Abstract
Background
Human immunodeficiency virus (HIV) and iron deficiency (ID) affect many African children. Both HIV and iron status interact with gut microbiota composition and related biomarkers. The study's aim was to determine the associations of HIV and iron status with gut microbiota composition, gut inflammation and gut integrity in South African school‐age children.
Methods
In this two‐way factorial case–control study, 8‐ to 13‐year‐old children were enrolled into four groups based on their HIV and iron status: (1) With HIV (HIV+) and ID (n = 43), (2) HIV+ and iron‐sufficient nonanaemic (n = 41), (3) without HIV (HIV−) and ID (n = 44) and (4) HIV− and iron‐sufficient nonanaemic (n = 38). HIV+ children were virally suppressed (<50 HIV RNA copies/ml) on antiretroviral therapy (ART). Microbial composition of faecal samples (16S rRNA sequencing) and markers of gut inflammation (faecal calprotectin) and gut integrity (plasma intestinal fatty acid–binding protein [I‐FABP]) were assessed.
Results
Faecal calprotectin was higher in ID versus iron‐sufficient nonanaemic children (p = 0.007). I‐FABP did not significantly differ by HIV or iron status. ART‐treated HIV (redundancy analysis [RDA] R 2 = 0.009, p = 0.029) and age (RDA R 2 = 0.013 p = 0.004) explained the variance in the gut microbiota across the four groups. Probabilistic models showed that the relative abundance of the butyrate‐producing genera Anaerostipes and Anaerotruncus was lower in ID versus iron‐sufficient children. Fusicatenibacter was lower in HIV+ and in ID children versus their respective counterparts. The prevalence of the inflammation‐associated genus Megamonas was 42% higher in children with both HIV and ID versus HIV− and iron‐sufficient nonanaemic counterparts.
Conclusions
In our sample of 8‐ to 13‐year‐old virally suppressed HIV+ and HIV− children with or without ID, ID was associated with increased gut inflammation and changes in the relative abundance of specific microbiota. Moreover, in HIV+ children, ID had a cumulative effect that further shifted the gut microbiota to an unfavourable composition.
Keywords: gut microbiota, HIV, inflammation, iron deficiency, school‐age children, South Africa
Key points
-
1.
Iron deficiency (ID) was associated with higher gut inflammation measured by faecal calprotectin.
-
2.
Gut mucosal integrity measured by intestinal fatty acid–binding protein did not differ by human immunodeficiency virus (HIV) or iron status.
-
3.
In HIV+ children, ID had a cumulative effect that promoted an unfavourable gut microbiota composition.
Abbreviations
- 3TC
lamivudine; ABC abacavir
- AGP
α‐1‐acid glycoprotein
- ANCOVA
analysis of covariance
- ART
antiretroviral therapy
- ASV
Amplicon Sequence Variants
- ATV/r
atazanavir boosted with ritonavir
- AZT
zidovudine
- BRINDA
Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia
- CRP
C‐reactive protein
- EFV
efavirenz
- FTC
emtricitabine
- Hb
haemoglobin
- HDI
highest density intervals
- HIV
human immunodeficiency virus
- HIV−
living without HIV
- HIV+
living with HIV
- ID
iron deficiency
- I‐FABP
intestinal fatty acid–binding protein
- IQR
interquartile range
- LPV/r
lopinavir boosted with ritonavir
- NNRTI
non‐nucleoside reverse‐transcriptase inhibitor
- NRTI
nucleoside reverse‐transcriptase inhibitors
- NVP
nevirapine
- PCoA
principal coordinate analysis
- PF
plasma ferritin
- PI/r
ritonavir‐boosted protease inhibitor
- RDA
redundancy analysis
- SD
standard deviation
- sTfR
soluble transferrin receptor
- TDF
tenofovir
INTRODUCTION
Globally, ~37.7 million people are living with human immunodeficiency virus (HIV+), and two‐thirds of this population are from sub‐Saharan Africa. The number of HIV+ children <15 years is estimated at 1.7 million, of whom 310,000 (18%) are from South Africa. 1 In many African countries, an additional challenge is malnutrition, with 52% of African children estimated to be iron deficient. 2 Because HIV is associated with chronic systemic inflammation, 3 , 4 , 5 HIV+ children are more vulnerable to iron deficiency (ID) than children without HIV (HIV−). Systemic inflammation can down‐regulate iron absorption, adversely affect iron status and cause anaemia of inflammation. 6 This is especially detrimental in resource‐poor settings where iron intake from plant‐based diets is low and poorly bioavailable, which in turn causes nutritional anaemia. 7
The interplay between colonic iron availability and compositional changes in the gut microbiota has become a topic of interest, though more frequently discussed in the context of an iron surplus. However, in vitro colonic fermentation and animal studies have found that very low colonic iron conditions significantly alter gut bacteria composition and function, especially butyrate producers and short‐chain fatty acid metabolism. 8 , 9 A potential explanation is that enzymes in the butyrate production pathway are iron dependent. 10 It is unclear whether these findings are applicable to ID children, where complex human–microbiota interactions and fine regulation of iron absorption come into play.
The gut is also the compartment most persistently affected by HIV in the long term, despite viral suppression. Soon after HIV infection, the gut‐associated lymphoid tissue is severely depleted of CD4+ T cells, including T17 helper cells that are essential to maintain the gut mucosal barrier. 11 The early loss of CD4+ T cells marks the beginning of HIV‐associated enteropathy. 12 Although antiretroviral therapy (ART) suppresses HIV replication and reverses CD4+ T‐cell depletion in the peripheral blood compartment, its kinetics are slower in the gut mucosa. This leads to unsuccessful or partial replenishment of the gut environment. Consequences of HIV‐associated enteropathy include gut inflammation and increased intestinal permeability, despite ART. 13 A loss of gut mucosal integrity could facilitate microbial translocation, with chronic immune activation, poorer restoration of CD4+ T cells and disturbances of the host–microbiota homeostasis as potential consequences. 14 , 15 , 16
Studies suggest that ART alters gut microbiota composition to a bacterial community structure distinct from HIV−, as well as ART‐naive HIV+ reference groups. 16 , 17 In HIV+ populations, gut microbiota composition appears to shift towards higher proportions of proinflammatory and lower proportions of anti‐inflammatory bacterial species. 16 , 18 , 19 This state of dysbiosis promotes pathogenic and inflammatory pathways. 20 , 21
Current and future paediatric HIV populations will increasingly receive ART from early infancy. Thus, understanding the effects of prolonged ART exposure is becoming more important. Both HIV and iron status interact with gut inflammation, gut integrity and microbiota composition, yet to our knowledge, associations between iron status and gut health in the context of HIV have not been studied. Considering the high burden of HIV and ID in sub‐Saharan Africa, it is important to examine the relationship of both HIV and iron status with gut health, as well as the potential interactions between HIV and iron status. Therefore, the aim of this study was to determine the associations of HIV and iron status with gut microbiota composition, gut inflammation and gut integrity in South African school‐age children.
METHODS
Study design and participants
This two‐way factorial case–control study was performed within a cohort of 8‐ to 13‐year‐old HIV+ and HIV− children at the Family Centre for Research with Ubuntu in Cape Town, South Africa. 22 Children were eligible if they reported no recent acute illness, if they reported no iron supplementation use in the past 3 months and, in the HIV+ children, if they were virally suppressed (<50 HIV RNA copies/ml plasma) based on routine annual monitoring data obtained from the National Health Laboratory Service electronic portal. In HIV− children, the absence of HIV was confirmed using a rapid HIV assay (First Response HIV Card 1–2.0, Premier Medical Corporation Pvt Ltd). Children were excluded if severely underweight or obese (body‐mass‐index‐for‐age Z‐score <−3 or >2) 23 and severely anaemic (haemoglobin [Hb] <80 g/L). 7 Screening measurements included serum ferritin and Hb, and based on their HIV and iron status, 180 children were enrolled in the following four groups: (1) HIV+ and ID (n = 45), (2) HIV+ and iron‐sufficient nonanaemic (n = 45), (3) HIV− and ID (n = 45) and (4) HIV− and iron‐sufficient nonanaemic (n = 45). For enrolment purposes, ID was defined as inflammation‐unadjusted ferritin ≤40 µg/L, iron sufficiency as unadjusted ferritin >40 µg/L and the absence of anaemia as Hb ≥115 g/L.
For this present study, further exclusion criteria included (1) detectable HIV viral load, (2) antibiotic use in the 4 weeks prior to faecal sample collection, (3) probiotic use 1 week prior to faecal sample collection, (4) vegetarianism or veganism and (5) self‐reported gastrointestinal disorders. HIV viral load was measured (Roche COBAS AmpliPrep/TaqMan HIV‐1 Test, v2, Hoffmann‐La Roche, Basel, Switzerland), and six children were excluded because of viral loads ≥50 copies/ml. Three children were excluded because of antibiotic use. Of the remaining 171 children, 5 children provided an insufficient faecal sample for the necessary measurements, resulting in a final subsample of 166 children. The number of participants per group was (1) HIV+ and ID (n = 43), (2) HIV+ and iron‐sufficient nonanaemic (n = 41), (3) HIV− and ID (n = 44) and (4) HIV− and iron‐sufficient nonanaemic (n = 38). Based on the two‐way factorial design and assuming 80% power and a type I error rate of 5%, the final sample size allowed us to detect an effect size of 0.3 between groups.
Participant, socio‐demographic, anthropometric, dietary intake, anaemia, iron status and systemic inflammation indicators were collected with the using detailed methods previously described. 22 In brief, socio‐demographic and HIV information was obtained using a structured questionnaire, and weight and height were measured using a Micro 1023 electronic platform scale and stadiometer (Scalerite) and standardised techniques. 24 Habitual dietary intake information was collected using an abbreviated quantified food frequency questionnaire. The questionnaire was developed for the specific study population using a methodical multiphase approach with the using details previously described. 25 Hb concentrations were measured in whole blood using a Siemens Advia 2120i Haematology System (Siemens), and plasma ferritin (PF), soluble transferrin receptor (sTfR), C‐reactive protein (CRP) and α‐1‐acid glycoprotein (AGP) were measured using a multiplex immunoassay. 26 PF values were adjusted for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia (BRINDA) correction approach. 27 This approach uses linear regression to adjust PF using the CRP and AGP concentrations. Intestinal fatty acid–binding protein (I‐FABP) was measured using an ELISA (enzyme‐linked immunosorbent assay) immunoassay (Hycult Biotech).
Faecal sample collection and analyses
Faecal samples were collected at home the night or morning before the study visit. The children received a lined plastic container with a lid that sealed airtight and an OxoidTM AnaeroGenTM 2.5‐L Sachet (ThermoFisher Scientific Inc.) to generate an anaerobic environment in the container after sample deposit. A cooler bag, icepacks and illustrated instructions in their home language were provided in addition. On the day of the visit, stool samples were aliquoted and frozen at −70°C for the calprotectin and gut microbiota analyses. Faecal calprotectin was measured using an ELISA immunoassay (Eurospital). Elevated gut inflammation was classified as faecal calprotectin >200 μg/g. 28
DNA extraction, library preparations and sequencing
DNA extraction was performed using the MagAttract PowerMicrobiome DNA/RNA Kit (Qiagen). For microbiota analysis, the V4 region of the 16S rRNA gene was amplified with the primer pairs 515F and 806R (GTGYCAGCMGCCGCGGTAA and GGACTACNVGGGTWTCTAAT, respectively), modified to contain a barcode sequence between each primer and the Illumina adaptor sequences to produce dual‐barcoded libraries. Followed by size selection using Agencourt AMPure to remove fragments below 200 bases, 16S rRNA sequencing was performed on an Illumina MiSeq platform (MiSeq Reagent Kit v2, 500 cycles, 15.38% PhiX, 2 × 250 PE) at the VIB Nucleomics core laboratory (Leuven, Belgium). De‐multiplexing was performed using LotuS. This was followed by quality inspection; the removal of chimeras, primers and the first 10 bases following the primer; and the merging of paired sequences using DADA2 (v1.6). The resulting sequences were further grouped into Amplicon Sequence Variants (ASV). 29 Finally, taxonomy was assigned to all ASVs (using Ribosomal Database Project's trainset 16) and agglomerated to genus level.
Statistical analysis
Participant characteristics and gut health markers
Statistical analyses were performed using IBM SPSS Statistic software, version 27 (IBM Corp.). Normally distributed continuous variables were described using means and standard deviations (SD), non‐normally distributed variables with medians and interquartile ranges and categorical variables with frequencies and percentages. Non‐normally distributed outcome variables were log‐transformed prior to analysis. The characteristics of the four groups were compared using two‐way factorial analysis of variance for continuous variables and two‐way binary logistic regression for categorical variables. The associations of HIV and iron status with gut health markers were assessed using two‐way factorial analysis of covariance (ANCOVA) for continuous outcome variables and two‐way binary logistic regression for categorical outcome variables, adjusting for age, sex, ethnicity and deworming. If the ANCOVA or logistic regression did not show a significant interaction effect, it was repeated without the interaction factor. In the case of a significant interaction effect (observed only for a categorical outcome variable in this study), between‐group differences were analysed using a χ2 test with Bonferroni adjustment for multiple comparisons. Statistical significance was set at p < 0.05.
Gut microbiota
Statistical analyses were performed using R statistical software (http://www.r-project.org/). Genera with low prevalence (detected in less than 20% of the samples) were excluded from the analysis. The α‐diversity for each sample was calculated using the Shannon diversity index upon the rarefied abundances. A principal coordinate analysis (PCoA), using the Bray–Curtis distance, was carried out on 16S rRNA gene abundances after aggregating counts at the genus level (using DADA2). The Kruskal–Wallis test with post hoc Dunn's test (with fdr_bh to correct for multiple testing) was used to test median differences of α‐ and β‐diversity between groups. Enterotypes were obtained by combining 16S rRNA gene data from this sample with data from the Flemish Gut Flora Project 30 and applying an approach based on Dirichlet multinomial mixtures. 31
Redundancy analysis (RDA) was used to identify variables in the metadata that explained the variance in gut microbiota composition between the four groups. The independent effect size of significant variables on microbial composition was determined using the function capscale (using Euclidean distance on centred log‐ratio‐transformed abundance data), part of the vegan package, whereas the nonredundant effect was obtained combining the rda and ordiR2step from the same package. Statistical significance was set at a false discovery rate <0.1. To force a feature to be considered before others, this procedure is split into two steps: first, ordiR2step is run with a null model (without features) to a model with features that need to be accounted first (HIV status in this study – see ‘Results’ section). Next, ordiR2step is run again starting from the model with the forced features to the model with all features. Finally, the output from both runs is merged.
To assess the associations of HIV and iron status with various genera, a probabilistic model was used implemented in Python (version 3.10.5) with PyMC (version 4.0.0). 32 Here five models, based on negative binomial distributions, with various degrees of complexity, were used. The simplest model contains a single feature (p base) to model the number of reads found from a given genus in all samples which is used with a binomial likelihood. For more complex models, additional features (m hiv, m id and m interaction) that allow HIV and iron status to affect the binomial likelihood's probability p were included.
Given HIV and iron status are encoded as 0 or 1 (for absent and present, respectively), the models’ probabilities were defined as follows:
p = p base
p = p base + m hiv × HIV status
p = p base + m id × iron status
p = p base + m hiv × HIV status + m id × iron status
p = p base + m hiv × HIV status + m id × iron status + m interaction × (HIV status × iron status)
All five models were run on all genera using PyMC's No‐U‐Turn sampler 33 with 4000 samples, 2000 tuning steps and 4 chains. Noninformative priors were used for all features. For each genus, the simplest model with the best fit was selected by visual inspection of the model performance using the function plot_compare from ArViz (version 0.12.1). 34 Genera where either HIV or iron status was retained as a component of the model were considered for further analysis. Probability density functions and highest density intervals (HDI) for the models inspected in detail were generated using plot_trace and summary from the ArViz package.
To assess if a difference in the prevalence of participants with Megamonas better explained our observations for that genus than differences in abundance between participant groups, another model was used. In this model each of the four groups was assigned two weights w for participants with and without Megamonas using a Dirichlet distribution with a flat prior. Two possible binomial distributions, one with the probability of success set to zero (for participants lacking the genus) and the other with the success probability set to a variable p base (from a HalfNormal distribution with sigma 0.001), were combined with the weights w for each group and compared with the data using a mixture likelihood. Deterministic variables were set to extract the difference in prevalence of participants with Megamonas between HIV− and iron‐sufficient nonanaemic participants and participants from the other groups. Sampling was performed using the same settings as the previous analysis except for the parameter target_accept which was increased to 0.9.
All figures for this study were generated using Python 3.10.5 with Seaborn 0.11.2 and Matplotlib 3.5.1. Statsannotations 0.4.4 was used to include results from statistical tests in box plots.
RESULTS
Participant characteristics
Participant characteristics are summarised in Table 1. Compared with HIV− children, HIV+ children were from smaller households (p = 0.040) had lower height‐for‐age Z‐scores (p < 0.001), and higher sTfR levels (p = 0.001), CRP (p = 0.007) and AGP concentrations (p = 0.031). HIV+ children reported lower intake of animal protein (p < 0.001) and haem iron (p = 0.002) compared with HIV− children. Compared with iron‐sufficient nonanaemic children, more ID children received care from a single primary caregiver (p = 0.028). isiXhosa African children comprised 43% of the study population, and 57% of the children were of Capetonian mixed ancestry. The proportions of these two ethnic groups differed significantly between groups, with fewer isiXhosa African children in the HIV− iron‐sufficient nonanaemic group compared with the other three groups. Of all children, 96% had been dewormed in the past 6 months. In the HIV+ children, ritonavir‐boosted protease‐inhibitor‐based ART regimens were more common (71%) than non‐nucleoside reverse‐transcriptase inhibitor‐based regimens (29%).
Table 1.
Characteristics of the four groups of South African children enrolled based on HIV and iron status.
| HIV+ and ID | HIV+ and iron‐sufficient nonanaemic | HIV− and ID | HIV− and iron‐sufficient nonanaemic | p‐Values* | HIV × iron status | ||
|---|---|---|---|---|---|---|---|
| n = 43 | n = 41 | n = 44 | n = 38 | HIV | Iron status | ||
| Participant information | |||||||
| Age (y), median (IQR) | 11.6 (9.8–12.5) | 11.4 (10.8–12.4) | 11.2 (9.7–12.3) | 10.6 (9.5–12.2) | 0.06 | 0.94 | 0.23 |
| Male/female, n (%) | 20 (47)/23 (54) | 27 (66)/14 (34) | 21 (48)/23 (52) | 20 (53)/18 (47) | 0.46 | 0.12 | 0.34 |
| isiXhosa African/Capetonian mixed ancestry, n (%)† | 18 (42)/25 (58)a | 27 (66)/14 (34)a | 23 (52)/21 (48)a | 3 (8)/35 (92)b | 0.33 | 0.029 | <0.001 |
| Dewormed in the past 6 months, n (%) | 40 (93) | 40 (98) | 43 (98) | 37 (97) | 0.42 | 0.47 | 0.50 |
| HIV RNA (copies/ml) | <50 | <50 | – | – | – | – | – |
| Age at antiretroviral therapy start (y), median (IQR) | 1.0 (0.0–2.5) | 1.0 (0.0–1.0) | – | – | – | 0.94 | – |
| NNRTI‐based/PI/r‐based, n (%)‡ | 14 (33)/29 (67) | 10 (24)/30 (73) | – | – | – | 0.45 | – |
| Household information | |||||||
| Formal/informal housing, n (%)§ | 26 (61)/17 (40) | 23 (56)/18 (44) | 30 (68)/14 (32) | 26 (68)/12 (32) | 0.19 | 0.78 | 0.77 |
| Number of household members, median (IQR) | 5 (4–6) | 5 (4–6) | 6 (4–7) | 6 (5–7) | 0.040 | 0.47 | 1.00 |
| Primary caregiver single/in partnership, n (%) | 24 (56)/19 (44) | 16 (39)/25 (61) | 19 (43)/25 (57) | 10 (26)/28 (74) | 0.09 | 0.028 | 0.91 |
| Breadwinner unemployed, n (%) | 20 (47) | 17 (42) | 22 (50) | 10 (26) | 0.48 | 0.06 | 0.20 |
| Anthropometry | |||||||
| Height‐for‐age Z‐score, mean ± SD | −1.4 ± 1.0 | −1.1 ± 0.9 | −0.7 ± 1.0 | −0.4 ± 0.9 | <0.001 | 0.12 | 0.98 |
| Body‐mass‐index‐for‐age Z‐score, mean ± SD | −0.4 ± 1.0 | −0.4 ± 1.0 | −0.2 ± 1.2 | −0.1 ± 1.0 | 0.12 | 0.70 | 0.57 |
| Anaemia and iron status | |||||||
| Haemoglobin (g/L), mean ± SD | 119 ± 11 | 126 ± 8 | 121 ± 8 | 124 ± 8 | 0.74 | <0.001 | 0.23 |
| Plasma ferritin (adjusted for inflammation) (μg/L),‖ median (IQR) | 17 (13–27) | 38 (27–66) | 20 (17–26) | 35 (27–49) | 0.88 | <0.001 | 0.07 |
| Soluble transferrin receptor (mg/L), median (IQR) | 7.0 (6.0–8.9) | 7.0 (5.4–8.7) | 6.5 (5.8–7.2) | 6.1 (5.3–6.9) | 0.001 | 0.23 | 0.41 |
| Systemic inflammation | |||||||
| C‐reactive protein (mg/L), median (IQR) | 0.12 (0.02–1.14) | 0.04 (0.03–1.04) | 0.04 (0.02–0.35) | 0.04 (0.02–0.43) | 0.007 | 0.73 | 0.89 |
| α‐1‐acid glycoprotein (g/L), median (IQR) | 0.6 (0.5–0.8) | 0.6 (0.4–0.9) | 0.5 (0.4–0.8) | 0.5 (0.4–0.7) | 0.031 | 0.78 | 0.47 |
| Selected daily nutrient intake | |||||||
| Total protein (g), median (IQR) | 69 (53–76) | 62 (52–76) | 80 (62–98) | 81 (66–102) | 0.003 | 0.73 | 0.47 |
| Animal protein (g), median (IQR) | 32 (22–39) | 27 (21–37) | 40 (26–51) | 44 (34–64) | <0.001 | 0.55 | 0.15 |
| Plant protein (g), median (IQR) | 34 (29–45) | 35 (25–42) | 36 (30–45) | 34 (22–41) | 0.69 | 0.07 | 0.69 |
| Total iron (mg), median (IQR) | 16 (14–21) | 17 (14–21) | 18 (15–21) | 17 (13–22) | 0.84 | 0.19 | 0.92 |
| Haem iron (mg), median (IQR) | 2.3 (1.6–4.0) | 2.0 (1.5–3.4) | 3.3 (2.0–4.3) | 3.2 (2.5–5.1) | 0.002 | 1.00 | 0.19 |
| Nonhaem iron (mg), median (IQR) | 13 (12–18) | 14 (11–17) | 15 (12–17) | 12 (9–18) | 0.44 | 0.13 | 0.73 |
| Total fibre (g) median (IQR) | 25 (20–31) | 25 (19–30) | 28 (22–36) | 25 (18–36) | 0.32 | 0.12 | 0.90 |
Abbreviations: HIV, human immunodeficiency virus; ID, iron deficient; IQR, interquartile range; NNRTI, non‐nucleoside reverse‐transcriptase inhibitor; PI/r, ritonavir‐boosted protease inhibitor; SD, standard deviation.
Non‐normally distributed outcome variables were log‐transformed prior to analysis. Associations of the factors HIV and iron status with household and socio‐demographic characteristics were assessed using two‐way analysis of variance for continuous variables and two‐way logistic regression analysis for categorical variables.
Between‐group differences were analysed using a χ2 test with Bonferroni adjustment for multiple comparisons. Values in a row without a common letter (a, b) differ significantly (p < 0.05).
HIV+ and iron‐sufficient nonanaemic (n = 40), incomplete information. All regimens included two nucleoside reverse‐transcriptase inhibitors (NRTIs) in combination with either a non‐nucleoside reverse‐transcriptase inhibitor (NNRTI‐based) or a ritonavir‐boosted protease inhibitor (PI/r‐based). NRTIs included abacavir (ABC), lamivudine (3TC), zidovudine (AZT), tenofovir (TDF) and emtricitabine (FTC); NNRTIs included efavirenz (EFV) and nevirapine (NVP); and PIs included lopinavir boosted with ritonavir (LPV/r) and atazanavir boosted with ritonavir (ATV/r).
Formal housing represents a brick house, whereas informal housing represents a Wendy house or dwelling built with scrap building material and typically not equipped with water and/or electricity.
Plasma ferritin adjusted for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia correction approach. 27
Gut inflammation and gut integrity
Table 2 presents the measured gut health‐related biomarkers. Faecal calprotectin was significantly higher in ID children than in iron‐sufficient nonanaemic children (p = 0.007). I‐FABP did not significantly differ by HIV or iron status.
Table 2.
Gut inflammation and gut integrity.
| HIV+ and ID | HIV+ and iron‐sufficient nonanaemic | HIV− and ID | HIV− and iron‐sufficient nonanaemic | p‐Valuesa | |||
|---|---|---|---|---|---|---|---|
| n = 43 | n = 41 | n = 44 | n = 38 | HIV | Iron status | HIV × iron status | |
| Faecal calprotectin (µg/g) median (IQR)b | 22 (7–57) | 6 (3–18) | 20 (3–75) | 5 (1–50) | 0.85 | 0.006 | 0.77 |
| 50–200 µg/g, n (%) | 7 (17) | 4 (10) | 8 (18) | 7 (18) | 0.36 | 1.00 | 0.36 |
| >200 µg/g, n (%) | 4 (10) | 4 (10) | 5 (11) | 2 (5) | 0.99 | 0.50 | 0.45 |
| Intestinal fatty acid–binding protein (pg/ml), median (IQR) | 859 (482–1164) | 799 (366–1110) | 633 (457–1010) | 935 (603–1219) | 0.47 | 0.44 | 0.09 |
Abbreviations: HIV, human immunodeficiency virus; ID, iron deficient; IQR, interquartile range.
Non‐normally distributed outcome variables were log‐transformed prior to analysis. Associations of the factors HIV and iron status with gut health markers were assessed using two‐way analysis of covariance for continuous variables and two‐way logistic regression analysis for categorical variables, adjusting for age, sex, ethnicity and deworming.
Total study population n = 162, HIV+ and ID n = 42, HIV+ and iron‐sufficient nonanaemic n = 39 and HIV− and ID n = 43, because insufficient stool sample sizes were provided in four cases.
Gut microbiota composition
There was a high relative abundance of Prevotella in all four groups (Figure 1a). This was also observed during enterotyping, with 96.4% of all children presenting as the Prevotella enterotype and 3.6% as the Bacteroides 2 enterotype. The PCoA (Figure 1b) revealed that along the first axis there was little variation between the four groups. However, along the second axis there was a clear downward shift for HIV+ samples, with samples from ID children spread out more. There were no significant differences in α‐diversity between the four groups (p = 0.99) (Figure 1c). β‐diversity (inter‐individual differences) based on mean Bray–Curtis distances was significantly higher in HIV+ than in HIV− children (p < 0.001) (Figure 1d). Because all HIV+ children were on ART, the effects of HIV status and ART cannot be uncoupled in this analysis.
Figure 1.
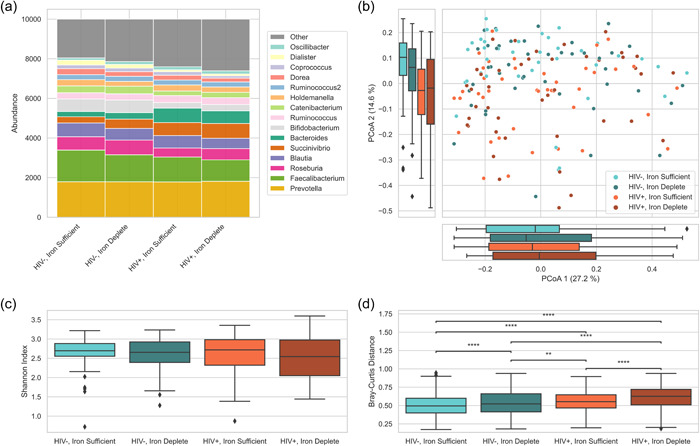
Relative abundance and diversity comparisons in virally suppressed HIV+ and HIV− children with sufficient iron stores (inflammation‐unadjusted plasma ferritin >40 µg/L) and insufficient iron stores (inflammation‐unadjusted plasma ferritin ≤40 µg/L). All children with sufficient iron stores were also nonanaemic (haemoglobin ≥115 g/L). (a) Relative abundance of faecal bacterial taxa at the genus level by HIV and iron status. Genera with low prevalence (detected in less than 20% of the samples) were excluded from the analysis. (b) Principal coordinate analysis of inter‐individual differences by Bray–Curtis dissimilarity. (c) Alpha diversity using the Shannon index in different groups; no significant difference was observed (Kruskal–Wallis). (d) Within‐group inter‐individual Bray–Curtis distance (β‐diversity); annotations above the box plots indicate significant difference between groups (Kruskal–Wallis followed by a post hoc Dunn's test with correction for multiple testing): **0.001 < p ≤ 0.01 and ****p ≤ 0.0001.
The RDA, which unveils features in the metadata that drive variation in the microbiota, revealed that from a total of 35 variables (Supporting Information, Supplementary Table 1a and b), age was the only microbiota covariate (RDA R 2 = 0.016, p = 0.008). However, age showed a borderline difference (p = 0.06) by HIV status in this sample and may therefore capture some of the same variation as ART‐treated HIV. HIV status, when forced for first consideration, was a significant factor from the metadata (RDA R 2 = 0.009, p = 0.029), and age remained a significant factor (RDA R 2 = 0.013, p = 0.004).
Using probabilistic models with various degrees of complexity (see ‘Methods’ section), we examined the associations of HIV and iron status with the gut microbiota. Table 3 presents the differences in relative abundance explained by HIV status only (with no iron‐status effect). In contrast with traditional statistical methods, probabilistic methods do not provide p‐values but aim to generate a distribution of plausible values for a given parameter of interest. These distributions can be summarised by the smallest interval that contains 94% of those plausible values, the highest density interval (HDI) along with the mean and SD of those values.
Table 3.
Genera with their relative abundance confidently linked with HIV status (without an effect of iron status) with their percentage change due to HIV status (mean and standard deviation from the probabilistic model) and the 94% highest density interval.
| Genus | Relative abundance in children with no HIV or ID (per 10,000 reads) | Relative increase or decrease in abundance when HIV+ (%) | Highest density interval |
|---|---|---|---|
| More abundant in HIV+ | |||
| Butyricimonas | 9.5 | 67 | 52–81 |
| Sutterella | 13.2 | 102 | 87–116 |
| Desulfomicrobium | 7.2 | 105 | 85–124 |
| Bacteroides | 309.7 | 112 | 109–115 |
| Alistipes | 42.7 | 130 | 121–139 |
| Clostridium_XlVb | 8.5 | 144 | 123–165 |
| Barnesiella | 14.3 | 150 | 134–167 |
| Desulfovibrio | 19.0 | 154 | 140–169 |
| Parabacteroides | 52.0 | 160 | 151–169 |
| Clostridium_XVIII | 16.2 | 180 | 163–197 |
| Bilophila | 4.5 | 202 | 168–237 |
| Phascolarctobacterium | 12.8 | 236 | 213–258 |
| Subdoligranulum | 1.5 | 253 | 189–324 |
| Odoribacter | 8.4 | 263 | 233–295 |
| Fusobacterium | 0.6 | 10,993 | 8108–14,058 |
| Less abundant in HIV+ | |||
| Turicibacter | 25.6 | 81 | 80–83 |
| Romboutsia | 37.3 | 71 | 69–73 |
| Intestinibacter | 18.2 | 63 | 60–67 |
| Clostridium_sensu_stricto | 58.0 | 57 | 55–59 |
| Terrisporobacter | 7.4 | 56 | 50–62 |
| Olsenella | 55.0 | 56 | 54–58 |
| Dialister | 208.2 | 55 | 54–56 |
| Bifidobacterium | 615.9 | 49 | 48–50 |
| Roseburia | 694.1 | 25 | 24–26 |
| Dorea | 264.2 | 17 | 16–19 |
| Faecalibacterium | 1437.8 | 16 | 16–17 |
Models convincingly show that the relative abundances of Anaerostipes (Figure 2a) and Anaerotruncus (Figure 2b) shift by iron status but not by HIV status. The relative abundance of Anaerostipes and Anaerotruncus was 45% ± 1 (HDI 43–47) and 56% ± 2 (HDI 52–60) lower, respectively, in children with ID. Fusicatenibacter (Figure 2c) differed by HIV status and by iron status but with no interaction effect. Fusicatenibacter was 29% ± 1 (HDI 27–30) and 35% ± 1 (HDI 34–37) lower in children with HIV and in children with ID, respectively. There was an HIV × iron status interaction effect for Megamonas (Figure 2d). Given the low prevalence of Megamonas, an alternative model was tested. This model assumes a constant abundance across the different groups; however, the prevalence of people carrying Megamonas can differ between groups. This was a better fit for our data than the previous model, and although we cannot confidently say that Megamonas prevalence is affected by HIV or iron status alone, the prevalence was 42% ± 9 (HDI 25%–59%) higher in children with both HIV and ID than in HIV− and iron‐sufficient nonanaemic counterparts.
Figure 2.
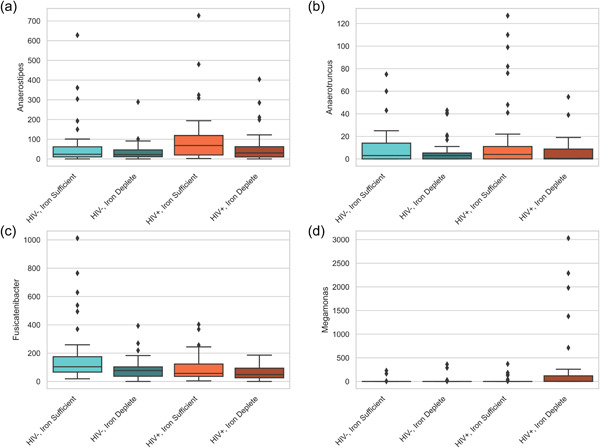
Example of four genera's relative abundance for which the probabilistic models confidently found a shift in abundance based on HIV and/or iron status. (a, b) Anaerostipes and Anaerotruncus are associated with iron status, (c) Fusicatenibacter is associated with both HIV and iron status independently and (d) Megamonas is the only genus where an HIV × iron status interaction effect was noted. The prevalence of Megamonas was higher in children with both HIV and ID versus HIV− and iron‐sufficient nonanaemic counterparts.
DISCUSSION
Our aim was to better comprehend the associations of HIV and iron status with the gut health of 8‐ to 13‐year‐old South African children. We found that gut mucosal integrity and gut microbial α‐diversity measures did not significantly differ by HIV or iron status. ID was associated with higher gut inflammation, and both HIV and ID were associated with higher gut microbial β‐diversity. ART‐treated HIV and age explained the variance in gut microbiota composition across the four groups. In the HIV+ children, the relative abundance of 15 microbial genera was higher and that of 12 microbial genera was lower than in the HIV− children. In ID children, the relative abundance of Anaerostipes, Anaerotruncus and Fusicatenibacter was lower than in the iron‐sufficient nonanaemic children. There was an HIV × iron status interaction effect for Megamonas, and the prevalence‐based model confidently demonstrated that the prevalence of Megamonas was higher in children with both HIV and ID compared with HIV− and iron‐sufficient nonanaemic counterparts.
Encouragingly, we found no indication of gut mucosal barrier disruption according to plasma I‐FABP concentrations. Several studies have reported a loss of gut integrity in HIV+ individuals of varying age groups and support the chain of gastrointestinal events after HIV acquisition. 12 , 35 , 36 However, I‐FABP is not consistently elevated in children and adults across studies. Our finding of no difference in I‐FABP by HIV status is consistent with previous studies in African adults 37 and infants. 38
Although increased faecal calprotectin was only suggestive of subclinical gut inflammation, the disparity between ID and iron‐sufficient children was significant, and ID, but not HIV, was associated with an increased faecal calprotectin. Previous studies have observed a positive association between faecal calprotectin and dysbiosis in the context of gastrointestinal diseases. 39 , 40 In our study, both HIV and ID contributed to dysbiosis (discussed further), and therefore, we expected an increase in faecal calprotectin in both HIV+ and ID children. Possibly, the mild gut inflammation observed was not a consequence of ID but rather a contributor to the depleted iron stores in ID children by impairing dietary iron absorption.
The gut microbiota diversity indices suggest that bacterial richness and evenness were similar between the HIV+ and HIV− children and independent of iron status. Previously, Abange et al. reported a lower Shannon index in Cameroonian HIV+ children and adolescents compared with HIV− counterparts, 41 whereas this was not the case in a Zimbabwean study by Flygel et al. 42 However, the latter group reported a decrease in α‐diversity in HIV+ children based on other diversity indices. Although these two studies included children and adolescents on ART, a substantial proportion were not virally suppressed. The participants either initiated treatment during study enrolment, or their time spent on ART was not sufficient to achieve viral suppression. Furthermore, one of these studies implemented a higher threshold for viral suppression (1000 HIV RNA copies/ml) 42 than ours (50 HIV RNA copies/ml). Nowak et al. reported a positive correlation between gut microbial diversity and CD4+ T‐cell count, which is restored when viral load is suppressed. 43 The comparable bacterial richness between HIV+ and HIV− children in our study may be the result of successful viral suppression, as observed in previous studies. 44 , 45
On the contrary, we detected a higher β‐diversity (dissimilarity between participants) in HIV+ than HIV− children, consistent with several paediatric and adult studies. 21 , 41 , 42 , 43 Within both the HIV+ and HIV− groups, ID children displayed significantly higher β‐diversity, suggesting that both HIV and ID can disrupt the microbiota and increase the variation within the composition. Thus, HIV and ID may additively contribute to dysbiosis.
Although Prevotella was previously associated with HIV, 46 , 47 our observation of a high relative abundance of Prevotella was not limited to HIV+ but observed in all four groups. Another recent study in young Capetonian children also reported a high relative abundance of Prevotella. 48 This was not surprising as Prevotella can be enriched in individuals from nonindustrialised countries whose diets are high in fibre and low in protein. 49
The RDA revealed that ART‐treated HIV and age were the only significant factors for explaining the variance in gut microbiota across the four groups. This was also confirmed by more significant differences in the relative abundances of bacterial genera across the four groups by HIV status than by iron status (27 versus 4 genera). HIV and ART are known modulators of the gut microbiota. 19 , 50 HIV‐related variation in gut microbial profiles differ by viraemia 51 , 52 , 53 as well as by ART regimen. 50 , 54 Compared with recent HIV infection, an HIV‐specific gut microbiota signature depleted of Akkermansia, Anaerovibrio, Bifidobacterium and Clostridium appears to develop over time, becoming evident on long‐term ART. 55 Of these genera and compared with HIV− counterparts, lower relative abundances of Bifidobacteria and Clostridium were noted in our sample of virally suppressed HIV+ children on ART. An interesting observation by HIV status was the steep increase in the relative abundance of Fusobacterium in the HIV+ children versus very low abundances in the HIV− children in our sample. Fusobacterium is a pathogenic bacterium with virulence factors that could trigger gut inflammation and disease. 56 An increase in the relative abundance of Fusobacterium was previously associated with suboptimal immune recovery and functioning despite ART. 57
In vitro and animal studies have highlighted the adverse effects of low colonic iron availability on butyrate‐producing bacteria and short‐chain fatty acid metabolism. 8 , 9 In our sample, the relative abundance of both Anaerostipes and Anaerotruncus was lower in ID children compared with iron‐sufficient nonanaemic counterparts. Anaerostipes and Anaerotruncus are butyrate‐producing probiotic bacteria strongly and positively correlated with Hb and serum ferritin in rats. 58 The short‐chain fatty acid butyrate has anti‐inflammatory effects and is beneficial to intestinal health. 59 Similar to our findings, a previous study among children with inflammatory bowel disease reported a lower abundance of butyrate‐producing microbiota when faecal calprotectin was elevated. 39 Furthermore, our observation of a lower abundance of Fusicatenibacter in HIV+ as well as in ID children may also relate to gut inflammation, as a decrease in Fusicatenibacter has been associated with ulcerative colitis 60 and Crohn's diseases, 61 both chronic inflammatory diseases of the gut.
An HIV × iron status interaction effect was observed only for the genus Megamonas, with a higher prevalence of Megamonas in children with both HIV and ID than without HIV and ID. Although an increase in the relative abundance of Megamonas in ART‐treated HIV+ adults has been reported, 62 literature associating Megamonas with ID is scarce. A study in women with gestational anaemia reported gut microbial enrichment of Megamonas. 63 In HIV+ adults either on ART or ART naive, Megamonas was correlated significantly with interleukin‐6, a systemic inflammatory cytokine. 64 In our study, HIV was significantly associated with increases in both CRP and AGP concentrations. Although the levels of inflammation were low, if considered together with the iron status–associated increase in gut inflammation, and HIV and iron status–associated changes in gut microbiota composition, our findings support an interplay between HIV, iron status, gut health and systemic inflammation.
Our study had several strengths. We investigated the relationship of two factors, HIV and iron status, with gut health. By using probabilistic models adapted specifically for this study design, interpretable results for the specific research questions could be obtained. Furthermore, the partially pooled models allow maximum information to be used from each participant's sample, reducing uncertainty of the results. A limitation of this study is that it was observational, and therefore, conclusions of causality or temporality cannot be drawn.
In conclusion, in 8‐ to 13‐year‐old virally suppressed HIV+ and HIV− children with or without ID, ID was associated with increased gut inflammation and changes in the relative abundance of specific microbiota. Moreover, in HIV+ children, ID had a cumulative effect that further shifted the gut microbiota to an unfavourable composition. Preventing ID in HIV+ children may benefit gut health, and supporting optimal iron status with context‐appropriate interventions should be prioritised.
AUTHOR CONTRIBUTIONS
Charlene Goosen designed the study and conducted the research. Charlene Goosen, Kashish Mallick and Jeannine Baumgartner analysed the data (other than the microbiota data). Sebastian Proost performed the microbiota analysis and visualisation. Raul Y. Tito processed 16S sequencing data. Charlene Goosen and Kashish Mallick prepared the original draft of the paper. Shaun L. Barnabas, Mark F. Cotton and Michael B. Zimmermann provided study resources. Jeroen Raes supervised the microbiota analysis and provided study resources. Renée Blaauw supervised the research study and provided study resources. All authors reviewed the paper and read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/jhn.13171.
ETHICAL APPROVAL
The study was approved by the health research ethics committees of ETH Zurich (EK 2018‐N‐40) and Stellenbosch University (M18/05/017 and S18/06/136).
TRANSPARENCY DECLARATION
The lead authors affirm that this manuscript is an honest, accurate and transparent account of the study being reported. The reporting of this work is compliant with STROBE guidelines. The lead authors affirm that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained. The study cohort was from a trial registered at clinicaltrials.gov as NCT03572010.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
We thank the study participants and their caregivers; the clinical, laboratory and support staff from the Family Centre for Research with Ubuntu, the Division of Human Nutrition at Stellenbosch University and the Infectious Diseases Outpatient Unit at Tygerberg Hospital; C. Brand (Stellenbosch University, South Africa), C. Verspecht, L. Rymenans and L. De Commer (VIB and KU Leuven, Belgium); N. Mikulic (ETH Zurich, Switzerland) and J. Erhardt (Willstaett, Germany) for supporting sample processing and laboratory analyses; and R. Laubscher (SAMRC Biostatistics Unit) for nutrient conversions. This research study used a cohort of children that was funded by the Thrasher Research Fund, USA (14199). The Raes lab is supported by KU Leuven, VIB and the Rega Institute. Raul Y. Tito is funded by a postdoctoral fellowship from the Research Foundation Flanders (FWO‐Vlaanderen, grant number: 1234321N). Charlene Goosen is supported by the L'Oréal‐UNESCO for Women in Science Sub‐Saharan Africa Fellowship Programme, the Harry Crossley Foundation and the Ernst and Ethel Eriksen Trust. This work was also made possible through funding from the South African Medical Research Council (SAMRC) through its Division of Research Capacity Development under the SAMRC Bongani Mayosi National Health Scholars Programme with funding received from the South African National Treasury. The content here is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Biographies
Charlene Goosen is a registered dietitian (SA) and researcher at Stellenbosch University. After a career in HIV policy development and support, she obtained her PhD in nutritional sciences. Her research is focused on paediatric HIV co‐morbidities and nutrition, in particular iron deficiency and oral iron interventions.
Sebastian Proost is a senior postdoctoral fellow at KU Leuven and the VIB. Since the start of his PhD in 2007 he has leveraged analysis of (large‐scale) data to further our understanding of biology. Currently, his research is focused on the interplay between the gut microbiome and the host's health.
Jeannine Baumgartner is a lecturer in nutritional sciences at King's College London and was previously senior scientist at ETH Zurich with a PhD in human nutrition.
Kashish Mallick is a researcher at ETH Zurich and a master of health science and technology graduate.
Raul Y. Tito is a molecular biologist at KU Leuven with a PhD in bioinformatics/metagenomics.
Shaun L. Barnabas is a paediatric infectious diseases specialist at Stellenbosch University with a PhD in immunology.
Mark F. Cotton is a paediatric infectious diseases specialist and emeritus professor at Stellenbosch University.
Michael B. Zimmermann is a medical doctor and full professor at ETH Zurich.
Jeroen Raes is co‐director of the VIB‐KU Leuven Center for Microbiology and full professor at KU Leuven.
Renée Blaauw is a registered dietitian (SA) and full professor at Stellenbosch University.
Goosen C, Proost S, Baumgartner J, Mallick K, Tito RY, Barnabas SL, et al. Associations of HIV and iron status with gut microbiota composition, gut inflammation and gut integrity in South African school‐age children: A two‐way factorial case–control study. J Hum Nutr Diet. 2023;36:819–832. 10.1111/jhn.13171
Charlene Goosen and Sebastian Proost have contributed equally to this study.
Contributor Information
Charlene Goosen, Email: charleneg@sun.ac.za.
Jeroen Raes, Email: jeroen.raes@kuleuven.be.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Joint United Nations Programme on HIV/AIDS . UNAIDS data 2021. https://www.unaids.org/en/resources/documents/2021/2021_unaids_data (2021). Accessed 28 Nov 2022.
- 2. Muriuki JM, Mentzer AJ, Webb EL, Morovat A, Kimita W, Ndungu FM, et al. Estimating the burden of iron deficiency among African children. BMC Med. 2020;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV‐related anemia in 2013. Hematology Am Soc Hematol Educ Program. 2013;2013:377–81. [DOI] [PubMed] [Google Scholar]
- 4. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148–57. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization . Nutritional anaemias: tools for effective prevention and control. Geneva: WHO. https://www.who.int/nutrition/publications/micronutrients/anaemias-tools-prevention-control/en/ (2017). Accessed 28 Nov 2022.
- 8. Dostal A, Fehlbaum S, Chassard C, Zimmermann MB, Lacroix C. Low iron availability in continuous in vitro colonic fermentations induces strong dysbiosis of the child gut microbial consortium and a decrease in main metabolites. FEMS Microbiol Ecol. 2013;83:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, et al. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2012;142:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, et al. Iron modulates butyrate production by a child gut microbiota in vitro. mBio. 2015;6:e01453–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of mucosal CD103+ DCs and IL‐17+ and IL‐22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirajlal‐Fargo S, El‐Kamari V, Weiner L, Shan L, Sattar A, Kulkarni M, et al. Altered intestinal permeability and fungal translocation in Ugandan children with Human Immunodeficiency Virus. Clin Infect Dis. 2020;70:2413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crakes KR, Jiang G. Gut microbiome alterations during HIV/SIV infection: implications for HIV cure. Front Microbiol. 2019;10:e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral‐treated HIV infection. J Infect Dis. 2009;199:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trøseid M, Manner IW, Pedersen KK, Haissman JM, Kvale D, Nielsen SD. Microbial translocation and cardiometabolic risk factors in HIV infection. AIDS Res Hum Retroviruses. 2014;30:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV‐induced alteration in gut microbiota. Gut Microbes. 2014;5:562–70. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen QT, Ishizaki A, Bi X, Matsuda K, Nguyen LV, Pham HV, et al. Alterations in children's sub‐dominant gut microbiota by HIV infection and anti‐retroviral therapy. PLoS One. 2021;16:e0258226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, et al. Alterations in the gut microbiota associated with HIV‐1 infection. Cell Host Microbe. 2013;14:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vujkovic‐Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vázquez‐Castellanos JF, Serrano‐Villar S, Latorre A, Artacho A, Ferrús ML, Madrid N, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV‐infected individuals. Mucosal Immunol. 2015;8:760–72. [DOI] [PubMed] [Google Scholar]
- 22. Goosen C, Baumgartner J, Mikulic N, Barnabas SL, Cotton MF, Zimmermann MB, et al. Examining associations of HIV and iron status with nutritional and inflammatory status, anemia and dietary intake in South African schoolchildren. Nutrients. 2021;13:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Onis M, et al. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . Training course on child growth assessment. Geneva: WHO. https://www.who.int/publications/i/item/9789241595070 (2008). Accessed 28 Nov 2022.
- 25. Goosen C, Blaauw R. The development of a quantified food frequency questionnaire for assessing iron nutrition in schoolchildren from resource‐limited settings in Cape Town, South Africa. Ecol Food Nutr. 2021;60:707–21. [DOI] [PubMed] [Google Scholar]
- 26. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. J Nutr. 2004;134:3127–32. [DOI] [PubMed] [Google Scholar]
- 27. Namaste SM, Ou J, Williams AM, Young MF, Yu EX, Suchdev PS. Adjusting iron and vitamin A status in settings of inflammation: a sensitivity analysis of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) approach. Am J Clin Nutr. 2020;112:458S–467S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2017;13:53–6. [PMC free article] [PubMed] [Google Scholar]
- 29. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falony G, Joossens M, Vieira‐Silva S, Wang J, Darzi Y, Faust K, et al. Population‐level analysis of gut microbiome variation. Science. 2016;352:560–4. [DOI] [PubMed] [Google Scholar]
- 31. Holmes I, Harris K, Quince C. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS One. 2012;7:e30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salvatier J, Wiecki TV, Fonnesbeck C. Probabilistic programming in Python using PyMC3. PeerJ Computer Science. 2016;2:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffman MD, Gelman A. The No‐U‐Turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res. 2014;15:1593–623. [Google Scholar]
- 34. Kumar R, Carroll C, Hartikainen A, Martin O. ArviZ a unified library for exploratory analysis of Bayesian models in Python. J Open Source Softw. 2019;4:1143. [Google Scholar]
- 35. Isnard S, Ramendra R, Dupuy FP, Lin J, Fombuena B, Kokinov N, et al. Plasma levels of C‐type lectin REG3α and gut damage in people with human immunodeficiency virus. J Infect Dis. 2020;221:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prendergast AJ, Chasekwa B, Rukobo S, Govha M, Mutasa K, Ntozini R, et al. Intestinal damage and inflammatory biomarkers in Human Immunodeficiency Virus (HIV)‐exposed and HIV‐infected Zimbabwean infants. J Infect Dis. 2017;216:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olwenyi OA, Naluyima P, Cham F, Quinn TC, Serwadda D, Sewankambo NK, et al. Brief report: differential associations of interleukin 6 and intestinal fatty acid‐binding protein with progressive untreated HIV‐1 infection in Rakai, Uganda. J Acquir Immune Defic Syndr. 2016;72:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koay WLA, Lindsey JC, Uprety P, Bwakura‐Dangarembizi M, Weinberg A, Levin MJ, et al. Intestinal integrity biomarkers in early antiretroviral‐treated perinatally HIV‐1‐infected infants. J Infect Dis. 2018;218:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolho KL, Korpela K, Jaakkola T, Pichai MVA, Zoetendal EG, Salonen A, et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015;110:921–30. [DOI] [PubMed] [Google Scholar]
- 40. Klingberg E, Magnusson MK, Strid H, Deminger A, Ståhl A, Sundin J, et al. A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin. Arthritis Res Ther. 2019;21:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abange WB, Martin C, Nanfack AJ, Yatchou LG, Nusbacher N, Nguedia CA, et al. Alteration of the gut fecal microbiome in children living with HIV on antiretroviral therapy in Yaounde, Cameroon. Sci Rep. 2021;11:7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flygel TT, Sovershaeva E, Claassen‐Weitz S, Hjerde E, Mwaikono KS, Odland JØ, et al. Composition of gut microbiota of children and adolescents with perinatal Human Immunodeficiency Virus infection taking antiretroviral therapy in Zimbabwe. J Infect Dis. 2020;221:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV‐1 infection. AIDS. 2015;29:2409–18. [DOI] [PubMed] [Google Scholar]
- 44. Ji Y, Zhang F, Zhang R, Shen Y, Liu L, Wang J, et al. Changes in intestinal microbiota in HIV‐1‐infected subjects following cART initiation: influence of CD4+ T cell count. Emerg Microbes Infect. 2018;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villanueva‐Millán MJ, Pérez‐Matute P, Recio‐Fernández E, Lezana Rosales JM, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV‐infected patients. J Int AIDS Soc. 2017;20:21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bandera A, De Benedetto I, Bozzi G, Gori A. Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Curr Opin HIV AIDS. 2018;13:73–80. [DOI] [PubMed] [Google Scholar]
- 47. Kaur US, Shet A, Rajnala N, Gopalan BP, Moar P, D H, et al. High abundance of genus Prevotella in the gut of perinatally HIV‐infected children is associated with IP‐10 levels despite therapy. Sci Rep. 2018;8:17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nel Van Zyl K, Whitelaw AC, Hesseling AC, Seddon JA, Demers AM, Newton‐Foot M. Association between clinical and environmental factors and the gut microbiota profiles in young South African children. Sci Rep. 2021;11:15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Goffau MC, Jallow AT, Sanyang C, Prentice AM, Meagher N, Price DJ, et al. Gut microbiomes from Gambian infants reveal the development of a non‐industrialized Prevotella‐based trophic network. Nat Microbiol. 2022;7:132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinto‐Cardoso S, Klatt NR, Reyes‐Terán G. Impact of antiretroviral drugs on the microbiome: unknown answers to important questions. Curr Opin HIV AIDS. 2018;13:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou Y, Ou Z, Tang X, Zhou Y, Xu H, Wang X, et al. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med. 2018;22:2263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cook RR, Fulcher JA, Tobin NH, Li F, Lee D, Javanbakht M, et al. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. AIDS. 2019;33:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serrano‐Villar S, Vázquez‐Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando‐Martínez S, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV‐infected subjects. Mucosal Immunol. 2017;10:1279–93. [DOI] [PubMed] [Google Scholar]
- 54. Pinto‐Cardoso S, Lozupone C, Briceño O, Alva‐Hernández S, Téllez N, Adriana A, et al. Fecal Bacterial Communities in treated HIV infected individuals on two antiretroviral regimens. Sci Rep. 2017;7:43741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rocafort M, Noguera‐Julian M, Rivera J, Pastor L, Guillén Y, Langhorst J, et al. Evolution of the gut microbiome following acute HIV‐1 infection. Microbiome. 2019;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ohkusa T, Yoshida T, Sato N, Watanabe S, Tajiri H, Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J Med Microbiol. 2009;58:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee SC, Chua LL, Yap SH, Khang TF, Leng CY, Raja Azwa RI, et al. Enrichment of gut‐derived Fusobacterium is associated with suboptimal immune recovery in HIV‐infected individuals. Sci Rep. 2018;8:14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. He H, Teng H, Huang Q, He D, An F, Chen L, et al. Beneficial effects of AOS‐iron supplementation on intestinal structure and microbiota in IDA rats. Food Sci Hum Wellness. 2021;10:23–31. [Google Scholar]
- 59. Meijer K, De Vos P, Priebe MG. Butyrate and other short‐chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–21. [DOI] [PubMed] [Google Scholar]
- 60. Gryaznova MV, Solodskikh SA, Panevina AV, Syromyatnikov MY, Dvoretskaya YD, Sviridova TN, et al. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon. 2021;7:e06432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qiu X, Zhao X, Cui X, Mao X, Tang N, Jiao C, et al. Characterization of fungal and bacterial dysbiosis in young adult Chinese patients with Crohn's disease. Therap Adv Gastroenterol. 2020;13:1756284820971202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie Y, Sun J, Wei L, Jiang H, Hu C, Yang J, et al. Altered gut microbiota correlate with different immune responses to HAART in HIV‐infected individuals. BMC Microbiol. 2021;21:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wei H, Deng S, Qin Y, Yang X, Chen T, Wang X, et al. Insight into the potential value of gut microbial signatures for prediction of gestational anemia. Front Cell Infect Microbiol. 2021;11:734561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ling Z, Jin C, Xie T, Cheng Y, Li L, Wu N. Alterations in the fecal microbiota of patients with HIV‐1 infection: an observational study in a Chinese population. Sci Rep. 2016;6:30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


