Abstract
The Mango I and II RNA aptamers have been widely used in vivo and in vitro as genetically encodable fluorogenic markers that undergo large increases in fluorescence upon binding to their ligand, TO1-Biotin. However, while studying nucleic acid sequences, it is often desirable to have trans-acting probes that induce fluorescence upon binding to a target sequence. Here, we rationally design three types of light-up RNA Mango Beacons based on a minimized Mango core that induces fluorescence upon binding to a target RNA strand. Our first design is bimolecular in nature and uses a DNA inhibition strand to prevent folding of the Mango aptamer core until binding to a target RNA. Our second design is unimolecular in nature, and features hybridization arms flanking the core that inhibit G-quadruplex folding until refolding is triggered by binding to a target RNA strand. Our third design builds upon this structure, and incorporates a self-inhibiting domain into one of the flanking arms that deliberately binds to, and precludes folding of, the aptamer core until a target is bound. This design separates G-quadruplex folding inhibition and RNA target hybridization into separate modules, enabling a more universal unimolecular beacon design. All three Mango Beacons feature high contrasts and low costs when compared to conventional molecular beacons, with excellent potential for in vitro and in vivo applications.
Keywords: G-quadruplex folding regulation, RNA Mango aptamer, fluorescent reporter, molecular beacon, nucleic acid reporting
INTRODUCTION
Molecular beacons (MBs) (Tyagi and Kramer 1996) have been used extensively in vitro (Vet et al. 1999) and in vivo (Mao et al. 2020a) as a gold standard for nucleic acid detection. But their application is restricted by inherent design challenges. These chemically synthesized reporters achieve low fluorescence in their “OFF” state by colocalizing fluorophores and quenchers on the 5′ and 3′ ends of a DNA hairpin (Fig. 1A). MBs can result in fluorescence enhancements of one to two orders of magnitude upon successful target binding and physical separation of the fluorophore and quencher, but are fundamentally limited by the quenching efficiency of the fluorophore (Tsourkas et al. 2003; Zheng et al. 2015). Further, owing to the complex synthesis, conjugation, and purification steps required to produce MBs, they can be costly. For in vivo studies, MBs cannot cross the negatively charged plasma membrane without assistance, requiring laborious methods such as microinjection (Chen et al. 2016), electroporation (Mao et al. 2020b), or reverse transfection (Yang et al. 2023). And, once inside cells, they are susceptible to unintentional fluorescence emergence due to nuclease degradation or protein binding (Mao et al. 2020a). Thus, there is a need for improved nucleic acid detection methods with low cost, high fluorescence enhancement, potential for in situ generation, and improved biological integration.
FIGURE 1.
Fluorescence emergence strategies for MBs and novel Mango Beacons. (A) Fluorescence emergence in a conventional MB, triggered by binding to a complementary target sequence, which separates the chemically conjugated fluorophore (green circle) and quencher (gray square). (B) Bimolecular Mango Beacon formed by appending target binding hybridization arms (blue) onto a minimal Mango I or II core (black). Folding of the beacon is inhibited by a DNA suppressor (red), which is released upon binding to a trigger sequence (orange), allowing for the Mango core to fold and bind to its ligand TO1-Biotin (green). (C) Simple unimolecular Mango Beacon, which shows minimal folding until the addition of a trigger. (D) Self-inhibited unimolecular Mango Beacon, which has an inhibition domain (red) that deliberately hybridizes to the Mango core, reducing folding in the absence of a trigger.
Fluorogenic RNA aptamers such as Mango (Autour et al. 2018a), Spinach (Paige et al. 2011), and Broccoli (Filonov et al. 2014) have attracted interest as an alternative method to visualize nucleic acids (Lu et al. 2023). Upon binding to their innately dim fluorogenic ligands, they induce increases in brightness by up to three orders of magnitude. In their unmodified forms, these aptamers can be genetically encoded into an RNA of interest, creating cis-acting probes that are transcribed in situ, requiring only the addition of a fluorogenic ligand to produce fluorescence. The high fluorescence contrast afforded by RNA aptamers has been applied in vivo for single-molecule imaging (Cawte et al. 2020a) and to quantify RNA polymerase transcription activity (Song et al. 2017). In vitro, fluorogenic aptamers have been used as amplification reporters for viral detection assays (Abdolahzadeh et al. 2019), as monitoring units for transcription (Sando et al. 2008), as tags for ribonucleoprotein purification (Panchapakesan et al. 2017), and as reporters for electrochemical biosensing (Ferapontova et al. 2008). Recently, the modification of these aptamers to produce conditionally triggered trans-acting sensors has resulted in a series of “turn-on” molecular sensors that compare favorably with MBs in terms of cost, fluorescence enhancement, and in vivo application (Bouhedda et al. 2018). These sensors typically rely on modifying an existing fluorogenic aptamer to inhibit their function until the addition of a target sequence, which restores fluorescence. Using the Spinach family of aptamers, this has been realized by appending sequences that disrupt folding of the aptamer core (Bhadra and Ellington 2014; Soni et al. 2019), removing domains to destabilize folding (Aw et al. 2016; Ong et al. 2017), or by inhibition using a secondary sequence that binds and interferes with the aptamer (Ji et al. 2019).
In this work, we report the structure-guided development of a suite of high contrast turn-on fluorogenic aptamer sensors (Mango Beacons) based on RNA Mango I and II (Fig. 2). These aptamers contain potassium-dependent three-tiered G-quadruplex structures that bind to their fluorogenic ligand TO1-Biotin with nanomolar affinity, causing a 103-fold increase in ligand brightness (Trachman et al. 2017, 2018). Using a truncated version of Mango that minimally preserves G-quadruplex folding, we form target binding probes that maintain low fluorescence until the addition of a “trigger” sequence that is complementary to two programmable hybridization arms immediately upstream and downstream from the Mango core.
FIGURE 2.

Schematic for the development of Mango Beacon structures (MIB/MIIB). Starting with the wild-type Mango RNA aptamer, removing the stem–loop results in a minimal Mango core with marginally reduced fluorescence. The addition of hybridization arms results in sequence-targeting Mango Beacon constructs, but with variable inhibition of G-quadruplex core folding efficiency. The GAA^A loop adapter found in Mango I and II is indicated in purple.
We first designed a bimolecular construct that inhibits quadruplex core folding by hybridizing a “suppressor” DNA oligonucleotide across one of the Beacon's hybridization arms and into the Mango core (Fig. 1B). The addition of a target, “trigger” strand that is fully complementary to the Beacon's arms displaced this suppressor oligo, enabling rapid folding of the Mango G-quadruplex core and triggering the rapid emergence of fluorescence. In a second unimolecular design, the suppression of aptamer folding was achieved by adding noncomplementary hybridization arms flanking the minimal core (Fig. 1C), and is similar in spirit to the approaches taken by Ellington and Sharma with the Spinach and Baby Spinach aptamers (Bhadra and Ellington 2014; Soni et al. 2019). Building upon this design, our third strategy deliberately incorporated a small inhibition domain into the 3′ hybridization arm, which was engineered to inhibit aptamer folding by base-pairing onto residues involved in G-quadruplex formation. The addition of a trigger sequence disrupts this cis base-pairing, allowing for the G-quadruplex core to fold and trigger the emergence of fluorescence (Fig. 1D). These Mango Beacon designs, which are easily synthesized by in vitro transcription, show ON/OFF contrast ratios superior to both conventional fluorescence-quenched MBs and existing turn-on aptamer sensors.
RESULTS AND DISCUSSION
Curiously, the crystal structures of both the Mango I and II aptamers showed the presence of a GNRA stem–loop-like adapter that connects the G-quadruplex aptamer core, responsible for fluorophore ligand binding, to an arbitrary external sequence. However, removing this motif from Mango I causes only a twofold to eightfold decrease in binding affinity and a 20%–30% decrease in final fluorescent brightness compared to the wild-type aptamer (Trachman et al. 2017). Using this minimal Mango core, we added single-stranded flanking arms that are fully customizable and complementary to a target sequence of interest (Fig. 2). This replacement was found to have a strong inhibitory effect on Mango I and II fluorescence, making this morphology a good baseline for the formation of sequence-dependent RNA Mango Beacons (MIB and MIIB, respectively).
DNA oligonucleotide-inhibited Mango II Beacons
Our first strategy for creating a “turn-on” Mango Beacon was to introduce a 12 nt DNA “suppressor” sequence that stabilizes the unfolded state of MIIB by hybridizing to one of the flanking hybridization arms and part of the Mango II G-quadruplex core, resulting in a dark “OFF” state (Fig. 1B). However, the addition of a target “trigger” sequence that is complementary to the upstream and downstream Mango Beacon arms can remove this “suppressor” using the well-established toehold-mediated displacement method (Zhang and Winfree 2009), reforming the fluorescent “ON” state.
As binding and displacement of the suppressor sequence are expected to impact both the dimness of the aptamer in the “OFF” state and the brightness in the “ON” state, we evaluated a series of inhibitor DNA sequences, all of the same length but that hybridize to different regions of the Mango Beacon. This allowed us to investigate the optimal position for the suppressor sequence that sufficiently inhibits folding of the aptamer G-quadruplex core, while still allowing for displacement upon target strand binding. To evaluate the performance of these constructs, Mango Beacons and suppressor sequences were added to a solution of TO1-Biotin fluorophore and buffer. The beacons were then incubated for 60 min at room temperature to detect even small amounts of prefolding, as monitored by the emergence of fluorescence, before the addition of a trigger RNA strand complementary to the beacon arms. We defined contrast as the ratio between fluorescence in the “ON” state after the addition of the trigger, divided by fluorescence in the “OFF” state immediately before the addition of the trigger. The best constructs should have both a high contrast and a high maximum fluorescence.
It was found that by positioning the 3′ end of the 12 nt DNA suppressor sequence between positions 2–8 and 24–28 of the MIIB sequence, effective MIIBs could be formed (Fig. 3A,B). Inhibition at these positions led to contrast values of at least 20 between the “ON” and “OFF” states, suggesting that hybridization of the suppressor sequence successfully inhibited G-quadruplex core folding and fluorescence emission, while hybridization of a trigger RNA sequence rescued folding. Positioning the 3′ terminus of the suppressor DNA oligo at nucleotide position 6 of MIIB (suppressor 6, Fig. 3C) resulted in the highest contrast ratio of 107 with a high final emission intensity ∼70% of the reported wild-type Mango II aptamer (Trachman et al. 2018). This fluorescence enhancement is on par with the best “turn-on” aptamer sequences, as well as conventional MBs (Zheng et al. 2015). Sequence analysis shows that this suppressor oligo hybridizes entirely to the first G-quadruplex stack and partially to the second (..GGAGAGGAG.., underlined with G-quadruplex stacks in bold, Fig. 3C). This is expected to fully disrupt the folding of the G-quadruplex aptamer core, while still allowing toehold displacement of the DNA suppressor by target RNA hybridization to the 5′ hybridization arm. DNA sequences binding substantially to either the 5′ or the 3′ hybridization arms of the MIIB favored the “ON” state and had poor contrast upon the addition of the RNA trigger strand. Likewise, DNA sequences binding predominantly within the G-quadruplex core favored the “OFF” state and could not be turned “ON,” also resulting in poor contrast. Only those DNA sequences that simultaneously spanned one of the hybridization arms and a portion of the G-rich quadruplex core could be “OFF” initially and then turn “ON” by the addition of the RNA trigger strand.
FIGURE 3.

Optimization of bimolecular Mango II Beacons by modulating the position of the DNA oligo suppressor. (A, B) Aligned with respect to C, which shows the positioning and sequence of suppressor 6, which produced the highest contrast between “ON” and “OFF” states. (A) Maximum fluorescence of bimolecular Mango Beacon constructs following the addition of a trigger sequence. Numbers along the x-axis represent the 3′ position of a complementary DNA suppressor. The standard deviation of three replicates is shown (error bars). (M) Mango II control, (C) MIIB in the absence of a DNA suppressor oligo. (B) Contrast ratios of bimolecular Mango Beacon constructs, defined as the ratio of fluorescence before and after the addition of a trigger strand. The contrast and fluorescence values for the construct with the highest contrast (inhibited with DNA suppressor 6) are highlighted in red. (C) Sequence of Mango Beacon (MIIB) inhibited by suppressor 6. The Mango core is underlined, to highlight the two noncore linker A's. G-stacks for a given column of G residues in the G-quadruplex structures are highlighted in gray. The Mango Beacon arms are highlighted in blue, and are complementary to the trigger sequence used (shown in orange).
The simultaneous requirement for the DNA suppressor strand to inhibit folding of the G-quadruplex core, and to be easily displaced by the RNA trigger strand, suggests that improved beacons can be obtained by increasing the length of the DNA suppressor. We explored this question by extending the Beacon's upstream and downstream hybridization arms, and inhibiting initial fluorescence using 18-nt DNA suppressor sequences, in contrast to the 12-mers previously evaluated (Supplemental Fig. S1). The 12-nt suppressor 6 oligo, which provided the highest contrast previously, showed a slightly higher contrast of 113 ± 4 on this construct. However, the 18-nt long suppressor “A,” which extends further into the 5′ hybridization arm, was found to have an improved contrast of 126 ± 2 and slightly higher brightness than the initial design. The contrast was enhanced even further to 176 ± 10 using suppressor “C,” which extended the DNA:RNA hybridization into the G-quadruplex core region by 1 nt. However, this came with a consequent loss in final brightness. Further modifications to the suppressor sequence are presented in Supplemental Figure S1, highlighting the interplay between the suppression of G-quadruplex folding and the ability for toehold-mediated displacement to occur. From this data, it is clear that for a given Mango Beacon sequence, significant optimization of the DNA suppressor hybridization length and region is possible. Designing a DNA suppressor remains simple, however, and several of these constructs resulted in similar, if not better, contrasts compared to reported MBs (Tsourkas et al. 2003; Zheng et al. 2015; Bidar et al. 2021).
The Mango Beacon design described here differs from a previously reported bimolecular Universal Baby Spinach-based Probe (Ji et al. 2019). This turn-on sensor also uses a DNA suppressor to prevent the folding of an unmodified Baby Spinach aptamer, but differs by inserting a target binding sequence into the suppression strand. A fluorescent signal is obtained when target binding causes detachment of this suppressor sequence from the Spinach aptamer. This strategy uniquely allows for the detection of several substrates, such as Hg2+ ions, thrombin, and miRNA. However, in contrast to our design above, the Universal Baby Spinach-based Probe has significantly lower contrast and does not associate with its target strand, precluding its use in, for example, RNA localization studies.
Unimolecular constructs
While the oligo-inhibited Mango Beacons featured impressive fluorescence enhancements that are on par with the best MB technologies, the need to hybridize a suppressor oligo to preform the “OFF” state adds an additional step that could challenge their use for in vivo applications, where the beacon constructs must be transcribed within living cells. We, therefore, set out to create a series of unimolecular constructs that exhibit similar target-mediated structural rearrangements upon binding to a sequence of interest, but that lack a bimolecular DNA inhibitor sequence. We appended the same 10 nt upstream and downstream hybridization arms that were used in the bimolecular constructs to the minimal Mango I and II cores, which served to both mediate target specificity and interfere with the folding of the aptamer core until a trigger is bound (Fig. 1C).
Given that a GNRA tetraloop domain was selected for in both the Mango I and II aptamers, despite its minimal impact on fluorescence, we hypothesized that this sequence may serve to isolate the G-quadruplex core from potentially interfering upstream and downstream sequences and ensure the aptamer's proper folding. Therefore, by removing this tetraloop, we speculated that we could develop Mango Beacons that are susceptible to interference from surrounding sequences until the addition of a trigger strand that base-pairs to the interfering arms. With this in mind, we developed constructs that mimicked the geometry of a GNRA tetraloop to facilitate proper folding upon binding to their target. We investigated whether the addition of either A or U residues between the Mango I core and hybridization arms was beneficial, and tested each of these constructs against trigger sequences that were complementary to the hybridization arms, with either 0, 1, or 2 A residues inserted between the two arm sequences. Fluorescence and contrast ratios were evaluated for each of the 18 resulting constructs using the protocol described in the Materials and Methods section, with the exception that a plate reader was used for fluorescence measurements (Supplemental Fig. S2). Mango I constructs with A residues flanking the 5′ and 3′ sides of the core performed best along with trigger sequences that did not have any adenosine added. This is consistent with crystallographic data, which shows that the distances between the two A residues on either side of the GAA^A tetraloop flanking the aptamer core are 8.0 Å and 5.9 Å in Mango I and II, respectively (Trachman et al. 2017, 2018). Such distances overlap with the ∼6.1 Å backbone spacing found in A-form dsRNA (Wang et al. 2019) and suggest why Mango core folding is so compatible with the simple RNA trigger constructs used in this study. This trigger morphology is also ideal for the universal targeting of sequences, in contrast to the Pandan light-up sensor, which was found to require a target with two U residues between the flanking arms to stabilize aptamer folding (Aw et al. 2016). This optimal MIB construct, containing two flanking adenosine residues and a fully complementary trigger sequence, was found to have a contrast of 23 ± 6, which is comparable to the performance of many quenched and conventional MBs. But the final brightness was only ∼30% of the wild-type Mango I aptamer, indicating room for further optimization.
To improve the brightness of our MIB Beacons, which are limited by the intrinsic brightness of the Mango I aptamer, we investigated the use of Mango II which is 1.5 times brighter in its native form. Given the similarities between the G-quadruplex structures for the Mango I and II aptamers, we constructed and evaluated a unimolecular Mango II Beacon construct (MIIB) using the same approach described previously (Fig. 4A). Remarkably, the MIIB demonstrated a fluorescence intensity (159 ± 6) four times higher than the MIB construct (38 ± 3); however, the contrast was diminished significantly (C = 2.2 ± 0.2) due to substantial prefolding in the absence of trigger sequence, which was not observed for the MIB construct (Fig. 4B; Table 1). This suggested that the two hybridization arms were not interacting as effectively with the Mango II core as the Mango I core, leading to a dimmer “OFF” state of MIIB. We therefore set out to modify the 3′ arm in our unimolecular MIIB construct to deliberately inhibit prefolding, while maintaining a high final brightness.
FIGURE 4.
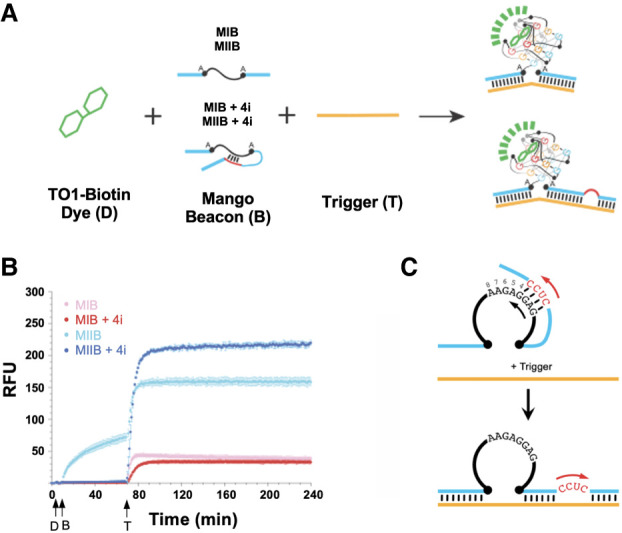
Inclusion of self-inhibiting nucleotides in the hybridization arm improves contrast. (A) Experimental scheme used to measure fluorescence emergence. (B) Comparison of the fluorescence emergence for the Mango I Beacon and Mango II Beacon with a 4-nt inhibitory domain (MIB + 4i and MIIB + 4i, respectively) and without this domain (MIB and MIIB, respectively). Time courses were performed as follows: TO1-Biotin solution (D, 100 nM final, added at t = 5 min) is incubated with a Mango Beacon construct (B, 50 nM final concentration, added at t = 10 min) and then a trigger sequence added (T, 100 nM final concentration, added at t = 70 min). (C) Cartoon showing the design of the MIIB + ni construct, where n refers to the number of base pairs of hybridization, as indicated by the arrow.
TABLE 1.
Self-inhibiting, unimolecular Mango Beacon and trigger sequences for Figure 5 with contrast and relative Fmax
| Identity | Sequence | Contrasta | F max b |
|---|---|---|---|
| MIB | GGA CAA UAG CAG GGA CGG UGC GGA GAG GAG AAC GUA GCC U | 23 ± 6 | 38 ± 3 |
| MIB + 4i | GGA CAA UAG CAG GGA CGG UGC GGA GAG GAG AAC GUA GCC UGC UCC GCU AA | 85 ± 23 | 33 ± 3 |
| MIIB | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CU | 2.2 ± 0.2 | 159 ± 6 |
| MIIB + 4i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CGC UAA | 78 ± 10 | 252 ± 3 |
| MIIB + 5i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CUG CUA A | 234 ± 24 | 181 ± 5 |
| MIIB + 6i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CUC GCU AA | 138 ± 51 | 183 ± 8 |
| MIIB + 7i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CUC UGC UAA | 163 ± 15 | 168 ± 10 |
| MIIB + 8i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CUC UUG CUA A | 57 ± 13 | 76 ± 2 |
| Trigger | GGG UUA GCC AGG CUA CGU GCU AUU GUC C | n/a | n/a |
Adding the inhibiting domain increases the contrast ratio by decreasing the “OFF” state fluorescence values. Nucleotides in bold indicate Mango core sequences with the additional flanking As; nucleotides that are in bold and italicized indicate inhibitory nucleotides (+ ni); underlined regions indicate potential core nucleotides to which a region in one of the flanking arms hybridizes. Trigger sequences are shown starting with 5′ end.
aObtained by taking the average of the fluorescence values at t = 238–240 min and dividing it by the average of t = 67–69 min, which are the fluorescent values immediately before the trigger was added at 70 min.
bObtained by taking the average of the fluorescence values at t = 238–240 min. The errors are from the standard deviation of the triplicate data.
Self-inhibiting unimolecular constructs
We hypothesized that the contrast of the unimolecular Mango Beacons could be improved by rationally inserting an inhibition domain into the 3′ hybridization arm that is able to hybridize with residues involved in G-quadruplex formation within the Mango I or II core. In the absence of a target, we expected such constructs to effectively inhibit the folding of the Mango core, resulting in the potential for a darker “OFF” state and therefore higher contrast upon the addition of a trigger sequence. When a trigger is added, this inhibition domain simply forms a bulge on the 3′ arm of the beacon where it is not complementary to the trigger, and therefore does not limit the scope of target sequences. This design is an improvement from our previous MIB and MIIB constructs, which did not intentionally feature sequences complementary to the core, and instead relied on indiscriminate interactions of the target-specific hybridization arms with the aptamer core to achieve a dark “OFF” state. It was found that adding a 4 bp sequence into the 3′ arm that is able to hybridize to the fourth G-quadruplex stack nearly fully suppressed problematic prefolding of the aptamer core. For the MIIB construct, a remarkable increase, in contrast, was observed from 2.2 ± 0.2 to 78 ± 10 when using the version with a 4 bp inhibition sequence. The MIIB + 4i beacon also became 1.4 times brighter than MIIB in its “ON” state (Fig. 4B), suggesting that the addition of this inhibition sequence both prevents prefolding of the Mango II aptamer core before the addition of a trigger strand, and improves folding upon the addition of a trigger strand. Consistent with our hypothesis, the addition of an inhibition sequence also improved the contrast for the MIB construct from 23 to 85. However, a slight decrease in the final construct brightness was observed, compared to the MIB construct without the 4 bp inhibition sequence.
We sought to further optimize the design of this loop-back inhibition sequence by varying the number of residues that hybridize to the core. Using the brighter MIIB beacon, we created a series of MIIB + ni constructs, where n specifies the number of bp of hybridization into the G-quadruplex core (4, 5, 6, 7, or 8) (Figs. 4C and 5A–D; please note that MIIB + 4i is a replicate of the data presented in Fig. 4B). This resulted in constructs with contrast ratios of C = 78 ± 10, 234 ± 24, 138 ± 51, 163 ± 15, and 57 ± 13, respectively (Fig. 5C). It was found that 4 bp of inhibition was optimal for achieving high brightness, while ≥5 bp was best for optimizing contrast by inhibiting fluorescence before the addition of a trigger sequence. However, increasing the number of bp of hybridization to the core also slowed the rate of fluorescence emergence and decreased the final brightness achieved (Fig. 5D; Table 1). For a beacon of this sort to be useful, there must be a balance between low background fluorescence, fast interaction time, and high fluorescence. The 4, 5, and 6 bp inhibition designs were found to be optimal in these regards.
FIGURE 5.

Contrast and fluorescence optimization of self-inhibiting domains. (A) Fluorescence emergence (measured as described previously) of self-inhibiting unimolecular Mango Beacons with increasing base pairs of self-inhibition (n = 4–8). Note, MIIB + 4i is a replicate of the data presented in Figure 4B. (B) Prefolding region of plot A is highlighted for clarity. (C) Contrast observed for self-inhibiting Mango Beacons, where n = 0 is equivalent to the MIIB construct. (D) Maximum fluorescence intensity observed for self-inhibiting Mango Beacons, where n = 0 is equivalent to the MIIB construct.
Sequence dependence
To evaluate the impact of the target sequence on beacon function, we evaluated a series of MIB + 4i and MIIB + 4i type reporters, generated in vitro, able to hybridize to RNA sequences derived from Mouse, Drosophila, Chicken, and Human (Table 2). These sequences represent diverse potential targets that could be detected using Mango Beacons. Contrast ratios from 0.24 to 275 (median = 49, based on nine constructs) were observed for MIIB + 4i, while contrast ratios from 60 to 107 (median = 87.5, based on four constructs) were observed for MIB + 4i, making it clear that both the target and complementary hybridization arm sequence compositions play a role in inhibiting or assisting the Mango Beacon folding. From this, we hypothesized that Mango Beacon constructs could detect single-point mutations in the target trigger sequence. MIIB + 4i was evaluated against trigger strands with a series of point mutations on their 3′ end. While these mutations did affect the brightness, no clear pattern was observed that explains why some worked better than others (Supplemental Fig. S3; Table 3). We expect that mutations on the 5′ end of the trigger sequence would provide equally unpredictable results, but these were not evaluated. Overall, this observation suggests that Mango Beacons may be used to detect point mutations in some sequences, but more experiments are needed to determine if there is a generalizable pattern.
TABLE 2.
Mango Beacon and trigger sequences with contrast and Fmax
| Identity | Type | Sequence | Contrast | F max |
|---|---|---|---|---|
| Beacon MI.B2 Trigger MI.B2 |
MIB + 4i | UUG CAC AUG CCG AGG GAC GG UGC GG AGA GG AGA GAG CCG UUG U CUCC ACG ACC AGC GC AAC GUG UAC GGC CUC GGC AAC AGC UGC UGG UCG CGG G |
107 | 321a |
| Beacon MI.B3 Trigger MI.B3 |
MIB + 4i | CGA CCA GCG CAG AGG GAC GG UGC GG AGA GG AGA CGA UAU CGU CAU CUCC A UGG C GCU GGU CGC GUC GCU AUA GCA GUA GGU ACC GGG |
91 | 261a |
| Beacon MI.B4 Trigger MI.B4 |
MIB + 4i | ACU UCA GGG UCA AGG GAC GG UGC GG AGA GG AGA GGA UAC CUC UCU CUCC CUC UGG GCC UCG UGA AGU CCC AGU CCU AUG GAG AGA ACG AGA CCC GGA GCG G |
60 | 169a |
| Beacon MI.B5 Trigger MI.B5 |
MIB + 4i | AAG GGU GUA AAA AGG GAC GG UGC GG AGA GG AGA CGC AGC UCA GUA CUCC A GUC CGC CUA UUC CCA CAU UUU GCG UCG AGU CAU UGU CAG GCG GAUGG |
84 | 210a |
| Beacon MII.CBA1 Trigger MII.CBA1 |
MIIB + 4i | GGA UCC UGA GUC AGG AGA GGA GAG GAA GAG GAG AAA GCG CCA AA CUCC UAG AAA AAA CA UAG GAC UCA GUU CGC GGU UUU CUU UUU UGU GG |
115 | 149 |
| Beacon MII.CBA2 Trigger MII.CBA2 |
MIIB + 4i | GGC AUA CCG GAG AGG AGA GGA GAG GAA GAG GAG ACC AUU GUC AA CUCC UCA ACG AGC GC GUA UGG CCU CGG UAA CAG UUG UUG CUC GCG GG |
45 | 142 |
| Beacon MII.O1 Trigger MII.O1 |
MIIB + 4i | GGA AAA GCG GAA GGA GAG GAG AGG AAG AGG AGA AAA GUU UGA CUCC UA GAG AA UUU UCG CCU UUU CAA ACU UCU CUU CAA AAG UCG ACG AAC GCG AAU AGG |
0.24 | 19 |
| Beacon MII.O2 Trigger MII.O2 |
MIIB + 4i | GGA AAA GCG GAA AAA GGA GAG GAG AGG AAG AGG AGA GUU UGA AGA GAA GUU CUCCUU UCA GCU GCU UGC GCU U UUU UCG CCU UUU CAA ACU UCU CUU CAA AAG UCG ACG AAC GCG AAU AGG |
53 | 211a |
| Beacon MII.O4 Trigger MII.O4 |
MIIB + 4i | GGC GCA UUU ACG CUG AGG AGA GGA GAG GAA GAG GAG AGC UUG CUG GUA GAA ACU
CCU UUG UUG AGA U CCG CGU AAA UGC GAC CGA ACG ACC AUC UUU AAC AAC UCU AGG |
37 | 787a |
| Beacon MII.HBA1 Trigger MII.HBA1 |
MIIB + 4i | GGA CUU CAG GGU GAA GGA GAG GAG AGG AAG AGG AGA GGA UGC CUC UCU CUCC UC UCU GGG CCU CG UGA AGU CCC ACU CCU ACG GAG AGA ACG AGA CCC GGA GCG G |
275 | 217 |
| Beacon MII.HBA2 Trigger MII.HBA2 |
MIIB + 4i | GGC AAU GAU CUU AGG AGA GGA GAG GAA GAG GAG AGA UCU UCA UU CUCC UGU GCU GGG UG GUU ACU AGA ACU AGA AGU AAC ACG ACC CAC GG |
3 | 4 |
| Beacon MII.HBA3 Trigger MII.HBA3 |
MIIB + 4i | GGC CGA CUG CUG AGG AGA GGA GAG GAA GAG GAG AUC ACC UUC AC CUCC UCG UUC CAG UU GGC UGA CGA CAG UGG AAG UGG CAA GGU CAA GG |
195 | 214 |
| Beacon MII.HBAZ1 Trigger MII.HBAZ1 |
MIIB + 4i | GGG AAA GGG UGU AAA GGA GAG GAG AGG AAG AGG AGA CGC AAC UAA GUC CUCC UU CCG CCU A CUU UCC CAC AUU GCG UUG AUU CAG AGG CGG AUG G |
40 | 205 |
MI and MII denote either a Mango I- or Mango II-based Beacon reporter. Beacon sequences are shown starting with 5′ end, trigger sequences are shown starting with 3′ end.
aThe cuvette used had a 5× larger path length. Reactions were performed in singlet—no errors are presented. Trigger sequences are bolded. Fmax here represents the final fluorescence following between 2 and 4 h of incubation with the trigger. These Beacons were designed to target various RNA from Mouse, Drosophila, Chicken, and Human by varying sequences of the hybridization arms.
TABLE 3.
Trigger sequences for Supplemental Figures S3 and S5 with contrast and relative Fmax
| Identity | Sequence | Contrast | F max |
|---|---|---|---|
| MIIB + 4i Construct 2 |
GGA CAA TAG CAG GAG AGG AGA GGA AGA GGA GAA CGT AGC CTG CTC CGC TCC | n/a | n/a |
| Trigger A (WT) | GGG UUA GCC AGG CUA CGU GCU AUU GUC C | n/a | n/a |
| Trigger B | GGG UUA GCC CCA GGC UAC GUG CUA UUG UCC | n/a | n/a |
| Trigger C | GGG UUA GCC CAG GCU ACG UGC UAU UGU CC | n/a | n/a |
| MIIB + 4i | GGA CAA UAG CAG GAG AGG AGA GGA AGA GGA GAA CGU AGC CUG CUC CGC UAA | 85 ± 23 | 33 ± 3 |
| C20A | GGG UUA GCC AGG CUA CGU GAU AUU GUC C | 113 ± 22 | 191 ± 11 |
| C20U | GGG UUA GCC AGG CUA CGU GUU AUU GUC C | 72 ± 22 | 130 ± 5 |
| U21G | GGG UUA GCC AGG CUA CGU GCG AUU GUC C | 79 ± 8 | 96 ± 7 |
| U23G | GGG UUA GCC AGG CUA CGU GCU AGU GUC C | 117 ± 7 | 130 ± 2 |
Residues in bold indicate mutations. Trigger sequences are shown starting with 5′ end.
Kinetics
One concern for both the unimolecular and bimolecular constructs is that multiple hybridization events are required for the beacons to function. Firstly, a suppression event must inhibit the folding of the aptamer core (either by self-interaction or the addition of an external suppressor sequence), forming an “OFF” state. Secondly, an interaction with a target “trigger” sequence must initiate rapid quadruplex folding, forming the fluorescent “ON” state. If trigger on-rates could, in fact, be measured, this would imply that the rate of quadruplex folding and ligand binding must be quite rapid relative to the rate of trigger strand hybridization. Therefore, to compare our RNA Mango Beacons to established MBs, we measured the rate of fluorescence emergence as a function of trigger strand concentration for both the bimolecular MIIB construct (inhibited with suppressor D5, which is positioned with its 3′ end at nucleotide 5) and the self-inhibitory construct MIIB + 4i. We found on-rate values of 1.2 ± 0.1 × 104 M−1 sec−1 and 1.9 ± 0.12 × 104 M−1 sec−1, respectively (Supplemental Fig. S4). Such rates are directly comparable to traditional MBs, where on rates are reported in the 0.4–1.4 × 104 M−1 sec−1 range (Table 4). This implies that quadruplex folding rates and TO1-B ligand binding must be quite rapid (on the minute time scale or faster) as slower folding rates would have resulted in distinctive kinetic behavior.
TABLE 4.
Mango Beacon on-rates
| Identity | # Nucleotides of hybridizationa | On-rate Kon (M−1 sec−1) |
|---|---|---|
| MIIB + 4i | 25 | 1.2 × 104 ± 1.0 × 103 |
| MIIB + DNA inhibition (5D) | 20 | 1.9 × 104 ± 1.2 × 103 |
| Molecular beacon (Tsourkas et al. 2003)b | 17–19 | 4.10 × 103 to 7.4 × 103 |
| Linear dual labeled probe (Tsourkas et al. 2003)c | 17–19 | 1.0 × 104 to 1.4 × 104 |
aThe number of nucleotides of hybridization to target.
bRange of six reported rates, no error reported.
cRange of three reported rates, no error reported.
Structural analysis
To investigate the folding of the self-inhibiting unimolecular constructs, a 32P labeled Mango II type beacon (MIIB + 4i) was incubated with its trigger sequence, and then loaded into a native gel. The number of folding states was identified by counting the radioactive bands present, and whether or not these states were correctly folded was evaluated using a previously established TO1-Biotin staining method to observe in-gel Mango fluorescence (Yaseen et al. 2019). One comigrating radioactive and fluorescent band was observed, which suggests that only a single, correctly folded, state of the Mango II core was formed (Trigger A, Supplemental Fig. S5A; Table 3). No evidence for incorrectly folded states was observed. When MIIB + 4i was incubated with a DNA trigger, no in-gel fluorescence was observed. Fluorescence assays performed with a DNA trigger, double-stranded RNA trigger, or the complementary sequence of Trigger A also showed inhibited performance consistent with this data (Fig. 6; Table 5).
FIGURE 6.

Mango Beacons display specificity to RNA single-stranded nucleic acid triggers. Mango Beacon MIIB + 4i fluorescence after the addition of an RNA, DNA, reverse sequence of the RNA (Reverse RNA), or a double-stranded RNA (dsRNA). (D) Addition of TO1-Biotin dye (100 nM final), (S) addition of Mango Beacon (50 nM final), (T) addition of Trigger (100 nM final). Sequences are shown in Table 5.
TABLE 5.
Trigger sequences for Figure 6 with contrast and relative Fmax
| Trigger identity | Sequence | Contrasta | F max b |
|---|---|---|---|
| RNA (WT) | GGG UUA GCC AGG CUA CGU GCU AUU GUC C | 96 ± 42 | 219 ± 3 |
| DNA | GGG TTA GCC AGG CTA CGT GCT ATT GTC C | 92 ± 8 | 126 ± 8 |
| Reverse RNA (+GGG) | GGG CCU GUU AUC GUG CAU CGG ACC GAU UGG G | 1.6 ± 0.2 | 2.3 ± 0.3 |
| dsRNA | WT + reverse complement | 2.4 ± 1.3 | 5.5 ± 2.4 |
aObtained by taking the average of the fluorescence values at t = 243–247 min and dividing this by the average of t = 65–69 min, which are the fluorescent values immediately before the trigger was added at 70 min.
bObtained by taking the average of the fluorescence values at t = 243–247 min. The errors are from the standard deviation of the triplicate data.
While it is not surprising that dsRNA and a noncomplementary RNA sequence were unable to trigger fluorescence, it is curious that the DNA trigger was much less potent relative to an RNA trigger. A ninefold decrease in initial folding rate and a twofold loss in fluorescence maximum was observed compared to its RNA counterpart. This indicates that a DNA trigger is substantially less effective than the equivalent RNA trigger at stabilizing G-quadruplex formation. This may be due to differences in the properties of DNA–RNA duplexes compared to RNA–RNA duplexes. Recently, a computational analysis of DNA–RNA duplexes by Tan and coworkers showed that properties such as stretch modulus and base-pair inclination are often between the values expected for B-form DNA and A-form RNA. Further, DNA–RNA duplexes show very low twist-stretch coupling compared to their homopolymeric counterparts (Liu et al. 2019). Therefore, it is not entirely unexpected that a DNA–RNA duplex would not stabilize the Mango G-quadruplex as efficiently compared to an RNA–RNA duplex. However, further analysis is required to elucidate the cause of the low fluorescence observed here.
We performed DMS protection analysis to evaluate if beacon folding with and without a trigger present was comparable to previous patterns observed for the Mango core (Supplemental Fig. S6). As DMS modifies guanine residues at the N7 position by methylation unless hydrogen bonding is present, such as when G-tetrad structures form, a DMS-Aniline cleavage assay can be used to investigate whether or not the G-quadruplex has formed. The parental Mango II aptamer incubated with potassium and ligand showed strong protection of the G residues, as previously reported (Autour et al. 2018b). The MIIB + 5i beacon showed a similar response only when the trigger was also present, as expected. This strongly implicates a pronounced refolding of the Mango II core into a quadruplex structure after the addition of an appropriate trigger strand, consistent with the gel mobility data.
Hybridization of MIIB + 4i with its trigger creates a CUCC bulge in the 3′ beacon arm, resulting from noncomplementarity of the inhibition domain with the trigger. To further study the folding dynamics of the beacon, we designed two new trigger sequences, Triggers B and C, that feature one or two bulged cytosine residues opposite to the CUCC bulge (Supplemental Fig. S5B). These residues were chosen as they can thermodynamically compete for binding to the Mango core with cytosine residues in the beacon arm. Incubation of the beacons with these new trigger sequences resulted in three bands in the native gel, indicating that at least two additional misfolding states were being formed. Indeed, using a TO1-Biotin stained native gel experiment (Yaseen et al. 2019b), we found that the new alternative folds were not strongly fluorescent, consistent with aptamer misfolding (Supplemental Fig. S5B). We hypothesize that the noncomplementary cytosine residues in Triggers B and C can interact with guanine residues in the Mango core during the refolding events that must take place to form both the G-quadruplex core and RNA duplex in the hybridization arms. Interestingly, as our kinetic data showed that trigger–beacon interactions occur very quickly, this data suggests that the trigger sequences must be positioned closely to the G-quadruplex core during the folding process, in such a way that it forms nonfluorescent misfolded states. This, together with previous evidence, indicates that Mango G-quadruplex core folding is highly contextual in nature.
Quadruplex beacon technology and future developments
Although genetically encoded Mango aptamers have been used previously as cis-acting probes for the localization of RNA in live and fixed cells (Autour et al. 2018b; Cawte et al. 2020b), having a versatile trans beacon is undoubtedly useful both in vitro and in vivo. Mango Beacons can be generated in situ, but have the potential to hybridize to biological RNAs without requiring endogenous sequence modifications. This is particularly important for studying sequences where the inclusion of a genetic tag is at risk of modifying its function. Further, these probes can be produced in vivo using well-established plasmid methods, precluding the need for complex genetic engineering. Remarkably, we have shown that Mango Beacons produce fluorescence contrasts that are comparable, or even exceed, conventional MBs (Tsourkas et al. 2003), while being bioproducible and inexpensive.
Given our success in developing Mango Beacons based on the Mango I and II RNA aptamers, it is possible that similar reporters can be generated from Mango III (Trachman et al. 2019) and the natively dimeric Mango IV aptamers (Trachman et al. 2020). While these aptamers display significant sequence and structural differences compared to Mango I and II, crystal structures show that these aptamers also contain stem-to-G-quadruplex adaptor sequences that might be utilized in a similar fashion to this work. These aptamers have additional benefits, such as high magnesium resistance and improved brightness (Autour et al. 2018b) over the Mango I and II systems, and would be worthy of further exploration. Furthermore, Mango aptamers can bind a variety of fluorescently distinct green (TO1), orange (YO3), and red (TO3) dyes, which suggests that Mango Beacons can be used in conjunction with other aptamer reporting systems for multicolor detection. The newly developed Peach aptamers (Kong et al. 2021), one of which appears to contain a GNRA stem–loop motif, favor binding to red-shifted TO3-B over TO1-B, making it probable that orthogonal ligand binding beacons for multicolor detection and imaging can be made in the future.
In this study, we present Mango Beacons with short upstream and downstream sequence arms that can be programmed to detect nucleic acid targets. Future optimization could vary such arm lengths, and in vitro selection could be used to fully optimize junction nucleotides between these arms and the fluorogenic aptamer core. Mango Beacons bring unique benefits compared to the current chemically synthesized technology, and we hope that their use will enable advancements in the field of nucleic acid biology and biochemistry.
MATERIALS AND METHODS
RNA generation and purification
RNA Mango Beacons and triggers were produced using in vitro run-off transcription. Reactions were carried out using 1 µM DNA template (IDT), 2 U T7 RNA polymerase (Applied Biological Materials), 8 mM GTP, 5 mM CTP, 5 mM ATP, 2 mM UTP, 40 mM TRIS buffer pH 7.9, 2.5 mM spermidine, 26 mM MgCl2, and 0.01% Triton X-100. RNA was purified via 5% PAGE (19:1 acrylamide:bis). The resolved RNA band was excised and rotated overnight in 300 mM NaCl. The RNA transcript was recovered by ethanol precipitation and resuspended in water. Concentrations were determined using optical density at 260 nm with a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific) and IDT's online nearest neighbor estimate for extinction coefficient.
Initial Mango I Beacon screening
Mango Beacons (50 nM) were incubated with or without their trigger sequences (100 nM) in 100 nM TO1-Biotin, 15 mM HEPES pH 7.5, and 90 mM KCl buffer. A Spectramax M5 Plate Reader with excitation at 495 nm and emission at 535 nm was used to assess fluorescence. Samples were incubated at room temperature for 10 min before taking measurements. Measurements were taken at room temperature.
Fluorescence kinetics
Samples were excited at 510 nm and fluorescence was detected at 535 nm using a Varian Cary Eclipse Spectrofluorometer, unless otherwise stated in the Materials and Methods. In a quartz cuvette (Starna Cell), buffer solution (10 mM sodium phosphate buffer pH 7.2, 140 mM KCl, 1 mM MgCl2, 0.05% Tween-20) was incubated for 5 min before adding TO1-Biotin ligand at a final concentration of 100 nM. At t = 10 min, Mango Beacon RNA was added to a final concentration of 50 nM. At t = 70 min, the trigger sequence was added to a final concentration of 100 nM. Contrast ratios were obtained by averaging the final fluorescence values from each experiment (t = 240 min for all fluorescence curves shown) and dividing this value by the average of the final three readings obtained before adding the trigger sequence (t = 67–69 min).
Kon determination
Experiments were performed as described above with 50 nM beacon (either MIIB or MIIB + 4i), 100 nM TO1-B, and 0–256 nM of trigger RNA (Table 1). Fluorescence was measured as described previously, except for MIIB whose fluorescence in the absence of trigger was not monitored. Kinetic data immediately after the addition of trigger sequence fit well to a linear parametrization using the following equation:
Here, A is the y-intercept, B is the rate in RFU/min, and t is the time in minutes. The RFU/min rate was converted to M/s, by taking the beacon concentration and dividing it by the plateau fluorescence of the experiment. This molar rate of fluorescence emergence was then plotted against trigger concentration and fit to a straight line. The resulting slope was divided by the concentration of the Mango Beacon to determine the on rate (Kon) (Table 4).
DNA oligo inhibition
Reactions were prepared as described in the fluorescent kinetics method using a Varian Cary Eclipse Spectrofluorometer, with the alteration that 100 nM 12 nt DNA oligo inhibitor (final concentration) was incubated with the beacon before use in the reaction. Samples were prepared in triplicate and only single-point readings were performed for the OFF measurement without trigger (t = 69 min into reaction) and ON readings after the addition of trigger (t = 240 min). A series of 31 one-DNA oligos were tested against the MIIB beacon and final relative fluorescence and contrast are reported in Figure 4.
DMS probing of Mangos
DMS probing consisted of four main steps:
DMS (denaturing): 50 nM of RNA was 3′-end-labeled with 32P pCp and gel-purified. The resulting RNA was incubated in 50 mM HEPES pH 7.5 (50 µL) at room temperature for 30 min. After incubation, 10 µg carrier RNA was added. The sample was then heated to 90°C for 3 min before the addition of 0.5 µL of 25% DMS (diluted in ethanol) and heated to 80°C for 1 min, 150 µL ice-cold ethanol, and 5 µL 3 M NaCl was then immediately added and the sample moved to −20°C for 30 min. DMS-modified RNA was pelleted by centrifuge at 16,300 RCF at 4°C for 20 min.
DMS (native): 50 nM 3′-end-labeled RNA was incubated in 50 mM HEPES pH 7.5, 1 mM MgCl2, 140 mM either KCl or NaCl, with or without 500 nM TO1-B (final volume 50 µL) at room temperature for 30 min. After incubation, 10 µg carrier RNA was added. The sample was then incubated at room temperature for 15 min after the addition of 0.5 µL of 100% DMS. Ice-cold ethanol of 150 µL and 5 µL 3 M NaCl was then immediately added and pelleted for the denaturing DMS protocol.
Reduction: Pellets were resuspended in 10 µL 1 M Tris buffer pH 8, and 10 µL of freshly prepared 0.2 M sodium borohydride was added. The reaction was carried out on ice and in the dark for 30 min. Reactions were stopped by ethanol precipitation as above.
Aniline cleavage: To the resulting pellet, 20 µL (one part aniline, seven parts ddH2O, three parts glacial acetic acid) were added and incubated at 60°C for 15 min in the dark. Samples were flash-frozen by placing tubes in liquid nitrogen and lyophilized by speed vacuum centrifuge. Once dry, 20 µL ddH2O was added, and the sample was refrozen and lyophilized once again. The pellet was resuspended in a 50% formamide denaturing solution before being loaded on a 15% polyacrylamide gel (19:1 acrylamide:bis).
T1 RNase ladder and alkaline hydrolysis ladder
A total of 200 pmol 3′-end-labeled RNA was incubated in 20 mM sodium citrate, 6.3 M urea, and 1 U/µL T1 RNase (Thermo Fisher Scientific) at 50°C for 10 min. Samples were flash-frozen in liquid nitrogen for 5 min and heat denatured in denaturing solution at 95°C for 5 min before gel loading. Hydrolysis ladders were generated by incubating RNA in 50 mM NaHCO3 at 90°C for 20 min and neutralizing using 1 M Tris-HCl.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We would like to thank the National Science and Engineering Research Council of Canada for an operating grant to P.J.U.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.079833.123.
REFERENCES
- Abdolahzadeh A, Dolgosheina EV, Unrau PJ. 2019. RNA detection with high specificity and sensitivity using nested fluorogenic Mango NASBA. RNA 25: 1806–1813. 10.1261/rna.072629.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autour A, Jeng S, Cawte A, Abdolahzadeh A, Galli A, Panchapakesan SSS, Rueda D, Ryckelynck M, Unrau PJ. 2018a. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat Commun 9: 656. 10.1038/s41467-018-02993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autour A, Jeng S, Cawte A, Abdolahzadeh A, Galli A, Panchapakesan SSS, Rueda D, Ryckelynck M, Unrau PJ. 2018b. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat Commun 9: 656. 10.1038/s41467-018-02993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw SS, Tang MX, Teo YN, Cohen SM. 2016. A conformation-induced fluorescence method for microRNA detection. Nucleic Acids Res 44: e92. 10.1093/nar/gkw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra S, Ellington AD. 2014. A Spinach molecular beacon triggered by strand displacement. RNA 20: 1183–1194. 10.1261/rna.045047.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidar N, Amini M, Oroojalian F, Baradaran B, Hosseini SS, Shahbazi M-A, Hashemzaei M, Mokhtarzadeh A, Hamblin MR, De La Guardia M. 2021. Molecular beacon strategies for sensing purpose. TrAC Trends Anal Chem 134: 116143. 10.1016/j.trac.2020.116143 [DOI] [Google Scholar]
- Bouhedda F, Autour A, Ryckelynck M. 2018. Light-up RNA aptamers and their cognate fluorogens: from their development to their applications. Int J Mol Sci 19: 44. 10.3390/ijms19010044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawte AD, Unrau PJ, Rueda DS. 2020a. Live cell imaging of single RNA molecules with fluorogenic Mango II arrays. Nat Commun 11: 1283. 10.1038/s41467-020-14932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawte AD, Unrau PJ, Rueda DS. 2020b. Live cell imaging of single RNA molecules with fluorogenic Mango II arrays. Nat Commun 11: 1283. 10.1038/s41467-020-14932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu J, Hong Y. 2016. The morpholino molecular beacon for specific RNA visualization in vivo. Chem Commun 52: 3191–3194. 10.1039/C5CC07124K [DOI] [PubMed] [Google Scholar]
- Ferapontova EE, Olsen EM, Gothelf KV. 2008. An RNA aptamer-based electrochemical biosensor for detection of theophylline in serum. J Am Chem Soc 130: 4256–4258. 10.1021/ja711326b [DOI] [PubMed] [Google Scholar]
- Filonov GS, Moon JD, Svensen N, Jaffrey SR. 2014. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J Am Chem Soc 136: 16299–16308. 10.1021/ja508478x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Li Z, Kwok CK. 2019. Rational design and development of a universal baby spinach-based sensing platform for the detection of biomolecules. Analyst 144: 7173–7177. 10.1039/C9AN02061F [DOI] [PubMed] [Google Scholar]
- Kong KYS, Jeng SCY, Rayyan B, Unrau PJ. 2021. RNA Peach and Mango: orthogonal two-color fluorogenic aptamers distinguish nearly identical ligands. RNA 27: 604–615. 10.1261/rna.078493.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-H, Xi K, Zhang X, Bao L, Zhang X, Tan Z-J. 2019. Structural flexibility of DNA-RNA hybrid duplex: stretching and twist-stretch coupling. Biophys J 117: 74–86. 10.1016/j.bpj.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kong KYS, Unrau PJ. 2023. Harmonizing the growing fluorogenic RNA aptamer toolbox for RNA detection and imaging. Chem Soc Rev 52: 4071–4098. 10.1039/D3CS00030C [DOI] [PubMed] [Google Scholar]
- Mao S, Ying Y, Wu R, Chen AK. 2020a. Recent advances in the molecular beacon technology for live-cell single-molecule imaging. iScience 23: 101801. 10.1016/j.isci.2020.101801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Ying Y, Wu X, Chen AK. 2020b. Delivering molecular beacons via an electroporation-based approach enables live-cell imaging of single RNA transcripts and genomic loci. In RNA chaperones (ed. Heise T.), Vol. 2106 of Methods in molecular biology, pp. 241–252. Springer, New York: http://link.springer.com/10.1007/978-1-0716-0231-7_15 (accessed September 1, 2023). [DOI] [PubMed] [Google Scholar]
- Ong WQ, Citron YR, Sekine S, Huang B. 2017. Live cell imaging of endogenous mRNA using RNA-based fluorescence “turn-on” probe. ACS Chem Biol 12: 200–205. 10.1021/acschembio.6b00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Wu KY, Jaffrey SR. 2011. RNA mimics of green fluorescent protein. Science 333: 642–646. 10.1126/science.1207339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchapakesan SSS, Ferguson ML, Hayden EJ, Chen X, Hoskins AA, Unrau PJ. 2017. Ribonucleoprotein purification and characterization using RNA Mango. RNA 23: 1592–1599. 10.1261/rna.062166.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando S, Narita A, Hayami M, Aoyama Y. 2008. Transcription monitoring using fused RNA with a dye-binding light-up aptamer as a tag: a blue fluorescent RNA. Chem Commun 33: 3858. 10.1039/b808449a [DOI] [PubMed] [Google Scholar]
- Song W, Filonov GS, Kim H, Hirsch M, Li X, Moon JD, Jaffrey SR. 2017. Imaging RNA polymerase III transcription using a photostable RNA–fluorophore complex. Nat Chem Biol 13: 1187–1194. 10.1038/nchembio.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R, Sharma D, Krishna AM, Sathiri J, Sharma A. 2019. A highly efficient Baby Spinach-based minimal modified sensor (BSMS) for nucleic acid analysis. Org Biomol Chem 17: 7222–7227. 10.1039/C9OB01414D [DOI] [PubMed] [Google Scholar]
- Trachman RJ III, Demeshkina NA, Lau MWL, Panchapakesan SSS, Jeng SCY, Unrau PJ, Ferré-D'Amaré AR. 2017. Structural basis for high-affinity fluorophore binding and activation by RNA Mango. Nat Chem Biol 13: 807–813. 10.1038/nchembio.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Abdolahzadeh A, Andreoni A, Cojocaru R, Knutson JR, Ryckelynck M, Unrau PJ, Ferré-D'Amaré AR. 2018. Crystal structures of the Mango-II RNA aptamer reveal heterogeneous fluorophore binding and guide engineering of variants with improved selectivity and brightness. Biochemistry 57: 3544–3548. 10.1021/acs.biochem.8b00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Autour A, Jeng SCY, Abdolahzadeh A, Andreoni A, Cojocaru R, Garipov R, Dolgosheina EV, Knutson JR, Ryckelynck M, et al. 2019. Structure and functional reselection of the Mango-III fluorogenic RNA aptamer. Nat Chem Biol 15: 472–479. 10.1038/s41589-019-0267-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Cojocaru R, Wu D, Piszczek G, Ryckelynck M, Unrau PJ, Ferré-D'Amaré AR. 2020. Structure-guided engineering of the homodimeric Mango-IV fluorescence turn-on aptamer yields an RNA FRET pair. Structure 28: 776–785.e3. 10.1016/j.str.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourkas A, Behlke MA, Rose SD, Bao G. 2003. Hybridization kinetics and thermodynamics of molecular beacons. Nucleic Acids Res 31: 1319–1330. 10.1093/nar/gkg212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S, Kramer FR. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol 14: 303–308. 10.1038/nbt0396-303 [DOI] [PubMed] [Google Scholar]
- Vet JAM, Majithia AR, Marras SAE, Tyagi S, Dube S, Poiesz BJ, Kramer FR. 1999. Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc Natl Acad Sci 96: 6394–6399. 10.1073/pnas.96.11.6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams B, Chirasani VR, Krokhotin A, Das R, Dokholyan NV. 2019. Limits in accuracy and a strategy of RNA structure prediction using experimental information. Nucleic Acids Res 47: 5563–5572. 10.1093/nar/gkz427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Jo J, Tabata Y. 2023. A reverse transfection system with cationized gelatin nanospheres incorporating molecular beacon as a tool to visualize cell function. ACS Appl Bio Mater 6: 3363–3375. 10.1021/acsabm.2c00944 [DOI] [PubMed] [Google Scholar]
- Yaseen IM, Ang QR, Unrau PJ. 2019a. Fluorescent visualization of Mango-tagged RNA in polyacrylamide gels via a poststaining method. J Vis Exp 59112. 10.3791/59112-v [DOI] [PubMed] [Google Scholar]
- Zhang DY, Winfree E. 2009. Control of DNA strand displacement kinetics using toehold exchange. J Am Chem Soc 131: 17303–17314. 10.1021/ja906987s [DOI] [PubMed] [Google Scholar]
- Zheng J, Yang R, Shi M, Wu C, Fang X, Li Y, Li J, Tan W. 2015. Rationally designed molecular beacons for bioanalytical and biomedical applications. Chem Soc Rev 44: 3036–3055. 10.1039/C5CS00020C [DOI] [PMC free article] [PubMed] [Google Scholar]



