Abstract
Background
Electrosurgical devices are commonly used during mastectomy for simultaneous dissection and haemostasis, and can provide potential benefits regarding vessel and lymphatic ligation. The aim of this prospective RCT was to assess whether using a vessel-sealing device (LigaSure™) improves perioperative outcomes compared with monopolar diathermy when performing simple mastectomy.
Methods
Patients were recruited prospectively and randomized in a 1 : 1 manner to undergo simple mastectomy using either LigaSure™ or conventional monopolar diathermy at a single centre. The primary outcome was the number of days the drain remained in situ after surgery. Secondary outcomes of interest included operating time and complications.
Results
A total of 86 patients were recruited (42 were randomized to the monopolar diathermy group and 44 were randomized to the LigaSure™ group). There was no significant difference in the mean number of days the drain remained in situ between the monopolar diathermy group and the LigaSure™ group (7.75 days versus 8.23 days; P = 0.613) and there was no significant difference in the mean total drain output between the monopolar diathermy group and the LigaSure™ group (523.50 ml versus 572.80 ml; P = 0.694). In addition, there was no significant difference in the mean operating time between the groups, for simple mastectomy alone (88.25 min for the monopolar diathermy group versus 107.20 min for the LigaSure™ group; P = 0.078) and simple mastectomy with sentinel lymph node biopsy (107.20 min for the monopolar diathermy group versus 114.40 min for the LigaSure™ group; P = 0.440).
Conclusion
In this double-blinded single-centre RCT, there was no difference in the total drain output or the number of days the drain remained in situ between the monopolar diathermy group and the LigaSure™ group.
Registration number
EudraCT 2018-003191-13 BEAUMONT HOSPITAL REC 18/66.
In this double-blinded single-centre RCT, there was no difference in the total drain output or the number of days the drain remained in situ between the monopolar diathermy group and the LigaSure™ group. Monopolar diathermy is at least as effective and significantly cheaper compared with LigaSure™.
Introduction
Breast cancer is the most common cancer in women worldwide and surgery remains a crucial component of multimodal management1. While there has been a shift in contemporary surgical practice towards breast-conserving surgery, sometimes this is not clinically feasible2. In such patients, simple mastectomy is often performed. Simple mastectomy has several associated complications, with seroma formation being the most common3,4.
A seroma is a fluid collection that can accumulate in the dead space created when breast tissue is removed. This is partly due to lymphatic vessels being cut during surgery, resulting in lymphatic fluid leakage and subsequent accumulation5,6. The current rate of seroma formation is estimated to be 15.0%4. Seroma formation can lead to a prolonged hospital stay, discomfort, and impaired wound healing, and can be a source of delay for adjuvant therapy, particularly radiotherapy7–10. To prevent or minimize the risk of seroma formation, various techniques can be employed, including placing drains in the surgical bed, quilting sutures to close the dead space, and applying compression dressings. Whilst closed-suction drain placement is currently routinely used, with the aim of reducing seroma formation, there is a paucity of evidence to support this11. Conversely, prolonged drain placement can negatively affect a patient’s quality of life and risks introducing infection12. The choice of electrosurgical device has been shown to influence and potentially reduce seroma formation in other surgical procedures13. An electrothermal bipolar vessel-sealing system has been shown in some studies to decrease blood loss and drainage volume compared with electrocautery13–17. Other common complications after simple mastectomy include haematoma formation and surgical-site infection (rates of 0.6–7% and 5–6% respectively)4. Complications can also impact the length of time that postoperative drains remain in situ, reoperation rates, and postoperative pain, as well as contribute to delays in patients receiving adjuvant treatment.
Whereas the standard reference technique for simple mastectomy involves the use of monopolar electrocautery, newer electrosurgical devices, such as LigaSure™ and the harmonic scalpel, are commonly used during simple mastectomy for simultaneous dissection and haemostasis. Their potential benefits regarding vessel and lymphatic sealing are not known18,19. LigaSure™ (Medtronic, Dublin, Ireland) is an electrothermal bipolar vessel-sealing system that achieves haemostasis using a combination of pressure and electrothermal energy by denaturing collagen and elastin within the vessel wall and surrounding connective tissue. This haemostatic device ensures vessel sealing, with minimal thermal spread and limited tissue charring19–21.
The aim of this prospective RCT was to assess whether using a vessel-sealing device (LigaSure™) improves perioperative outcomes compared with monopolar diathermy when performing simple mastectomy.
Methods
Study design and participants
The CONSORT guidelines for randomized studies were utilized for this prospective single-centre RCT22. See the Supplementary material for the CONSORT checklist. This RCT was a standard two-group parallel-designed trial conducted at a high-volume surgical centre (Beaumont Hospital) in Dublin in the Republic of Ireland. Patients were randomized in a 1 : 1 ratio. All potential participants received an information leaflet and explicit informed written consent was obtained before enrolment. Patient demographics and biometrics, including sex, age, and BMI, were recorded at the time of enrolment. Ethical approval was obtained from the Beaumont Hospital Research Ethics Committee (REC: 18/66). This RCT opened for recruitment on 15 August 2019. The study completed recruitment on 11 November 2022 and the follow-up interval was completed on 23 November 2022.
Inclusion and exclusion criteria
Patients aged 18 years or over, presenting for simple mastectomy, with or without a sentinel lymph node biopsy (SLNB), were considered for inclusion. All patients had to be classified as ASA grade I or II to warrant inclusion23. Exclusion criteria included axillary lymph node dissection, immediate breast reconstruction, and pregnancy/lactation.
Randomization
After the consent process, randomization occurred using a digital randomization tool. A custom design to stratify for BMI greater than and equal or less than 25 kg/m2 was used, while maintaining a 1 : 1 randomization. This was developed to ensure equality in both groups. The patient and the nurse collecting postoperative drain outputs were blinded to the assigned study arm.
The operating surgeon was informed of the electrosurgical device to which the patient was assigned before surgery and proceeded according to specific and predefined guidelines.
Simple mastectomy techniques
The procedure for simple mastectomy as per the study protocol involved the following steps. The surgeon makes an elliptical incision encompassing the nipple-areolar complex that can vary based on the size and location of the tumour. The surgeon then dissects the subcutaneous tissue to expose the breast tissue using the assigned diathermy device in the standard mastectomy plane down to the chest wall. The breast tissue is then dissected free from the pectoral muscles and chest wall. The specimen is then removed en bloc and sent as a histopathological specimen with standard suture markings of Long Lateral (LL), Short Superior (SS), and Double Deep (DD). The surgeon inserts a 7 mm Jackson–Pratt drain into the breast cavity to remove any excess fluid that can accumulate after surgery. The drain is typically placed through a separate incision in the anterior chest wall. Finally, the drain is secured to the skin using a silk 2-0 suture. Closure is completed in the usual manner using absorbable sutures, Steri-Strips™, and waterproof adhesive dressing.
Both groups received standard postoperative care that involved patients being educated in drain management and discharged with the drain in situ. Drains were removed in the outpatient department by a nurse when draining less than 30 ml per 24 h. Patients were educated before discharge on documenting the total drain volume in a daily drainage diary. Patient follow-up was in the outpatient department on postoperative day 5 and then weekly until drain removal.
Outcomes
The primary outcome was the number of days the drain remained in situ after surgery. Secondary outcomes included operating time and complications, including seroma formation, haematoma formation, and wound infection.
Power calculation
Sample size calculation was based on the primary outcome measure, that is the mean number of days the drain remained in situ. A 3-day difference in duration of drain in situ was considered clinically meaningful. The sample size calculation was based on the assumption that patients operated on with LigaSure™ would have a shorter duration of drain in situ. A pragmatic sample size was determined to test the hypothesis. Using Stata’s power calculation command, assuming a significance level of α = 0.05 and a power of 80%, a sample size of 86 participants was required (power two mean(s.d.) 14 11(5.5), one-sided). This sample size also supported analysis of the secondary outcomes that included operating time and complications.
Statistical analyses
Continuous data are summarized as mean(s.d.) and categorical data as n (%). Analyses were performed on an intention-to-treat principle, retaining all participants in their randomized groups. Clinical and demographic information was correlated with the electrosurgical device used, using chi-squared and two-tailed t tests, as appropriate. The Shapiro–Wilk test was used to assess the distribution of data. All significance tests were two-tailed, with P < 0.05 indicating statistical significance. Data were analysed using SPSS® (IBM, Armonk, New York, USA; version 26.0).
Results
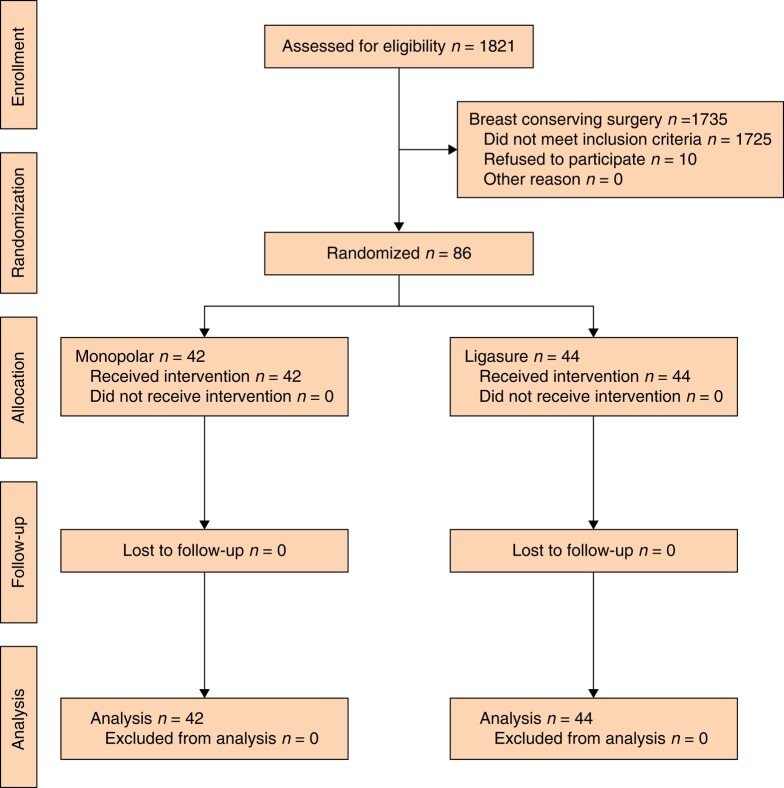
A total of 86 patients were recruited, all of whom were followed up and analysed. Details of enrolment, allocation, follow-up, and analysis are summarized in the CONSORT diagram (Fig. 1). After randomization, 42 patients were assigned to the monopolar diathermy group and 44 to the LigaSure™ group. Patients in both groups had similar demographics (mean age, mastectomy indication, number of patients undergoing SLNB, mean BMI, and mean breast weight), as summarized in Table 1.
Fig. 1.
CONSORT diagram of the progress through the phases of this RCT
Table 1.
Patient demographics
| All (n = 86) | Monopolar diathermy (n = 42) | LigaSure™ (n = 44) | |
|---|---|---|---|
| Age (years), mean (sd) | 60.79 (1.6) | 62.4 (13.3) | 58.8 (16.7) |
| Mastectomy indication | |||
| Completion | 19 (22.1) | 11 (26.1) | 8 (18.2) |
| Primary | 64 (74.4) | 30 (71.4) | 34 (77.3) |
| Prophylactic | 3 (3.5) | 1 (2.4) | 2 (4.5) |
| SLNB | 65 (75.6) | 33 (78.6) | 32 (72.7) |
| No SLNB | 21 (24.4) | 9 (21.4) | 12 (27.3) |
| BMI (kg/m2), mean (sd) | 27.89 (5.22) | 27.61 (5.31) | 28.15 (5.19) |
| Breast weight (g), mean (sd) | 877.3 (515.30) | 902.5 (575.09) | 850.5 (460.5) |
Values are n (%) unless otherwise indicated. SLNB, sentinel lymph node biopsy.
Perioperative outcomes
There was no significant difference in mean total drain output between the monopolar diathermy group and the LigaSure™ group (523.5 ml versus 572.8 ml respectively; P = 0.694) (Fig. 2a). There was also no significant difference in the mean number of days the drain remained in situ between the monopolar diathermy group and the LigaSure™ group (7.75 days versus 8.23 days respectively; P = 0.61) (Fig. 2b).
Fig. 2.
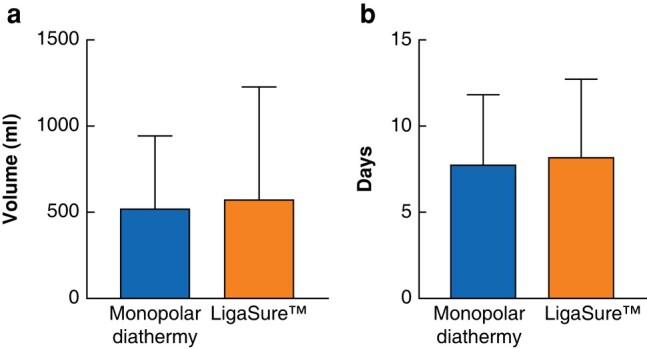
Drain output and number of days drain in situ
a Mean total drain output. Monopolar diathermy = 523.5 ml and LigaSure™ = 572.8 ml; P = 0.694. b Mean number of days to drain removal. Monopolar diathermy = 7.75 days and LigaSure™ = 8.23 days; P = 0.613.
Operating time
There was no significant difference in the mean operating time between the groups, for simple mastectomy alone (88.25 min for the monopolar diathermy group versus 107.5 min for the LigaSure™ group; P = 0.078) and simple mastectomy with SLNB (107.2 min for the monopolar diathermy group versus 114.4 min for the LigaSure™ group; P = 0.448). See Table 2. Notably, the time taken to carry out a simple mastectomy was a mean of 19 min longer using LigaSure™.
Table 2.
Secondary outcomes in Monopolar versus LigaSure
| All (n = 86) | Monopolar diathermy (n = 42) | LigaSure™ (n = 44) | P | |
|---|---|---|---|---|
| Operating time (min), mean | ||||
| Mastectomy only | – | 88.25 | 107.5 | 0.078 |
| Mastectomy + SLNB | – | 107.2 | 114.4 | 0.448 |
| Complication | ||||
| Seroma formation | 4 (4.7) | 2 (4.8) | 2 (4.5) | 0.944 |
| Haematoma formation | 2 (2.3) | 2 (4.8) | 0 (0) | 0.139 |
| Wound infection | 5 (5.8) | 2 (4.8) | 3 (6.8) | 0.683 |
Values are n (%) unless otherwise indicated. SLNB, sentinel lymph node biopsy.
Complications
There was no significant difference in complication rates between the monopolar diathermy group and the LigaSure™ group. See Table 2. Both the monopolar diathermy group and the LigaSure™ group had a similar rate of seroma formation (4.8% and 4.5% respectively; P = 0.944). While not statistically significant, the rate of haematoma formation was higher in the monopolar diathermy group compared with the LigaSure™ group (4.8% versus 0% respectively; P = 0.139). The rate of wound infection requiring treatment with antibiotics was also not significantly different between the monopolar diathermy group and the LigaSure™ group (4.8% versus 6.8% respectively; P = 0.683).
Discussion
Despite rapid advances in surgical haemostasis technology, the current surgical paradigm fails to provide a standardized approach for tissue cutting and sealing devices in breast cancer. Several factors currently influence a surgeon’s choice of diathermy equipment, such as ergonomics, cost, and outcomes. While several non-randomized studies have investigated the optimal sealing device for axillary surgery in breast cancer, there is a paucity of information available breast surgeons regarding which device makes a meaningful difference to patient outcomes for simple mastectomy. In one RCT by Park et al.24, LigaSure™ was found to significantly improve drainage volume and duration in comparison with monopolar diathermy. Park et al.24 showed favourable outcomes for the LigaSure™ group; however, owing to the diversity of procedures performed by Park et al.24, such as the inclusion of immediate reconstruction and/or axillary dissection, it was still unknown whether LigaSure™ improves outcomes for patients undergoing simple mastectomy alone.
In terms of the primary outcome of interest, there was no difference in the number of days the dain remained in situ between monopolar diathermy and LigaSure™ in the present trial. In keeping with this finding, the total drain output was similar in both study arms. Similarly, there were no statistically significant differences in the secondary outcomes between the monopolar diathermy group and the LigaSure™ group (that is rates of seroma formation, haematoma formation, and wound infection). The 2.3% and 5.8% overall rates of postoperative haematoma formation and wound infection respectively are in keeping with current international guidelines and demonstrate acceptability of either practice.
Another essential consideration is operating time and this has implications for both the patient and the surgeon25. Monopolar diathermy traditionally represents a relatively quick and easy method of achieving haemostasis26. Conversely, LigaSure™, with its smaller surface area and the need for opening and closing the device with each cut, logically represents a slower device. Although no statistically significant increase in operating time was observed in the present study, performing a mastectomy using LigaSure™ was a mean of almost 20 min slower than performing a mastectomy using monopolar diathermy (mean of 107.5 min versus 88.25 min respectively; P = 0.41). Maximum efficiency without compromising patient outcomes is the goal of most surgeons. The findings from the present study place monopolar diathermy ahead of the more modern cutting and sealing LigaSure™ device, with no compromise with regard to seroma formation.
In addition, monopolar diathermy saves over €300 in comparison with LigaSure™ (€5 versus €340 respectively); this, coupled with its ease of use, low complication rates, and faster operating time, make monopolar diathermy an attractive option. These are essential considerations for surgeons, as cost savings and health economics are increasingly important in modern healthcare resource management.
There are some limitations, which must be taken into consideration when interpreting the results. Whereas the patient is blinded to the arm to which they are allocated, the operating surgeon is aware of the participant’s arm, making them subject to unintentional bias. A degree of information bias must be considered present within the present study, as drain outputs were self-reported by participants. Similarly, the decision to drain a seroma is made by an individual consultant based on their clinical acumen, which may introduce a degree of observer bias. Notwithstanding these limitations, the single-centre double-blinded methodology increases the robustness of the results of the present trial, thus supporting the routine use of monopolar diathermy over the vessel-sealing device LigaSure™ when performing simple mastectomy.
Simple mastectomy continues to be a commonly performed procedure for invasive and in situ breast cancer, as well as risk-reducing surgery. Methods to improve postoperative complication rates are important for this patient cohort and evidence-based surgery should be adhered to when introducing new medical devices. This RCT shows that, whilst both monopolar diathermy and the vessel-sealing device LigaSure™ are safe and effective for carrying out simple mastectomy, there are no significant differences in rates of seroma formation, haematoma formation, and wound infection between these devices. Monopolar diathermy represents a safe, cost-effective, and well-established sealing method that should remain as the first-line choice for surgeons when performing simple mastectomy.
Supplementary Material
Contributor Information
Stephen Keelan, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Gavin P Dowling, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Trudi Roche, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Aisling Hegarty, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Matthew G Davey, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Amenah A Dhannoon, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Sorcha O’Grady, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Eithne Downey, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Jarlath Bolger, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Michael Boland, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Jan Sorensen, Health Outcomes Research Centre, School of Population Health, RCSI University of Medicine and Health Sciences, Dublin, Ireland.
Colm Power, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Abeeda Butt, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Chwanrow Baban, Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Arnold D K Hill, Department of Surgery, RCSI University of Medicine and Health Sciences, Dublin, Ireland; Beaumont RCSI Cancer Centre, Beaumont Hospital, Dublin, Ireland.
Funding
The authors have no funding to declare.
Author contributions
Stephen Keelan (Data curation, Formal analysis, Investigation, Writing—review & editing), Gavin P. Dowling (Data curation, Formal analysis, Investigation, Writing—review & editing), Trudi Roche (Data curation), Aisling Hegarty (Data curation, Writing—review & editing), Matthew G. Davey (Formal analysis), Amenah A. Dhannoon (Data curation, Methodology), Sorcha O’Grady (Data curation), Eithne Downey (Data curation, Methodology, Project administration), Jarlath Bolger (Methodology), Michael Boland (Investigation, Writing—review & editing), Jan Sorensen (Formal analysis), Colm Power (Investigation), Abeeda Butt (Investigation), Chwanrow Baban (Investigation), and Arnold D. K. Hill (Investigation, Supervision)
Disclosure
The authors declare no conflict of interest. Consort diagram was created with BioRender.com.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data will remain within the Department of Surgery, RCSI for an interval of 7 years after study closure. Data available for audit/interrogation. Participants were asked to consent to future research within this field of research. Data can be made available for future research in this capacity.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249 [DOI] [PubMed] [Google Scholar]
- 2. Keelan S, Flanagan M, Hill ADK. Evolving trends in surgical management of breast cancer: an analysis of 30 years of practice changing papers. Front Oncol 2021;11:622621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nespoli L, Antolini L, Stucchi C, Nespoli A, Valsecchi MG, Gianotti L. Axillary lymphadenectomy for breast cancer. A randomized controlled trial comparing a bipolar vessel sealing system to the conventional technique. Breast 2012;21:739–745 [DOI] [PubMed] [Google Scholar]
- 4. Calpin GG, McAnena PF, Davey MG, Calpin P, Kerin MJ, McInerney N et al. The role of tranexamic acid in reducing post-operative bleeding and seroma formation in breast surgery: a meta-analysis. Surgeon 2023;21:e183–e194 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma formation following breast cancer surgery. Breast J 2003;9:385–388 [DOI] [PubMed] [Google Scholar]
- 6. Tejler G, Aspegren K. Complications and hospital stay after surgery for breast cancer: a prospective study of 385 patients. Br J Surg 1985;72:542–544 [DOI] [PubMed] [Google Scholar]
- 7. Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World J Surg Oncol 2004;2:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woodworth PA, McBoyle MF, Helmer SD, Beamer RL. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg 2000;66:444–450; discussion 450–451 [PubMed] [Google Scholar]
- 9. Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer 2012;15:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain P, Sowdi R, Anderson A, MacFie J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br J Surg 2004;91:54–60 [DOI] [PubMed] [Google Scholar]
- 11. Adrien C, Katia M, Marie-Lucile B, Alice R, Claire B, Roman R. Prevention of lymphocele or seroma after mastectomy and axillary lymphadenectomy for breast cancer: systematic review and meta-analysis. Sci Rep 2022;12:10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vos H, Smeets A, Neven P, Laenen A, Vandezande L, Nevelsteen I. Early drain removal improves quality of life and clinical outcomes in patients with breast cancer—results from a randomised controlled trial. Eur J Oncol Nurs 2018;36:112–118 [DOI] [PubMed] [Google Scholar]
- 13. Park HS, Lee J, Kim JY, Park JM, Kwon Y. A prospective randomized study to compare postoperative drainage after mastectomy using electrosurgical bipolar systems and conventional electro-cautery. J Breast Cancer 2022;25:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manouras A, Markogiannakis H, Genetzakis M, Filippakis GM, Lagoudianakis EE, Kafiri G et al. Modified radical mastectomy with axillary dissection using the electrothermal bipolar vessel sealing system. Arch Surg 2008;143:575–580; discussion 581 [DOI] [PubMed] [Google Scholar]
- 15. Khan S, Khan S, Chawla T, Murtaza G. Harmonic scalpel versus electrocautery dissection in modified radical mastectomy: a randomized controlled trial. Ann Surg Oncol 2014;21:808–814 [DOI] [PubMed] [Google Scholar]
- 16. Chang YW, Kim HS, Jung SP, Woo SU, Lee JB, Bae JW et al. Comparison of skin-sparing mastectomy using LigaSure™ small jaw and electrocautery. World J Surg Oncol 2017;15:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortadellas T, Córdoba O, Espinosa-Bravo M, Mendoza-Santin C, Rodríguez-Fernández J, Esgueva A et al. Electrothermal bipolar vessel sealing system in axillary dissection: a prospective randomized clinical study. Int J Surg 2011;9:636–640 [DOI] [PubMed] [Google Scholar]
- 18. Porter KA, O'Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. Am J Surg 1998;176:8–11 [DOI] [PubMed] [Google Scholar]
- 19. Sutton PA, Awad S, Perkins AC, Lobo DN. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel™ and the Ligasure™. Br J Surg 2010;97:428–433 [DOI] [PubMed] [Google Scholar]
- 20. Palazzo FF, Francis DL, Clifton MA. Randomized clinical trial of Ligasure™ versus open haemorrhoidectomy. Br J Surg 2002;89:154–157 [DOI] [PubMed] [Google Scholar]
- 21. Jayne DG, Botterill I, Ambrose NS, Brennan TG, Guillou PJ, O'Riordain DS. Randomized clinical trial of Ligasure™ versus conventional diathermy for day-case haemorrhoidectomy. Br J Surg 2002;89:428–432 [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abouleish AE, Leib ML, Cohen NH. ASA provides examples to each ASA physical status class. ASA Monitor 2015;79:38–49 [Google Scholar]
- 24. Park HS, Lee J, Kim JY, Park JM, Kwon Y. A prospective randomized study to compare postoperative drainage after mastectomy using electrosurgical bipolar systems and conventional electro-cautery. J Breast Cancer 2022;25:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson TD, Wannares JJ, Lancaster RT, Rattner DW, Hutter MM. Does speed matter? The impact of operative time on outcome in laparoscopic surgery. Surg Endosc 2011;25:2288–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Massarweh NN, Cosgriff N, Slakey DP. Electrosurgery: history, principles, and current and future uses. J Am Coll Surg 2006;202:520–530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will remain within the Department of Surgery, RCSI for an interval of 7 years after study closure. Data available for audit/interrogation. Participants were asked to consent to future research within this field of research. Data can be made available for future research in this capacity.