participants and 18 (60%) LSALT participants experienced an AE. Of these, 21 (67.7%) and 17 (56.7%), respectively, were TEAEs and 4 were severe in the placebo group and 3 were severe in the LSALT group. Other measures of safety were similar between the two treatment groups. There were two deaths at separate study sites in the LSALT group and none in the placebo group. An 80-year-old male (BMI 25.8 kg/m2; PaO2/FiO2 ratio at baseline=63) died from multiple common COVID-19 complications including AKI, ARDS, superimposed secondary infection and septic shock. He had received 10 days of active therapy and died 21 days after the last dose and after day 28 of the study. The principal investigator assessed that this was possibly related to drug therapy. The other death was a 75-year-old male (BMI 45 kg/m2; PaO2/FiO2 ratio at baseline=182.2) who received 7 days of study therapy, had similar complications and died 9 days after study entry. This death was assessed by the PI to be unrelated to drug therapy.
Discussion
This placebo-controlled, exploratory Phase 2 study examined the safety and efficacy of the DPEP-1 inhibitor LSALT peptide in patients with moderate-to-severe COVID-19. The data support the safety and tolerance of LSALT peptide in patients with moderate-to-severe COVID-19 infection. Although there were no significant differences detected in primary or secondary endpoints, post hoc analysis suggested a greater number of ventilation-free days in LSALT peptide-treated patients compared with placebo and a lower need for intensive respiratory support. In addition, patients treated with LSALT peptide demonstrated a general trend towards declining inflammatory biomarker levels, as well as a significant decrease in CXCL10 at EOT. LSALT peptide was well tolerated with an AE rate similar to subjects receiving placebo. Collectively, these data support advancing LSALT peptide in a larger study to determine its efficacy in acute lung and kidney inflammation and injury.
The two participants who failed to meet the primary endpoint in the LSALT peptide-treated group also represented the only two deaths in the study. Both also had comorbidities and profiles that are now known to substantially increase the risk of death from COVID-19.1 12–16 Aside from these, there were very few outcomes involving organs other than the lung in the study cohort. Thus, the effect of LSALT peptide to mitigate complications such as AKI, cardiac or liver injury could not be determined. However, in the exploratory analysis of serum biomarkers, a greater proportion of inflammatory biomarkers, and in particular CXCL10, decreased in patients receiving LSALT peptide compared with placebo. The reduction of CXCL10 is potentially relevant since its function is in keeping with LSALT peptide’s mechanism of action to inhibit leucocyte recruitment in various vascular beds including the lungs and kidney.10
Although we tested a novel compound in the setting of COVID-19 to evaluate its ability to attenuate disease complications in a randomised trial, some limitations must be considered. As we did not have guiding efficacy estimates for our primary outcome, and had few adverse effects, the study is underpowered to evaluate key differences between LSALT peptide and placebo. Although in earlier phases of the pandemic there had been significant morbidity and mortality in hospitalised patients with COVID-19, we did not observe this in our study and this may have contributed to the underpowering of the study. The low number of severe outcomes may relate to improving the standard of care over the course of the pandemic, or a selection bias of the research as it is difficult to enrol patients who are or become critically ill in the first 24 hours of hospital admission. We did have some missing data in some secondary outcome measures, but this did not hinder our ability to evaluate the safety and primary outcomes of LSALT in this Phase 2 trial. Reassuringly, we had collected all safety and clinical endpoint data for the entire study period in all subjects. As the pandemic has evolved very quickly, the adaptability of these findings to other viruses beyond COVID-19 will need to be considered in future clinical evaluations. Finally, the 5 mg dosing for LSALT peptide was based on the maximal dose tested in the Phase 1 study and it is possible that a higher single daily dosing or multiple daily dosing may have provided a greater effect as suggested in the preclinical studies.10
Our randomised Phase 2 trial is the first to explore safety and efficacy in a patient population dosed with the specific DPEP-1 inhibitor, LSALT peptide. Armed with efficacy information from preclinical models, an excellent safety profile in healthy male and female subjects, and now a potential signal of drug effect in attenuated lung injury and inflammation, the rationale exists to advance LSALT peptide to larger powered trials. As immunity to SARS-CoV-2 increases in the global population through vaccination and natural infection, the severity of COVID-19 is diminishing. Thus, conducting a large study for the LSALT peptide in COVID-19 is probably no longer feasible. In fact, based on the preliminary data from this study, LSALT peptide was entered as an arm in the Canadian Treatments for COVID-19 study in 2022 but did not meet its enrolment target due to an overall decrease in eligible patients. However, given the similarity in biological mechanisms between COVID-19 and other critical illnesses such as sepsis or pneumonia, the results from this study can be applied as rationale to assess the efficacy of LSALT peptide in other clinical conditions that involve lung inflammation and injury.
In conclusion, our randomised Phase 2 trial of LSALT peptide further demonstrated safety and tolerance in a cohort of hospitalised persons with moderate-to-severe COVID-19 infection. With few organ failure events, the study was underpowered to evaluate significant differences between treatment groups. Future-powered Phase 3 studies in severe respiratory infections can better delineate the efficacy profile of LSALT peptide.
Supplementary Material
Acknowledgments
We would like to express our thanks and acknowledge the dedication of our clinical monitoring groups, including Monitor CRO in Turkey under the leadership of Dr Oğuz Akbaş, Syneos Health in the USA under the leadership of Juan Torres and Catherine St. George in Canada. Further, we appreciate the expertise and diligence of the Data and Safety Monitoring Board, chaired by Dr Nadir Ulu.
Footnotes
Contributors: The Sponsor (refer to author list affiliations) and affiliated monitoring groups hired by the Sponsor were involved in the study design, in the collection, analysis and interpretation of the data. RS, RG, OFT, MH, NG, SAC, SDK, KG, SMR, DLS, DS, RKSL, JL, FE, RK, DM and AT were involved in the acquisition of data, interpretation of data and drafting or revising the manuscript critically. RS, DM and DRL drafted the manuscript with input from the coauthors; RS and DM finalised the manuscript and managed all revisions. All authors contributed substantially to the work and approved the final version of the manuscript. DM is the guarantor.
Funding: This Phase 2 trial was funded by Arch Biopartners Inc. and by a grant from Innovation Science and Economic Development Canada, Strategic Innovation Fund, Countermeasures for COVID-19 (Grant # 811-513415). There were no other funding sources. Clinicaltrials.gov identifier, NCT04402957.
Competing interests: DRL, DS, KG and AL are employed by Arch Biopartners Inc. DRL, AL, SMR and DLS hold equity positions in Arch Biopartners. SMR, DLS, AL and DM have patents issued and pending in the areas of dipeptidase-1 and the LSALT peptide. DM is the acting chief science officer for Arch Biopartners and is compensated with an equity position. DM has received research funding from Arch Biopartners. All other authors have no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data relevant to the study are included in the article. The protocol and analysis can be found on clinicaltrials.gov. Deidentified participant data may be requested from study investigators with approval obtained from the relevant ethics boards.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki, in compliance with the approved protocol, good clinical practice and applicable regulatory requirements. The study protocol, associated amendments and informed consents in the USA and Canada were approved by each hospital’s institutional Research Ethics Committee (ethics approval number: Advarra CIRB MOD00749358 20 August 2020) and respective national regulatory agencies before enrolment began. Ethics approval in Turkey was issued by the Ministry of Health and the two participating institutions. Participants gave informed consent to participate in the study before taking part.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. Forchette L, Sebastian W, Liu T. A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr Med Sci 2021;41:1037–51. 10.1007/s11596-021-2395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murakami N, Hayden R, Hills T, et al. Therapeutic advances in COVID-19. Nat Rev Nephrol 2023;19:38–52. 10.1038/s41581-022-00642-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali K, Azher T, Baqi M, et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ 2022;194:E242–51. 10.1503/cmaj.211698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Consortium WHOST . Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet 2022;399:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Link-Gelles R, Levy ME, Natarajan K, et al. Estimation of COVID-19 mRNA vaccine effectiveness and COVID-19 illness and severity by vaccination status during omicron BA.4 and BA.5 sublineage periods. JAMA Netw Open 2023;6:e232598. 10.1001/jamanetworkopen.2023.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epidemiology C, Surveillance T. COVID-19 Australia: epidemiology report 71: reporting period ending 12 February 2023. Commun Dis Intell 2018:47. [DOI] [PubMed] [Google Scholar]
- 9. Malahe SRK, Hoek RAS, Dalm V, et al. Clinical characteristics and outcomes of immunocompromised patients with coronavirus disease 2019 caused by the omicron variant: a prospective, observational study. Clin Infect Dis 2023;76:e172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choudhury SR, Babes L, Rahn JJ, et al. Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell 2019;178:1205–21. 10.1016/j.cell.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 11. Lau A, Rahn JJ, Chappellaz M, et al. Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury. Sci Adv 2022;8:eabm0142. 10.1126/sciadv.abm0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and influenza - veterans health administration, United States. MMWR Morb Mortal Wkly Rep 2018;69:1528–34. 10.15585/mmwr.mm6942e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One 2021;16:e0247461. 10.1371/journal.pone.0247461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Chi J, Lv W, et al. Obesity and diabetes as high-risk factors for severe Coronavirus disease 2019 (COVID-19). Diabetes Metab Res Rev 2021;37:e3377. 10.1002/dmrr.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020;81:e16–25. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076142supp002.pdf (150.6KB, pdf)
bmjopen-2023-076142supp001.pdf (170.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data relevant to the study are included in the article. The protocol and analysis can be found on clinicaltrials.gov. Deidentified participant data may be requested from study investigators with approval obtained from the relevant ethics boards.