Abstract
A series of adenosine deaminase (ADA) retroviral vectors were designed and constructed with the goal of improved performance over the PA317/LASN vector currently used in clinical trials. First, the bacterial selectable-marker neomycin phosphotransferase (neo) gene was removed to create a “simplified” vector. Second, the Moloney murine leukemia virus long terminal repeat (LTR) promoter used for ADA expression was replaced with either the myeloproliferative sarcoma virus (MPSV) or SL3-3 LTR. Supernatant from each ADA vector was used to transduce ADA-deficient (ADA−) B- and T-cell lines as well as primary peripheral blood mononuclear cells (PBMC) from an ADA− severe combined immunodeficiency patient. Total ADA enzyme activity and ADA activity per integrant in the transduced cells demonstrated that the MPSV LTR splicing vector design provided the highest level of ADA expression per cell. This ADA(MPSV) vector was then tested in packaging cell lines containing either the gibbon ape leukemia virus envelope (PG13 cells), the murine amphotropic envelope (FLYA13 cells), or the feline endogenous virus RD114 envelope (FLYRD18 cells). The results indicate that FLYRD18/ADA(MPSV), a simplified ADA retroviral vector with the MPSV LTR, provides a 17-fold-higher level of ADA expression in human lymphohematopoietic cells than the PA317/LASN vector currently in use.
Retroviral vectors have been the most common gene transfer vehicles in clinical gene therapy trials (15). These vectors can integrate into the host genome to provide permanent transgene expression in the targeted cells (20). The first generation of retroviral vectors have been useful in demonstrating the feasibility of gene therapy approaches, but vectors capable of higher levels of gene transfer and transgene expression would be beneficial. For example, gene transfer levels achieved by first-generation retroviral vectors in large mammals (28) and in human gene therapy trials (7, 13) have been disappointing. There are at least two avenues for improving retroviral vectors. First, molecular changes can be made in the retroviral vector sequence. Second, different packaging cell lines could be tested to modify the host range, increase transduction in a given cell type, and/or render the virions resistant to inactivation by human complement.
A clinically useful model for improving retroviral vector design is the vector LASN packaged in the amphotropic line PA317. PA317/LASN was the first therapeutic vector used in a gene therapy clinical trial (1). This vector has yielded gene transfer levels of generally less than 10% in peripheral blood T cells of adenosine deaminase-deficient (ADA−) severe combined immunodeficiency (SCID) patients. Two possibilities to improve this vector include eliminating the dominant selectable marker gene and changing the long terminal repeat (LTR) promoter to optimize expression. LASN, like many of the retroviral vectors used in clinical trials to date, contains two genes: the therapeutic gene (the ADA gene) and a dominant selectable marker gene (the bacterial neomycin phosphotransferase II gene; neo). Dominant selectable marker genes have historically been included to facilitate the generation, isolation, and titration of retroviral producer cell clones and to permit the evaluation and selection of successfully targeted cells. neo is the most commonly used selectable marker gene, although other genes have been used, including a mutant dihydrofolate reductase gene (dhfr) (19), the multidrug resistance gene (mdr) (10), and genes for cell surface markers such as cd24 (24) and the human nerve growth factor receptor (2). Vectors carrying dominant selectable marker genes, particularly those of nonhuman origin, have two theoretical disadvantages. First, careful analysis of some patients has revealed an immune response directed against the dominant selectable marker protein expressed from the retroviral integrant (20a, 25). Second, the more complex retroviral genomes required to express two separate genes may result in lower titers or suboptimal expression of the therapeutic gene product due to promoter interference (8, 29). On the other hand, cloning and determining the titers of useful retroviral vectors without selectable markers have been laborious. Using a recently developed rapid-screening procedure, we have been able to identify a number of “simple” ADA retroviral vectors which lack dominant selectable markers (23).
Different packaging cell lines may also improve gene transfer of retroviral vectors into specific target cells. Retroviral vectors are limited by the host range specified by the envelope protein on the surface of the retrovirus. Most gene therapy trials have used retroviruses with a murine amphotropic (4070A) host range. However, packaging cell lines with the gibbon ape leukemia virus (GALV) envelope (PG13 cells) (18) and the cat endogenous virus RD114 envelope (FLYRD18 cells) (5) have become available; these may improve transduction frequencies into various target cell populations. For example, there is evidence that GALV-pseudotyped retroviral vectors may facilitate gene transfer into human peripheral blood T cells with greater efficiency than vectors with an amphotropic envelope (3). Packaging cell lines derived from murine cells have the additional disadvantage that they produce retroviruses which are inactivated by complement in human sera. Packaging cell lines of human origin (FLYA13 and FLYRD18) (5) produce vectors which are complement resistant. Testing both new simple retroviral vector designs and new packaging cells may therefore improve retrovirus-mediated gene transfer.
We report the construction and characterization of three simplified ADA vectors by using either the Moloney murine leukemia virus (MLV) LTR, the myeloproliferative sarcoma virus (MPSV) LTR, or the SL3-3 LTR. We tested these vectors to determine which LTR provided the highest level of ADA expression in our target cells of interest: human ADA− lymphohematopoietic cells. The ADA retroviral vector with the highest level of transduction/expression was then evaluated in different packaging cell lines including PG13, FLYA13, and FLYRD18.
MATERIALS AND METHODS
Retroviral vector construction.
The retroviral vector plasmids containing the ADA cDNA expressed from the three chimeric LTRs (MLV, MPSV, and SL3-3 LTRs) were constructed as follows. All three ADA retroviral constructs are derived from pGCsap containing the MLV 3′ LTR with intact splice donor (SD) and acceptor (SA) sites for the generation of subgenomic mRNA (Fig. 1). The human ADA cDNA was cloned as an NcoI-NotI fragment into NcoI-NotI-digested pGCsap to make pGCsapADA(MLV) [referred to hereafter as pADA(MLV)]. The ADA cDNA fragment was obtained from pEMCADA and begins with the translation initiation site and includes 28 bp of the human ADA gene 3′ untranslated region. pADA(MLV) was made such that the ADA translational start site was positioned precisely where the envelope translational start site would be in the wild-type virus. The 3′ MLV LTR was replaced with either the MPSV or SL3-3 LTR as follows. pADA(MLV) was digested with ClaI and NdeI to remove the MLV fragment, which was replaced by the corresponding ClaI-NdeI MPSV or SL3-3 fragment. The resulting plasmids were pGCsapADA(MPSV) [pADA(MPSV)] and pGCsapADA(SL3-3) [pADA(SL3-3)]. Retroviral vector LASN has been previously described (11) and contains the human ADA cDNA expressed from the LTR and the bacterial neo gene expressed from an internal simian virus 40 (SV40) early promoter.
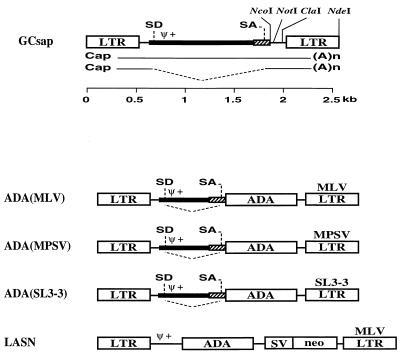
FIG. 1.
Structure of the simplified retroviral vector GCsap and the ADA retroviral vectors. GCsap has the MLV LTR with intact SD and SA sites. The ADA cDNA (ADA) was cloned between NcoI and NotI sites of GCsap to generate ADA(MLV). The 3′ MLV LTR was replaced by the corresponding ClaI-NdeI MPSV or SL3-3 fragment to make ADA(MPSV) or ADA(SL3-3), respectively. Gene sequences present in each vector are labeled as follows: Ψ+, packaging signal; SV, SV40 early promoter; neo, neomycin phosphotransferase gene sequence.
Cell culture.
An Epstein-Barr virus-transformed B lymphoblastoid cell line (B-LCL) from an ADA− SCID patient (22) was established and maintained in RPMI 1640 medium (Life Technologies, Gaithersburg, Md.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; HyClone, Logan, Utah) and 50 mM β-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.). A human T-cell leukemia virus type 1 (HTLV-1)-transformed ADA− SCID patient T-cell line (TJF-2) (12) was established and maintained in RPMI 1640 with 10% FCS containing 1,000 U of recombinant interleukin-2 (rIL-2) per ml. Producer cell lines derived from GP+E86 (16), PA317 (17), PG13, FLYA13, FLYRD18, and HeLa cells were cultured in Dulbecco’s modified Eagle medium (DMEM high glucose; 4.5 g/liter; Life Technologies) supplemented with 10% (vol/vol) heat-inactivated FCS. All cells were cultured in media supplemented with 100 U of penicillin G sodium and 100 μg of streptomycin sulfate per ml and 2 mM l-glutamine (Life Technologies).
Establishment of retrovirus-producing cell lines.
Retroviral producer cell lines were established as follows. First, 15 μg of vector plasmid DNA was transfected into GP+E86 cells by CaPO4 coprecipitation (mammalian transfection kit; Stratagene, La Jolla, Calif.). Sixteen hours later, supernatant from these transfected cells was harvested, filtered (0.45-μl-pore-size microfilter; Millex-Ha; Millipore, Bedford, Mass.), and used to transduce PG13 cells. Sixteen hours later these transduced PG13 cells were harvested and plated at 0.5 cells per well in flat-bottom 96-well plates (Costar no. 3596; Cambridge, Mass.). Supernatants obtained from the wells containing cells at 14 days were tested by RNA dot blot assay as previously described (23). All clones positive by RNA dot blot assay were transferred to six-well plates and expanded. To identify the clone with the highest titer, the positive clones were replated in six-well plates at equal cell numbers (3 × 105/well) and the RNA dot blot analysis was performed on resulting supernatants 48 h later. Clones with the highest relative titer by RNA dot blot analysis were expanded for subsequent experiments. Supernatant from the highest-titer PG13/ADA(MPSV) vector was used to transduce FLYRD18 cells. Transient supernatant harvested from these transduced FLYRD18 cells was then used to transduce FLYA13 cells. Limiting dilution of the FLYRD18 and FLYA13 cells followed by RNA dot blot analysis was used to identify individual high-titer clones from each producer cell line. The titers of PA317/LASN and PG13/LASN clones were determined by serial dilution of supernatant onto HeLa cells followed by G418 selection of resistant colonies (results are expressed as G418-resistant [G418R] CFU per milliliter) (4). No replication-competent helper virus was generated, as determined by a marker rescue assay (data not shown).
Transduction protocol.
The ADA− B-LCL and TJF-2 cells were transduced with supernatants from retroviral producer cells in the presence of 5 μg of protamine sulfate (Sigma) per ml with centrifugation (1,000 × g for 30 min at 32°C) followed by overnight exposure at 37°C. The transduced cells were harvested and prepared for ADA enzyme assay and Southern analysis 48 h later. Fresh ADA− SCID peripheral blood mononuclear cells (PBMC) were transduced as follows. First, PBMC were obtained from an ADA− SCID patient by apheresis and stimulated with rIL-2 (100 U/ml) and anti-CD3 antibody (OKT3; 100 U/ml) for 4 days, followed by transduction with the protocol described above.
RNA dot blot assay.
Nylon membranes (Hybond N+; Amersham Life Science, Arlington Heights, Ill.) were soaked for 10 min in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and then placed onto a Minifold slot blot apparatus (Schleicher & Schuell Inc., Keene, N.H.). Supernatant (180 μl) from the producer clones was harvested, transferred onto nylon membranes, and cross-linked by UV. Membranes were hybridized with an ADA cDNA probe randomly labeled with 32P. Membranes were then subjected to phosphorimager analysis, and dot densities were measured with the Bio imaging analyzer (BAS1500; Fuji Photo Film Co., Ltd., Tokyo, Japan).
Semi-quantitative PCR.
High-molecular-weight DNA was extracted from the transduced cells by standard techniques (27). A sense (5′-GAGGCTGTGAAGAGCGGCATTC-3′) primer from exon 7 and an antisense (5′-CGAATGACTGCATGCTCCGTGT-3′) primer from exon 9 of the human ADA gene were synthesized (National Human Genome Research Institute core facility, National Institutes of Health). PCR with these primers results in two bands in cells containing proviral integrants: a 496-bp band from the endogenous ADA gene and a 240-bp band from the proviral integrant. Reaction mixtures containing 0.5 μl (2.5 U) of Taq polymerase (TaKaRa Ex Taq; TaKaRa Shuzo Co., Ltd., Tokyo, Japan) were incubated for 30 cycles of 20 s at 98°C and 3 min at 68°C. The PCR products were separated through a 2% agarose gel and transferred onto a nylon membrane (Biotrace HP; Gelman Sciences, Ann Arbor, Mich.). The filters were then hybridized to ADA cDNA randomly labeled with 32P. The ratio of the vector-derived band to the endogenous band was determined by phosphorimager analysis to estimate the relative proviral copy number in the transduced cells.
Southern blot analysis.
Genomic DNA from the transduced cells was digested with KpnI, separated by 1.0% agarose gel, and transferred onto a nylon membrane (Biotrace HP). Filters were then hybridized to ADA cDNA randomly labeled with 32P. KpnI digestion of transduced cells generates two bands (2.6 and 9.2 kb) from the endogenous ADA gene and one band from the retroviral integrant [LASN, 3.1 kb; ADA(MLV) and ADA(MPSV), 1.90 kb; ADA(SL3-3), 1.97 kb). The ratio between the endogenous 2.6-kb band and the retroviral integrant band in each lane was calculated by phosphorimager analysis and used to normalize the percentage of transduced cells.
TLC ADA enzyme assay.
Transduced cells were washed twice with phosphate-buffered saline and then resuspended in 100 mM Tris, pH 7.4, containing 1% bovine serum albumin. Cell lysates were obtained by five rapid freeze-thaw cycles. Cellular debris was removed by centrifugation, and the supernatant was collected and stored at −80°C until it was assayed. ADA enzyme activity was determined by measuring the conversion of [14C]adenosine (Amersham Life Science) to [14C]-inosine after thin-layer chromatography (TLC) separation as previously described (12). Quantitation was performed by phosphorimager analysis. Results are expressed as nanomoles of inosine produced per minute per 108 cells.
Statistical analysis.
Student’s t test for comparison of means was used to compare groups. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Construction of simplified ADA retroviral vectors.
A series of considerations were incorporated to make second-generation ADA retroviral vectors that are more effective than the LASN vector currently used in gene therapy clinical trials. First, the vectors were simplified by eliminating the dominant selectable marker (Fig. 1). Second, the vectors include an SA sequence to allow splicing of a percentage of retroviral transcripts. According to the scanning model for translation, translational efficiency would be expected to be higher for these spliced transcripts because the translational start codon of each one is moved closer to the 5′ end of the message. Third, the ADA gene was cloned into the vectors such that the ADA translational start site was at the precise location of the env translational start site used in the wild-type virus. Fourth, a series of vectors containing different LTRs were engineered to compare the levels of expression from the LTRs in relevant target cell populations. The LTRs chosen were from MLV, MPSV, and SL3-3 (Fig. 1). These vectors were compared with LASN, which has ADA expressed from the MLV LTR and neo driven by the SV40e promoter. Finally, all constructs were packaged in the PG13 packaging cell line since this cell line has been reported to provide a higher level of transduction in human peripheral blood lymphocytes than the PA317 cell line (3).
Determining the titers of simplified ADA retroviral vector producer cell clones.
Using limiting dilution and RNA dot blot analysis of supernatant with the ADA cDNA as a probe, we identified a number of PG13 clones positive for ADA virus for each of the vectors. This first screen revealed positive clones but did not provide a direct comparison between clones because the cell number in each well was variable. To determine a relative titer for each clone, we replated the positive clones from the initial screen at equal cell numbers (3 × 105 cells/well in a six-well plate) and collected supernatant 48 h later. Clones with the highest dot intensity were expanded for further experiments. Once clones expressing the highest levels of vector transcript were identified, a final verification was made to confirm that the vector actually expressed a functional form of the gene product [see below; PG13/ADA(MLV)]. Using this methodology we identified the cell clones with the highest levels of production for LASN, ADA(MLV), ADA(MPSV), and ADA(SL3-3).
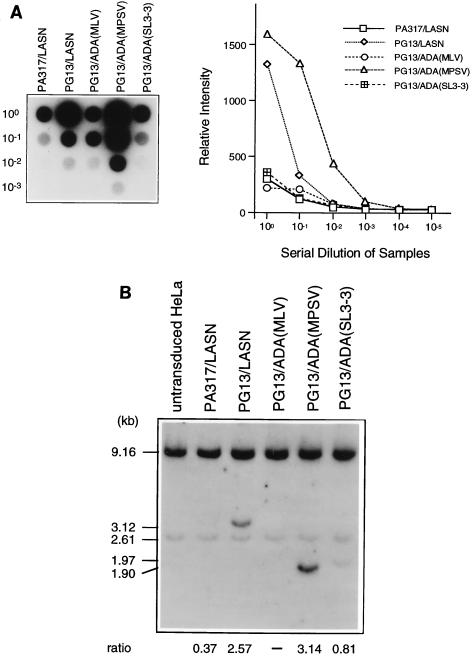
The highest-titer clones for each vector were then assayed relative to one another by RNA dot blot. Serial dilutions of these clones were performed to further validate the quantitative nature of the RNA dot blot procedure (Fig. 2A). Relative titers were also validated by transducing HeLa cells with equal volumes of supernatant from each clone. Southern analysis for ADA sequences was conducted on DNA extracted from these transduced HeLa cells (Fig. 2B). The intensity of each retrovirally derived ADA band was normalized to an endogenous ADA band to give the relative proviral copy number in the transduced cells. All methods for determining titers yielded consistent results, with the relative titers as follows (from lowest to highest): PG13/ADA(MLV), PA317/LASN, PG13/ADA(SL3-3), PG13/LASN, and PG13/ADA(MPSV). From a standard titer measurement by G418 selection we determined that PA317/LASN had a titer of 104 G418R CFU/ml and that PG13/LASN had a titer of 4 × 105 G418R CFU/ml on HeLa cells. Southern analysis of all the retroviral producer cell clones with EcoRV revealed full-length proviral integrants, suggesting no gross rearrangements or deletions (data not shown).
FIG. 2.
Characterization of simplified ADA retrovirus producer clones. (A) RNA dot blot analysis of serial dilutions of retroviral supernatant from each clone as a measure of relative titer (left). The graph depicts the dot intensity of each clone. (B) Titer estimation by Southern blot analysis of HeLa cells transduced with equal volumes of supernatant from each producer cell clone. Relative proviral copy number (ratio) is determined as the ratio of the phosphorimager signal of the viral ADA band (LASN, 3.12 kb; MLV and MPSV, 1.90 kb; SL3-3, 1.97 kb) to the intensity of the endogenous ADA band (2.61 kb).
Transduction of ADA− human lymphohematopoietic cell lines.
We wanted to assess the ability of these new vectors to transduce ADA− SCID lymphohematopoietic cells and to make functional ADA enzyme. Equal volumes of supernatant from each clone were used to transduce a human Epstein-Barr virus-transformed ADA− B-cell line (B-LCL). Cell extracts from the transduced B-LCL cells were subjected to a quantitative TLC assay for ADA enzyme activity. The relative amounts of ADA enzyme activity in the transduced B-LCL cells correlated well with the relative titers determined by RNA dot blot with the notable exception of that for ADA(MLV), which gave no ADA activity (Table 1). We assume that the lack of ADA activity from ADA(MLV) is the result of a mutation in the producer cell since these cells do make ADA retroviral particles detectable by RNA dot blot and show no gross rearrangement by Southern analysis.
TABLE 1.
ADA enzyme activitiesa in the transduced cells
| Vector | Measured ADA enzyme activity, avg proviral copy no./cell, calculated ADA enzyme activity/integrated provirus for:
|
||||||
|---|---|---|---|---|---|---|---|
| B-LCL cells
|
TJF-2 cells
|
||||||
| Expt 1 | Expt 2 | Avg ADA/provirusc | Expt 1 | Expt 2 | Expt 3 | Avg ADA/provirus | |
| Noneb | 1 | 2 | 5 | 2 | 1 | ||
| PA317/LASN | 8, 0.5, 16 | 16, 1.0, 16 | 16 | 14, 0.4, 32 | 30, 1.0, 30 | 28, 1.0, 28 | 30 |
| PG13/LASN | 51, 3.0, 17 | 24, 1.2, 20 | 19 | 155, 2.0, 76 | 99, 2.0, 50 | 43, 2.0, 43 | 49 |
| PG13/ADA(MPSV) | 81, 0.8, 101 | 79, 0.8, 98 | 100d | 189, 2.4, 79 | 118, 2.3, 52 | 136, 2.3, 60 | 64e |
| PG13/ADA(SL3-3) | 38, 0.9, 43 | 32, 0.9, 36 | 40 | 137, 1.4, 98 | 53, 1.3, 41 | 66, 1.1, 60 | 67e |
ADA enzyme activity is expressed as nanomoles of inosine produced per minute per 108 cells.
Values in this row are measured ADA enzyme activities.
Avg ADA/provirus, average value for calculated ADA enzyme activity per integrated provirus.
P < 0.05 compared with values for PA317/LASN, PG13/LASN, and PG13/ADA(SL3-3).
P < 0.05 compared with values for PA317/LASN and PG13/LASN.
To compare ADA expression from the different LTRs, we normalized the ADA enzyme activity to the relative copy number in the targeted B-LCL cells. Southern analysis of the transduced B-LCL cells for retroviral and endogenous ADA sequences was performed as described for the producer cell analysis. Dividing the overall ADA activity by the relative proviral copy numbers allowed us to compare expression levels from vectors with different LTRs. Our results suggest that the MLV promoter (as indicated by LASN) and the SL3-3 promoter are less efficient than the MPSV promoter in this B-cell line. Thus, we conclude that both PG13 and PA317 retroviral vectors are capable of transducing human B-cell lines but that PG13/ADA(MPSV) provides the highest levels of total and per-integrant ADA expression in B cells.
Supernatant from each of the ADA producer clones was next used to transduce an HTLV-1-transformed human ADA− T-cell line (TJF-2). Consistent with the relative titers determined by dot blot, the ADA enzyme activity by TLC and Southern analysis for proviral copy number revealed a strong correlation between relative dot blot titer and the resulting total ADA activity in transduced T cells (Table 1). The only exception to this trend was the vector PG13/ADA(SL3-3), which gave a higher level of expression than expected based on the titer results. This is consistent with the finding that the SL3-3 promoter is more active in T cells than MLV or MPSV (6). Again, both PA317 and PG13 vectors were able to transduce the T-cell line at levels proportional to their relative titers.
Transduction into patient primary human ADA− T cells.
We next tested the ability of the new ADA vectors to transduce primary T cells from an ADA− SCID patient. PBMC isolated from the patient by apheresis were stimulated with OKT3 and rIL-2 for 4 days and then were transduced with equal volumes of supernatant from PA317/LASN, PG13/LASN, PG13/ADA(MPSV), and PG13/ADA(SL3-3). All transductions were done in duplicate. ADA enzyme activity and relative proviral copy number determinations (Fig. 3) were performed as described in Materials and Methods. The results indicate that all of the PG13 vectors outperformed the PA317/LASN vector for ADA expression in the ADA− T cells (Table 2). In these primary patient T cells, unlike TJF-2 cells, the MPSV vector gave significantly higher levels of overall expression and expression per integrant than the SL3-3 vector. The reason for this discrepancy is unclear, although it may be that MPSV yields higher levels of expression in primary T cells while SL3-3 yields higher levels of expression in this HTLV-1-transformed T-cell line. ADA enzyme activity in normal peripheral T cells ranges from 62 to 103 U.
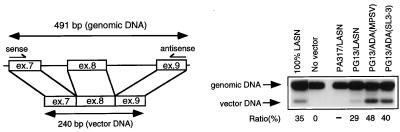
FIG. 3.
PCR analysis to evaluate the proviral copy number after retroviral transduction of PBMC from an ADA− SCID patient. PCR amplification with the sense and antisense primers indicated in the left panel yields two bands in cells containing proviral integrants: a 491-bp band from the endogenous ADA gene and a 240-bp band from the proviral integrant. The ratio values were determined by dividing the phosphoimager signal of the vector DNA band by that of the genomic DNA band. LASN-transduced, G418-selected HeLa cells (100% LASN) are used as the 100% transduced standard. ex, exon.
TABLE 2.
ADA enzyme activities in the transduced patient’s PBMC
| Vector | ADA enzyme activitya
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Avg | |
| None | 2 | 0 | 1 |
| PA317/LASN | 36 | 27 | 32 |
| PG13/LASN | 237 | 186 | 212 |
| PG13/ADA(MPSV) | 461 | 568 | 515 |
| PG13/ADA(SL3-3) | 80 | 79 | 80 |
ADA enzyme activity is expressed as nanomoles of inosine produced per minute per 108 cells.
Comparison of different packaging cell lines.
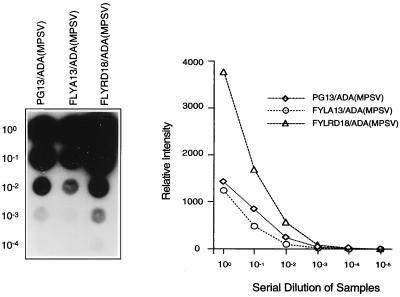
Having identified ADA(MPSV) as the best clone, we next used supernatant from this clone to transduce FLYA13 and FLYRD18 cells. Plating these cells in limiting dilution and screening the clones by RNA dot blot allowed us to identify the best clones for each producer cell line (Fig. 4). Equal volumes of supernatant from each clone were used to transduce TJF-2 cells, which were analyzed for ADA expression and relative proviral copy number 48 h later (Table 3). FLYRD18 cells showed the highest level of ADA activity in the transduced TJF-2 cells. This was consistent with Southern analysis of the transduced cells, which demonstrated that the FLYRD18 vector gave the highest relative proviral copy number (data not shown). Furthermore, all producer cell lines yielded similar levels of ADA enzyme activity per integrant since expression was driven by the same promoter (MPSV LTR) in all cases (experiment in Table 3).
FIG. 4.
Titer estimation by RNA dot blot assay of ADA(MPSV) clones in different packaging cell lines. The blot shows serial dilutions of supernatants from each producer cell clone and the graph depicts the phosphorimager intensity at each dilution.
TABLE 3.
ADA enzyme activities in the transduced TJF-2 cells
| Vector | ADA enzyme activitya
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3c | Avg | |
| Noneb | 4 | 4 | 3 | 4 |
| PG13/ADA(MPSV) | 138 | 100 | 98 (1.0) | 112 |
| FLYA13/ADA(MPSV) | 68 | 206 | 232 (2.0) | 167 |
| FLYRD18/ADA(MPSV) | 323 | 308 | 422 (4.8) | 351d |
ADA enzyme activity is expressed as nanomoles of inosine produced per minute per 108 cells.
Values in this row are measured ADA enzyme activities and proviral copy numbers.
Values in parentheses are average proviral copy numbers/cell.
P < 0.05 compared with the value for PG13/ADA(MPSV).
DISCUSSION
Gene therapy clinical trials using retroviral vectors have illuminated limitations with current retroviral vector technology. First, relatively low transduction frequencies, particularly in hematopoietic cells, continue to be problematic (7, 13). Second, immune responses against selectable markers of nonhuman origin which could lead to elimination of successfully targeted cells have been observed in some patients (25). In this study, we sought to ameliorate these problems by constructing retroviral vectors without dominant selectable markers and by testing these simplified vectors in different packaging cell lines.
Our goal was to design an ADA retroviral vector that was improved over the PA317/LASN vector currently used in clinical trials. To accomplish this, we eliminated the selectable marker gene, we tested different LTRs (MLV, MPSV, and SL3-3) for their abilities to express ADA, and we tested different packaging cell lines. This work supports the work of Riviere and colleagues (26) who generated simplified ADA vectors with a murine ecotropic host range for transduction into murine hematopoietic stem cells. Riviere’s studies demonstrated long-term marking and high levels of ADA expression from these simplified vectors. Our work extends these studies by generating simplified ADA vectors which are clinically applicable for human trials and by testing these vector supernatants in human ADA− lymphohematopoietic target cells. These simplified retroviral vectors should be less immunogenic since they lack a dominant selectable marker and may have the additional advantages of higher titers and higher levels of expression per integrant (14, 21).
Higher levels of expression over the first-generation vectors might also be achieved by providing a stronger promoter than the MLV LTR used in LASN. We made a series of simple retroviral vectors with ADA transcriptionally regulated by the MLV, MPSV, or SL3-3 LTR. MPSV is known to provide a high level of expression in progenitor type cells, including embryocarcinoma cells (9), while SL3-3 expresses well in T-cell lineages (6). These vectors were tested by transduction into ADA− hematopoietic cells and ADA enzyme activity, total and per integrant, was determined. Our results indicate that the MPSV promoter is clearly superior in human B-cell lines but that both SL3-3 and MPSV express well in human T cells. The MLV construct (LASN) gave the lowest level of ADA expression in both B and T cells. However, the LASN vector is not precisely analogous to the other simplified vectors tested, and expression might be influenced by factors other than the MLV LTR. The data confirms that MPSV vectors are good candidates for T-cell-directed gene therapy. Furthermore, since MPSV-based retroviral vectors provide expression in immature progenitor cell types, they may also be effective for gene therapy trials using hematopoietic stem cells.
Having identified the best vector, we wanted to determine which packaging cell line would provide the highest level of transduction into ADA− human lymphohematopoietic cells. We tested vectors pseudotyped with the murine amphotropic (4070A) envelope (FLYA13), the GALV (PG13) envelope, and the feline endogenous virus RD114 envelope (FLYRD18). Both FLY packaging cell lines have the additional advantage of producing retroviral vectors that are resistant to inactivation by human complement. Our results suggest that all three envelopes can mediate transduction into human T cells but that the FLYRD18-packaged viruses had the highest transduction efficiency.
Our initial goal to make an ADA retroviral vector that was an improvement over the PA317/LASN vector currently in use was realized. Our first advance was to eliminate the potential of an immune response against the bacterial protein neomycin phosphotransferase. The resulting simplified ADA vectors also had substantially higher titers than PA317/LASN. The simplified MPSV construct produced about a sevenfold-higher level of ADA expression than LASN in both B- and T-cell lines. In addition, MPSV showed a 17-fold-higher level of ADA expression in an ADA− SCID patient’s PBMC. Finally, by packaging the vector in FLYRD18, we made a vector which should be resistant to inactivation by human complement, with even better transduction into human ADA− T cells.
ACKNOWLEDGMENTS
Masdafumi Onodera and David M. Nelson contributed equally to this study.
We thank Linda Muul and Sherry Lau for reagents and technical advice.
REFERENCES
- 1.Blaese R M, Culver K W, Miller A D, Carter C S, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt J J, Rosenberg S A, Klein H, Berger M, Mullen C A, Ramsey W J, Muul L, Morgan R A, Anderson W F. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 2.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-vs-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 3.Bunnell B A, Muul L M, Donahue R E, Blaese R M, Morgan R A. High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1995;92:7739–7743. doi: 10.1073/pnas.92.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cone R D, Mulligan R C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci USA. 1984;81:6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couture L A, Mullen C A, Morgan R A. Retroviral vectors containing chimeric promoter/enhancer elements exhibit cell-type-specific gene expression. Hum Gene Ther. 1994;5:667–677. doi: 10.1089/hum.1994.5.6-667. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar C E, Cottler-Fox M, O’Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, Leitman S F, Wilson W H, Cowan K, Young N S, Nienhuis A W. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 8.Emerman M, Temin H M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984;39:459–467. [PubMed] [Google Scholar]
- 9.Franz T, Hilberg B, Seliger B, Stocking C, Ostertag W. Retroviral mutants efficiently expressed in embryonal carcinoma cells. Proc Natl Acad Sci USA. 1986;83:3292–3296. doi: 10.1073/pnas.83.10.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guild B C, Mulligan R C, Gros P, Housman D E. Retroviral transfer of a murine cDNA for multidrug resistance confers pleiotropic drug resistance to cells without prior drug selection. Proc Natl Acad Sci USA. 1988;85:1595–1599. doi: 10.1073/pnas.85.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hock R A, Miller A D, Osborne W R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989;74:876–881. [PubMed] [Google Scholar]
- 12.Kohn D B, Mitsuya H, Ballow M, Selegue J E, Barankiewicz J, Cohen A, Gelfand E, Anderson W F, Blaese R M. Establishment and characterization of adenosine deaminase-deficient human T cell lines. J Immunol. 1989;142:3971–3977. [PubMed] [Google Scholar]
- 13.Kohn D B, Weinberg K I, Nolta J A, Heiss L N, Lenarsky C, Crooks G M, Hanley M E, Annett G, Brooks J S, el-Khoureiy A, Lawrence K, Wells D, Moen R C, Bastian J, Williams-Herman D E, Elder M, Wara D, Bowen T, Hershfield M S, Mullen C A, Blaese R M, Parkman R. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krall W J, Skelton D C, Yu X J, Riviere I, Lehn P, Mulligan R C, Kohn D B. Increased levels of spliced RNA account for augmented expression from the MFG retroviral vector in hematopoietic cells. Gene Ther. 1996;3:37–48. [PubMed] [Google Scholar]
- 15.Marcel T, Grausz J. The TWC worldwide gene therapy enrollment report, end 1996. Hum Gene Ther. 1997;8:775–780. doi: 10.1089/hum.1997.8.6-775. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller A D, Law M F, Verma I M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985;5:431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan R C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 20a.Muul, L., and R. M. Blaese. Unpublished data.
- 21.Ohashi T, Boggs S, Robbins P, Bahnson A, Patrene K, Wei F S, Wei J F, Li J, Lucht L, Fei Y, Clark S, Kimak M, He H, Mowery-Rushton P, Barranger J A. Efficient transfer and sustained high expression of the human glucoceregrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc Natl Acad Sci USA. 1992;89:11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onodera M, Ariga T, Kawamura N, Kobayashi I, Otsu M, Yamada M, Tame A, Furuta H, Okano M, Matsumoto S, Kotani H, McGarrity G J, Blaese R M, Sakiyama Y. Successful peripheral T lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood. 1998;91:30–36. [PubMed] [Google Scholar]
- 23.Onodera M, Yachie A, Nelson D M, Welchlin H L, Morgan R A, Blaese R M. A simple and reliable method for screening retroviral producer cell clones without selectable markers. Hum Gene Ther. 1997;8:1189–1194. doi: 10.1089/hum.1997.8.10-1189. [DOI] [PubMed] [Google Scholar]
- 24.Pawliuk R, Kay R, Lansdorp P, Humphries R K. Selection of retrovirally transduced hematopoietic cells using CD24 as a marker of gene transfer. Blood. 1994;84:2868–2877. [PubMed] [Google Scholar]
- 25.Riddell S R, Elliott M, Lewinsohn D A, Gilbert M J, Wilson L, Manley S A, Lupton S D, Overell R W, Reynolds T C, Corey L, Greenberg P D. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. . (Comment.) [DOI] [PubMed] [Google Scholar]
- 26.Riviere I, Brose K, Mulligan R. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Van Beusechem V W, Valerio D. Gene transfer into hematopoietic stem cells of nonhuman primates. Hum Gene Ther. 1996;7:1649–1668. doi: 10.1089/hum.1996.7.14-1649. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Yee J K, Wolff J A, Friedman T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]