Abstract
Objectives
Transfusion of a high ratio of plasma to packed red blood cells (PRBCs), to treat or prevent acute traumatic coagulopathy, has been associated with survival after major trauma. However, the effect of prehospital plasma on patient outcomes has been inconsistent. The aim of this pilot trial was to assess the feasibility of transfusing freeze‐dried plasma with red blood cells (RBCs) using a randomized controlled design in an Australian aeromedical prehospital setting.
Methods
Patients attended by helicopter emergency medical service (HEMS) paramedics with suspected critical bleeding after trauma managed with prehospital RBCs were randomized to receive 2 units of freeze‐dried plasma (Lyoplas N‐w) or standard care (no plasma). The primary outcome was the proportion of eligible patients enrolled and provided the intervention. Secondary outcomes included preliminary data on effectiveness, including mortality censored at 24 h and at hospital discharge, and adverse events.
Results
During the study period of June 1 to October 31, 2022, there were 25 eligible patients, of whom 20 (80%) were enrolled in the trial and 19 (76%) received the allocated intervention. Median time from randomization to hospital arrival was 92.5 min (IQR 68–101.5 min). Mortality may have been lower in the freeze‐dried plasma group at 24 h (RR 0.24, 95% CI 0.03–1.73) and at hospital discharge (RR 0.73, 95% CI 0.24–2.27). No serious adverse events related to the trial interventions were reported.
Conclusions
This first reported experience of freeze‐dried plasma use in Australia suggests prehospital administration is feasible. Given longer prehospital times typically associated with HEMS attendance, there is potential clinical benefit from this intervention and rationale for a definitive trial.
INTRODUCTION
Hemorrhage is responsible for over 40% of all trauma‐related deaths, with nearly half occurring in the prehospital setting. 1 , 2 Among major trauma patients who reach emergency departments alive, in‐hospital transfusion of a high ratio, of at least 1 unit of plasma to every 2 units of red blood cells (RBCs), has been associated with survival. 3 This transfusion of plasma can prevent or treat the acute traumatic coagulopathy (ATC) that is observed in around 25% of major trauma patients. 4 , 5 Patients with ATC are three to four times more likely to die and up to eight times more likely to die within the first 24 h. 6 , 7 , 8 , 9 ATC on admission is also associated with development of acute kidney injury and multiple organ failure, fewer ventilator‐free days, and longer stays in the intensive care unit (ICU) and hospital. 10
One treatment of ATC is the infusion of fresh‐frozen plasma (FFP). However, there is limited evidence to support the resource investment necessary to provide FFP in the prehospital environment. The two randomized controlled trials using prehospital FFP differed in their conclusions. 11 , 12 An alternative to FFP that is particularly suitable for prehospital use is freeze‐dried plasma, but a recent United Kingdom trial of freeze‐dried plasma and RBCs compared to crystalloid in the prehospital setting did not demonstrate a difference in outcomes. 13
The Australian setting is different than that in the United Kingdom, with longer prehospital times due to vast geography and highly centralized trauma centers. No freeze‐dried plasma product is licensed for use in Australia. The feasibility of recruiting patients to a study of freeze‐dried plasma in this context is unknown. We therefore undertook a pilot randomized controlled trial of freeze‐dried plasma versus standard care to be administered prehospital to critically bleeding trauma patients receiving RBC transfusion. The aim was to determine feasibility for a definitive trial of prehospital freeze‐dried plasma, assessing prehospital times, numbers of eligible patients, and the proportion able to be recruited to the trial in this context, along with evaluation of clinically relevant effectiveness outcomes that might be employed in a subsequent study.
METHODS
Setting and population
Ambulance Victoria provides emergency medical services to the state of Victoria, Australia. It is the single provider of emergency aeromedical care in the state and operates five emergency helicopters from four bases across Victoria. The service conducts primary scene responses as well as interhospital transports, serving a population of almost 6.7 million people across an area of approximately 227,000 km2 (for comparison, the entire United Kingdom is approximately 244,000 km2). The helicopters are staffed by senior, postgraduate qualified intensive care flight paramedics, who each have at least a decade of prehospital experience. Each helicopter carries four units of group O D– RBCs. Patients eligible for RBCs (i.e., standard care) are those with suspected hemorrhage and hypovolemia after clinical judgment of the paramedic. RBCs are transfused to a target systolic blood pressure ≥70 mm Hg or if there is concurrent severe traumatic brain injury, a target systolic blood pressure ≥ 120 mm Hg. 14 Tranexamic acid is not administered in this service. The eligibility criteria for this trial were any adult patient receiving prehospital transfusion of RBCs for trauma and being transported to The Alfred Hospital, one of two adult major trauma centers in Victoria. Patients at extremes of age (<18 years or >90 years), who had no intravenous access, known pregnancy, or active palliative care, were not eligible.
Randomization and masking
This was an unblinded pilot randomized controlled trial. Randomization packs, containing allocation of freeze‐dried plasma or standard care were prepared by independent research staff using a computer‐generated sequence. Random permuted blocks with a block size of four were used. Trial packs were consecutively numbered, opaque and with a tamper‐proof seal to ensure allocation concealment. Each helicopter emergency medical service unit had 12 randomization envelopes supplied at the start of the trial.
Intervention
After the first unit of RBCs, patients were randomized in a 1:1 ratio to receive 2 units of freeze‐dried plasma or standard care. Lyoplas N‐w is a freeze‐dried plasma product manufactured by the German Red Cross, has similar coagulation factor activity to FFP and similar transfusion‐related complication rates, and has had no cases of virus transmission reported since 2007. 15 It has a shelf life of 15 months when stored between +2 and +25°C. The infusion of freeze‐dried plasma, once commenced, was continued in hospital until completion of the entire intervention dose. There were no other changes to management either prehospital or in hospital.
Comparator
Among patients allocated to the standard care arm, management was dictated by Ambulance Victoria clinical practice guidelines. 14 There were no prehospital plasma products available for patients randomized to the standard care group.
Outcomes
The primary outcome for this study was feasibility of intervention, measured by the proportion of eligible patients (meeting all the inclusion criteria and none of the exclusion criteria) who were randomized and completed the intervention. Secondary outcomes were mortality, censored at 24 h from the time of injury and at hospital discharge, hospital length of stay, requirement for ICU admission, coagulation status on arrival to hospital, measured as first international normalized ratio, platelet count, activated partial thromboplastin time, fibrinogen count, first lactate, blood component use in the first 24 h of hospital arrival, thromboembolic events (deep venous thrombus, pulmonary embolism, ischemic stroke, acute myocardial infarction, other arterial thrombus) diagnosed, and any serious adverse events reported. Secondary outcomes were measured for exploratory purpose only, as this pilot trial was not powered to detect any differences in these outcomes.
Statistical analysis
Baseline characteristics were summarized using frequency (percentage) or median (interquartile range [IQR]). The primary outcome of successful enrollment was reported using proportion with 95% confidence intervals (95% CIs). Secondary outcomes were reported by intention‐to‐treat subgroups. Count variables were compared with the unadjusted chi‐square test for equal proportions, with results reported as frequency (percentage) per treatment group with a relative risk (RR), accompanied by 95% CI. Secondary outcomes were presented using odds ratios (ORs) with 95% CIs. Blood biomarker observations and blood component use were summarized using median (IQR). As a feasibility study, a sample size of 20 patients was chosen and not adequately powered for statistical hypothesis testing. The data are reported as an intention‐to‐treat analysis. All analyses were conducted with Stata v 15.1. A two‐sided p‐value of <0.05 was used to indicate statistical significance.
RESULTS
During the study period of June 1 to October 31, 2022, there were 25 patients who received prehospital RBCs after trauma and were planned to be transported to The Alfred Emergency & Trauma Centre. Of these, 20 (80%; 95% CI 59.3–93.2) were enrolled in the trial and 19 (76%; 95% CI 54.9–90.6) received the allocated intervention after randomization. The intervention could not be delivered to one patient due to inadequate intravenous access that failed after randomization. There were nine patients randomized to the freeze‐dried plasma arm and 11 patients to the standard care arm (Figure 1).
FIGURE 1.
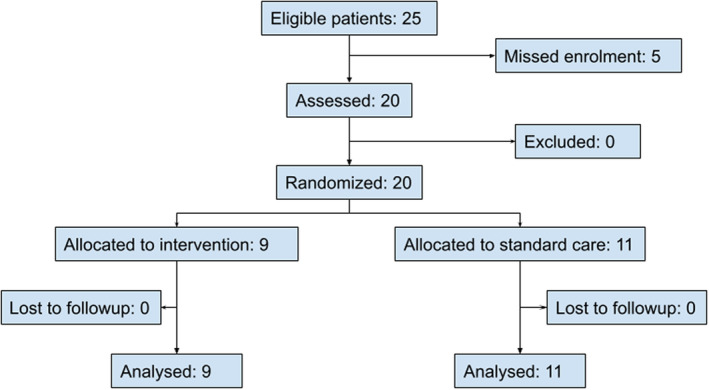
Patient enrollment.
Baseline characteristics of included patients are listed in Table 1. Overall median injury severity score was 61.5 (IQR 40–75) and median prehospital shock index was 1.4 (95% CI 1.1–1.6). Most patients presented with a low Glasgow Coma Scale score. The median (IQR) number of units of prehospital RBCs administered was 3 (2–4) and equal in both arms. Among the nine patients in the freeze‐dried plasma group, eight received 2 units of freeze‐dried plasma. Among patients transported to hospital (n = 16), median time from randomization to hospital arrival was 92.5 min (IQR 68–101.5 min), while median time from initial call to hospital arrival was 166.5 min (IQR 140–196.5 min). Time from randomization to hospital arrival was more than 20 min for all patients and time from initial call to hospital arrival was more than 100 min for all patients. Consistent with clinical practice guidelines, crystalloids were sparingly used, and administered to four patients of volumes less than 1 L.
TABLE 1.
Baseline characteristics of patients.
| Freeze‐dried plasma (n = 9) | Standard care (n = 11) | |
|---|---|---|
| Age (years) | 48 (40–51) | 34 (19–54) |
| Sex | ||
| Male | 7 (77.8) | 7 (63.6) |
| Female | 2 (22.2) | 4 (36.4) |
| Mechanism of injury | ||
| Motor vehicle crash | 4 (44.4) | 6 (54.5) |
| Motorcycle crash | 2 (22.2) | 1 (9.1) |
| Pedestrian | 1 (11.1) | 1 (9.1) |
| Bicycle crash | 1 (11.1) | 1 (9.1) |
| Fall | 0 | 1 (9.1) |
| Stabbing | 1 (11.1) | 0 |
| Crushed by object | 0 | 1 (9.1) |
| Injury severity score | ||
| ≤25 | 1 (11.1) | 2 (18.2) |
| 26–50 | 3 (33.3) | 2 (18.2) |
| >50 | 5 (55.6) | 7 (63.6) |
| Prehospital vital signs | ||
| Heart rate (beats/min) | 112 (92–134) | 123 (110–133) |
| Systolic blood pressure (mm Hg) | 81 (60–100) | 79 (0–86) |
| Prehospital GCS | ||
| 3–8 | 6 (66.7) | 10 (90.9) |
| 9–12 | 1 (11.1) | 1 (9.1) |
| 13–15 | 2 (22.2) | 0 |
| Time to ED (min) | ||
| From initial call | 183.5 (154–196.5) | 149.5 (123.5–149.5) |
| From randomization | 95.5 (65–109) | 90 (72.5–98) |
Note: Data are reported as median (IQR) or n (%).
Abbreviations: GCS, Glasgow Coma Scale; IQR, interquartile range.
Secondary outcomes are listed in Table 2 and mortality endpoints displayed in Figure 2. There were four patients who died prior to hospital arrival, one in the freeze‐dried plasma group and three in the standard care group. At the time of hospital discharge, there were eight (40%) deaths. Among the 16 patients who arrived alive to the ED, 13 received further RBCs and 11 received FFP. There were no serious adverse events reported.
TABLE 2.
Secondary outcomes.
| Freeze‐dried plasma (n = 9) a | Standard care (n = 11) a | Relative risk or median difference (95% CI) | |
|---|---|---|---|
| Mortality (censored at 24 h) | 1 (11.1) | 5 (45.4) | 0.24 (0.03 to 1.73) |
| Mortality (censored at hospital discharge) | 3 (33.3) | 5 (45.4) | 0.73 (0.24 to 2.27) |
| ICU admission | 6 (66.7) | 6 (54.5) | 1.2 (0.6 to 2.5) |
| Hemoglobin b (g/L) | 101.5 (83 to 125) | 142.5 (126 to 155) | −41.0 (−82.0 to 6.0) |
| Platelet count b (×109/L) | 206 (148.5 to 295.5) | 210.5 (156 to 296) | −4.5 (−318.1 to 300.1) |
| Fibrinogen b (g/L) | 2.0 (1.8 to 2.6) | 2.3 (1.9 to 2.3) | −0.3 (−2.6 to 2.4) |
| INR b | 1.3 (1.2 to 1.4) | 1.3 (1.2 to 1.5) | 0 (−62.9 to 62.9) |
| ≤1.3 | 5 (62.5) | 5 (62.5) | 1.0 (0.28 to 3.54) |
| >1.3 | 3 (37.5) | 3 (37.5) | |
| aPTT b (s) | 30.5 (24.8 to 34.2) | 32 (28.4 to 48.2) | −1.5 (−38.6 to 31.8) |
| Lactate b (mmol/L) | 2.8 (1.7 to 4.5) | 2.8 (1.8 to 6.5) | 0 (−5.0 to 4.6) |
| RBC units in 24 h b | 8 (2 to 8) | 6.5 (1 to 8.5) | 1.5 (−5.8 to 9.8) |
| FFP units in 24 h b | 4 (0 to 7) | 4 (0 to 5.5) | 0 (−5.6 to 5.6) |
| Platelets units in 4 h b , c | 1 (0 to 5) | 1 (0 to 1) | 0 (− 5.2 to 5.2) |
| Cryoprecipitate in 4 h | 0 (0 to 0) | 0 (0 to 0) | 0 (−6.9 to 6.9) |
| Hospital length of stay (days) | 19 (13 to 21) | 9 (0 to 30) | 10 (−14.3 to 34.3) |
| Thromboembolism | 1 (11.1) | 2 (18.2) | 0.61 (0.07 to 5.70) |
Abbreviations: aPTT, activated partial thromboplastin time; FFP, fresh‐frozen plasma; ICU, intensive care unit; INR, international normalized ratio; RBC, red blood cell.
Data are reported as median (IQR) or n (%).
Among 16 patients who arrived at the hospital.
One adult dose is either a platelet unit produced from a pool of buffy coats collected from four whole‐blood donors or an apheresis platelet unit.
FIGURE 2.
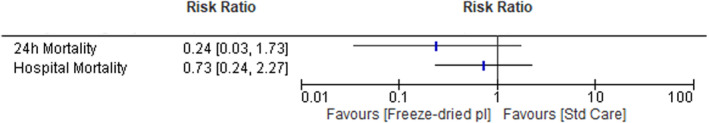
Mortality outcomes.
DISCUSSION
This pilot study is the first reported experience of freeze‐dried plasma in Australia. Prehospital randomization of critically bleeding trauma patients and delivery of freeze‐dried plasma was feasible, with enrollment of most eligible patients and successful delivery of the intervention in most patients. It provides preliminary data on effectiveness of prehospital freeze‐died plasma for critical bleeding after trauma. The results support a definitive randomized controlled trial of prehospital plasma for prehospital resuscitation of critical bleeding after trauma.
This pilot study was completed after the results of the resuscitation with Prehospital blood products (RePHILL) trial were published from the United Kingdom. 13 The RePHILL trial, to date, provides the highest level of evidence regarding the use of prehospital RBCs and freeze‐dried plasma for trauma resuscitation. In this multicenter trial across four prehospital services, adult patients with hypotension presumed due to hemorrhagic shock were randomized to receive 2 units of RBCs and 2 units of freeze‐dried plasma or 1 L crystalloid resuscitation. No significant differences in the primary composite outcome of mortality at hospital discharge or failure to reach lactate clearance, or secondary outcomes including mortality at hospital discharge, were observed.
Prior to the RePHILL trial, two randomized controlled trials (PAMPer and COMBAT) had examined the role of prehospital plasma, both using FFP in bleeding injured patients. 11 , 12 Pooled 24‐h mortality appears reduced in the plasma group (RR 0.69, 95% CI 0.48–0.99). However, prehospital plasma had no significant effect on 1‐month mortality (RR 0.86, 95% CI 0.68–1.11), acute lung injury (OR 1.03, 95% CI 0.71–1.50), or multiorgan failure rates (OR 1.30, 95% CI 0.92–1.86). 16 A key difference between the two studies was transport mechanisms to hospital, one being by road ambulance with shorter prehospital times, which did not show benefit from plasma administration, and the other being by aeromedical retrieval and longer (median 41 min) prehospital times, which concluded a clinically and statistically significant 9.8% absolute risk reduction of mortality at 30 days after administration of prehospital plasma.
A plausible explanation for the difference between these two well‐conducted trials involving FFP was that, like any prehospital intervention, there needs to be a sufficiently long window of opportunity for the plasma to achieve its intended effect. With very short prehospital times, prehospital plasma is unlikely to add benefit to the various interventions available upon hospital arrival. The benefits of prehospital plasma are therefore more likely in a setting such as Australia, with vast geography and centralized trauma services, resulting in long prehospital times for most patients. This is supported by a post hoc analysis of pooled data from the PAMPer and COMBAT trials assessing prehospital plasma in the United States, which concluded that there was a survival benefit when transport times are longer than 20 min. 17
A key difference between patients in the RePHILL trial and our pilot trial was prehospital time. Overall time from initial call to hospital arrival in the United Kingdom was around 90 min, compared to 166 min in Victoria. Time from randomization to hospital arrival in the United Kingdom was 36 min, compared to 92 min in Victoria. Therefore, despite the equivalence in outcome between the two groups of the RePHILL trial, the hypothesis that prehospital RBCs and plasma may improve outcomes for critically bleeding trauma patients in the Australian context remains plausible. A further unique characteristic of prehospital transfusion in Australia is the use of clinical judgment by senior prehospital clinicians, rather than an objective observation such as hypotension alone. This has the potential to select patients more likely to benefit from any intervention.
This pilot trial provides confirmation of feasibility of the study design, but is limited by uncertainty that consistent rates of enrollment, without trial fatigue, can be achieved in a longer term. 18 However, Australian prehospital services have a strong track record of completing trials enrolling critically unwell patients. 19 , 20 , 21 , 22 , 23 , 24 , 25 Eligibility for the trial was the pragmatic administration of RBCs, but not based on objective criteria. This provided evidence on the potential utility of plasma when administered with RBC transfusion. There was potential for imbalance between the two groups, particularly regarding a higher rate of traumatic brain injury in the standard care arm. The intervention was not blinded, but potentially possible through manufacture of an inert soluble powder of similar appearance. The point estimates for secondary outcomes are limited by wide CIs, but the observed mortality difference was consistent with the only prior randomized controlled trial of prehospital plasma during aeromedical transport. The safety profile of the freeze‐dried plasma product cannot be determined from this pilot study but extensive experience in other countries has not identified any significant safety concerns to date. 15 , 26 , 27 , 28 , 29 Finally, despite successful randomization and delivery of treatment, acceptability of this intervention among prehospital clinicians in Australia was not studied in this trial. No acceptability issues were anecdotally reported and this is the focus of a follow‐up study.
CONCLUSIONS
Prehospital transfusion of freeze‐dried plasma with red blood cells was feasible in the setting of a randomized trial. In Australia, where prehospital times are long, the potential benefits could be similar to those experienced by aeromedical services in the United States using fresh‐frozen plasma. These results provide strong support for a definitive trial of prehospital freeze‐dried plasma for patients who have critical bleeding after trauma.
AUTHOR CONTRIBUTIONS
Conceptualization: Biswadev Mitra, Michael C. Reade. Data curation and formal analysis: Olivia Bradley, Biswadev Mitra. Funding acquisition: Biswadev Mitra, Stephen Bernard, Marc Maegele, Russell L. Gruen, Erica M. Wood, Zoe K. McQuilten, MR. Investigation: Ben Meadley, Toby St. Clair, Andrew Webb, David Anderson. Methodology: Biswadev Mitra, Ben Meadley, Stephen Bernard, Marc Maegele, Russell L. Gruen, Erica M. Wood, Zoe K. McQuilten, David Anderson, Michael C. Reade. Project administration: Ben Meadley, Olivia Bradley. Writing: all authors.
FUNDING INFORMATION
The study was funded by a seed grant from the National Blood Authority, Commonwealth of Australia. The research was supported by the Australian National Health and Medical Research Council‐funded Blood Synergy program. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by The Alfred Human Research and Ethics Committee (Project No. 224/21) and Ambulance Victoria Research Committee (Project No. R21‐016). A requirement to seek informed consent from patients was waived on the basis that the treatment needed to be given urgently, coupled with the known safety profile of the product overseas. Patients or a person responsible were notified of enrollment at the earliest possible time, with the option to withdraw their data from analysis. The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12622000763741).
ACKNOWLEDGMENT
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Mitra B, Meadley B, Bernard S, et al. Pre‐hospital freeze‐dried plasma for critical bleeding after trauma: A pilot randomized controlled trial. Acad Emerg Med. 2023;30:1013‐1019. doi: 10.1111/acem.14745
Funding informationFunded by the National Blood Authority, Commonwealth of Australia.
Supervising Editor: Dr. Aaron Robinson.
REFERENCES
- 1. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3‐S11. [DOI] [PubMed] [Google Scholar]
- 2. Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population‐based assessment. World J Surg. 2010;34:158‐163. [DOI] [PubMed] [Google Scholar]
- 3. da Luz LT, Shah PS, Strauss R, et al. Does the evidence support the importance of high transfusion ratios of plasma and platelets to red blood cells in improving outcomes in severely injured patients: a systematic review and meta‐analyses. Transfusion. 2019;59:3337‐3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maegele M. Acute traumatic coagulopathy: incidence, risk stratification and therapeutic options. World J Emerg Med. 2010;1:12‐21. [PMC free article] [PubMed] [Google Scholar]
- 5. Moore EE, Moore HB, Kornblith LZ, et al. Trauma‐induced coagulopathy. Nat Rev Dis Primers. 2021;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127‐1130. [DOI] [PubMed] [Google Scholar]
- 7. MacLeod JBA, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39‐44. [DOI] [PubMed] [Google Scholar]
- 8. Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German trauma registry on 8724 patients. Injury. 2007;38:298‐304. [DOI] [PubMed] [Google Scholar]
- 9. Mitra B, Cameron PA, Mori A, Fitzgerald M. Acute coagulopathy and early deaths post major trauma. Injury. 2012;43:22‐25. [DOI] [PubMed] [Google Scholar]
- 10. Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680‐685. [DOI] [PubMed] [Google Scholar]
- 11. Moore HB, Moore EE, Chapman MP, et al. Plasma‐first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315‐326. [DOI] [PubMed] [Google Scholar]
- 13. Crombie N, Doughty HA, Bishop JRB, et al. Resuscitation with blood products in patients with trauma‐related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open‐label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9:e250‐e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical Practice Guidelines . Air Ambulance Victoria; 2022. Accessed May 9, 2023. https://www.ambulance.vic.gov.au/paramedics/clinical‐practice‐guidelines/ [Google Scholar]
- 15. Bux J, Dickhörner D, Scheel E. Quality of freeze‐dried (lyophilized) quarantined single‐donor plasma. Transfusion. 2013;53:3203‐3209. [DOI] [PubMed] [Google Scholar]
- 16. Coccolini F, Pizzilli G, Corbella D, et al. Pre‐hospital plasma in haemorrhagic shock management: current opinion and meta‐analysis of randomized trials. World J Emerg Surg. 2019;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pusateri AE, Moore EE, Moore HB, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2020;155:e195085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winkler G, Levin EA, Whalen EY, Larus J. Overcoming “trial fatigue”: a strategy for optimizing patient accrual speed and resource utilization. Drug Inf J. 1996;30:35‐40. [Google Scholar]
- 19. Bernard SA, Bray JE, Smith K, et al. Effect of lower vs higher oxygen saturation targets on survival to hospital discharge among patients resuscitated after out‐of‐hospital cardiac arrest: the EXACT randomized clinical trial. JAMA. 2022;328:1818‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitra B, Bernard S, Gantner D, et al. Protocol for a multicentre prehospital randomised controlled trial investigating tranexamic acid in severe trauma: the PATCH‐trauma trial. BMJ Open. 2021;11:e046522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard SA, Smith K, Finn J, et al. Induction of therapeutic hypothermia during out‐of‐hospital cardiac arrest using a rapid infusion of cold saline: the RINSE trial (rapid infusion of cold Normal saline). Circulation. 2016;134:797‐805. [DOI] [PubMed] [Google Scholar]
- 22. Stub D, Smith K, Bernard S, et al. A randomized controlled trial of oxygen therapy in acute myocardial infarction air verses oxygen In myocarDial infarction study (AVOID study). Am Heart J. 2012;163:339‐345.e1. [DOI] [PubMed] [Google Scholar]
- 23. Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252:959‐965. [DOI] [PubMed] [Google Scholar]
- 24. Bernard SA, Smith K, Cameron P, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out‐of‐hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122:737‐742. [DOI] [PubMed] [Google Scholar]
- 25. Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350‐1357. [DOI] [PubMed] [Google Scholar]
- 26. Gokhale SG, Scorer T, Doughty H. Freedom from frozen: the first British military use of lyophilised plasma in forward resuscitation. J R Army Med Corps. 2016;162:63‐65. [DOI] [PubMed] [Google Scholar]
- 27. Sunde GA, Vikenes B, Strandenes G, et al. Freeze dried plasma and fresh red blood cells for civilian prehospital hemorrhagic shock resuscitation. J Trauma Acute Care Surg. 2015;78:S26‐S30. [DOI] [PubMed] [Google Scholar]
- 28. Gellerfors M, Linde J, Gryth D. Helicopter In‐flight resuscitation with freeze‐dried plasma of a patient with a high‐velocity gunshot wound to the neck in Afghanistan – a case report. Prehosp Disaster Med. 2015;30:509‐511. [DOI] [PubMed] [Google Scholar]
- 29. Glassberg E, Nadler R, Gendler S, et al. Freeze‐dried plasma at the point of injury: from concept to doctrine. Shock. 2013;40:444‐450. [DOI] [PubMed] [Google Scholar]


